Abstract
Generally accepted methods for processing postmortem brains are lacking despite the efforts of pioneers in the field, and the growing awareness of the importance of brain banking for investigating the pathogenesis of illnesses unique to humans. Standardizing methods require compromises, institutional or departmental mindset promoting collaboration, and willingness for sharing ideas, information, and samples. A sound balance between competition and institutional interests is needed to best fulfill the tasks entrusted to health care institutions. Thus, a potentially widely accepted protocol design involves tradeoffs.
We successfully integrated brain banking within the operation of the department of pathology. We reached a consensus whereby a brain can be utilized for diagnosis, research, and teaching. Thus, routing brains away from residency programs is avoided. The best diagnostic categorization possible is being secured and the yield of samples for research maximized.
Thorough technical details pertaining to the actual processing of brains donated for research was recently published. Briefly, one-half of each brain is immersed in formalin for performing the neuropathologic evaluation, which is combined with the teaching task. The contralateral half is extensively dissected at the fresh state to obtain samples ready for immediate disbursement once categorized diagnostically. The samples are tracked electronically, which is crucial. This important tracking system is described separately.
This report focuses on key lessons learned over the past 25 years of brain banking including successful solutions to originally unforeseen problems.
Keywords: Brain Banking, Human Tissue, Methods, Neuropathology, Organization
Introduction
Brain banking must be accomplished expertly, and therefore, professionalized. Universally accepted guidelines are needed. Poorly prepared or inadequately categorized samples that may have been disbursed in the belief that they were genuinely eligible for specific studies may, in fact, not have been representative of the disease of interest. The ripple effects of such deeds on further, related studies could be disastrous. The time and endeavor investigators dedicate to projects is precious. The overall costs of investigations using human postmortem brain are enormous. This can be mostly avoided if appropriate methods of processing human brains donated for research are applied to optimize the tissue quality and diagnostic specificity of the samples provided to investigators.
Over the past 25 years we kept improving a protocol for processing human postmortem brains for research. We recently published the thorough description as to how these brains are actually processed successfully at the New York Brain Bank (NYBB). The currently applied protocol is fully integrated within the operation of a department of pathology.
The quintessence of this protocol is to perform a thorough, itemized dissection, and concomitant evaluation of one half of each brain donated for research from donors who have been clinically well characterized. The dissection occurs as soon as possible after death. Numerous samples whose anatomical origin is precisely recorded are being frozen at −180° C using liquid nitrogen vapor (LNV). The fresh frozen samples are electronically tracked (see related article in this issue of the journal). These samples are ready for immediate disbursement once categorized neuropathologically.
In the following section we briefly review the genesis of an efficient, successful, standardized protocol of brain banking. The important issue of organizing and tracking samples banked is addressed separately in this issue of the journal. This report deals with important lessons we learnt over the past 25 years during which we kept improving brain banking. A selection of practicable solutions to initially unexpected, but worsening problems are addressed.
Lessons of the past: The need for standardized, though flexible methods of brain banking
Professionalization of brain banks
Optimizing the quality and diagnostic categorization of brain samples donated for research is labor intensive and is crucial to generate reliable data on neurologic or psychiatric ailments. In addition, managing the inputs and outputs of diagnostically categorized brain samples made available to neuroscientists is complex. Thus, individuals involved in brain banking must have a good command of the protocol for processing brains, of the neuroanatomy, and pathology, including organizational and communicative skills.
Brain banking is now accepted as being a necessity to effectively make progress in the attempts to unveil the pathogenesis of a wide range of neurological diseases. However, it is costly. Therefore, dedicated financial and personnel commitments are key prerequisites for establishing a successful operation. An important helpful precondition is that the brain banks interact with pathology departments as much as possible even to the point of integration. This integration eases access to brains donated for research, minimizes postmortem intervals, and facilitates the combination of the findings of the general autopsy with those of the neuropathological examination. The specificity of diagnostic categorization is improved. The combined findings become part of the pathology report, thus improving the clinicopathologic correlation. From an operational point of view, the integration of the brain bank with pathology department reduces the costs of brain banking. Indeed, to some extent it promotes sharing hardware and manpower required for performing the neuropathological evaluation and for processing the tissue required for the microscopical examination and diagnostic categorization. As in our center, the neuropathological examination of brains destined to research is typically more extensive and systematic than usual, and the integration is in turn qualitatively beneficial to the autopsy service. Above all, it prevents that brain banking becomes detrimental to the teaching assignments of academic institutions by routing brains away from residency programs given the ongoing decline of autopsy rate.
Basic methodological issues with latent entanglements
The prosector processing the fresh brain must be well trained. Methodological deficits at the onset of processing fresh brains for research may have dire consequences. Indeed, the fresh brains, or fresh cut surfaces of the slabs, or blocks (depending whether the half brain prepared for research is being sectioned, and whether subsequently blocks are being harvested) must be carefully examined during the processing to rule out the presence of unexpected changes, which may no longer be discernable or identifiable once the freezing took place. The results obtained from inadequately prepared or categorized samples may be misleading and contribute to the increasing concern that in cutting edge research, false findings may be the majority of published research claims [Ioannidis, 2005].
Especially challenging is comparing data yielded by brain samples sharing the same diagnostic category, but which were obtained from different donors. Important variables to be considered while assessing the eligibility of a brain to be processed for research are those that pertain to the clinical status, including the premortem conditions of the donors [Monfort, et al., 1985]. Qualitative determinants include the lack of universally accepted norms that are relevant to the eligibility of donors, the processing of fresh brains, and the diagnostic categorization. These norms vary from center to center, and even within a center, depending on the prosectors involved and their level of training. Unsettled are confounding factors that encompass the variety of protocols, or distortion of a protocol applied for processing brains at the fresh state. Likewise challenging is the lack of methodological standards for the neuropathological evaluation. The threshold of detection of abnormal aggregates greatly depends on the techniques applied. The traditional silver impregnation of the tissue sections is capricious [Dickson, et al., 1996]. Immunohistochemistry techniques are more reliable than silver impregnation techniques, but their results depend in part on how antigen retrieval is performed, on the source of specific antibodies used, and on the neuropathologist, who evaluates the sections [Alafuzoff, et al., 2008, Cairns, et al., 2007, Dickson, et al., 1996].
Ground rule to manage the records of type, extent, and severity of the changes identified neuropathologically should be implemented. Upon reviewing autopsy reports of 189 patients affected with Creutzfeldt-Jakob disease (CJD) Brown and al. claimed that “the diverse formats of hospital neurohistopathology reports prevented a precise tabulation of the topographical distribution and extent of severity of the different lesions in the entire case series”.[Brown, et al., 1994]. Our neuropathology report lists the itemized parts of the brains twice symmetrically: 1) for recording the findings made on gross examination, and 2) for recording the microscopical findings. There is no need to read the entire report to find out details recorded on either gross or microscopical examination, or both, which pertain to any part of the brain.
These selected examples of variables, which in particular pertain to the methods of processing and diagnostic categorization of brain samples for research, and to record keeping must be addressed. In so doing, we were able to optimize the yield of data, and to facilitate sorting out, and accessing among more than 100,000 samples in storage, those that best match the requirements of specific investigations. We reduced the risk of misinterpreting the data obtained from the samples banked. We enhanced the validity of the data comparisons among ranges of findings gained from samples that originated from different brains carrying the same diagnosis, either at similar, or at different stages of the pathologic process; and with data gained from control samples.
Fresh frozen brain samples
The methods whereby fresh brains or parts are being frozen are diverse, which further complicates the interpretation of data and comparison of results [Gsell, et al., 1993, Duyckaerts, et al., 1993, Newcombe, et al., 1993, Hulette, et al., 1997, Hulette, 2003, Schmitt, et al., 2007, Vonsattel, et al., 1995, Grinberg, et al., 2007, Vonsattel, et al., 2008].
The techniques applied for freezing fresh brains include either placing a whole brain or half of it within a freezer, or placing it on dry ice, and then storing it in a −80° C freezer. Therefore, currently banked, thus in storage, are either whole brains or half brains that were frozen en bloc. Obviously, the neuropathologic assessment of a whole brain frozen en bloc is curtailed, which is no longer compatible with the current standards of research (see below). In other instances, the whole brain or one half brain is sectioned fresh, and the slabs frozen, and stored. There are instances in which to fulfill a request, “frozen hemispheres (−80° C) are thawed to −10° C to −5° C and dissected on a cooled metal plate (−20° C)…” [Gsell, et al., 1993]. Alternatively, once the fresh slabs are obtained, blocks are harvested, and then frozen with or without the severed slabs using either dry ice or nitrogen-cooled liquid isopentane. These methods of freezing brains often yield samples with erratic artifacts due to the formation of ice crystals within cells, and within the neuropil. Freezing artifacts are typically prevented or minimal when fresh blocks are immersed in nitrogen cooled liquid isopentane. However, if the blocks are larger than 2.5 – 3.0 cm they tend to fragment.
Whole or half brains frozen en bloc, or frozen slabs, which are eligible as source specimens for fulfilling specific requests are repeatedly subjected to a wide range of harmful temperature fluctuations. Indeed, to harvest the requested parts from such source samples, they must be momentary kept outside the cabinet freezer, and warmed-up to allow the relevant dissection, and then put back into the freezer. The overall operation is time consuming. Because of the cumbersomeness of storing and retrieving half brains frozen en bloc, or frozen coronal slabs, and because of the difficulty of harvesting the areas of interest out of them, we no longer use these methods. Instead, as mentioned, many itemized blocks are now obtained at the fresh state, precisely identified anatomically and diagnostically.
Storage of frozen brain samples
The organization of the freezer space is crucial to keep track of the samples that are available. The compartmentalization of the freezer cabinets is a function of the combined volume and shape of the parts in storage.
The wider the range of the volumes and shapes of the frozen samples in storage the more difficult is the design of the compartmentalization of the freezer cabinets, which in turn, complicates keeping track of the site, where each one of them is supposed to be. Often the organization of the storage of frozen samples is inadequate at the cost the freezer space with subsequent waste of energy. Thus, accessing target source brains for retrieving the parts of interest are delayed or occasionally even in vain. Freezers must be kept open during the attempts to locate the source brains, which causes temperature fluctuations throughout the freezer cabinet. Parallelly the efficiency of the operation decreases, while labor and the risk of mistakes increase. These shortcomings are no longer compatible with scientific-based ethics and code-of-conduct expected practice. How we successfully met the challenge of the important issue of tracking each sample in storage is detailed separately in this issue of the journal (Keller et al. 2008).
Terminological shortcomings
Causing additional intricacy is the usage of vague, technical, terms or anatomical identifiers, e.g., “cerebral hemisphere” instead of “half brain”; “frontal lobe” instead of referring to a specific Brodmann area; or “basal ganglia” in lieu of “striatum”, or “neostriatum”, or “globus pallidus”. For example, proceeding with the dissection of the mesencephalon according to the following, published description may not yield the expected outcome: “Then the brainstem with the cerebellum is cut at the level of the midbrain 0.5 cm above the substantia nigra…” In fact, the rostral tip of the pars compacta of the substantia nigra, which is not visible on external examination, extends into the caudal diencephalon. Thus, a cut performed 0.5 cm above it, would pass near the midpoint along the dorso-ventral axis of the thalamus. Furthermore, the plane of section that should be used is not specified. The next example emphasizes the need of using well defined anatomical terms: “Samples from the basal ganglia, thalamus, hippocampus, nucleus accumbens are prepared…” which implies that the nucleus accumbens is not part of the basal ganglia [Schmitt, et al., 2007]. The term “basal ganglia” lacks universally accepted definition; therefore, one should provide a definition pertaining to the particular publication.
Misuse of anatomical terms leads to sorting and shipping banked samples to investigators, which were found in retrospect not to be suited for the study for which the request was originally placed. To minimize the risks of misunderstanding between requestors of samples and the brain bank, we developed an interactive program for placing a request, which guides the investigators. More than 95 percent of requests placed electronically can be fulfilled without seeking additional clarifications directly or per phone.
Brains harvested outside the institution and uninitiated prosectors
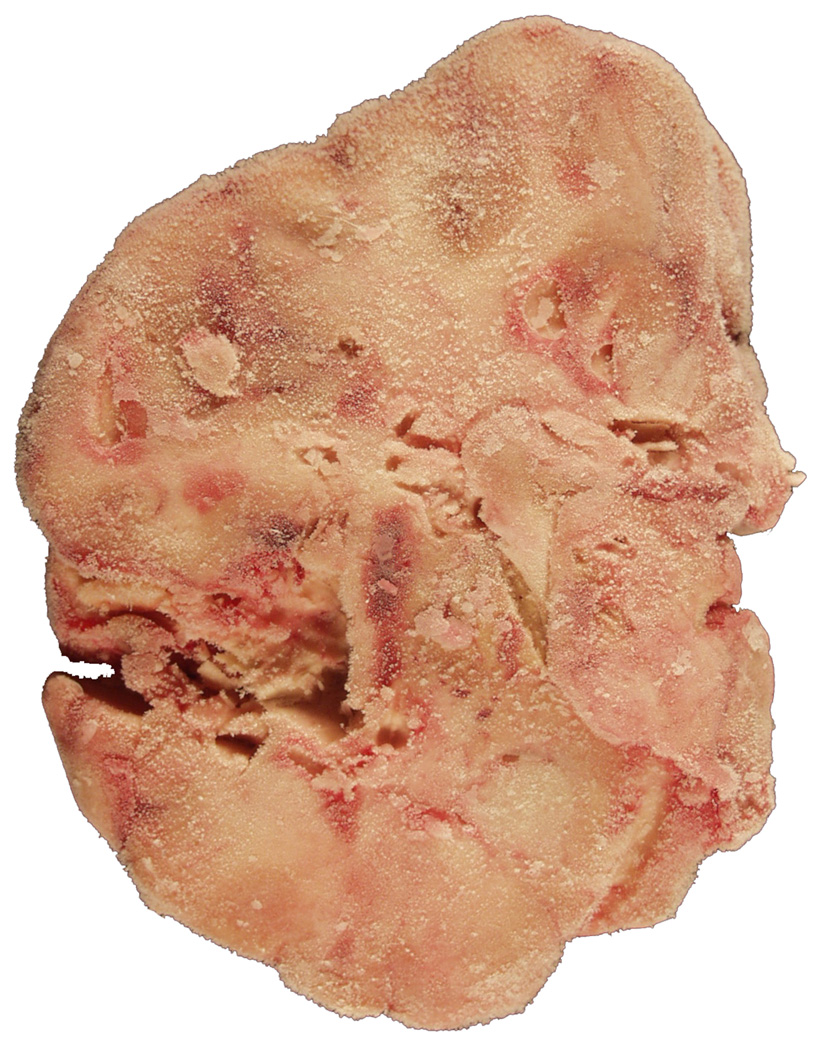
A subset of brain donors, especially those with neurodegenerative diseases, die in nursing homes, thus their brains may have to be harvested outside the institution harboring the bank suite. Our experience repeatedly showed that the results are disappointing when brains harvested outside the institution are partially processed in situ, that is at the site where the autopsy took place (Fig. 1a, b, c, d). Thus, this practice was abandoned.
Fig 1.

Results are often disappointing when brains are harvested outside the institution harboring the brain bank facilities, and when they are partially processed at the site of the autopsy by untrained prosectors (a – c). Uninitiated prosectors cut fresh brains regardless of their anatomical landmarks. The internal structures of the coronal slab (b) are no longer identifiable. Therefore, the presence or absence of gross lesions cannot be ruled out. Even a single requirement of splitting the brain sagittaly through the corpus callosum followed by the freezing of one half resulted in severe distortion (c). Anatomically and pathologically unidentifiable frozen part of a brain wrapped with aluminum foil (d).
If the body cannot be transported to the autopsy suite affiliated to the brain bank, a local pathologist harvests the brain as soon as possible after death. Then the intact, fresh brain is tightly double bagged, placed in a pail containing wet-ice and water, and immediately shipped to the NYBB. This tradeoff secures and optimizes the standardization of the processing (Fig. 2a, b, c, d).
Fig 2.
Example of a brain harvested in Kansas City by a local pathologist. The intact, fresh brain was inserted within tightly closed plastic bags, placed on wet ice, and then shipped to the NYBB, where it was photographed, at the time of its reception (a). The left half was banked (b). The coronal slabs obtained are free of distortion. The striatum and amygdala (c) or the thalamus and mammillothalamic tract are distinct (d). On gross examination, a thorough and reliable evaluation can be performed.
In fact, unless the prosector is familiar with both the neuroanatomy and the protocol, the dissection and subsequent freezing yields samples of relative reliability, which are often poorly identifiable (Fig. 1a, b). Uninitiated prosectors frequently cut the fresh brain regardless of anatomical landmarks. Therefore, thickness of the slabs, or the plane of the cuts of either coronal, or horizontal slabs, is not determined by anatomical features. The cut surfaces of the slabs tend to be irregular to the point that the internal structures are no longer identifiable (Fig. 1b). Furthermore, an uninitiated prosector is more likely to miss unexpected changes than a prosector trained in neuropathology. As mentioned, these changes may no longer be detectable once the slabs are frozen and often distorted, which raises the issue of safety and risks of contamination of unsuspected infectious diseases to whomever will use the samples especially when sharps are utilized.
A significant difference in detection of abnormalities in 100 brains was documented depending on whether the doctor had had formal postgraduate training in pathology. Furthermore, the difference was greater when the evaluation was done with fresh than with formalin-fixed brains [Katelaris, et al., 1994]. Practice clearly established that the probability of the occurrence of unexpected pathologic changes involving brains is proportional to the age of the donors at the time of death. This outcome is likely to worsen given the increasing life expectancy of donors. This trend coupled to the ever evolving, qualitative requirements of modern brain banking calls for thorough evaluation of the brain by well-trained prosectors with a solid background in neuropathology. These requirements must be fully operative while processing the fresh parts or fresh half brain, and during the neuropathological evaluation of the fixed parts or contralateral fixed half brain.
Risks secondary to curtailed neuropathological evaluation of brains banked
The criteria applied for the diagnostic categorization of the brains banked are variable. In some instances, notably when brains are frozen en bloc, the clinical diagnosis alone is used to determine whether a brain is eligible for a study relevant to a definite disease category. In other instances, the evaluation of the tissue is confined to the gross examination of the external surface of the brain or, in addition, of the cut surfaces of the slabs, and this with or without clinico-pathological correlation. Banking brains without or with minimal parts set aside for microscopic evaluation, which may be motivated by the discrepancy between availability and demand of samples from specific diagnoses, should not happen. Assessing a diagnostic categorization of each brain utilized for basic investigations is crucial. That brains are being obtained and kept for research and categorized diagnostically solely on the basis of clinical criteria, thus without or only with limited neuropathological evaluation, is no longer acceptable. Indeed, neurological disorders other than CJD of a cohort of 300 individuals with proven prion disease included multiple sclerosis, viral encephalitis, acute vascular accident, neoplasms, alcoholic abuse, olivopontocerebellar atrophy, Ramsay Hunt syndrome, presenile dementia, Alzheimer disease, and schizophrenia [Brown, et al., 1994]. CJD and Alzheimer disease share pathogenic, clinical, and postmortem findings, which further emphasizes the importance of performing accurate, neuropathological evaluations of brains donated for research [Hainfellner, et al., 1998, Leuba, et al., 2000]. Thorough neuropathological examination revealed that twenty-four among 100 patients clinically diagnosed as having Parkinson disease (PD) were misdiagnosed intra vitam [Hughes, et al., 1992]. Postmortem examinations and molecular analyses of 15 patients diagnosed with Gerstmann-Sträussler-Scheinker syndrome (GSS) included three patients who carried the clinical diagnoses of olivopontocerebellar atrophy, two patients that of Alzheimer disease, and one patient that of familial PD [Piccardo, et al., 1998]. Clinicopathological discrepancies of unsuspected, additional findings were discovered in 12 percent of brains collected for research over a 22-month period [Vonsattel, et al., 1995].
In summary, consensual, procedural, and diagnostic guidelines are needed and would greatly improve the services brain banks can offer to the neuroscientists and to the community.
New York Brain Bank – Taub Institute: Implementation of selected, practical solutions to initially unsuspected, gradually worsening problems of brain banking
In 2001, the Taub Institute at Columbia University provided the opportunity to construct a brain bank free of the framework of an ongoing operation (New York Brain Bank [NYBB] – www.nybrainbank.org - Taub Institute, Columbia University). Thus, we developed and are applying an efficient protocol for brain banking. The operation is integrated within the department of pathology, which strengthens the existing programs, widens connections to clinical programs, and fosters integration with the basic neuroscience research activities [Vonsattel, et al., 2008].
The protocol is the consequence of the implementation of a set of solutions to initially unanticipated problems, which were gradually identified over 25 years of undertaking in the field; and of suggestions made by investigators using the services of the brain bank. Indeed, while performing within an ongoing system, only a subset of solutions to gradually emerging problems related to the natural growth of the operation could be carried out. Impediments were mainly due to the logistics linked to the modus operandi whereby the samples that were frozen had wide range of sizes and shapes, which determined the subsequent organization of the freezer space for their storage. The samples consisted either of brains frozen en bloc, or half brains frozen en bloc, or frozen slabs, or blocks. The challenge of tracking the increasing number of frozen samples became barely manageable. Harvesting blocks of definite sizes at the fresh state and then tracking then electronically using a bar cod labeling solved the problem beyond our expectation. Detailed on the tracking system developed and used at the NYBB are provided elsewhere in this issue of the journal (Keller et al, 2008).
Collaboration between neuropathology department and brain bank: a critical condition
Early methodological flaws in brain banking were due in part to the enactment of improvised mechanisms impinging upon the operation of clinical neuropathology. The ensuing, nascent, physical organization of brain banks and, at that time, applicable methods were primeval, and idiosyncratic. Nonetheless, reaching an agreement was essential to figure out to which extent the tasks of brain banking could blend in those of the neuropathology department without jeopardizing the quality of the services to patients, clinicians, and to training programs.
In the beginning, the awareness of the significance of brain banking raised the question whether it was ethical to curtail the neuropathological evaluation since one part would be frozen, hence not evaluated as thoroughly as usual. Traditionally the entire brain had to be immersed in formalin during two to three weeks for fixation, and then methodically investigated to reach an accurate postmortem diagnosis. These methods nurtured clinicopathological correlations, which revealed fundamental data on the circuitry and functions, and morbidity of the central nervous system [Rebeiz, et al., 1968, Trétiakoff, 1919, Alzheimer, 1911, Bertrand, et al., 1925, Davison, 1941, Aström, et al., 1958, Kiesselbach, 1914, Blocq, et al., 1893]. The methods gained universal acceptance. They deeply molded the mindset of neuropathologists and the expectations of clinicians. Understandably efforts to implement the necessary innovations interfering with the steadfast and fruitful diagnostic paradigm of combined clinical, macroscopic, and microscopic observations met resistance. Nonetheless, the methods had to be updated so that emerging techniques could be applied in attempts to unveil brain diseases by using fresh, frozen samples. Unfortunately, the traditional fixation of brain (e.g., 10% buffered formalin phosphate solution) prevents most biochemical studies. Biochemical or molecular biology techniques using fresh frozen brain samples are essential, and now widely used in neuroscience research programs.
Repeated, well documented observations gradually confirmed that in neurodegeneration the morbid process involving one half brain faithfully mimics that involving the contralateral half, although with variable severity, as observed for example in corticobasal degeneration or in PD. Nonetheless, these observations convinced a subset of neuropathologists to set aside part of, or even one half brain for research. The impulse for such alterations of the traditional ways of evaluating the postmortem brains came in part from endocrinologists, neurologists, and biochemists, who emphasized the need and importance of the availability of fresh, frozen brain samples obtained from clinically well categorized patients [Tourtellotte, et al., 1993, Bird, et al., 1993, Ehringer, et al., 1998]. But, the startling discovery of the role of dopamine in PD was crucial in changing the neuropathologists’ frame of mind towards brain banking [Ehringer, et al., 1998, Birkmayer, et al., 1998, Hornykiewicz, 2004]. This favorable trend deeply influenced the scientific community, which was later reinforced with the advent of the importance of molecular biology in neurodegenerative diseases or metabolic dysfunction of the central nervous system [Swaab, et al., 1992, van de Nes, et al., 1993, van Zwieten, et al., 1991]. The incentives kept fostering the awareness and significance of brain donation for research. In 1992 and 1993, Ravid et al published seminal reports providing crucial methodological guidelines that deeply influenced the development and acceptance of brain banking, and brain donation for research [Ravid, et al., 1992, Ravid, et al., 1993].
The consensus to be reached to properly process fresh brains at the time of its harvest from the body geared toward fulfilling three aims: 1) to reach the best diagnostic categorization possible; 2) to obtain optimally prepared fresh frozen samples suitable for biochemical, molecular, or proteomic studies; and 3) to teach the neuroanatomy and neuropathology to the next generations of physicians and neuroscientists.
Steady accommodations of brain banking were driven by the challenges of fulfilling requests of samples. However, gradually the implementation of improving methodological measures could not be ideally fulfilled without disrupting the original operating construct of the brain bank, which became increasingly complex. Furthermore, changes were contingent upon the variable compliance of neuropathology units through which brains were and are channeled.
With the accruement of brain donation, a major growing challenge we faced, was mainly due to the range of the parts and sizes of brains that were frozen and stored in freezers. A subsequent issue of concern was how to track the samples within the freezer cabinets, and this according to a set of variables including diagnostic categorization, extend of pathological changes, age, gender; and how to match these variables independently with the ones pertaining to a specific research project.
With the accumulation of requests of samples from investigators, the mechanisms for disbursing the specified specimens had to be adjusted. The harvest of the requested parts from a pool of eligible source brains that were frozen en bloc, or from coronal slices, is labor intensive, time consuming, and often harmful to the tissue itself. Indeed, carving specific areas from a half brain frozen en bloc is extremely difficult and of questionable accuracy. Warming-up the frozen source-brains to ease the harvest of the samples of interest is deleterious. The practice of sawing the frozen brain to access the site of interest was excluded because the heat generated by the saw blade caused focal liquefaction of the parenchyma with subsequent contamination and alterations of the tissue around the margins of the cuts and beyond. The complexity of harvesting selected parts of interest from brain frozen en bloc lengthened the delay required for fulfilling the increasing number of requests of tissues. The record of the leftover was by itself complex and often vague. Eventually, this cumbersome practice, and the concerns about the accuracy and integrity of the samples obtained were incentive to design a new protocol. The latest version of this protocol was published recently [Vonsattel, et al., 2008]. In short, the brunt of the dissection is performed extensively at the fresh state and rigorously according to precise anatomical landmarks. The yield consists of discrete, frozen blocks, or pulverized aliquots of brain parenchyma contained in 1.8 ml vials, stored in freezers, ready for immediate disbursement. The delay for fulfilling requests of fresh frozen brain samples available is reduced from two months to five working days.
The New York Brain Bank as integral part of the department of pathology of the Presbyterian Hospital, Columbia University: practical issues
The advantage of our brain bank to be part of the department of pathology is manifold, as alluded to before.
That the autopsy suite is near the facility of the brain bank greatly promotes productive interactions between these two complementary components, which are involved in harvesting and processing brains donated for research. The accretion of samples for research is fostered. The administrative clearance for harvesting brains to be banked is facilitated, thus the clerical inertia is being virtually abolished. The postmortem interval is shortened. The ideal infrastructure, personal, and equipment are available to deal with the removal of brains. Appropriate measures can be immediately applied if unexpected findings upon the removal of brains or upon the processing of the full autopsy occur.
The integration eliminates the duplication of certain tasks that are common to the two parties. For example, not replicated for the diagnostic categorization are the following steps: processing of formalin phosphate fixed and paraffin embedded samples, staining of slides for general survey, and processing of slides subjected to specific antibodies.
One recalls, that one half brain of each one donated for research is formalin fixed and kept for performing a thorough neuropathological examination. To secure consistency, only a professional neuropathologist who has good command of the protocol and of the anatomy performs the examination. However, the examination is carried out in the presence of residents and fellows in training. Thus, the donation and subsequent preparation of a brain donated for research does not deprive physicians or neuroscientists in training of the opportunity of participating to the macroscopic and microscopic evaluations of a wide range of neuropathological changes. This is an important outcome of our protocol given the steady decrease in the rate of autopsies. Brain donation for research should not occur at the detriment of the teaching mission of academic institutions.
Conclusions
The gist of our protocol, which integrates practical solutions to a flow of challenges that emerged during 25 years of operation, is to harvest the brain as soon as possible after death, and to dissect one half of it precisely and extensively at the fresh state (Fig. 3). The samples harvested are carefully evaluated grossly, then immediately frozen using liquid nitrogen vapor (Fig. 3b, c, d). The anatomical origin, e.g., the Brodmann area for cortical samples, is one important identifier used. Therefore, to faithfully match the Brodmann areas with the blocks obtained, the prosector must have a good command of the neuroanatomy and neuropathology. As mentioned, assessing the fresh brain and the fresh cut surfaces of the slabs is more challenging than assessing fixed brains or fixed slabs [Katelaris, et al., 1994]. Therefore, expertise in neuropathology is necessary when processing the fresh half brain.
Fig 3.




Coronal slab of a seven month-old baby girl diagnosed with spino-muscular atrophy (a). A block including the lenticular nucleus with insula, and a block including the thalamus were obtained from (a), and then frozen at – 180 ° C (b). Two transverse blocks of the mesencephalon are shown fresh (c), and frozen (d). Note the absence of pigment of the pars compacta of the substantia nigra, as normally expected at that age.
The frozen samples are electronically tracked, and can be disbursed immediately once a diagnosis is assigned to the source brain, the fresh half of which is evaluated during the processing, and the contralateral half thoroughly examined neuropathologically in the presence of trainees.
The yield of fresh frozen samples ranges between 66 and 150 or more per half brain. The number of samples banked per brain depends on the inventory, and on the initial impression of the quality of the tissue. The number of samples in storage is continually monitored electronically.
The integration of the brain bank with the department of (neuro-) pathology offers reciprocal scientific and operational advantages, and lowers functional costs.
The methods and protocol developed and applied at New York Brain Bank – Taub Institute of Columbia University are efficient. The mean of the monthly number of samples disbursed inside and outside the USA is up to 500. Samples available for research are made available to investigators within five working days upon the receipt of the request.
Acknowledgements
This work was supported by grants from the National Institutes of Health and National Institute on Aging: P01-AG07232, R37-AG15473, and P50-AG08702, the Hereditary Disease Foundation, the Iseman Fund, and the Rudin Fund.
The authors are grateful to Mkeba Cason for her help.
The New York Brain Bank of the Taub Institute of the Columbia University is especially thankful to the numerous pathologists who referred case material, and to the families of the patients for providing brain tissue for research.
Bibliography
- Alafuzoff I, Parkkinen L, Al-Sarraf S, et al. Assessment of α-synuclein pathology: A study of the BrainNet Europe Consortium. Journal of Neuropathology and Experimental Neurology. 2008;67:125–143. doi: 10.1097/nen.0b013e3181633526. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Über eigenartige Krankheitsfälle des späteren Alters. Zeitschrift für die gesamte Neurologie und Psychiatrie (Berlin) 1911;4:356–385. [Google Scholar]
- Aström K-E, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy. A hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Bertrand I, van Bogaert L. La sclérose latérale amyotrophique (anatomie pathologique) Revue Neurologique. 1925;1:779–806. [Google Scholar]
- Bird ED, Vonsattel J-P. The development of a brain bank. Journal of Neural Transmission [Suppl] 1993;39:17–23. [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O. The effect of L-3,4-dihydroxyphenylalanine (= DOPA) on akinesia in parkinsonism. Parkinsonism and Related disorders. 1998;4:59–60. doi: 10.1016/s1353-8020(98)00013-3. [DOI] [PubMed] [Google Scholar]
- Blocq P, Marinesco G. Sur un cas de tremblement parkinsonien hémiplégique symptomatique d'une tumeur du pédoncule cérébral. Comptes Rendus Des Séances De La Société. De Biologie Et De Ses Filiales. 1893;5:105–111. [Google Scholar]
- Brown P, Gibbs CJ, Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Annals of Neurology. 1994;35:513–529. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neurochirurgica. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison C. Amyotrophic lateral sclerosis. Origin and extent of the upper motor neuron lesion. Archives of Neurology and Psychiatry. 1941;46:1039–1056. [Google Scholar]
- Dickson DW, Feany MB, Yen S-H, et al. Cytoskeletal pathology in non-Alzheimer degenerative dementia: new lesions in diffuse Lewy body disease, Pick's disease, and corticobasal degeneration. Journal of Neural Transmission [Suppl] 1996;47:31–46. doi: 10.1007/978-3-7091-6892-9_2. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Sazdovitch V, Seilhean D, et al. A brain bank in a neuropathology laboratory (with some emphasis on diagnostic criteria) Journal of Neural Transmission [Suppl] 1993;39:107–118. [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Parkinsonism and Related disorders. 1998;4:53–57. doi: 10.1016/s1353-8020(98)00012-1. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, de Lucena Ferretti RE, Farfel JM, et al. Brain bank of the Brazilian aging brain study group-a milestone reached and more than 1,600 collected brains. Cell Tissue Banking. 2007;8:151–162. doi: 10.1007/s10561-006-9022-z. [DOI] [PubMed] [Google Scholar]
- Gsell W, Lange KW, Pfeuffer R, et al. How to run a brain bank. A report from the Austro-German brain bank. Journal of Neural Transmission [Suppl] 1993;39:31–70. [PubMed] [Google Scholar]
- Hainfellner JA, Wanschitz J, Jellinger K, et al. Coexistence of Alzheimer-type neuropathology in Creutzfeldt-Jakob disease. Acta Neuropathologica. 1998;96:116–122. doi: 10.1007/s004010050870. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Oleh Hornykiewicz. The History of Neuroscience in Autobiography. 2004;4:242–281. [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulette CM. Brain banking in the United States. Journal of Neuropathology and Experimental Neurology. 2003;62:715–722. doi: 10.1093/jnen/62.7.715. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Crain B, et al. Rapid brain autopsy. The Joseph and Kathleen Bryan Alzheimer's Disease Research Center Experience. Archives of Pathology & Laboratory Medicine. 1997;121:615–618. [PubMed] [Google Scholar]
- Ioannidis JPA. Why most published research findings are false. PLoS Medicine. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katelaris A, Kencian J, Duflou J, et al. Brain at necropsy: to fix or not to fix? Journal of Clinical Pathology. 1994;47:718–720. doi: 10.1136/jcp.47.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesselbach G. Anatomischer Befund eines Falles von Huntingtonscher Chorea. Monatsschr.Psychiat.Neurol. 1914;35:525–543. [Google Scholar]
- Leuba G, Saini K, Savioz A, et al. Early-onset familial Alzheimer disease with coexisting β-amyloid and prion pathology. JAMA. 2000;283:1689–1691. doi: 10.1001/jama.283.13.1689-a. [DOI] [PubMed] [Google Scholar]
- Monfort JC, Javoy-Agid F, Hauw JJ, et al. Brain glutamate decarboxylase in Parkinson's disease with particular reference to a premortem severity index. Brain. 1985;108:301–313. doi: 10.1093/brain/108.2.301. [DOI] [PubMed] [Google Scholar]
- Newcombe J, Cuzner ML. Organization and research applications of the U.K. Multiple Sclerosis Society Tissue Bank. Journal of Neural Transmission [Suppl] 1993;39:155–163. [PubMed] [Google Scholar]
- Piccardo P, Dlouhy SR, Lievens PMJ, et al. Phenotypic variability of Gerstmann-Sträussler-Scheinker disease is associated with prion protein heterogeneity. Journal of Neuropathology and Experimental Neurology. 1998;57:979–988. doi: 10.1097/00005072-199810000-00010. [DOI] [PubMed] [Google Scholar]
- Ravid R, Swaab DF. The Netherlands brain bank-a clinico-pathological link in aging and dementia research. Journal of Neural Transmission [Suppl] 1993;39:143–153. [PubMed] [Google Scholar]
- Ravid R, van Zwieten EJ, Swaab DF. Brain banking and the human hypothalamus-factors to match for, pitfalls and potentials. Progress in Brain Research. 1992;93:83–95. doi: 10.1016/s0079-6123(08)64565-3. [DOI] [PubMed] [Google Scholar]
- Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Archives of Neurology. 1968;18:20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Bauer M, Heinsen H, et al. How a neuropsychiatric brain bank should be run: a consensus paper of Brainnet Europe II. Journal of Neural Transmission. 2007;114:527–537. doi: 10.1007/s00702-006-0601-8. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Grunke-Iqbal I, Iqbal K, et al. τ and ubiquitin in the human hypothalamus in aging and Alzheimer's disease. Brain Research. 1992;590:239–249. doi: 10.1016/0006-8993(92)91101-j. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WW, Rosario IP, COnrad A, et al. Human neuro-specimen banking 1961–1992. Journal of Neural Transmission [Suppl] 1993;39:5–15. [PubMed] [Google Scholar]
- Trétiakoff C. Contribution à l'étude de l'anatomie pathologique du locus niger de Soemmering avec quelques déductions relatives à la pathogénie des troubles du tonus musculaire et de la maladie de Parkinson. Journal(Issue) 1919 [Google Scholar]
- van de Nes JAP, Kamphorst W, Ravid R, et al. The distribution of Alz-50 immunoreactivity in the hypothalamus and adjoining areas of Alzheimer's disease patients. Brain. 1993;116:103–115. doi: 10.1093/brain/116.1.103. [DOI] [PubMed] [Google Scholar]
- van Zwieten EJ, Ravid R, van der Sluis PJ, et al. Increased vasopressin immunoreactivity in the rat brain after a postmortem interval of 6 hours. Brain Research. 1991;550:263–267. doi: 10.1016/0006-8993(91)91327-w. [DOI] [PubMed] [Google Scholar]
- Vonsattel J-PG, Aizawa H, Ge P, et al. An improved approach to prepare human brains for research. Journal of Neuropathology and Experimental Neurology. 1995;54:42–56. doi: 10.1097/00005072-199501000-00006. [DOI] [PubMed] [Google Scholar]
- Vonsattel JPG, Amaya M, Keller C. 21st Century Brain Banking: Processing brains for research. Acta Neuropathologica In Press. 2008 doi: 10.1007/s00401-007-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]