Abstract
Purpose
To define a prostate fossa clinical target volume (PF-CTV0 for Radiation Therapy Oncology Group (RTOG) trials utilizing post-operative radiotherapy for prostate cancer.
Methods and Materials
An RTOG sponsored meeting was held to define an appropriate PF-CTV after radical prostatectomy. Data was presented describing radiographic failure patterns after surgery. Target volumes used in previous trials were reviewed. Using contours independently submitted by 13 radiation oncologists, a statistical imputation method derived a preliminary “consensus” PF-CTV.
Results
Starting from the model derived CTV, consensus was reached for a CT image-based PF-CTV. The PF-CTV should extend superiorly from the level of the caudal vas deferens remnant to > 8-12mm inferior to vesicourethral anastomosis (VUA). Below the superior border of the pubic symphysis, the anterior border extends to the posterior aspect of the pubis and posteriorly to the rectum where it may be concave at the level of the VUA. At this level the lateral border extends to the levator ani. Above the pubic symphysis the anterior border should encompass the posterior 1-2cm of the bladder wall and posteriorly it is bounded by the mesorectal fascia. At this level the lateral border is the sacrorectogenitopubic fascia. Seminal vesicle remnants, if present, should be included in the CTV if there is pathologic evidence of their involvement.
Conclusions
Consensus on postoperative PF-CTV for RT after prostatectomy was reached and is available as a CT image atlas on the RTOG website. This will allow uniformity in defining PF-CTV for clinical trials that include post-prostatectomy RT.
Keywords: Prostate cancer, conformal radiation therapy, target volumes, postoperative
INTRODUCTION
Radiation therapy is being used with increasing frequency in the management of patients following radical prostatectomy for localized prostate cancer. Three prospective randomized clinical trials demonstrate a significant clinical advantage to the use of adjuvant radiation therapy to the surgical bed of patients who have extraprostatic tumor extension, seminal vesicle invasion, or positive surgical margins.1-3. Furthermore, there is substantial evidence that radiation therapy to the prostate fossa is an effective salvage therapy in men who have developed biochemical evidence of treatment failure following radical prostatectomy.4, 5
Radiation therapy to the prostatic fossa can be associated with urinary and bowel morbidity. Furthermore, the long term results of adjuvant and especially salvage radiation therapy demonstrate a substantial proportion of men recur despite treatment. The prospective randomized clinical trials of adjuvant radiation therapy and most retrospective series of salvage radiation therapy have utilized non-conformal radiation therapy methods. This may contribute to morbidity because of unnecessary normal tissue irradiation and to less than optimum tumor control due failure to accurately target areas harboring subclinical disease. This normal tissue radiation limits the radiation dose prescription to the target volume thereby reducing the potential effectiveness of radiation therapy.
As in men receiving primary radiation therapy for cancer of the prostate, there is strong rationale to utilize modern 3-D conformal or intensity-modulated radiation therapy techniques for patients following surgery. There is data that suggests that patients treated with conformal radiation therapy techniques in the definitive setting tolerate treatment better and this opens the possibility for radiation dose escalation.6
Until recently there has been little investigation into what constitutes the appropriate clinical target volume (CTV), the tissue volume at risk of subclinical microscopic and macroscopic tumor growth for the prostatic fossa following radical prostatectomy. Because the Radiation Therapy Oncology Group (RTOG) and other cooperative groups are interested in evaluating post-prostatectomy radiation therapy in clinical trials it is important to define the CTV for this special clinical situation. The objectives of this current study are to evaluate the agreement amongst genitourinary (GU) radiation oncologists and to develop an RTOG consensus for the CTV following radical prostatectomy.
METHODS AND MATERIALS
Measurement of agreement
This research was reviewed and approved by the Washington University Human Research Protection Office and all collaborators completed training in both human research and patient privacy at their respective institutions. Treatment planning computed tomography (CT) scans from two men with prostate cancer who had undergone prior radical prostatectomy were utilized for this study. The CT data sets were acquired with patients in the supine position. The men had empty rectums and partially full bladders at the time of treatment planning CT scanning. Non-contrast CT scans were obtained from the top of the iliac crest through the perineum in 3-millimeter slices. The CT data was anonymized and distributed to each collaborator by CD ROM or made available for download from the Image-Guided Therapy QA Center (ITC) website. Each case had a slightly different clinical scenario emphasizing either a positive margin at the apex or invasion of the seminal vesicles. Clinical synopses of each case were provided to a panel of participating physicians and these are summarized in Table 1.
Table 1.
|
Case 1 A 59-year-old gentleman with a preoperative clinical T1c, Gleason 7 (3+4) PSA 12 adenocarcinoma of the prostate. He is status post radical retro pubic prostatectomy and found to have pathologic T2c, Gleason Score 7 (3+4) disease. Pathology reveals margins positive bilaterally at the apex. His PSA went to undetectable for 2 years then rose to 0.2 and 6 months later was 0.3 ng/ml. Please draw the CTV-TB (CTV for the tumor bed/prostate bed for this case). |
|
Case 2 A 68-year-old gentleman with a preoperative clinical T2a, Gleason 8 (4+4) PSA 15 adenocarcinoma of the prostate. He is status post radical retro pubic prostatectomy and found to have pathologic T3b, pN0 (12 lymph nodes taken – all negative) Gleason Scores 8 (4+4) disease. Pathology reveals extracapsular extension at the right base and right seminal vesicle invasion. Surgical margins are negative. His PSA is undetectable and he is referred to you 90 days postoperatively. He has recovered urinary continence but is now impotent. Please draw the CTV-TB for this case. |
Each participating physician was asked to use their institutional treatment planning system to define a CTV for each clinical case. The CTs with contours were then returned to Washington University investigators by CD ROM or DICOM export to the ITC. Contours from each investigator were then imported into the Computerized Environment for Radiation Research (CERR), an open source Matlab-based radiation therapy planning analysis tool.7 Contours were then compared for agreement by using Matlab statistical software package.
Several algorithms were utilized to measure the level of agreement between physicians. The commonly used apparent volume overlap was calculated using the following formula:
| (1) |
Where pi is the agreement probability by which a voxel is selected by the experts. A volume – agreement cumulative histogram could be generated based on Eq. (1). In order to account for agreement by chance, a measure of corrected overlap agreement was determined by using generalized kappa statistics.8
| (2) |
Kappa statistics assume values between +1 (perfect agreement) through 0 (no agreement above chance) and −1 (complete disagreement). According to Landis and Koch kappa value of 0 is poor, 0.01 – 0.20 slight, 0.21 – 0.40 fair, 0.41 to 0.60 moderate, 0.61 – 0.80 substantial, and 0.80 – 1.00 almost perfect agreement.9
It was assumed that within the collection of submitted contours that a “true” CTV existed that represented the likely site of microscopic disease following radical prostatectomy. An estimation of the true CTV contours was determined using an imputation method of the Expected Maximum (EM) algorithms for simultaneous truth and performance level estimation (STAPLE).10 EM is a general method of finding the maximum likelihood estimate of parameters of an underlying distribution from a given dataset when the data is incomplete or has missing values.11 From the estimated true CTV contours we calculated the sensitivity and specificity that individual investigator contours would determine anatomical sites that could contain subclinical disease.
The results of the individual and composite contours were then returned to the original participants for review and comment. A consensus conference was held with eight of the original physician investigators or representatives from their institutions that were familiar with their CTV methodology and rationale.
At the consensus conference a presentation of patterns of failure, anatomy, and surgical findings at radical prostatectomy was done by one of the investigators (CM). Each clinical case was then reviewed and using the STAPLE contours as the starting point consensus contours were defined for each of the two cases. Anatomical descriptions of the CTV were created along with caveats for individual clinical findings. Following the conference, the consensus contours and their descriptions were then circulated for comments and feedback from a group of participants from the RTOG GU Committee. Based upon feedback, a teleconference was held to clarify some elements of the description of the CTV and this is reflected in the results.
RESULTS
CTV Variability
Thirteen physicians were asked to participate and eleven returned contour data sets. Significant variation in the definition of the CTV was seen amongst the participating physicians as demonstrated in Figure 1.
Figure 1.
Axial and sagittal CT reconstructions demonstrating individual contours from eleven physicians overlaid upon the CT data sets.
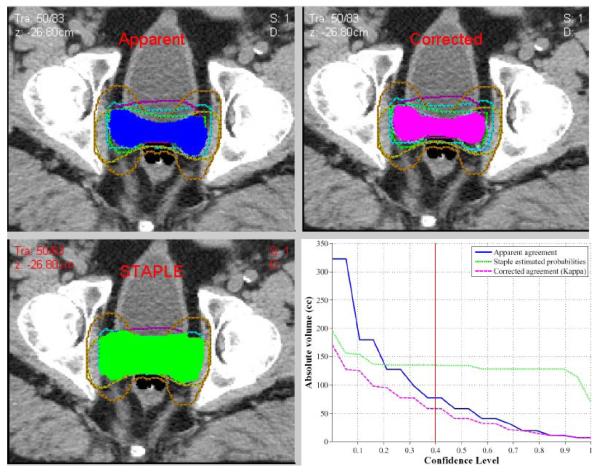
The variability of the contours is reported quantitatively in Table 2. A volume agreement cumulative histogram showing estimated agreement contouring by the investigators using the STAPLE estimated probability for (a) Case 1 and (b) Case 2. The volume of 100% agreement was only 6.8 and 13.4 cc and the volume of the union of all contours was 322.7 and 547.6 cc for case 1 and 2, respectively. According to kappa statistics, the overall agreement was judged to be moderate (kappa = 0.41 and kappa = 0.42) for case 1 and 2, respectively (p≤0.0001). The estimated experts sensitivities and specificities were 0.5 ±0.25 and 0.99 ± 0.23 for case 1 and 0.62 ± 0.22 and 0.96 ±0.07 for case 2 indicating higher agreement levels with increased volume as shown in Figure 2.
Table 2.
| Post-Op # 1 | Post-Op # 2 | |
|---|---|---|
| CTV-TB | CTV-TB | |
| Overall Kappa (p-value) | 0.41 (p < 0.0001) | 0.42 (p < 0.0001) |
| Sensitivity (Mean±SD) | 0.50 ± 0.25 | 0.62 ± 0.22 |
| Specificity ((Mean±SD)) | 0.99 ± 0.03 | 0.96 ± 0.07 |
| Min Volume (cc) | 23.5 | 31.8 |
| Max volume (cc) | 282 | 435 |
| Volume (Mean+/−SD) cc) |
88.5 ± 74.8 | 159.4 ± 116.6 |
| Total agreement volume (cc) | 6.8 | 13.4 |
| Union Volume (largest volume assuming outermost contours) (cc) |
323 | 548 |
Figure 2.
Model derived contours (a) apparent, (b) corrected and (c) STAPLE at the 40% confidence level, as an example. A (d) histogram of agreement amongst the participants.
CTV Consensus Development
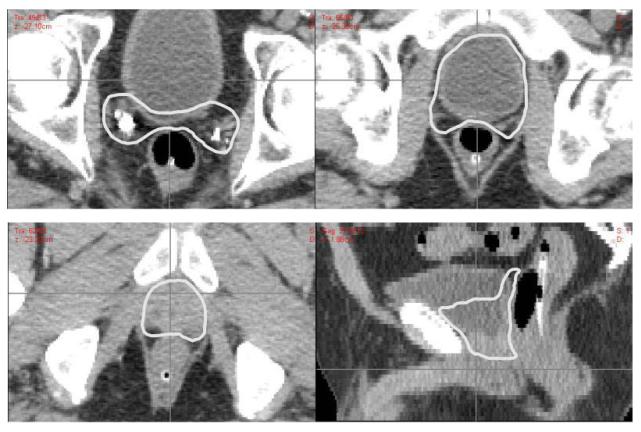
At the consensus conference, the contours were reviewed and discussed. It was agreed by all present that one of the submitted CTV contours was markedly larger than all the others. It was unclear if that submitting investigator intended to define the PTV or an ideal dose distribution rather than a CTV. This outlier was excluded from the STAPLE generated consensus contours because it would have created a CTV that exceeded most of the individual investigator contours. From the modified STAPLE contours, consensus for the two clinical scenarios was derived by slightly modifying the model contours to create smoothed edges. These consensus contours are displayed in Figure 3. The full CT data set with each contoured slice can be found on the RTOG website (www.RTOG.org).
Figure 3a.
Examples of consensus contours for patient with apical positive margins and biochemical recurrence
The anatomical boundaries of the CTV are listed in Table 3. With axial CT imaging, the vesicourethral anastomosis (VUA) can often be seen in the retropubic region as one slice below the most inferior urine-containing image. Magnetic resonance (MR) imaging defines this landmark more clearly with hyperintense urine signal on T2 images. Inferiorly, the border of the CTV should be at least 8-12 millimeters below the VUA. The position of the VUA can be difficult to visualize with CT, especially if the bladder is not filled with urine. A sagittal reconstruction can facilitate identification of the position of the VUA and establishing the inferior border of the CTV below it. If visualization of the VUA is problematic due to image quality or surgical clip artifacts, the inferior limit of the CTV can extend down to a level just above the penile bulb. The anterior border of the CTV extends to the posterior aspect of the pubic bone and the posterior border extends to the anterior muscular wall of the rectum. At the level of the VUA the CTV may curve concavely around the anterior surface of the rectal wall. Above the superior aspect of the pubic bone the anterior border of the CTV encompasses the posterior 1-2 cm of bladder wall and is bound posteriorly by the mesorectal fascia. At this level the lateral border is the sacrorectogenitopubic fascia. The superior aspect of the CTV should not extend above the transected remnant of the vas deferens. In some cases the vas deferens may be difficult to visualize or retract superiorly. Generally, unless there is gross disease or seminal vesicle remnant, the superior limit of the CTV need not extend more than 3-4 cm above the level of the pubic symphysis.
Table 3.
Anatomical Borders of Postoperative Clinical Target Volume for Prostate Cancer
| Below the superior edge of the symphysis pubis | Comments | |
|---|---|---|
| Anterior | Posterior edge of pubic bone | |
| Posterior | Anterior rectal wall | May need to be concave around lateral aspects |
| Inferior | 8-12 mm below VUA | May include more if concern for apical margin. Can extend to slice above penile bulb if VUA not well visualized |
| Lateral | Levator ani muscles, obturator internus | |
| Above the superior edge of the symphysis pubis | ||
|---|---|---|
| Anterior | Posterior 1-2cm of bladder wall | |
| Posterior | Mesorectal Fascia | |
| Superior | Level of cut end of vas deferens or 3- 4cm above top of symphysis |
Vas may retract postoperatively, Include seminal vesicle remnants if pathologically involved |
| Lateral | Sacrorectogenitopubic fascia | If concern about extraprostatic disease at base may extend to obturator internus |
In our CTV model, the superior aspect of the retropubic component of the CTV encompasses significant contents of the urinary bladder and bladder neck in order to treat. the circumference of the wall of the bladder neck from the anterior wall of the rectum to the immediate retropubic space, which is thought to be at risk. The ideal CTV would have the urinary contents subtracted from this volume. In practice, once a margin for PTV is added to this CTV, any sparing of the contents would be lost.
In case 1 the tumor involved the surgical margin at the apex. In this case the consensus panel felt the inferior border of the CTV could extend inferiorly more than 8-12 mm described above. Inferiorly the CTV can extend to the GU diaphragm but not into the penile bulb. In the case of apical involvement without extraprostatic extension at the base or involvement of the seminal vesicles the CTV need not include the entirety of the seminal vesicle remnants.
In case 2 extraprostatic extension at the base and involvement of the seminal vesicles required inclusion of the seminal vesicle remnants. The CTV extended more superiorly in this case. In both cases the CTVs encompassed surgical clips in the prostate and seminal vesicle bed. Above the level of the seminal vesicles it is common to see vascular clips left by the surgeon to control bleeding. It was not felt necessary to include all surgical clips in the pelvis as it is generally not the intent of the surgeon to leave them to mark sites of disease. These clips are placed to control bleeding from transected blood vessels in the course of the pelvic lymph node and prostate dissection that precedes the prostatectomy.
Discussion
There is a growing body of evidence supporting the use of radiation therapy in the postoperative setting for men with prostate cancer. Clinical trials and retrospective series have generally used non-conformal methods to administer this therapy.1, 2, 4 Three dimensional conformal radiation therapy and intensity modulated radiation therapy allow a reduction in the volume of bladder and rectum irradiated in the postoperative setting.6 This may improve treatment tolerance to the treatment and may open up the opportunity for radiation dose escalation in the postoperative setting.12
Patterns of clinical local failure following radical prostatectomy have been reported using a variety of imaging modalities. Using ultrasound, Connolly reported 53.5% of patients had biopsy evidence of local tumor recurrence in the anastomotic site (66%), the bladder neck (16%), and the retrotrigone region (13%).13 In a series of 35 patients with clinical suspicion of local recurrence, Silverman reported a sensitivity and specificity of 100% using transrectal contrast enhanced surface coil MRI to detect 31 recurrences. In that series the locations of the recurrences were at the posterior aspect of the VUA in 16, anterior to the VUA in 9 and both anterior and posterior to the VUA in 6 cases.14 Sella reported similarly high rates of sensitivity and specificity using endorectal coil MR imaging to detect 39 of 41 local recurrences in the prostate bed. Local recurrences were at the VUA in 19%, retrovesical in 40%, in retained seminal vesicles in 22% and at the anterior or lateral surgical margin in 9%.15 Leventis reported the location of local recurrences by ultrasound to commonly involve the VUA, bladder neck and retrovesical space.16
Several other groups have defined a 0radiation clinical target volume for patients following radical prostatectomy. 17-19 While there are some similarities to the current study, there are also many differences. As in our study, the published patterns of failure were reviewed by these investigators. In addition, some groups examined their own institutional experience with local recurrences and their locations by imaging. In the consensus paper by Wiltshire et al19, they identified at least one gross local recurrence in the retropubic region, anterior to the VUA. In the series from Miralbell17, sixty patients had abnormalities on contrast enhanced endorectal MRI. The location of the MRI abnormalities was determined to be at or near the VUA in all cases. They concluded that a cylindrically shaped (4cm high and 3cm) diameter CTV centered 0.5cm posterior and 3mm inferior to the VUA would encompass all these visualized sites of gross recurrence. Interestingly, they did not see recurrences in sites previously described by other authors such as the retrovesical space and their recommended CTV does not include these sites. Poortmans18 reported a similar consensus panel target volume for postoperative irradiation of prostate cancer patients to be utilized by EORTC sites. As in other published series, they used a pattern of failure review to determine the appropriate CTV in the postoperative setting. The published CTV from that series is subjectively smaller than that proposed by this group.
In this study, the approach differed from other groups because a consensus for the postoperative CTV was established by clinical investigators specializing in the treatment of prostate cancer. Anonymized clinical CT data sets were distributed to thirteen GU experts from RTOG institutions. While there was qualitatively substantial variation in the target volumes there were significant areas of agreement amongst these physicians. We then derived a CTV model using a statistically derived imputation method from the independently submitted contours. Starting from these STAPLE contours, a subgroup of participating investigators convened to define a consensus CTV for the two submitted clinical scenarios. This consensus CTV was then distributed for review and comments to the group of participants from the RTOG GU Committee. Whereas other CTV contours used a “bottom up” approach to create postoperative CTV from patterns of failure, our group used a “top down” approach to create a consensus CTV from individual submitted expert contours. It is possible that this method of target volume development could be applied to other disease sites. We are currently investigating this in breast and anal cancers.
The CTV defined by this group represents a minimum volume to be irradiated in a typical postoperative scenario. It is recognized that clinical judgment may require modification of the described CTV. As with any set of treatment guidelines, individual practitioners will have to consider individual patient characteristics. Variability in anatomy and co-morbidities are factors which may need to be considered in individual cases. The panelists felt that the pathology report should be available when defining the CTV with careful attention to areas of extraprostatic extension, seminal vesicle invasion or positive margins. Preoperative imaging, when available, can also help establish the superior limit of the CTV by referencing the superior extent of the prostate gland and seminal vesicles.
The inferior aspect of the CTV generated the most discussion and some disagreement amongst the participants. Wiltshire et al included urological surgeons in the development of their published postoperative CTV and they were emphatic that the penile bulb is never exposed to the surgical cavity.19 On the other hand, some of the radiation oncologists in our group expressed concern that apical tumors can extend into the GU diaphragm and inferior urethral sphincter. Tumor recurrences in this region are possible after either surgery or radiation therapy. For this reason, we agreed that the inferior aspect of the CTV should be allowed to extend to a level just above the penile bulb. This creates a CTV that could be 3 to 6 mm inferior to that described by Wiltshire.
Conclusion
Members of the RTOG GU Committee have demonstrated that substantial qualitative variability existed amongst prostate cancer experts supporting the need for the development of consensus guidelines. Using a model derived CTV from these expert contours, a consensus CTV has been established that is intended to be used in current and future RTOG clinical trials. An atlas of the postoperative CT generated from this consensus panel is available at http://www.rtog.org/PostoperativeProstateAtlas.
Figure 3b.
Examples of consensus contours for patient with extracapsular extension at base and involvement of seminal vesicle
ACKNOWLEDGEMENTS
Supported by grants from the National Cancer Institute, CA21661, CA32115, and CA37422
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT: There are no conflicts of interest associated with this manuscript by any of the authors.
References
- 1.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Jr., Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 3.Wiegel T, Bottke D, Willich N. Phase III results of adjuvant radiotherapy (RT0 versus “wait and see” (WS) in patients with pT3 prostate cancer following radical prostatectomy (RP) ASCO; Orlando, FL: 2005. [Google Scholar]
- 4.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cozzarini C, Fiorino C, Ceresoli GL, et al. Significant correlation between rectal DVH and late bleeding in patients treated after radical prostatectomy with conformal or conventional radiotherapy (66.6-70.2 Gy) Int J Radiat Oncol Biol Phys. 2003;55:688–694. doi: 10.1016/s0360-3016(02)04117-2. [DOI] [PubMed] [Google Scholar]
- 7.Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 8.Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed Wiley; New York: 1981. [Google Scholar]
- 9.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 10.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dempster A, Laird N, Rubin D. Maximum likelihood from incomplete data via the EM algorithm. J Royal Stat Soc, Series B. 1977;39:1–38. [Google Scholar]
- 12.Valicenti RK, Gomella LG, Ismail M, et al. Effect of higher radiation dose on biochemical control after radical prostatectomy for PT3N0 prostate cancer. Int J Radiat Oncol Biol Phys. 1998;42:501–506. doi: 10.1016/s0360-3016(98)00270-3. [DOI] [PubMed] [Google Scholar]
- 13.Connolly JA, Shinohara K, Presti JC, Jr., et al. Local recurrence after radical prostatectomy: characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology. 1996;47:225–231. doi: 10.1016/S0090-4295(99)80421-X. [DOI] [PubMed] [Google Scholar]
- 14.Silverman JM, Krebs TL. MR imaging evaluation with a transrectal surface coil of local recurrence of prostatic cancer in men who have undergone radical prostatectomy. Am J Roentgenol. 1997;168:379–385. doi: 10.2214/ajr.168.2.9016212. [DOI] [PubMed] [Google Scholar]
- 15.Sella T, Schwartz LH, Swindle PW, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004;231:379–385. doi: 10.1148/radiol.2312030011. Epub 2004 Apr 2002. [DOI] [PubMed] [Google Scholar]
- 16.Leventis AK, Shariat SF, Slawin KM. Local recurrence after radical prostatectomy: correlation of US features with prostatic fossa biopsy findings. Radiology. 2001;219:432–439. doi: 10.1148/radiology.219.2.r01ma20432. [DOI] [PubMed] [Google Scholar]
- 17.Miralbell R, Vees H, Lozano J, et al. Endorectal MRI assessment of local relapse after surgery for prostate cancer: A model to define treatment field guidelines for adjuvant radiotherapy in patients at high risk for local failure. Int J Radiat Oncol Biol Phys. 2007;67:356–361. doi: 10.1016/j.ijrobp.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 18.Poortmans P, Bossi A, Vandeputte K, et al. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol. 2007;84:121–127. doi: 10.1016/j.radonc.2007.07.017. Epub 2007 Aug 2013. [DOI] [PubMed] [Google Scholar]
- 19.Wiltshire KL, Brock KK, Haider MA, et al. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;69:1090–1099. doi: 10.1016/j.ijrobp.2007.04.068. [DOI] [PubMed] [Google Scholar]