Abstract
Integrin αvβ8 plays an important role in cerebral vascular development. It has been proven that αvβ8 is a key factor for transforming growth factor-β1 (TGF-β1) activation in epithelial cells. However, it is not clear whether αvβ8 can activate TGF-β1 and play a role in protection during neonatal hypoxic-ischemic brain injury. In this study, we investigated the relationship between αvβ8 and TGF-β1 activation, and thus the effects of TGF-β1 activation in the protection of neurons after hypoxia-ischemia (HI). Astrocytes and neurons from rat brains were cultured and then subjected to oxygen–glucose deprivation to generate HI model in vitro. β8 expression was determined using immunocytochemistry, western blot, and reverse-transcriptase polymerase chain reaction. TGF-β1 activation was determined by TGF-β bioassay in a tested cell (astrocyte) and a reporter cell co-culture system. The pro-apoptotic protein, cleaved caspase-3, and the anti-apoptotic protein, Bcl-2 and Bcl-xL, were detected using western blot. Cellular apoptosis was detected with TUNEL. We found that β8 expression was stronger in astrocytes than that in neurons under normoxia. HI resulted in a rapid and persistent increase of β8 expression in astrocytes, but only in a slight and transient increase in neurons. Astrocytes β8 could induce TGF-β1 leading to upregulation of Bcl-2 and Bcl-xL, and thus attenuated neuronal apoptosis. The present findings suggest that β8 protecting the brain against neonatal HI injury through TGF-β1 signaling pathway, which may have implications for the treatment of HI brain injury.
Keywords: Integrin, Transforming growth factor-β, Astrocyte, Hypoxia-ischemia, Neuronal apoptosis
Introduction
The neonatal brain can initiate a series of responsive mechanisms that may help the recovery of brain function after hypoxia-ischemia (HI). Astrocytes, the most numerous cells in CNS, have functions in supporting neuronal integrity, cerebral vascular development, and neuroprotection (Kirchhoff et al. 2001; Garcia et al. 2004; Gabryel and Trzeciak 2001). Understanding how astrocyte regulates these functions is important for providing targets for recovery following HI.
Recent studies have shown that astrocytes are involved in cell–matrix and cell–cell interactions (Milner et al. 2008; Wagner et al. 1997). In these interactive processes, integrins and cytokines play important roles. Integrins are cell-surface receptors expressed as heterodimers consisting of α and β subunits. At present, integrins are classified as three subfamilies: β1, β2, and αv. Among these subfamilies, αv integrins are necessary for the interactions between cerebral blood vessels and brain parenchymal cells. Deletion of αv gene results in a phenotype with severe cerebral hemorrhage and neonatal death (Bader et al. 1998; McCarty et al. 2002). The αv subfamily has five members: αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8. All of the members are extensively expressed in brain except αvβ6. Only deletion of β8 but not β1, β3, or β5 gene results in the similar phenotype as αv deletion. β3 and β5 subunits are not likely to be associated with cerebral vascular development, as no defective brain vessel formation is observed in β3 and β5 mutants (Zhu et al. 2002a; Proctor et al. 2005; Reynolds et al. 2002). β8 integrin is mainly expressed on glia, particularly astrocytes, with a slight expression in neurons but not in the endothelium (Nishimura et al. 1998). As one of the main integrins expressed in astrocytes, αvβ8 may be involved in the regulation of astrocyte functions.
αvβ8 is a key factor for the activation of transforming growth factor-β (TGF-β) (Mu et al. 2002; Cambier et al. 2005). TGF-β, a multi-functional cytokine, consists of three subfamilies, TGF-β1, TGF-β2, and TGF-β3, playing important roles in cell growth, differentiation, inflammation, and apoptosis in mammalian CNS (Dhandapani and Brann 2003; Brionne et al. 2003; Makwana et al. 2007). TGF-β1, the major form of the three subfamilies, is normally secreted as an inactive (latent) precursor with non-covalent association with a latency-associated peptide-β1 (LAP-β1). Therefore, latent TGF-β1 must be activated to promote transcription of its target genes such as vascular endothelial growth factor (VEGF), and brain-derived neurotrophic factor (BDNF), which are beneficial for angiogenesis and neuroprotection.
TGF-β1 activation needs either a nonproteolytic or a proteolytic process, for which integrins are required (Keski-Oja et al. 2004; Sheppard 2005). However, this activation process is not completely understood. At least four of αv integrins, αvβ3, αvβ5, αvβ6, and αvβ8 are capable of activating TGF-β1. Among the αv integrins, αvβ5 and αvβ6 are involved in the nonproteolytic mechanism by conformational change of latent TGF-β1 (Asano et al. 2006; Munger et al. 1999), whereas αvβ3 and αvβ8 are involved in proteolytic activation of latent TGF-β1 (Asano et al. 2005a; Mu et al. 2002). In CNS, αvβ5 and αvβ8, but not αvβ3 and αvβ6, are detectable in primary cultured astrocytes derived from postnatal animals (Milner et al. 2001). Furthermore, αvβ8 but not αvβ5 is proven to be capable of activating TGF-β1 in cultured astrocytes, which is related to angiogenesis (Cambier et al. 2005).
Although integrin αvβ8 is critical for angiogenesis during embryonic development (Zhu et al. 2002a; Proctor et al. 2005), it is unclear whether αvβ8 can mediate TGF-β1 activation and thus plays a neuroprotective role in neonatal brain following HI. We therefore hypothesized that integrin αvβ8 could activate TGF-β1 in CNS after HI, and thus regulates the downstream effects of TGF-β1 pathway, which may be beneficial for neuroprotection. To test this hypothesis, we detected β8 expression and its roles in TGF-β1 activation and neuronal apoptosis using in vitro HI models.
Materials and Methods
Cell Culture
Sprague-Dawley (SD) rats were ordered from Medical Animal Center of Sichuan Province. All animal research was approved by the Sichuan University Committee on Animal Research.
Astrocytes and neurons were prepared from primary cell cultures of cortical tissues from postnatal day 3 (P3) and embryonic day 16 (E16) pups, respectively, as described previously (Wang et al. 2008; Schmuck et al. 2002). Briefly, brains were cleaned of meninges and blood vessels. Cortical tissues were dissected and then incubated with 0.25% trypsin at 37°C for 10 min. After addition of fetal bovine serum (FBS), the dissociated cells were forced through 70 μm mesh. Cell pellets were collected and plated in poly-L-lysine (PLL) coated flasks in DMEM with 10% FBS (Hyclone) for astrocytes, in Neurobasal (Gibco) with 2% B27 (Gibco) for neurons, with the final cell densities adjusted to 1 × 106/ml. The purity of astrocytes and neurons were assessed separately by staining with GFAP (1:200, Santa Cruz Biotechnology) and NeuN (1:200, Chemicon) using immunocytochemistry.
Construction of β8 siRNA Expression Vectors
The expression vector pSicoR (gift from Jacks lab, MIT) was used for expression of β8 siRNA. The gene-specific inserts specifying a 19-nucleotide sequence corresponding to nucleotides 1172–1190 (GCATCAATGCACAATAATA) of the β8 cDNA of rat was ordered from Integrated DNA Technologies (IDT) and ligated into pSicoR vector (pSicoR–β8). A control vector (pSicoR–control) was constructed using a 19-nucleotide sequence (GTTCTATTTCTGCTCGGCG) with no homology to any mammalian gene sequence. pSicoR–β8 and pSicoR–control were sequenced. The correct insert sequences were used for transfection. Since β8 is uniquely paired with αv, it was detected to represent αvβ8 in this study.
Lentivirus Packaging and Infection with Astrocytes
Lentivirus was packaged and astrocytes were infected as described previously (Janas et al. 2006). Briefly, viral particles were produced by transfection of pSicoR–β8 or pSicoR–control along with packaging constructs in human embryonic kidney (HEK) 293T cells, using FuGENE HD Transfection Reagent according to manufacturer’s instructions (Roche). Medium containing viral particles was collected 2 days after transfection and then centrifuged at 2,000 × g to remove cell debris. Medium containing β8 siRNA or control siRNA virus was filtered through a 0.45 μm filtration unit. β8 siRNA or control siRNA virus were used to infect 80% confluent astrocytes at MOI of 10. Astrocytes were collected at 1, 2, 3, 5, and 7 days after the infection to evaluate β8 mRNA and protein expression. Astrocytes infected with β8 siRNA virus were named astrocytes, while those infected with control siRNA virus were named astrocytes.
Hypoxia-Ischemia and Reoxygenation
We performed oxygen–glucose deprivation (OGD) to simulate HI conditions in vitro as previously described (Hamrick et al. 2005). Astrocytes and neurons were cultured separately at day in vitro (DIV) 15 and 7, the medium was replaced with no glucose DMEM (Sigma), and then placed in an incubator attached to a hypoxia chamber with 5% CO2, 95% N2 at 37°C for 6 h as described previously (Fu et al. 2007). After 6 h of OGD treatment, the medium was replaced with original medium and then cultured in an incubator with normal condition to form reoxygenation.
Inmmunocytochemical Analysis
Immunocytochemistry was used to detect the distribution of β8 protein in cultured astrocytes and neurons. Cell monolayers grown on sterile coverslips were washed with 0.01 M PBS (pH 7.4). Four percent paraformaldehyde was used to fix antigens. Endogenous peroxidase was blocked with 0.3% H2O2. Goat serum (1:10) served as blocking agent before incubation with rabbit anti-β8 antibody (1:100, Santa Cruz Biotechnology). After incubated overnight at 4°C, biotin labeled goat anti-rabbit IgG was loaded, followed by freshly prepared avidin biotinylated enzyme complex (ABC, VECTOR Laboratories) and finally 3, 3′-diaminobenzidine (DAB, KPL) was used as chromogen.
Western Blot Analysis
To detect β8 protein expression, we developed a β8 polyclonal antibody with KLH ligated antigen sequence “EE-IKMDISKLNA” immunized in rabbits as previously described (Nishimura et al. 1998). Western blot was performed to quantify β8 protein expression in neurons, non-infected astrocytes, as well as and astrocytes. Total cell protein was isolated with RIPA and quantitated with bicinchoninic acid Kit (BCA, Bioteke). An equal amount of protein was separated by 8% sodium dodecyl-sulfate (SDS)-polyacrymide gel and then transferred to a PVDF membrane (Roche). Membranes were blocked with 5% milk for 1 h and then incubated with rabbit anti-β8 polycolonal antibody (1:100) or mouse anti-β-actin antibody (1:1,500, Santa Cruz Biotechnology) overnight at 4°C. The membranes were washed and then incubated with horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG (1:1,500, Santa Cruz Bio-technology). Antigens were identified by enhanced chemical luminescence (ECL, Keygen). Signal intensities of β8 and β-actin bands were scanned and measured using a densitometer and a computer-assisted Gel-Pro image analyzer (Omega).
Reverse-Transcriptase PCR, Real-Time PCR
Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed to semi-quantify β8 gene expression in non-infected astrocytes and neurons. Real-time PCR was used to detect the efficiency of RNA interference (RNAi) between and astrocytes. Total RNA was isolated using RNA extraction Kit (Watson). cDNAs were generated from 2 μg of total RNA using Revert Aid First Strand cDNA Synthesis Kit (Fermentas). PCR was performed according to the instruction of the manufacturers (Fermentas).
The oligonucleotide primers were as follows—β8: 5′-CTGGGTATTTTCACTTGTTCT-3′ (forward), 5′-CAGTATCACAACGTTCACTT-3′ (reverse); β-actin (for RT-PCR): 5′-ACACTGTGCCCATCTAGGAGG-3′ (forward), 5′-AGGGGCCGGACTCGTCATACT-3′ (reverse); β-actin (for Real-Time PCR): 5′-GCCAACACAGTGCTGTCT-3′ (forward); 5′-AGGAGCAATGATCTTGATCTT-3′ (reverse); TaqMan probe for β8: 5′-FAM-CCTCTTGAACACACCATCCAC-TAMRA-3′; TaqMan probe for β-actin: 5′-FAM-ATCTCCTTCTGCATCCTGTC-TAMRA-3′.
The reaction initially started with a 94°C denaturation for 4 min, followed by 38 cycles of 94°C/45 s, 55°C/45 s, 72°C/45 s. Levels of β8 mRNA expression were shown as relative copy ratios of β8/β-actin.
TGF-β Bioassay
TGF-β bioassay was performed as described previously (Mu et al. 2002). Briefly, TMLC, a mink lung epithelia reporter cell, was stably transfected with a portion of the plasminogen activator inhibitor 1 (PAI-1) promoter that can be activated by active TGF-β1. Therefore, while TGF-β1 was activated and released, PAI-1 promoter could be activated and then promoted its downstream gene, luciferase expression. In this system, TMLC were co-cultured with or astrocytes in the presence or absence of anti-TGF-β blocking antibody 1d11 (10 μg/ml, R&D systems). Meanwhile, since GM6001 was proven to be an effective general matrix metalloproteases (MMPs) inhibitor (Mu et al. 2002; Cambier et al. 2005), GM6001 (10 μmol/l, Millipore) was used to inhibit MMPs in the co-culture system. TGF-β1 activity was detected after co-cultured cells were lysed using a Luciferase Assay System (Pro-mega) with luminometer. Relative luciferase units (RLU) were defined as luciferase units (LU) minus the background activity of the TMLC reporter cells.
Establishment of Astrocyte/Neuron Co-culture System
We developed an astrocyte/neuron co-culture system using 0.4 μm PET transwell inserts (Millipore) in six-well plates. Primary cultured neurons were plated on six-well plates or sterile coverslips with 5 μg/ml of Ara-c to inhibit astrocytes for 1 day and then changed the media back to normal culture without Ara-c for another 4 days. Transwells containing or astrocytes were inserted into the six-well plates when cultured neurons reached to maturity. Neurons were co-cultured with astrocytes in the presence or absence of 1d11 (10 μg/ml) to detect whether blocking TGF-β1 activity can influence neuronal apoptosis in the system. Medium for co-culture was DMEM with 20% FBS and 10% horse serum (Hyclone). After co-culture for 1 day, cells underwent OGD and reoxygenation treatment as described above.
Neuronal Apoptosis Assay
After reoxygenation for 1 day, neuronal apoptosis was detected using In Situ Cell Death Detection Kit (Roche) for TUNEL detection in astrocyte/neuron co-culture system as previously described (Li et al. 2009). Briefly, neuron monolayers grown on sterile coverslips were washed in PBS and fixed in 4% paraformaldehyde. Then, incubated at 37°C for 1 h with biotinylated nucleotide and the terminal deoxynucleotidyl transferase, recombinant (rTdT) enzyme. After washes, cells were incubated with Streptavidin HRP solution and detected with 0.05% DAB. As negative controls, alternate coverslips were processed in parallel without rTdT enzyme. The apoptotic index (AI) was calculated as follows: AI = (number of apoptotic cells/total number counted) × 100%.
In this co-culture system, primary cultured neurons in six-well plates were collected to detect the expression of cleaved caspase-3 (CC3), a specific apoptotic protein, as well as the expression of Bcl-2 and Bcl-xL, anti-apoptotic proteins by western blot. Protein levels were normalized to β-actin as a loading control. Antibodies were used as follows: rabbit anti-CC3 (1:500, Cell Signaling Technology), rabbit anti-Bcl-2(1:200, Santa Cruz Biotechnology), rabbit anti-Bcl-xL(1:1,000, Cell Signaling Technology), mouse anti-β-actin antibody (1:1,500, Santa Cruz Biotechnology), goat anti-rabbit IgG, and rabbit anti-mouse IgG (1:1,500, Santa Cruz Biotechnology).
Statistical Analysis
Data were represented as mean ± standard deviation (SD). Statistical differences between controls and each group were compared using ANOVA with Bonferroni/Dunn post hoc tests. Statistical differences between two groups were compared using t tests. A value of P < 0.05 was considered statistically significant.
Results
Distribution and Expression of β8 in Astrocytes and Neurons
To determine the distribution and expression of β8 in normoxic cultured astrocytes and neurons, we performed immunocytochemistry using astrocyte and neuron monolayers grown on coverslips under normoxia. We found that β8 was strongly expressed in astrocytes (Fig. 1b) but weakly expressed in neurons (Fig. 1d). The positive immunoreactivity was mainly located in the membrane, cytoplasm, and neurites of the cells (Fig. 1b, d). There was no positive immunoreactivity in negative controls (Fig. 1a, c).
Fig. 1.
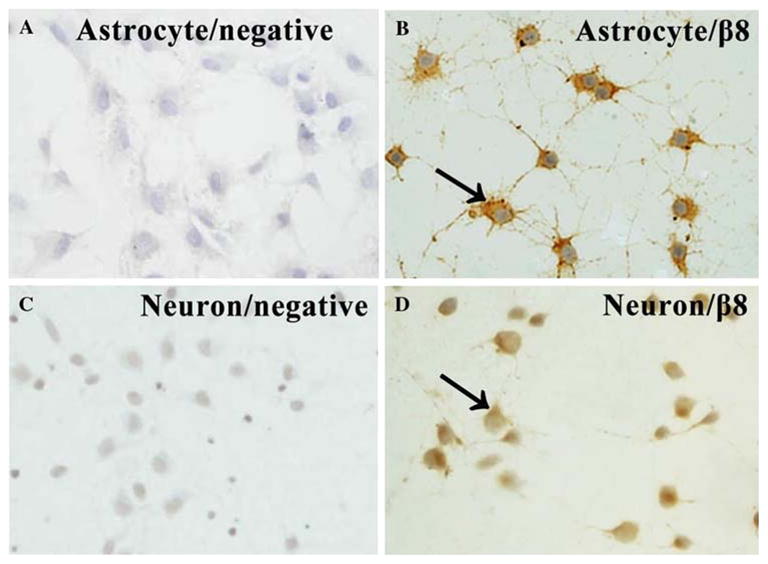
Immunoreactivity of β8 in normoxic cultured astrocytes and neurons (N = 5). β8 was strongly expressed in astrocytes (b) but with a weak expression in neurons (d). The positive immunoreactivity was mainly located in the membrane, cytoplasm, and neurites of the cells (b, d). There was no positive immunoreactivity in negative controls (a, c). Arrows show the positive staining cells. × 400
Expression of β8 in Astrocytes and Neurons After OGD
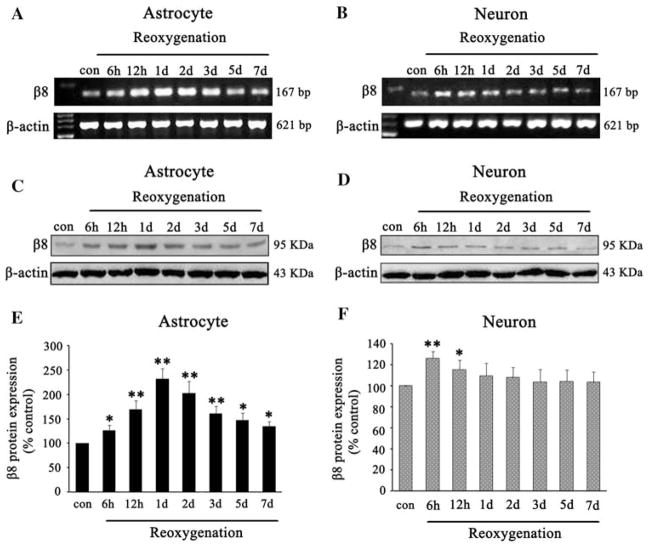
To investigate the effects of HI on β8 expression, we collected the samples at 6, 12 h, and 1, 2, 3, 5, and 7 days after OGD following reoxygenation and detected β8 mRNA and protein expression using RT-PCR and western blot analyses. We found that β8 mRNA expression was increased at 6 h, peaked at 1 day and then slowly decreased but still maintained at a high level at 7 days in astrocytes after reoxygenation (Fig. 2a). Meanwhile, we found that β8 mRNA expression was transiently increased at 6 h, declined at 1 day, and returned to the baseline at 2 days in neurons after reoxygenation (Fig. 2b). To quantify β8 expression, we performed western blot analysis and found that β8 protein expression was increased at 6 h, peaked at 1 day, and started to decline at 2 days but still maintained at a high level at 7 days after reoxygenation (Fig. 2c). After normalized with β-actin, we found an approximately 1.3- and 2.2-fold increase of β8 protein at 6 h and 1 day in astrocytes (F = 76.75, P < 0.05, Fig. 2e) after reoxygenation. The β8 protein expression was increased at 6 h in neurons but quickly returned to control level within 1 day after reoxygenation (Fig. 2d). After normalized with β-actin, there was approximately 1.3-fold increase of β8 protein in neurons at 6 h (t = 6.82, P < 0.05, Fig. 2f) after reoxygenation compared with that in normoxic controls.
Fig. 2.
The effects of HI on β8 expression in cultured astrocytes and neurons. β8 mRNA expression was increased at 6 h after reoxygenation, peaked at 1 day, then slowly decreased but still maintained at high level at 7 days in astrocytes compared with controls (a). HI also resulted in an increase of β8 mRNA expression in neurons at 6 h, but declined at 12 h and returned to baseline within 2 days after reoxygenation (b). Analysis showed that the β8 protein was increased at 6 h, and maintained for at least 7 days after reoxygenation in astrocytes (c), but slightly and transiently increased in neurons at 6 h after reoxygenation and quickly returned to baseline within 1 day (d). Quantification of β8 expression in astrocytes and neurons, respectively, in HI groups and normoxic controls (e, f). Results were normalized to controls and represented as mean ± SD. For each column, N = 4, * P < 0.05, ** P < 0.01 versus control
The Effects of siRNA on β8 Expression in Astrocytes
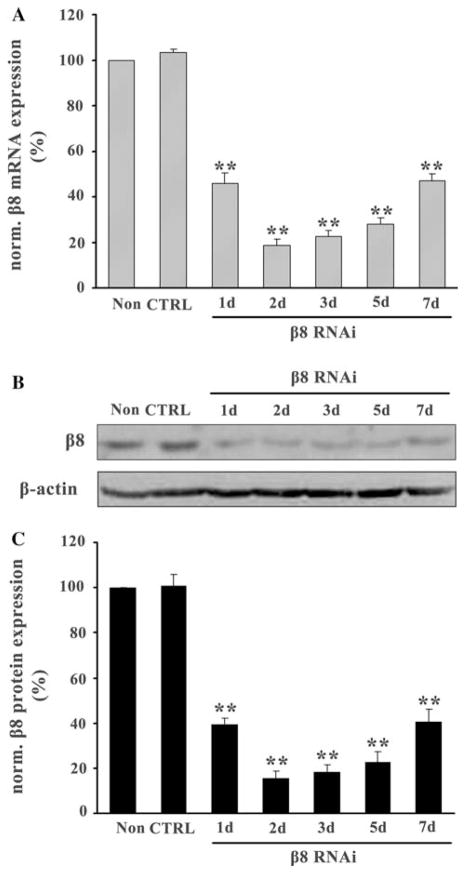
To determine the effects of β8 RNAi on β8 expression in astrocytes, β8 mRNA, and protein expression in β8 siRNA virus infected ( ) and control siRNA virus infected ( ) astrocytes were detected by real-time PCR and western blot at 1, 2, 3, 5, and 7 days after infection. The levels of β8 mRNA and protein were normalized to non-infected astrocytes (Non). We found that β8 mRNA expression was significantly inhibited at 1 day and reached the lowest at 2 days after infection. After quantification, we found that the highest inhibition rate of astrocytes was around 80% at 2 days (P < 0.05, Fig. 3a). Similarly, we found that β8 protein expression was also significantly inhibited in astrocytes with a maximal inhibition rate around 84% at 2 days after infection (P < 0.05, Fig. 3b, c). There was no inhibition of β8 expression in astrocytes (CTRL) (data not shown). Since the maximal inhibition was at 2 days after infection, 2 day of β8 RNAi was used for the following experiments for astrocytes.
Fig. 3.
Effects of RNAi on β8 expression in astrocyte cultures. Real-time RT-PCR and western blot analysis were used to detect the expression of β8 in cultured astrocytes after infection. The relative amounts of β8 mRNA in astrocytes treated with β8 siRNA (β8 RNAi) or control siRNA (CTRL) virus were determined by using the standard-curve method. The highest inhibition rate was around 80% at 2 days (a). Western blot analysis showed β8 protein expression was significantly inhibited in β8 knockdown astrocytes with a maximal inhibition rate around 84% at 2 days (b, c). Results were normalized to the non-infected astrocytes (Non) and represented as mean ± SD. For each column, N = 3, ** P < 0.01 versus Non & CTRL
The Association Between β8 and TGF-β1 Activation
Mink lung epithelia cells (TMLC) were stably transfected with a TGF-β1 responsive fragment of the PAI-1 promoters that can drive its downstream gene, luciferase expression. To test whether astrocytes can activate TGF-β1, we co-cultured astrocytes and TMLC and measured the activation of luciferase using RLU or LU as a readout. RLU was defined as LU minus the background activity of the TMLC reporter cells. The RLU or LU would increase while active TGF-β1 interacts with PAI-1. Therefore, RLU or LU were used to represent TGF-β1 activation in this assay. We found that TGF-β1 activation was increased significantly after co-cultured with astrocytes under normoxic condition (t = 22.35, P < 0.05, Fig. 4a). This upregulation of TGF-β1 was specific because anti-TGF-β antibody could inhibit the upregulation (t = 19.92, P < 0.05, Fig. 4a).
Fig. 4.
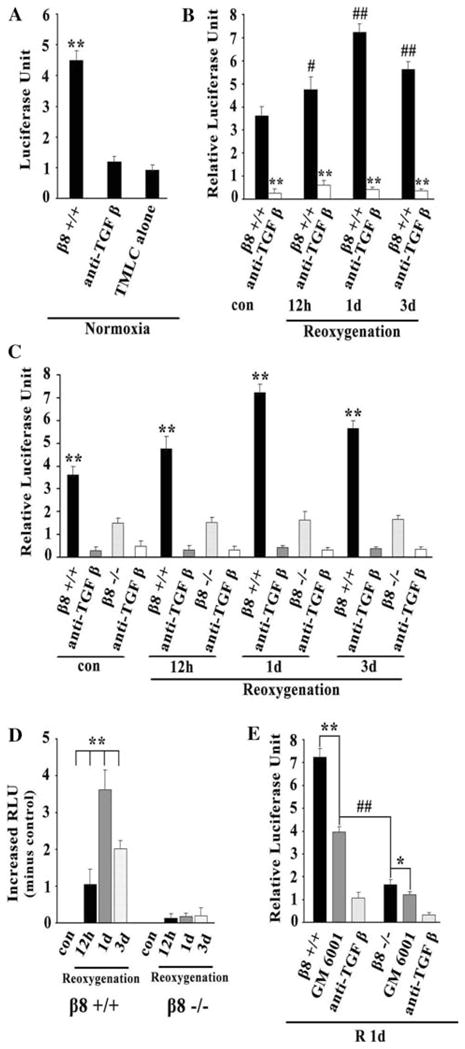
Astrocytes β8 mediates activation of TGF-β1 after HI following reoxygenation. TGF- β 1 activation was increased significantly after co-cultured with astrocytes under normoxic condition (a). TGF- β 1 activation was increased after reoxygenation compared with the normoxic controls (b). TGF- β 1 activation was significantly lower in astrocytes than that in astrocytes (c). TGF- β 1 activation was significantly increased in astrocytes but not in astrocytes after reoxygenation (d). TGF- β 1 activation was inhibited while treated with GM6001, especially by β 8 knockdown ( ) (e). For each column, N = 5, ** P < 0.01 versus anti-TGF β & TMLC alone (a); ** P < 0.01 versus , P < 0.05, ##P < 0.01 versus control (b); ** P < 0.01 versus (c); **P < 0.01 versus control (d); ** P < 0.01 versus GM6001, *P < 0.05 versus GM6001 ##P < 0.01versus (e)
Next, we investigated whether astrocytes with HI could promote TGF-β1 activation. TMLC and astrocytes were co-cultured and then submitted to OGD treatment. We found that TGF-β1 activation increased approximately 1.3-, 1.8-, and 1.5-fold at 12 h, 1 day, and 3 days (F = 74.92, P < 0.05, Fig. 4b) separately after HI following reoxygenation compared with that in the normoxic controls. This TGF-β1 activation induced by HI was also TGF-β1 specific since anti-TGF-β antibody could counteract this upregulation (P < 0.05, Fig. 4b).
To investigate whether TGF-β1 activation was related to β8 expression on astrocytes, TMLC, and β8 knockdown ( ) astrocytes were used for co-culture studies. We found that TGF-β1 activation was significantly increased in astrocytes after co-culture and reoxygenation. However, TGF-β1 activation was not induced significantly in astrocytes. The TGF-β1 activation is significantly lower in astrocytes than that in astrocytes (P < 0.05, Fig. 4c).
To further clarify whether TGF-β1 activation after reoxygenation was induced by increased β8 expression in astrocytes, RLU values at 12 h, 1 day, and 3 days after reoxygenation were normalized by subtraction of the RLU in normoxic controls to obtain the absolute increased RLU value induced by increased β8 after reoxygenation. We noticed that TGF-β1 activation was significantly increased in astrocytes with a peak at 1 day compared with that in normoxic controls (F = 90.73, P < 0.05, Fig. 4d). This TGF-β1 activation was consistent with the above finding that β8 expression peaked at 1 day after HI following reoxygenation in astrocytes (Fig. 2c, e). However, TGF-β1 activation did not show significant difference in astrocytes at different time points after reoxygenation (F = 2.66, P > 0.05, Fig. 4d). These results suggested that increased TGF-β1 activation after reoxygenation was induced by increased β8 expression in astrocytes.
Metalloproteases were proven to be an important factor in the activation of TGF-β1 by β8 in epithelial cells (Mu et al. 2002). To test whether metalloproteases were involved in the activation of TGF-β1 in CNS, GM6001, a metalloproteases inhibitor, was employed in this study. We found that the increased TGF-β1 activation was significantly inhibited in both astrocytes and astrocytes with GM6001 application. After statistical analysis, we found that TGF-β1 activation was inhibited approximately by 45% in astrocytes (t = 9.72, P < 0.05, Fig. 4e) and by 27% in astrocytes (t = 3.67, P < 0.05, Fig. 4e) at 1 day after reoxygenation, respectively. TGF-β1 activation was significantly inhibited by approximately 77% in astrocytes (t = 10.46, P < 0.05, Fig. 4e) compared with that in GM6001 treated astrocytes (by 45%).
The Effects of TGF-β1 Activation on Neuronal Apoptosis After HI
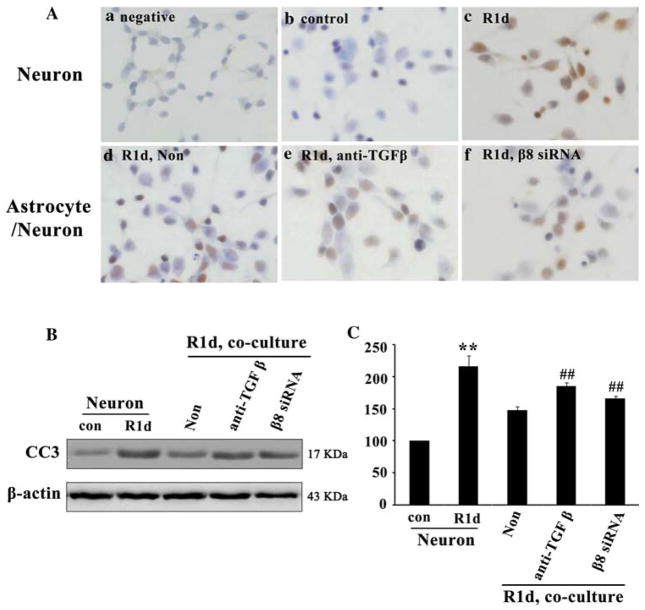
A previous study has shown that TGF-β1 was upregulated in response to CNS injuries (Yu and Fahnestock 2002), which suggests a possible role of TGF-β1 in neuroprotection. We have shown that TGF-β1 was activated by β8 after HI. Therefore, we determined whether β8/TGF-β1 pathway was involved in the protection of neuronal death after HI. Since TUNEL is a method that can provide accurate and rapid detection of apoptotic cells, we measured neuronal apoptosis in co-culture system with TUNEL. We found most of the neurons cultured alone were TUNEL positive with an AI about 60% at 1 day after HI following reoxygenation (Fig. 5Ac, R1d ) compared with the negative and normoxia controls with an AI less than 3% (Fig. 5Aa, Ab). However, when neurons were co-cultured with astrocytes, TUNEL positive cells were significantly decreased with an AI about 40% (Fig. 5Ad, Non) compared with that seen in neurons cultured alone (Fig. 5Ac, R1d). After blocking TGF-β1 with TGF-β antibody or blocking β8 with astrocytes in the co-culture system, we found that TUNEL positive cells with AI about 50% (Fig. 5Ae, Af) was increased than that in non-treated astrocytes (Fig. 5Ad, Non).
Fig. 5.
Integrin β 8/TGF- β 1 signaling pathway protected neurons from apoptosis after OGD. TUNEL positive cells expressed stronger in neurons at 1 day after reoxygenation (Ac, R1d) compared with the neurons in normoxic controls (Ab, con) and in negative controls (Aa). The expression of TUNEL positive cells were significantly decreased while co-cultured with astrocytes (Ad, Non). However, after blocking TGF-β 1 or blocking β 8, the number of TUNEL positive cells was increased (Ae, Af). Western blot analysis showed that CC3 expression was significantly increased at 1 day after reoxygenation in neurons cultured alone (B, R1d) compared with the normoxic controls. CC3 expression was significantly decreased while co-cultured with astrocytes (B, Non), but was rescued while blocking TGF-β (B, anti-TGF β) or β 8 (B, β8 siRNA). Quantification of CC3 expression in neurons cultured alone and neurons co-cultured with astrocytes (C). Results were normalized to normoxic controls and represented as mean ± SD. For each column, N = 4, **P < 0.01 versus con & Non, ## P < 0.01 versus Non
Besides TUNEL staining, caspase-3 expression is a typical method for apoptosis detection (Kothakota et al. 1997). Therefore, cleaved caspase-3 (CC3) protein, an active form of caspase-3, was used to quantify neuronal apoptosis in this study. Similarly, we found that CC3 expression was significantly increased at 1 day after reoxygenation in neurons cultured alone (Fig. 5b, R1d) compared with that in normoxic controls (Fig. 5b). However, CC3 expression was significantly decreased in neurons while co-cultured with astrocytes (Fig. 5b, Non) than that in neurons cultured alone (Fig. 5b, R1d). The decreased CC3 expression was rescued while blocking TGF-β1 using TGF-β antibody or blocking β8 using β8 siRNA treatment (Fig. 5b) in the co-culture system. After normalized with β-actin, we found that CC3 expression was increased approximately 2.2-fold (t = 12.20, P < 0.05, Fig. 5c, R1d) in neurons cultured alone at 1 day after reoxygenation compared with that in normoxic controls (Fig. 5c). However, the increased CC3 was significantly decreased by 45% (t = 6.97, P < 0.05, Fig. 5c, Non) while co-cultured with astrocytes (Fig. 5c). Furthermore, this decreased CC3 expression was significantly rescued by 25% (t = 8.46, P < 0.05, Fig. 5c) or 12% (t = 4.77, P < 0.05, Fig. 5c), respectively, while treated with TGF-β antibody or β8 siRNA in the co-culture system compared with non-treated astrocytes (Fig. 5c, Non).
TGF-β1 Activation and Cell Death Pathways After HI
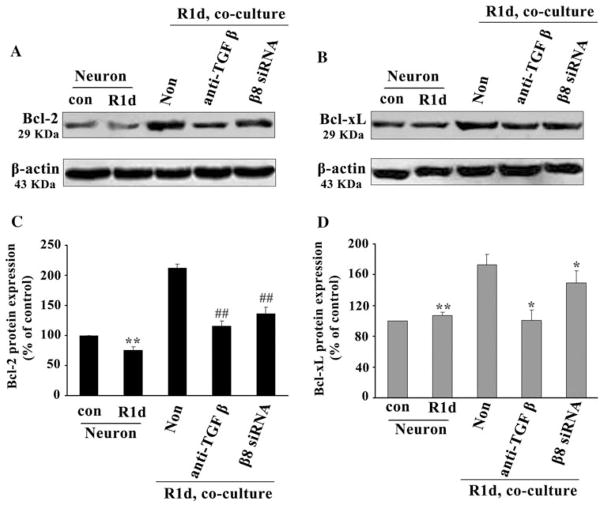
TGF-β1 activation by β8 could inhibit CC3 expression and cellular apoptosis. We then investigate the mechanisms of TGF-β1 activation in the protection of neurons from apoptosis. Since Bcl-2 and Bcl-xL have been proven to be typically anti-apoptotic proteins of the Bcl-2 family members (Burlacu 2003), we detected Bcl-2 and Bcl-xL expression in astrocyte/neuron co-culture system. We found that Bcl-2 protein expression was decreased in neurons cultured alone at 1 day after HI following reoxygenation (Fig. 6a, R1d) compared with the normoxic controls (Fig. 6a). However, Bcl-2 was significantly increased while co-cultured with astrocytes (Fig. 6A, Non) compared with that in neurons cultured alone (Fig. 6A, R1d). This increased Bcl-2 could be partially inhibited by application of either TGF-β antibody or β8 siRNA in co-culture system (Fig. 6a). Even though, Bcl-2 expression still maintained at a higher level than that in neurons cultured alone at 1 day after reoxygenation (Fig. 6a, R1d). After normalized with β-actin, we found an approximately 2.8-fold (t = 67.99, P < 0.05, Fig. 6c, Non) increase of Bcl-2 expression in neurons while co-cultured with astrocytes than that in neurons cultured alone (Fig. 6c, R1d). Meanwhile, we found the increased Bcl-2 protein expression (Fig. 6c, Non) was significantly inhibited by approximately 50% (t = 46.39, P < 0.05, Fig. 6c) or 35% (t = 11.31, P < 0.05, Fig. 6c) after application of TGF-β antibody or β8 siRNA compared with non-treated astrocytes in co-culture system (Fig. 6c, Non). In contrast to Bcl-2, there was no significant change of Bcl-xL protein expression in neurons cultured alone at 1 day after reoxygenation (Fig. 6b, R1d) compared with normoxic controls (Fig. 6b). However, Bcl-xL was significantly increased in neurons while co-cultured with astrocytes (Fig. 6b, Non). This increased Bcl-xL could be reduced after application of TGF-β antibody or β8 siRNA in the co-culture system (Fig. 6b). After normalizing with β-actin, we found an approximately 1.6-fold (t = 8.17, P < 0.05, Fig. 6d, Non) increase of Bcl-xL in neurons co-cultured with astrocytes than that in neurons cultured alone (Fig. 6d, R1d). Meanwhile, the increased Bcl-xL protein expression was inhibited by approximately 30% (t = 4.67, P < 0.05, Fig. 6d) or 15% (t = 3.72, P < 0.05, Fig. 6d) after application of TGF-β antibody or β8 siRNA compared with non-treated astrocytes in co-culture (Fig. 6d, Non).
Fig. 6.
The integrin β8/TGF-β1 signaling pathway protected neurons from apoptosis by upregulating anti-apoptotic protein expression after HI. Western blot analysis showed that Bcl-2 expression was decreased in neurons cultured alone at 1 day after reoxygenation (a, R1d) compared with controls (a, con). However, Bcl-2 was significantly increased while co-cultured with astrocytes (a, Non) but could be partially inhibited by either TGF-β antibody (a, anti-TGF β) or β8 knockdown (a, β8 siRNA). Bcl-xL did not show significant changes in neurons cultured alone at 1 day after reoxygenation (b, R1d) compared with controls (b, con). Bcl-xL was significantly increased while co-cultured with astrocytes (b, Non) but could be reduced by TGF-β antibody (b, anti-TGF β) or β8 knockdown (b, β8 siRNA). Quantification of Bcl-2 and Bcl-xL expression (c, d). Data were normalized to controls and represented as mean ± SD. For each column, N = 5, ** P < 0.01, ## P < 0.01 versus Non (c); **P < 0.01, * P < 0.05 versus Non (d)
Discussion
In this study, we show for the first time that integrin β8 expressed in astrocytes could be upregulated and activate TGF-β1, and thus play an important role in the protection of neurons after HI. This result is consistent with previous findings that TGF-β1 plays neuroprotective roles in CNS after injury (Boche et al. 2003; Zhu et al. 2002b).
Integrin αvβ8 is a cell-surface receptor, which is widely distributed in various tissues including brain. β8 subunit is expressed at a high level in CNS (Nishimura et al. 1998), indicating a specific function of β8 in the brain. However, it is unclear whether β8 can be induced by HI in CNS. In this study, we found that β8 was strongly expressed in astrocytes, and was highly upregulated and maintained at a high level at 7 days after HI following reoxygenation. These findings suggest that the astrocyte is the major source of β8 that might be involved in the signaling downstream effects elicited by growth factors and cytokines in CNS after HI.
TGF-β1, one of the cytokines, is secreted in an inactive form, which is associated with a LAP-β1. Studies have shown that TGF-β1 is activated while dissociated from LAP-β1 through proteolysis or non-proteolysis processes. Many factors such as integrins and MMPs are involved in the activation of TGF-β1 in the proteolysis process (Sheppard 2005; Jenkins 2008). In this study, we found that TGF-β1 activation was upregulated after co-culture with astrocytes, especially after OGD. This TGF-β1 upregulation was associated with astrocytes β8 since β8 knockdown could significantly inhibit TGF-β1 activation. Furthermore, the increased TGF-β1 activation caused by HI was also associated with the increased astrocytes β8 expression since β8 knockdown could eradicate the persistent TGF-β1 activation following reoxygenation. Our findings suggest that astrocytes β8 is a key factor for the persistent TGF-β1 activation in CNS under HI conditions, which is consistent with our previous findings that integrin β8 plays an important role in TGF-β1 activation in epithelial cells (Mu et al. 2002).
Besides the integrins, MMPs are reported to be involved in the activation of TGF-β1 (Jenkins 2008). We employed GM6001, an inhibitor of MMPs, to inhibit the activities of MMPs and found that GM6001 could inhibit TGF-β1 activation in this astrocytes co-culture system after OGD, which agrees with previous reports in epithelial cells and human astrocytes in normoxic cultures (Mu et al. 2002; Cambier et al. 2005). However, the inhibition of TGF-β1 activation by GM6001 was lower (45%) compared to β8 knockdown (77%) in this study. This difference may due to the limited inhibitory efficiency of GM6001, which is just an inhibitor for metalloproteases. There may be other enzymes besides metalloproteases that might be involved in the activation of TGF-β1 by β8 in CNS after HI.
In this study, β8 knockdown could not completely eradicate TGF-β1 activation. The maximal inhibition rate by β8 is not more than 80% compared with the inhibition rate by TGF-β antibody (up to 95%). This difference may due to the TGF-β1 activation generated from other integrins such as αvβ5. Integrin αvβ5 has been proven to be involved in TGF-β1 activation in epithelia cell lines (Asano et al. 2005b; Asano et al. 2006). Furthermore, in primary cultures of postnatal astrocytes, both αvβ5 and αvβ8 were found to be highly expressed (Milner et al. 2001). Although there are no evidence for αvβ5 induced TGF-β1 activation in CNS yet, we could not exclude the possibility that integrin αvβ5 or other integrins may play a role in the activation of TGF-β1 in CNS after HI.
TGF-β1 has been shown to protect neurons from excitotoxic injury (Boche et al. 2003), but the mechanisms of TGF-β1 in the protection of neurons are not clear. We, therefore, studied whether TGF-β1 can protect cultured neurons from HI injury and the cell death signaling. We found that neuronal apoptosis was significantly reduced after co-cultured with astrocytes. Blocking of either β8 or TGF-β1 in co-culture system could increase neuronal apoptosis. These findings suggest that β8/TGF-β1 signaling pathway plays an important role in protecting neurons from apoptosis after HI injury.
Whether β8/TGF-β1 signaling pathway is involved in the regulation of anti-apoptotic proteins is of interest. Bcl-2 and Bcl-xL, both typical anti-apoptotic proteins (Burlacu 2003), were chosen to further probe the possible mechanisms of TGF-β1 activation in neuroprotection after HI following reoxygenation. We found that neuronal Bcl-2 protein was significantly decreased at 1 day after reoxygenation compared with normoxic controls, which was consistent with our previous findings that neuronal apoptosis was obvious at 1 day. These findings suggest that decreased Bcl-2 contributed to neuronal apoptosis. In contrast to the decreased expression of Bcl-2, Bcl-xL protein expression was not significantly decreased at 1 day after reoxygenation. Although anti-apoptotic Bcl-xL did not decrease, its isoform, Bcl-xS, a pro-apoptotic protein might increase and contribute to the neuronal apoptosis in this model as shown by other reports in cancer cell lines and germ cells (Zhang et al. 2008; Mishra et al. 2005).
Interestingly, we found that Bcl-2 and Bcl-xL expression were significantly increased in neurons after co-cultured with astrocytes, which could be inhibited by both TGF-β antibody and β8 knockdown. These findings suggest that β8/TGF-β1 signaling pathway is involved in protecting neurons from apoptosis by upregulating anti-apoptotic proteins Bcl-2 and Bcl-xL expression.
In conclusion, we found that integrin β8 was mainly expressed in astrocytes and could activate TGF-β1 after HI. Activated TGF-β1 mediated Bcl-2 and Bcl-xL upregulation leads to neuron survival. These findings suggest that upregulation of integrin β8 may protect neurons against HI, which may be beneficial for the treatment of hypoxic-ischemic brain injury.
Acknowledgments
We sincerely appreciate Dr. Tyler Jack for providing the expression vector pSicoR (MIT Center for Cancer Research, Cambridge, MA). This work was supported by grants from National Natural Science Foundation of China (No.30825039, No.30973236, No.30770748), China Medical Board of New York (00-722), Ministry of Education of China (2006331-11-7 and 20070610092), and Science and Technology Bureau of Sichuan province (JY029-067).
References
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005a;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 2005b;52:2897–2905. doi: 10.1002/art.21246. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168:499–510. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alphav integrin. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Boche D, Cunningham C, Gauldie J, Perry VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003;23:1174–1182. doi: 10.1097/01.WCB.0000090080.64176.44. [DOI] [PubMed] [Google Scholar]
- Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys. 2003;39:13–22. doi: 10.1385/CBB:39:1:13. [DOI] [PubMed] [Google Scholar]
- Fu X, Li Q, Feng Z, Mu D. The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia. 2007;55:935–941. doi: 10.1002/glia.20515. [DOI] [PubMed] [Google Scholar]
- Gabryel B, Trzeciak HI. Role of astrocytes in pathogenesis of ischemic brain injury. Neurotox Res. 2001;3:205–221. doi: 10.1007/BF03033192. [DOI] [PubMed] [Google Scholar]
- Garcia CM, Darland DC, Massingham LJ, D’Amore PA. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res. 2004;152:25–38. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM. A role for hypoxia-inducible factor-1alpha in desferox-amine neuroprotection. Neurosci Lett. 2005;379:96–100. doi: 10.1016/j.neulet.2004.12.080. [DOI] [PubMed] [Google Scholar]
- Janas J, Skowronski J, Van Aelst L. Lentiviral delivery of RNAi in hippocampal neurons. Methods Enzymol. 2006;406:593–605. doi: 10.1016/S0076-6879(06)06046-0. [DOI] [PubMed] [Google Scholar]
- Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40:1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J, Koli K, von Melchner H. TGF-beta activation by traction? Trends Cell Biol. 2004;14:657–659. doi: 10.1016/j.tcb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Dringen R, Giaume C. Pathways of neuron-astrocyte interactions and their possible role in neuroprotection. Eur Arch Psychiatry Clin Neurosci. 2001;251:159–169. doi: 10.1007/s004060170036. [DOI] [PubMed] [Google Scholar]
- Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero D, Mu D. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009 Jul 22; doi: 10.1038/jcbfm.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makwana M, Jones LL, Cuthill D, Heuer H, Bohatschek M, Hristova M, Friedrichsen S, Ormsby I, Bueringer D, Koppius A, Bauer K, Doetschman T, Raivich G. Endogenous transforming growth factor beta 1 suppresses inflammation and promotes survival in adult CNS. J Neurosci. 2007;27:11201–11213. doi: 10.1523/JNEUROSCI.2255-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective association between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Relvas JB, Fawcett J, ffrench-Constant C. Developmental regulation of αv integrins produces functional changes in astrocyte behavior. Mol Cell Neurosci. 2001;18:108–118. doi: 10.1006/mcne.2001.1003. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra DP, Pal R, Shaha C. Changes in cytosolic Ca2+ levels regulate Bcl-xS and Bcl-xL expression in spermatogenic cells during apoptotic death. J Biol Chem. 2005;281:2133–2143. doi: 10.1074/jbc.M508648200. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Nishimura SL, Boylen KP, Einheber S, Milner TA, Ramos DM, Pytela R. Synaptic and glial localization of the integrin alphavbeta8 in mouse and rat brain. Brain Res. 1998;791:271–282. doi: 10.1016/s0006-8993(98)00118-8. [DOI] [PubMed] [Google Scholar]
- Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Schmuck G, Röhrdanz E, Tran-Thi QH, Kahl R, Schlüter G. Oxidative stress in rat cortical neurons and astrocytes induced by paraquat in vitro. Neurotox Res. 2002;4:1–13. doi: 10.1080/10298420290007574. [DOI] [PubMed] [Google Scholar]
- Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Wang Y, Weiss MT, Yin J, Tenn CC, Nelson PD, Mikler JR. Protective effects of N-methyl-D-aspartate receptor antagonism on VX-induced neuronal cell death in cultured rat cortical neurons. Neurotox Res. 2008;13:163–172. doi: 10.1007/BF03033500. [DOI] [PubMed] [Google Scholar]
- Yu G, Fahnestock M. Differential expression of nerve growth factor transcripts in glia and neurons and their regulation by transforming growth factor-beta1. Brain Res Mol Brain Res. 2002;105:115–125. doi: 10.1016/s0169-328x(02)00399-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cheng C, He CL, Zhou YJ, Cao Y. The expression of Bcl-XL, Bcl-XS and p27Kip1 in topotecan-induced apoptosis in hepatoblastoma HepG2 cell line. Cancer Invest. 2008;26:456–463. doi: 10.1080/07357900701683968. [DOI] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schimidt A, Reichardt LF. Beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002a;129:1903–2891. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J Neurosci. 2002b;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]