Abstract
Levels of the necessary nutrient vitamin C (ascorbate) are tightly regulated by intestinal absorption, tissue accumulation, and renal reabsorption and excretion. Ascorbate levels are controlled in part by regulation of transport through at least 2 sodium-dependent transporters: Slc23a1 and Slc23a2 (also known as Svct1 and Svct2, respectively). Previous work indicates that Slc23a2 is essential for viability in mice, but the roles of Slc23a1 for viability and in adult physiology have not been determined. To investigate the contributions of Slc23a1 to plasma and tissue ascorbate concentrations in vivo, we generated Slc23a1–/– mice. Compared with wild-type mice, Slc23a1–/– mice increased ascorbate fractional excretion up to 18-fold. Hepatic portal ascorbate accumulation was nearly abolished, whereas intestinal absorption was marginally affected. Both heterozygous and knockout pups born to Slc23a1–/– dams exhibited approximately 45% perinatal mortality, and this was associated with lower plasma ascorbate concentrations in dams and pups. Perinatal mortality of Slc23a1–/– pups born to Slc23a1–/– dams was prevented by ascorbate supplementation during pregnancy. Taken together, these data indicate that ascorbate provided by the dam influenced perinatal survival. Although Slc23a1–/– mice lost as much as 70% of their ascorbate body stores in urine daily, we observed an unanticipated compensatory increase in ascorbate synthesis. These findings indicate a key role for Slc23a1 in renal ascorbate absorption and perinatal survival and reveal regulation of vitamin C biosynthesis in mice.
Introduction
Vitamin C (ascorbate) is synthesized by most mammals. Humans lack the terminal enzyme gulonolactone oxidase in the synthesis pathway and rely on dietary intake for ascorbate (1). Ascorbate is indispensable: without it, the fatal deficiency disease scurvy inexorably occurs (2). Until 2000, scurvy prevention with an estimated safety margin was the basis for vitamin C recommended dietary allowances (RDAs; refs. 3, 4). For many decades, the essential criterion for all RDAs was “the amount of nutrient required to prevent the appearance of signs and symptoms caused by a lack of the nutrient,” based on nutrient intakes from foods (ref. 5; italics in original). Intake recommendations for vitamin C can be thought of as that amount of ingested vitamin that produces a concentration in vivo associated with a functional outcome. Until 2000, that functional outcome for vitamin C was prevention of scurvy with an arbitrary margin of safety (6).
We proposed that nutrient recommendations and public health would be better served by having a concentration-function basis for intake recommendations, independent of clinical deficiency as a functional outcome. These principles have been incorporated into dietary recommendations for vitamin C and other vitamins (4, 7, 8). However, other than preventing scurvy, in vivo functional consequences of different ascorbate concentrations remain poorly characterized (8).
Because ascorbate is accumulated in cells against a concentration gradient by all tissues other than red blood cells (9), characterizing ascorbate transport properties is a prerequisite for understanding concentration-function relationships in vivo. In humans, the issues are studied, indirectly, by describing dose-concentration relationships and the underlying physiology. In animals, the issues can be studied directly by disrupting candidate transporters and observing physiologic consequences.
In humans, dose-concentration data show that ascorbate concentrations appear to be mediated by 3 mechanisms: intestinal absorption of ingested vitamin, tissue accumulation, and renal reabsorption and excretion (9–11). If ascorbate ingestion in humans is less than 100 mg daily, or less than 2–3 servings of fruits and vegetables, there is a steep relationship between ingested dose and the concentration achieved in plasma and tissues. If ascorbate ingestion is at least 200 mg daily, or 4–5 servings of fruits and vegetables, then tissue concentrations are saturated, usually at millimolar concentrations, and plasma concentrations are saturated at approximately 80–90 μM (4, 10, 11). When daily ingested ascorbate is 200 mg and higher, concerted regulation of plasma and tissue concentrations of ascorbate occurs by the 3 mechanisms above and is termed tight control (10–12). Ingestion of higher amounts of ascorbate at amounts found in foods has minimal effects on plasma and tissue concentrations.
In both humans and animals, the observed physiology of tight control is explained by ascorbate transport and tissue accumulation. Ascorbate is transported as such by at least 2 known sodium-dependent ascorbate transporters: Slc23a1 and Slc23a2 (also referred to as Svct1 and Svct2; refs. 13, 14). In selective cell types, ascorbate may also be accumulated via oxidation (15). In this mechanism, ascorbate oxidizes extracellularly to dehydroascorbic acid, which is transported by facilitated glucose transporters and then reduced intracellularly to ascorbate (15–18). An essential functional requirement for Slc23a2 was revealed in Slc23a2–/– mice, which had virtually undetectable ascorbate tissue concentrations compared to Slc23a2+/+ and Slc23a2+/– littermates, and died within minutes after birth (14). These data indicated that Slc23a2 was responsible for ascorbate tissue accumulation, necessary for tight control, and needed for survival. These data also indicated that ascorbate accumulation via dehydroascorbic acid was not an essential mechanism for generalized vitamin C accumulation, although a role for dehydroascorbic acid transport is possible in other tissues not studied. If dehydroascorbic acid transport was widespread, it should have continued to occur in Slc23a2–/– mice and prevented the severe tissue deficiencies observed. Unfortunately, because Slc23a2–/– mice die at birth, functional outcomes in relation to tissue transport cannot be studied further.
Our aim here was to determine the functional role of the paralogous epithelial transporter Slc23a1 in vivo. By creating and studying Slc23a1–/– mice, we investigated whether Slc23a1 contributes to ascorbate renal reabsorption and maintenance of ascorbate homeostasis.
Results
Creation of Slc23a1–/– mice.
Northern blot analysis showed abundant Slc23a1 mRNA expression in murine kidney, liver, and small intestine, using β-actin gene expression as control (Figure 1A). The major transcript was approximately 2.2 kb, with minor transcripts of approximately 1.5 and 2.5 kb. The observed distribution of Slc23a1 gene transcription in these tissues was consistent with the putative role of Slc23a1 as an epithelial transporter.
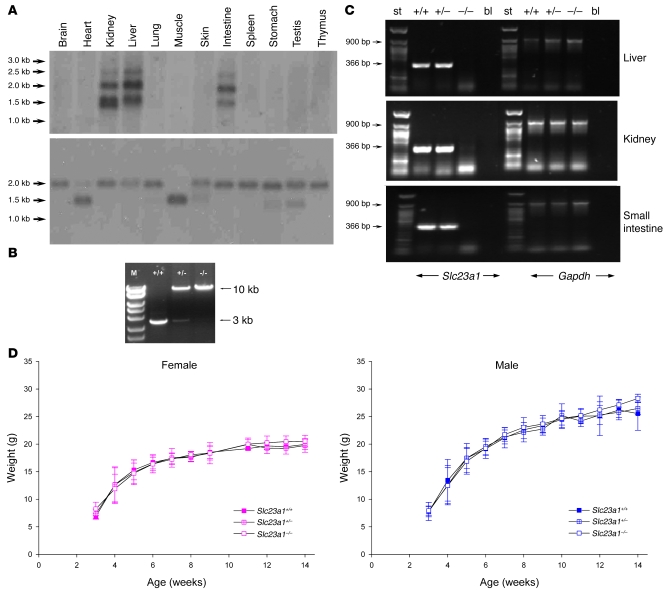
Figure 1. Design, preparation, and confirmation of Slc23a1–/– mice.
(A) Northern blot analysis of mouse Slc23a1 gene expression. Multitissue mouse Northern blot panel probed with [α32P]-d-CTP–labeled mouse Slc23a1 cDNA (top), normalized to β-actin gene expression (bottom). (B) Genomic DNA PCR analyses. DNA obtained from Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– littermates was analyzed by PCR. The wild-type allele was predicted to be a 3-kb fragment; the deletion allele was predicted to be a 10-kb fragment. M, DNA marker standards. (C) RT-PCR analysis of Slc23a1 gene expression in progeny from heterozygous crosses. Gene expression was assessed in liver, kidney, and small intestine from Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice. A 366-bp fragment was amplified by RT-PCR in Slc23a1+/+ and Slc23a1+/– RNA, but not in RNA isolated from Slc23a1–/– mice. As an internal control, gene expression of Gapdh was assessed by amplifying a 900-bp GAPDH PCR product in all tissues analyzed. bl, blank control; st, DNA standards. (D) Body weight as a function of time in Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice. Female (n = 3–19) and male (n = 4–29) littermates were weighed at weaning (3 weeks) and weekly thereafter. P = NS.
To determine whether Slc23a1 participated in maintaining plasma and tissue ascorbate concentrations in vivo, we created Slc23a1–/– mice in which exons 1–12 of the Slc23a1 gene were replaced by a NeoR gene. The mouse Slc23a1 gene was identified (Ensembl gene no. ENSG00000170482) and isolated, and a pPNT targeting construct was generated containing 2 Slc23a1 genomic arms flanking the NeoR gene. One arm was designed to contain 6.8 kb of Slc23a1 genomic sequence, including exons 13–15, and the other was designed to contain 6.1 kb of genomic sequence proximal to Slc23a1 gene from C57BL/6J genomic DNA. Homologous recombination into HGTC-8 ES (14) deletes exons 1–12, which contain 1,474 bp of 1,818 bp of open reading frame (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI39191DS1). The targeting construct was incorporated by electroporation into ES cells, and homologous recombination was determined. Genomic DNA from ES cell lysates was digested and analyzed by Southern blotting, indicating the presence of the targeted mutant allele (Supplemental Figure 2). Stem cells containing the mutant allele were injected into Balb/C blastocysts to generate chimeric mice, which were then crossed with C57BL/6J mice. Germline transmission of the mutant allele from chimeric mice to their F1 offspring was confirmed by long-range PCR genotyping. F1 Slc23a1+/– animals were intercrossed to generate F2 Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice. Long-range PCR genotyping showed that wild-type (3-kb PCR product) and mutant (10-kb PCR product) alleles were present (Figure 1B). RT-PCR analyses of Slc23a1 gene expression confirmed ablation in Slc23a1–/– mice using kidney, small intestine, and liver samples (Figure 1C). Body weights as a function of time were indistinguishable in male and female Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice (Figure 1D). Slc23a1–/– mice had normal growth and development without evidence of scurvy. On pathologic examination, no gross or histologic differences were noted between 12-week-old male and female Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– littermates in liver, kidney, gastrointestinal tract, skin and connective tissues, heart, spleen, endocrine tissues (pancreas, pituitary, adrenal, reproductive, and thyroid), skeletal muscle, brain, and spinal cord. Chemistry and hematology screens were within normal ranges for all groups (data not shown).
Slc23a1 and ascorbate renal reabsorption.
By measuring ascorbate in plasma and urine spot samples from male and female Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice, we determined that Slc23a1 participated in renal reabsorption of the vitamin (Figure 2A). In Slc23a1–/– females, plasma ascorbate concentrations decreased approximately 3-fold, with an equivalently increased concentration in urine. Compared with male Slc23a1+/+ mice, Slc23a1–/– males had 2-fold lower plasma ascorbate concentrations and 3-fold higher urine ascorbate concentrations. In Slc23a1+/– males and females, values were intermediate for plasma and urine concentrations.
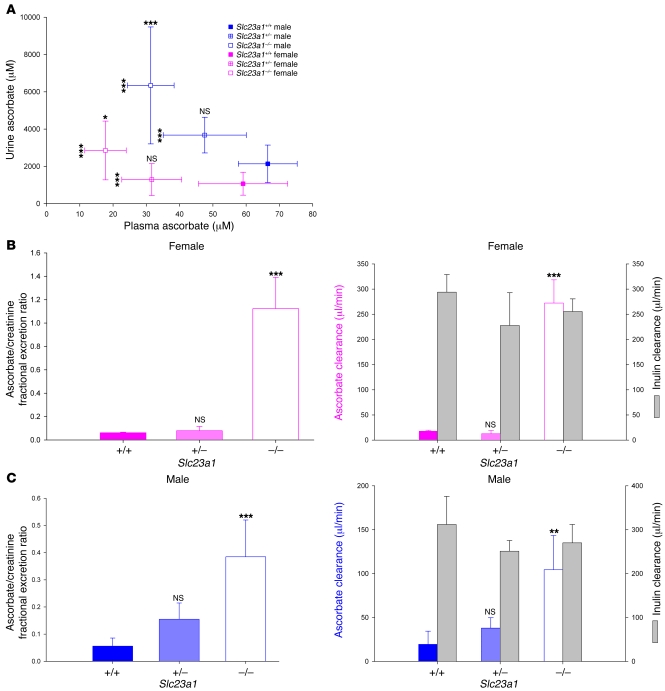
Figure 2. Role of Slc23a1 in ascorbate renal reabsorption and tight control.
(A) Urine ascorbate as a function of plasma ascorbate concentrations. Plasma and urine samples were obtained at the same time in female and male Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice (n = 6–18). Ascorbate concentrations were determined by HPLC as described in Methods. (B and C) Ascorbate fractional excretion and clearance. Plasma and urine was obtained at the same time from individual female (B) and male (C) mice (n = 6 per sex and genotype). Ascorbate, creatinine, and inulin were analyzed, and fractional excretion and clearance values were calculated as described in Methods. Differences between inulin clearances for genotypes of each sex were not statistically significant. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 versus respective control.
To further explore the role of Slc23a1 in vivo, we measured ascorbate fractional excretion relative to creatinine fractional excretion (Figure 2, B and C). For females, values were 0.06 for Slc23a1+/+; 0.07 for Slc23a1+/–; and 1.12 for Slc23a1–/–; moreover, ascorbate clearance in female Slc23a1+/+ mice was approximately 6% of inulin clearance (19, 20), whereas in Slc23a1–/– females, inulin and ascorbate clearances were indistinguishable (Figure 2B).
In male Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice, ascorbate fractional excretion relative to creatinine fractional excretion was 0.05, 0.15, and 0.38, and ascorbate clearances compared with inulin were 6%, 15%, and 39%, respectively (Figure 2C). Considering all data, calculations indicated that ascorbate clearance and fractional excretion increased in Slc23a1–/– mice 16- to 18-fold and 6- to 7-fold (for females and males, respectively) compared with Slc23a1+/+ mice. We conclude that ascorbate reabsorption was completely abolished in females, but only partially abolished in males. However, calculations based on creatinine clearance values are biased in males as a result of their greater muscle mass (19, 21), resulting in an underestimation of calculated male ascorbate clearance and fractional excretion compared with females. Consistent with this, urine/plasma creatinine ratios for male Slc23a1–/– mice were approximately 50% higher than for female Slc23a1–/– mice (data not shown). Inulin clearances were not significantly different between wild-type and knockout animals of each sex, indicating that the observed changes in kidney ascorbate reabsorption were a consequence of targeted disruption of Slc23a1, and not a general effect on kidney function.
Slc23a1 activity in small intestine and liver.
Slc23a1 was also expressed in small intestine and liver (Figure 1A). We assessed the impact of Slc23a1 deletion on small intestinal and liver ascorbate transport by administering the ascorbate analog 6-bromo-6-deoxy-L-ascorbate, a specific substrate for ascorbate transporters (22). Use of this compound permits analysis of intestinal uptake in vivo, without interference from endogenous ascorbate. In intestinal mucosa, accumulation of 6-bromo-6-deoxy-L-ascorbate was similar regardless of genotype (Figure 3A). After absorption, initial portal venous 6-bromo-6-deoxy-L-ascorbate levels should be much higher than subsequent values in the general circulation. The best chance for 6-bromo-6-deoxy-L-ascorbate to enter liver is when its concentration is highest, so that entry is not competed by endogenous ascorbate. In liver, analog accumulation was either drastically reduced or undetectable in male and female Slc23a1–/– mice compared with respective controls (Figure 3B). Slc23a1+/– males had intermediate analog uptake in liver, also consistent with their ascorbate levels in plasma and urine samples (Figure 2A). Urinary excretion of the analog in male and female Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice was similar (Figure 3C), providing additional evidence that 6-bromo-6-deoxy-L-ascorbate was absorbed. Together, these data suggest that another pathway independent of Slc23a1 mediates ascorbate intestinal transport, whereas ascorbate accumulation via the hepatic portal system is mediated primarily by Slc23a1.
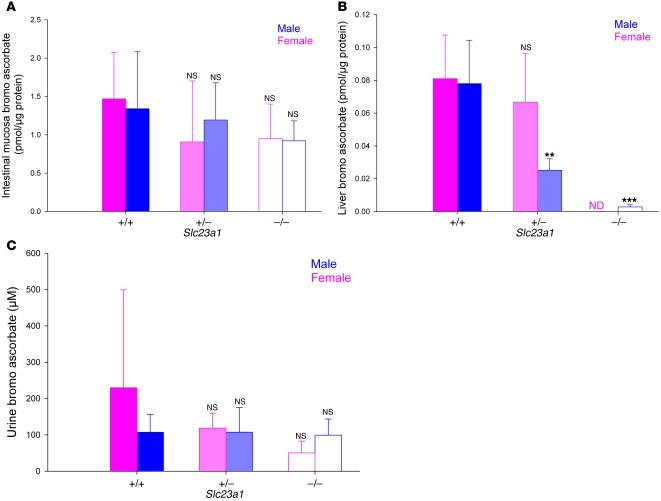
Figure 3. Ascorbate transport activity in intestine and liver of male and female Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice.
(A–C) Biodistribution of orally administered 6-bromo-6-deoxy-L-ascorbate. Male and female Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice received 0.3 mg 6-bromo-6-deoxy-L-ascorbate by gavage. After 30 minutes, urine was collected by bladder massage, and mice were immediately sacrificed. 6-Bromo-6-deoxy-L-ascorbate was measured in (A) intestinal mucosa (n = 5), (B) liver (n = 5), and (C) urine (n = 6). ND, no peak detected. **P ≤ 0.01, ***P ≤ 0.001 versus respective control.
Urine excretion of 6-bromo-6-deoxy-L-ascorbate indicated that intestinal absorption occurred, but this is a qualitative rather than a quantitative measure of absorption. After 6-bromo-6-deoxy-L-ascorbate is absorbed, its tissue transport and renal reabsorption is competitively inhibited by endogenous ascorbate. Competitive kinetics for these substrates were not comparable between Slc23a1+/+ and Slc23a1–/– mice because their endogenous ascorbate plasma concentrations were substantially different (Figure 2A). Ascorbate absorption via drinking water was quantitated in male and female Slc23a1–/– mice and was not statistically different from that in Slc23a1+/+ controls (data not shown). Together, these data suggest that Slc23a1–/– mice have an alternate mechanism for ascorbate absorption.
Slc23a1 and perinatal mortality.
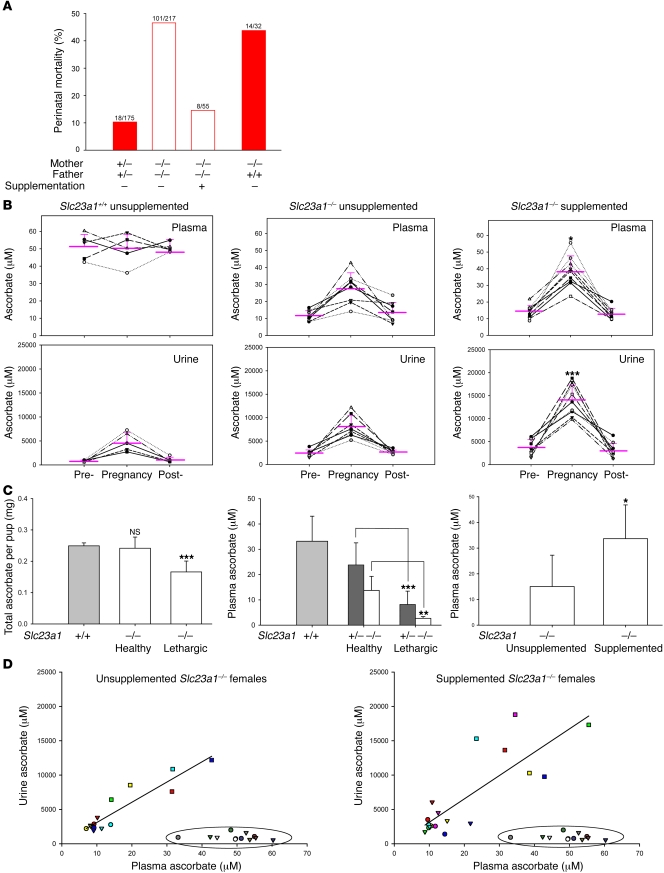
Although controversial, some human studies suggest an association between low intake of vitamins C and E and increased perinatal morbidity and/or mortality (23–28). To explore this, controls were performed to assess mortality in newborn pups. Male and female Slc23a1+/– mice were crossed, and mortality of newborn pups was approximately 10% (Figure 4A). Male and female Slc23a1–/– mice were then crossed, and Slc23a1–/– females were observed through pregnancy and delivery. In the perinatal period, 45% of pups died (Figure 4A). Of the 101 deaths, 72% occurred within 72 hours of birth, and the remaining deaths were either stillbirths (11%) or retained pups (17%). Deceased pups had no obvious pathologic abnormalities. Stomach milk was absent in pups that died after birth, indicating these pups did not nurse. We hypothesized that ascorbate supplementation of pregnant Slc23a1–/– mice would decrease mortality of newborn pups. Slc23a1–/– pregnant females were supplemented with 330 mg/l ascorbate in drinking water daily, an amount previously used to maintain L-gulono-γ-lactone oxidase–deficient (gulo–/–) mice, which are unable to synthesize ascorbate (29). Perinatal mortality was reversed with ascorbate supplementation (Figure 4A). Next, we investigated whether mortality would occur if the dams were knockouts but the pups were not. Slc23a1+/+ male and Slc23a1–/– female mice were crossed: the pups, all heterozygous, had mortality similar to that of pups born to Slc23a1–/– females crossed with Slc23a1–/– males (Figure 4A).
Figure 4. Dam and pup ascorbate and perinatal survival.
(A) Perinatal mortality in offspring from Slc23a1–/– dams compared with Slc23a1+/– controls. Perinatal mortality was determined after crossing unsupplemented or supplemented mice. Numbers denote deceased pups relative to total pups. (B) Pregnancy and maternal ascorbate concentrations. Plasma and urine ascorbate concentrations in spot samples from female mice (n = 5–9). Samples obtained prior to pregnancy (pre-), within 72 hours before delivery (pregnancy), and 3 weeks postpartum (post-). *P ≤ 0.05, ***P ≤ 0.001 versus unsupplemented Slc23a1–/–. (C) Ascorbate in newborn Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– pups. All samples were obtained less than 24 hours postpartum. Total ascorbate (n = 5–8) and plasma ascorbate concentrations (n = 6) were determined. Additionally, Slc23a1–/– mice were crossed, with dams unsupplemented or supplemented, and plasma ascorbate values were determined (n = 5). (D) Ascorbate urine concentrations as a function of ascorbate plasma concentrations in Slc23a1–/–, Slc23a1–/– supplemented, and Slc23a1+/+ (encircled) female mice. Shown are ascorbate levels in plasma and urine samples from B. Individual dams are represented by different colors, with symbols distinguishing prepregnant (triangle), pregnant (square), and postpartum (circle) levels. Left: r2 = 0.86; x = –0.56 when y = 0 (renal threshold). Right: r2 = 0.67; x = 0.77 when y = 0 (renal threshold).
Ascorbate in plasma and urine was measured before and after pregnancy and during pregnancy within 72 hours of delivery in Slc23a1–/– females mated with Slc23a1–/– males; Slc23a1–/– ascorbate-supplemented females mated with Slc23a1–/– males; and Slc23a1+/+ females mated with Slc23a1+/+ males (Figure 4B). Plasma ascorbate concentrations increased in unsupplemented pregnant Slc23a1–/– mice compared with prepregnancy levels in the same mice (Figure 4B), although ascorbate was undetectable in mouse chow (data not shown). Ascorbate-supplemented Slc23a1–/– pregnant mice had a further increase in plasma ascorbate (Figure 4B), which prevented perinatal mortality (Figure 4A). As expected, urine ascorbate concentrations increased in Slc23a1–/– pregnant mice as plasma concentrations increased (Figure 4B). Increased plasma and urine ascorbate concentrations in supplemented pregnant Slc23a1–/– mice were statistically significant compared with unsupplemented pregnant Slc23a1–/– mice (P < 0.05 and P < 0.001, respectively).
We tested whether low ascorbate measurements in newborn pups (less than 24 hours postpartum) were associated with morbidity and mortality. Male and female Slc23a1–/– mice were mated, and ascorbate values were measured in their progeny. Slc23a1–/– pups displayed no signs of distress (i.e., were healthy) or were lethargic. Healthy nursing Slc23a1–/– pups had higher total body and plasma ascorbate values compared with lethargic Slc23a1–/– pups (Figure 4C). Consistent with our earlier finding that Slc23a1+/– mice conserved ascorbate better than did Slc23a1–/– mice (Figure 2A), when Slc23a1–/– dams were crossed with Slc23a1+/+ males, newborn Slc23a1+/– pups had higher plasma values than did newborn Slc23a1–/– pups (Figure 4C). Despite their increased ability to conserve ascorbate, lower ascorbate values in Slc23a1+/– pups were again associated with increased morbidity: these pups were lethargic. Lethargic Slc23a1+/– and Slc23a1–/– pups all died within 72 hours of birth.
To test whether there is a role of Slc23a1 in ascorbate placental transfer, Slc23a1–/– mice were crossed, and pregnant mice were either unsupplemented or supplemented with ascorbate. Ascorbate plasma values of newborn pups born to ascorbate-supplemented Slc23a1–/– dams were double those of newborn pups born to unsupplemented Slc23a1–/– dams (Figure 4C). These data are also consistent with rescue of Slc23a1–/– pups by ascorbate supplementation to Slc23a1–/– dams (Figure 4A). Using quantitative RT-PCR, we found Slc23a1 mRNA to be present in placenta from Slc23a1+/+ mice, but not Slc23a1–/– mice (data not shown). Taken together, the data suggest that Slc23a1 is not essential for placental ascorbate transfer, although a role for Slc23a1 cannot be excluded in wild-type mice. Placental transport of ascorbic acid is believed to be dependent on Slc23a2 (14), but another transporter cannot be ruled out. Ascorbate must be provided during pregnancy to Slc23a1–/– dams if Slc23a1–/– pups are to have a high survival rate during the perinatal period (Figure 4A). Although endogenous ascorbate synthesis in the fetus begins a few days before birth (30–32), it is insufficient.
The lethargy observed in Slc23a1+/– and Slc23a1–/– pups born to Slc23a1–/– dams was most likely due to low ascorbate concentrations in pups (Figure 4C), and was prevented by ascorbate supplementation to Slc23a1–/– dams (Figure 4A). These data are also consistent with increased plasma ascorbate concentrations in Slc23a1–/– pups born to supplemented Slc23a1–/– dams compared with values in Slc23a1–/– pups born to unsupplemented Slc23a1–/– dams (Figure 4C). After mating with Slc23a1–/– males, pregnant Slc23a1–/– dams were supplemented with carnitine in drinking water, but pup mortality was approximately 70%. Expression of carnitine transporters Slc22a4 and Slc22a5, determined by RT-PCR, was similar in liver, kidney, and placenta of Slc23a1+/+ and Slc23a1–/– dams, and in liver and kidney of Slc23a1+/+ and Slc23a1–/– pups (data not shown). These data suggest that carnitine transport was not disrupted in Slc23a1–/– mice and that carnitine deficiency was not responsible for the observed lethargy in newborn pups. Collagen synthesis was indistinguishable in Slc23a1+/+ and Slc23a1–/– pups (data not shown).
Several conclusions can be made from the data in Figure 4, A–D. First, Slc23a1–/– females lost their renal threshold for ascorbate, as seen when urine ascorbate was plotted against plasma ascorbate in unsupplemented and supplemented pregnant Slc23a1–/– mice (Figure 4D). Second, there must be an alternate means of intestinal absorption other than Slc23a1, as supplemented pregnant Slc23a1–/– mice had increased plasma ascorbate compared with their unsupplemented counterparts, with reversal of perinatal mortality (Figure 4, A–C). Third, Slc23a1 was not required for placental ascorbate transfer. Placental ascorbate transport occurred in supplemented Slc23a1–/– dams, as evidenced by both increased ascorbate plasma values and perinatal survival in pups born to supplemented Slc23a1–/– dams (Figure 4, A and C). Fourth, ascorbate plasma concentrations were associated with a functional outcome distinct from scurvy. Scurvy is expected only when ascorbate plasma concentrations are maintained below 5–10 μM for several weeks in mice unable to synthesize ascorbate (29, 33). However, increased demise of newborn pups of Slc23a1–/– dams occurred even though ascorbate plasma concentrations in dams were approximately 2.5- to 5-fold higher, at 27 μM (Figure 4, A and B). Fifth, despite their increased ability to conserve ascorbate, Slc23a1+/– pups born to Slc23a1–/– dams had the same perinatal mortality as did Slc23a1–/– pups that were the product of Slc23a1–/– parents. Coupled to the finding that perinatal mortality in Slc23a1–/– pups born to Slc23a1–/– dams was prevented by ascorbate supplementation during pregnancy (Figure 4A), we deduce that ascorbate provided by the dam influences perinatal survival. Sixth, the data suggest that ascorbate biosynthesis was upregulated in pregnant mice (Figure 4B). Plasma ascorbate concentrations in pregnant Slc23a1–/– dams increased more than 2-fold compared with Slc23a1+/+ mice, all of whom were fed chow in which ascorbate was undetectable using an ultrasensitive ascorbate assay (22, 34). Although plasma concentrations were constant in Slc23a1+/+ mice before and during pregnancy, urine excretion significantly increased during pregnancy, again consistent with increased ascorbate synthesis.
Clinical implications: SNPs and ascorbate intake.
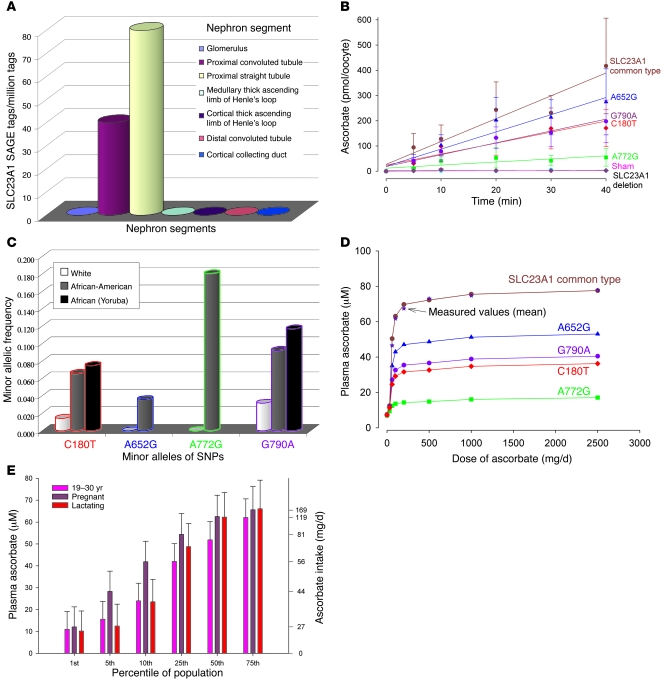
There is potential clinical relevance if disrupted Slc23a1 function in mice is echoed in humans by SNPs in SLC23A1 (35). SLC23A1 was expressed in human kidney and localized to proximal convoluted and straight tubule segments, based on high-resolution mapping of gene expression patterns (Figure 5A). Although preliminary experiments suggested that SNPs did not affect SLC23A1 activity (36), activity was quantitated in more detail here. Using cRNA-injected Xenopus laevis oocytes, SLC23A1 transported ascorbate robustly, whereas an SLC23A1 knockout construct and sham injections lacked activity. We found 1 synonymous and 3 nonsynonymous human SNPs had diminished transport, including an 80% reduction for SNP A772G rs35817838 (Figure 5B). SLC23A1 genotype analyses showed the relative occurrence of these SNPs in African-Americans was 6%–17%; SNP A772G rs35817838, with the largest reduction in ascorbate transport, had the highest prevalence (Figure 5C). For 2 SNPs with available data, frequencies were similar in Africans and African-Americans. In humans, tight control of ascorbate concentrations in relation to dose results in part from ascorbate excretion in urine when oral doses exceed 100 mg daily (10–12), presumably because renal SLC23A1 transport activity approaches Vmax as plasma concentrations exceed 50–60 μM. If SLC23A1 transport activity were impaired, plasma concentrations in relation to dose should decrease as a result of diminished renal reabsorption. To test this, we applied observed transport activities of SLC23A1 with and without nonsynonymous SNPs to a clinical model of ascorbate pharmacokinetics (Figure 5D). As a control, modeled data without SNPs were compared with measurements from healthy women (11), and results were superimposable. The model showed SNP A772G rs35817838 produced an approximate 75% decline in plasma ascorbate concentrations across the dose range, and declines of 40%–50% were produced by SNPs G790A rs33972313, C180T rs6886922, and A652G rs34521685. These data suggest that disruption of tight control that occurs in Slc23a1–/– mice might be recapitulated in humans by some SNPs of SLC23A1.
Figure 5. SLC23A1 SNPs and ascorbate intake.
(A) Distribution of SLC23A1 in kidney nephron segments. Serial Analysis of Gene Expression (SAGE) libraries GSM10419 and GSM10423–GSM10429 (53) containing expression data from microdissected glomeruli and 6 different nephron segments were interrogated (54); values are expressed as tags per million. (B) Effect of SLC23A1 SNPs on ascorbate transport. X. laevis oocytes were microinjected with the following SLC23A1 cRNAs: common type; sham injected; human deletion construct; and SNPs A652G rs34521685, G790A rs33972313, A772G rs35817838, and C180T rs6886922. (C) Population prevalences of SLC23A1 polymorphisms. Shown are averaged minor allelic frequencies of SLC23A1 genotypes in African (n = 48), American-African (n = 438), and white (n = 1,874) individuals, using pooled genotype data (35, 55, 56). (D) Modeled effects of SLC23A1 polymorphisms on plasma ascorbate concentrations in humans. Values in healthy young women for common type SLC23A1 are measured (stars; ref. 11) and calculated fasting steady-state plasma ascorbate concentrations. For women with SNPs, values are calculated. (E) Percentiles of 19- to 30-year-old, pregnant, and lactating women in relation to range of ascorbate plasma concentrations and intake. Plasma concentrations as a function of intake were calculated based on dose concentration pharmacokinetics data (D and ref. 11). Percentiles of women with varying intakes used food intake data (8); the y axis is not continuous because of the sigmoid relationship between ascorbate intake and plasma concentration (D and ref. 11).
Independent of SNPs, it is possible that findings in pregnant mice may have clinical relevance. Perinatal mortality of 45% in Slc23a1–/– pregnant mice was associated with ascorbate plasma concentrations of approximately 27 μM. It is unknown whether rodent findings in pregnancy apply to humans. Given this uncertainty, we further determined that similar ascorbate concentrations were reported in approximately 5% of pregnant US women (Figure 5E). Only a small increase in ascorbate intake in women (Figure 5E), less than 20 mg or one-third a serving of orange juice, produces plasma concentrations that we found sufficient to correct perinatal mortality in mice (Figure 4A).
Ascorbate biosynthesis.
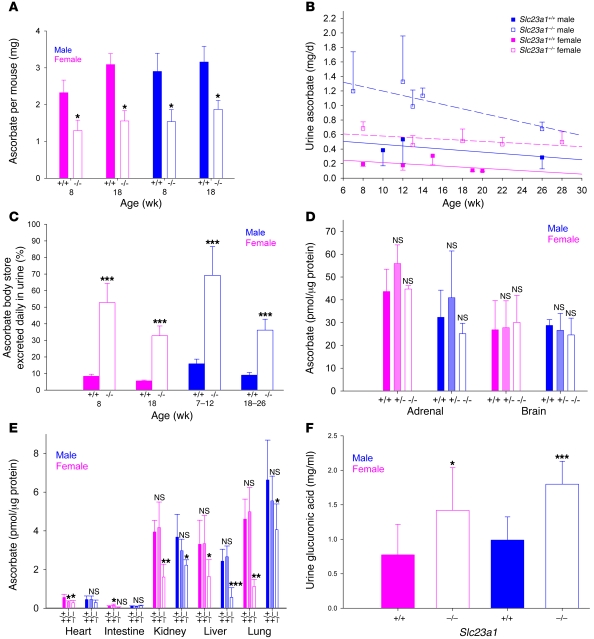
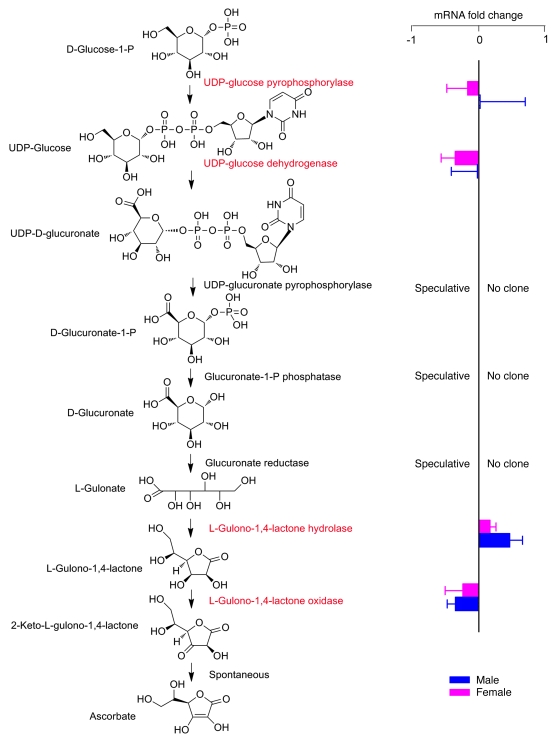
We investigated whether increased ascorbate synthesis that occurred during pregnancy was a general phenomenon in Slc23a1–/– mice. The fraction of total body ascorbate excreted in urine daily by Slc23a1+/+ and Slc23a1–/– mice was determined (Figure 6, A–C). Over several months, Slc23a1+/+ males excreted approximately 10%–17% of total body ascorbate daily, but Slc23a1–/– males excreted as much as 70% of total body ascorbate daily. Slc23a1+/+ females excreted approximately 5%–8% daily, but Slc23a1–/– females excreted as much as 50% daily (Figure 6C). For daily urinary ascorbate loss as a function of age (Figure 6B), we tested whether slopes of all lines were statistically different than 0. The only significantly non-0 slope was that for Slc23a1+/+ females, which may represent a nonspecific age-dependent decrease in glomerular filtration rate. Ascorbate tissue amounts were measured in male and female Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice (Figure 6, D and E). Ascorbate tissue amounts were lower in Slc23a1–/– than in Slc23a1+/+ mice in liver, kidney, and lung; were not lower in adrenal, brain, heart, and intestine; and in no cases were at scorbutic levels (14, 29, 33). Massive ascorbate urinary loss in Slc23a1–/– mice fed ascorbate-deficient chow, without development of scurvy based on ascorbate tissue concentrations (Figure 6, D and E) and with normal growth (Figure 1F), is consistent with upregulation of ascorbate biosynthesis. In agreement, excretion of glucuronic acid, an upstream precursor in the synthesis pathway for ascorbate, was doubled in Slc23a1–/– compared with Slc23a1+/+ mice (Figure 6F). We investigated whether increased ascorbate biosynthesis in Slc23a1–/– mice was caused by increased transcription of ascorbate biosynthetic enzymes. mRNA expression for enzymes in the ascorbate biosynthetic pathway was similar in Slc23a1+/+ and Slc23a1–/– mice (Figure 7), although transcripts are not known for all enzymes. Upregulated ascorbate synthesis may be due to either increased transcription of one of the enzymes not yet cloned or a posttranslational mechanism.
Figure 6. Role of Slc23a1 in ascorbate body content, urinary loss, tissue distribution, and upregulation of synthesis.
(A) Total body ascorbate content of male and female Slc23a1+/+ and Slc23a1–/– mice (n = 6). (B) Amount of ascorbate excreted in urine, as a function of age, by male and female Slc23a1+/+ and Slc23a1–/– littermates aged 7–28 weeks. For clarity, error bars are unidirectional. The slope of all lines except that for Slc23a1+/+ females (P < 0.05) was not significantly different from 0. (C) Percent ascorbate body stores excreted in urine by male and female Slc23a1+/+ and Slc23a1–/– mice. Calculations based on data in A and B. (D and E) Ascorbate in tissues isolated from male and female Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– littermates. Tissues with the highest ascorbate concentrations are grouped in D; others are shown in E. (F) Urinary glucuronic acid excretion (n = 8). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 versus respective control.
Figure 7. Differential expression of genes in the ascorbic acid biosynthesis pathway in livers of male and female Slc23a1–/– and Slc23a1+/+ mice.
Differences were not statistically significant.
Discussion
We generated mice with targeted deletion of Slc23a1 to investigate the mechanisms underlying the remarkably tight control of plasma ascorbate concentrations in vivo. Slc23a1–/– mice lost the ability to reabsorb ascorbate filtered by the kidneys, with ascorbate clearance into urine increasing 18-fold in female mice. Plasma ascorbate concentrations in Slc23a1–/– mice were reduced 50%–70% compared with Slc23a1+/+ mice, with a 3-fold increase in ascorbate urinary loss. These data support the conclusion that Slc23a1 is essential for renal ascorbate reabsorption and tight control of ascorbate plasma concentrations. Despite loss of intestinal Slc23a1, Slc23al–/– mice given supplemental ascorbate or the analog 6-bromo-6-deoxy-L-ascorbate excreted both compounds, which indicates that an alternate mechanism of ascorbate intestinal absorption either exists or is induced in these mice.
Perinatal mortality increased nearly 5-fold in pups born to Slc23a1–/– dams. Increased perinatal mortality was a consequence of low ascorbate concentrations secondary to absent renal ascorbate reabsorption, because perinatal mortality was reversed with maternal oral ascorbate supplementation to compensate for renal loss. Perinatal mortality occurred when maternal ascorbate concentrations were approximately 27 μM and was reversed when these concentrations were increased to approximately 40 μM. Perinatal mortality occurred when the dam was Slc23a1–/–, independent of whether pups were Slc23a1+/– and could partially reabsorb ascorbate or were Slc23a1–/– and could not reabsorb ascorbate. The totality of the data indicated that ascorbate provided by the dam influenced perinatal survival of newborn pups and that low ascorbate in newborn pups was associated with mortality. Mortality of newborn Slc23a1–/– pups was not caused by defective collagen biosynthesis, consistent with normal 4-hydroxyproline concentrations despite very low tissue concentrations of ascorbate in Slc23a2–/– mice (14). Although mRNA for carnitine transporters was expressed in placenta, Slc23a1–/– pups could not be rescued by feeding carnitine to Slc23a1–/– dams throughout pregnancy. These data are consistent with observations that mice unable to synthesize ascorbate have normal carnitine synthesis (37). There was no evidence of scurvy on histopathologic examination of newborn Slc23a1–/– pups compared with Slc23a1+/+ littermates. Deceased newborn pups did not nurse, based on the absence of milk in their stomachs. Lethargy is the first symptom — albeit nonspecific — of vitamin C deficiency in humans (10, 38), and the reason for lethargy remains unknown.
Can the findings in pregnant mice be translated to humans? In some pregnant women, perinatal complications were diminished when plasma ascorbate concentrations were increased from approximately 30 to 45 μM, although a vitamin E supplement was coadministered and study subject numbers were relatively low (24). Vitamin E and vitamin C supplements had no effect on perinatal complications in 2 additional studies (26, 27), but the relationship between ascorbate intake and plasma concentrations was not determined. Ascorbate concentrations at study entry were estimated to be above 50 μM (28), so that additional supplementation would have minimal effects on plasma concentrations (4, 11). Based on our present findings, it is also possible that SLC23A1 SNPs could lower plasma ascorbate concentrations via renal loss, particularly in susceptible populations with marginal ascorbate intake. Obviously, there are myriad differences between mice and humans, as well as transporter expression and activity in vivo versus in Xenopus oocytes, and translational conclusions are absolutely limited by these differences. Whether low ascorbate concentrations in pregnant women may contribute to either perinatal morbidity or mortality can only be determined by obtaining appropriate clinical data.
Concentration-function relationships provide a quantitative and ideal basis of nutrient recommendations (6, 7, 39), although in practice, they are difficult to determine other than using clinical deficiency as an outcome measure. Perinatal mortality associated with low plasma ascorbate concentrations in Slc23a1–/– dams is a new example of a concentration-dependent functional outcome that is not scurvy, although deficiency still appeared to be causally related to death in pups born to these dams. Exploration of concentration-function relationships for vitamin C, as well as other nutrients, that are independent of clinical deficiency has promise to provide new guidelines for nutrition intake recommendations (6, 40, 41).
Based on multiple datasets, an unexpected, major conclusion was that ascorbate synthesis increased in Slc23a1–/– mice and in all pregnant dams. To our knowledge, regulation of ascorbate synthesis in an animal as part of physiology is unprecedented. In agreement with our observations, ascorbate and glucuronic acid excretion increased many-fold in rats given phenobarbital, consistent with upregulation of ascorbate biosynthesis in response to the drug (42). These findings and our present data imply that ascorbate sensing and response mechanisms exist and may be hormonally mediated in animals that make ascorbate. Could a parallel ascorbate sensing system translate to humans, who lack gulonolactone oxidase and are unable to synthesize vitamin C? It is unknown whether the ascorbate synthesis pathway proximal to gulonolactone (Figure 7) remains intact in humans. It is theoretically possible that although humans cannot synthesize ascorbate itself, proximal precursor concentrations are inversely correlated with those of ascorbate. Mechanisms underlying this unanticipated ascorbate sensing system are unknown and deserve to be explored.
Methods
Creation of Slc23a1+/– mouse ES cells and Slc23a1–/– mice.
Mouse Slc23a1 was identified using human SLC23A1 cDNA sequence and the Ensembl Mouse gene view search engine. Using the NCBI BAC clone finder, BAC clone RP23-461O23 from RPCI-23 Mouse BAC Clone library (genomic DNA origin, female kidney/brain C57BL/6J, in vector pBelo Bac11) was obtained from Caltech Children’s Hospital Oakland Research Institute and purified. A targeting vector was constructed that replaced Slc23a1 exons 1–12 with the aminoglycoside phosphotransferase gene (NEOr) conferring neomycin resistance (14). ES cell colonies resistant to G418 (280–300 μg/ml, Geneticin; Invitrogen) and sensitive to 1-(2′-deoxy-2′-fluoro-b-D-arabinofuranosyl)-5-iodouracil (0.2 μM) were screened by Southern blotting to identify correctly targeted cell clones. Next, 2 clones were used for blastocyst injections to establish 2 chimeric lines of mice, which were crossed with inbred 129s6/SvEvTac mice to generate F1 and F2 offspring, which were genotyped by PCR (14, 43). All animal experiments were conducted according to protocols approved by the Animal Care and Use Committee of NIDDK, NIH. Mice were fed chow that had no detectable ascorbate as measured by HPLC. For all experiments, unless otherwise indicated, Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– mice were 12–16 weeks of age.
PCR genotyping of F1 and F2 mouse colonies.
Genomic DNA was isolated from tail biopsies as described previously (44). To detect wild-type allele, primers 5′ exon 3 TATGGTCCAGGTTCAGGACA, 3′ exon 11 TGCAGGAAGCCAAGGACTGGGTAG, mutant allele 5′ NeoR GGTGGAGAGGCTATTCGGCTATGA, and 3′ new cmSlc23a1 CGAATTCAAGGCCAGCTGGTTACAT at final concentration 0.2 μM were used with 1 ng genomic DNA template and TaKaRa one shot LA PCR kit master mix (Takara Bio). Amplification of PCR products was achieved under the following thermo cycling conditions: 98°C for 1 minute; 32–35 cycles of 94°C for 10 seconds and 68°C for 11 minutes; 72°C for 10 minutes; and 4°C for 20 minutes.
RNA purification and reverse transcription.
Total RNA was prepared from small intestine, liver, and kidney from F2 Slc23a1+/+, Slc23a1+/–, and Slc23a1–/– littermates using TRIzol (Invitrogen). To detect Slc23a1 and Gadph gene expression, 1 μg total RNA was used with the following primers: 5′ Slc23a1, CAGCAGGGACTTCCACCAGGGAC; 3′ Slc23a1, CCAGTTACCGTAGATCTCTTC; 5′ Gapdh, GGTCTTACTCCTTGGAGGCCATGT; 3′ Gapdh, GACCCCTTCATTGACCTCAACTACA. Slc23a1 primers were separated by 1.43 kb of genomic DNA and flanked exons 2 and 5, a region deleted in the mutant allele. One-tube long-range RT-PCR Kit (Invitrogen) amplification of PCR products was achieved under the following thermo cycling conditions: 50°C for 30 minutes; 94°C for 2 minutes; 40 cycles of 94°C for 2 minutes, 55°C for 30 seconds, and 68°C for 30 seconds; and 72°C for 10 minutes. PCR products were resolved on a 1% agarose gel. Slc23a1 and Gapdh gene expression was determined by the presence of 366- and 900-bp PCR products, respectively.
Northern blotting.
Mouse Multi-tissue Northern blot panels (Sigma-Aldrich) were probed overnight with [α32P]-d-CTP–labeled mouse Slc23a1, Slc23a2, and β-actin at 60°C in hybridization solution, which contained 4× SSC (150 mM NaCl and 15 mM Na citrate), 150 mM NaCl, 50% formamide, 5× Denhardts, and 100 μg/ml salmon sperm. Filters were then washed in 0.1× SSC and 0.1% SDS for 30 minutes at room temperature and at 50°C. Filters were autoradiographed at –70°C overnight and developed.
Measurements of ascorbate, 6-bromo-6-deoxy-L-ascorbate, creatinine, protein, glucuronic acid, and collagen synthesis.
Blood samples collected from mandibular or cardiac puncture were centrifuged in heparin treated plasma collector tubes (Becton-Dickinson) for 10 minutes at 1,000 g at 4°C. Urine was collected either directly from the bladder or by gently agitating mice until they urinated. Plasma and urine samples were diluted 1:10 and 1:1,000, respectively, in 90% methanol plus 1 mM EDTA, then centrifuged at 25,000 g at 4°C for 15 minutes, and supernatants were frozen at –80°C. 6-Bromo-6-deoxy-L-ascorbate was synthesized as described previously (22). Ascorbate and 6-bromo-6-deoxy-L-ascorbate were analyzed by HPLC with coulometric electrochemical detection (22). Tissue samples (≤100 mg) were harvested from mice and homogenized on ice in 100 μl (adrenal) or 1,000 μl (all other tissues) in ice-cold 90% methanol plus 1 mM EDTA. Samples were then centrifuged at 25,000 g at 4°C for 15 minutes. Supernatants were collected and diluted in 1:10 (heart) or 1:100 (adrenal, small intestine, brain, liver, lung, and kidney) in 90% methanol plus 1 mM EDTA for ascorbate analysis. Pellets were diluted in 1 ml CHAPS for protein assay (Pierce). Mouse chow was analyzed for ascorbate in the same manner as tissue samples. Glucuronic acid in urine was measured as described previously (45). Collagen synthesis was measured using Masson and picrosirius red staining in multiple sections from each of 5 Slc23a1+/+ and 5 Slc23a1–/– newborn pups (46). Creatinine in plasma and urine was measured by HPLC (47, 48), with modifications (49).
General pathologic examination.
Slc23a1–/– mice (12 weeks old) were sacrificed using carbon dioxide inhalation, and newborn pups were sacrificed by cervical dislocation. After sacrifice, incisions were made over the skull and into the thoracic and abdominal cavities. Whole animals were immediately fixed in Bouin’s solution for 48 hours and then transferred to 70% ethanol solution until sectioning and analysis. Blood was obtained immediately at sacrifice for standard serum chemistries (albumin, alkaline phosphatase, alanine aminotransferase, amylase, aspartate aminotransferase, blood urea nitrogen, calcium, cholesterol, gamma-glutamyl transferase, glucose, lactate dehydrogenase, inorganic phosphorous, total bilirubin, total protein, triglycerides, and uric acid) and complete blood counts (white and red blood cell counts, white blood cell differential, platelet count, hemoglobin, hematocrit, and red blood cell indices).
Intestinal and liver absorption.
Mice were anesthetized with isoflurane and received by gavage 0.3 mg of 6-bromo-6-deoxy-L-ascorbate in 100 μl PBS. After 30 minutes, animals were sacrificed, and blood, urine, and liver samples were collected. Approximately 15–20 cm of jejuna was removed, and the intestinal lumen was washed several times with ice-cold PBS. The luminal surface was then exposed, and mucosal scrapes were obtained. Blood, urine, and tissue samples were processed for ascorbate and 6-bromo-6-deoxy-L-ascorbate analysis.
To measure bioavailability, Slc23a1+/+ and Slc23a1–/– male and female mice were not supplemented or were supplemented with ascorbate in drinking water at a dose of 660 mg/l, so that ascorbate urinary excretion would be substantially higher than in unsupplemented mice. As noted above, mice were fed chow that had no detectable ascorbate as measured by HPLC. Volumes of water consumed and urine excretion over 24 hours were measured. Samples were processed for ascorbate analysis. Bioavailability was calculated as follows (all measurements in μg), then expressed as a percent, with 100% equivalent to maximal absorption: (Urine ascorbate excretionsupplemented – urine ascorbate excretionunsupplemented)/oral amount ingested.
Pregnancy supplementation studies.
Where indicated, Slc23a1–/– females from the time of mating until delivery were supplemented with 330 mg/l ascorbate in drinking water, the amount previously used to supplement gulo–/– mice (29). Water was changed daily. For carnitine supplementation, Slc23a1–/– females from the time of mating until delivery were supplemented with 5 g/l carnitine in drinking water, similar to amounts used by others (50).
Total ascorbate body stores.
After isoflurane anesthesia and cervical dislocation, Slc23a1–/– mice were cut into 2 pieces; tail were removed, and brain tissue was exposed. Mouse parts were then submerged in 200 ml of 90% methanol plus 1 mM EDTA cooled for at least 1 hour on dry ice. As an internal control for oxidation, 3 mg 6-bromo 6-deoxy-L-ascorbate was added to mice 7 weeks or older, and 0.3 mg was added to pups. Mouse tissues were homogenized (Pro 250 homogenizer; Pro Scientific) at the lowest setting for 1 minute and at full power for 9 minutes. Homogenate (1–2 ml) was centrifuged at 25,000 g for 15 minutes at 4°C. Supernatants were diluted 1:10 in 90% methanol plus 1 mM EDTA for ascorbate and 6-bromo 6-deoxy-L-ascorbate analysis. Total body ascorbate values were corrected based on oxidation of added 6-bromo 6-deoxy-L-ascorbate.
Urine measurements: fractional excretion, glomerular filtration, clearance, and excretion measurements.
Urine and plasma samples for fractional excretion were obtained at the same time and analyzed by HPLC for ascorbate and creatinine. Plasma was obtained by centrifugation of capillary tubes containing whole blood, acquired by mandibular venous puncture. Urine was obtained by bladder massage. Fractional excretion of ascorbate relative to creatinine was based on the calculation shown in Equation 1.
(Equation 1)

Glomerular filtration rate in conscious mice was measured by the technique of single injection fluorescein isothiocyanate–labeled inulin clearance with minimal plasma volume sampling (19, 20, 51). In brief, 5% fluorescein isothiocyanate–labeled inulin was dialyzed overnight against 0.9% NaCl, resulting in a final concentration of approximately 3% fluorescein isothiocyanate–labeled inulin. Dialyzed compound (3.7 μl/g body weight) was injected into the retro-orbital plexus during brief isoflurane anesthesia, from which the animals recovered within about 20 seconds. At 3, 7, 10, 15, 35, 55, and 75 minutes after injection, mice were placed in a restrainer, and approximately 2 μl blood was drawn from the tail vein using a 30-gauge atraumatic needle. Samples were centrifuged, and 500 nl plasma was transferred into a microcapillary tube and diluted 1:10 in 500 mmol HEPES (pH 7.4). To generate a standard curve, 1 μl of approximately 3% fluorescein isothiocyanate–labeled inulin was diluted 1:50, 1:100, and 1:500 in 500 mmol HEPES (pH 7.4). Fluorescence was determined in 1.7 μl of each sample in a Nanodrop-ND-3300 fluorescence spectrometer (Nanodrop Technologies). Glomerular filtration rate was calculated using a 2-compartment model of 2-phase exponential decay.
Ascorbate clearance was calculated as: Fluorescein isothiocyanate–labeled inulin clearance × ascorbate fractional excretion.
Ascorbate excretion per day was calculated as: [Ascorbate (μg)/μl urine] × [24-hour urine volume (μl)].
Ascorbate was measured in spot urine samples obtained by bladder massage. 24-hour urine samples were collected using metabolic cages and were 1.1 ± 0.16 ml/d in Slc23a1+/+ mice (n = 5) and 1.11 ± 0.08 ml/d in Slc23a1–/– mice (n = 6).
Real-time quantitative RT-PCR.
Total RNA was prepared from snap-frozen mouse tissue using TRIzol extraction (Invitrogen) and RNeasy (Qiagen) cleanup following the manufacturers’ protocols. First-Strand cDNA was synthesized using random primers and Superscript III RT (Invitrogen) in a 20 μl reaction. Reactions were diluted 10 fold, and 1–4.5 μl was used as template for each quantitative PCR. TaqMan primers and probes were as supplied (Applied Biosystems). 10-μl reactions were performed in triplicate by using the ABI PRISM 7900 Sequence Detection System or the Step One System (Applied Biosystems). Cycling conditions were 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 0.15 minute and 60°C for 1 minute. For liver tissue, relative amounts of mRNA, normalized by eukaryotic 18S rRNA, were calculated from threshold cycle numbers (i.e., 2–ΔΔCT), according to the manufacturer’s suggestions. As an internal control for all other tissues, expression of the mouse β-actin gene was determined in the same reactions by duplexing using the assay Mm00607939_s1.
X. laevis oocyte transport assay.
Human SLC23A1 cRNA SNP variants and an equivalent deletion construct (based on the mouse Slc23a1–/– construct described above) were prepared by in vitro transcription reaction using the SP6 mMessage mMachine (Ambion). The 3 SNPs A652G rs34521685, G790A rs33972313, and A772G rs35817838 were nonsynonymous, and SNP C180T rs6886922 was synonymous. X. laevis oocytes were isolated and injected with cRNAs as described previously (22). Briefly, ovaries were resected from adult female frogs anesthetized with 3-aminobenzoic acid ethyl ester (2 g per 750 ml; Sigma-Aldrich) in ice water. Ovarian lobes were opened and incubated in 2 changes of OR-2 without calcium (5 mM HEPES, 82.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1 mM Na2HPO4, and 100 μg/ml gentamicin, pH 7.8), plus collagenase (2 mg/ml; Sigma-Aldrich), for 30 minutes each at 23°C. Individual oocytes (stages V and VI) were isolated from connective tissue and vasculature, transferred to calcium-containing OR-2 (1 mM CaCl2), and maintained at 18°C–20°C until injection with cRNA. Oocytes were injected with a Nanoject II injector (Drummond Scientific). Injection volumes were 36.8 nl, and cRNA concentrations 1 ng/nl. Sham-injected oocytes were injected with 36.8 nl water. After injection, oocytes were maintained at 18°C–20°C until experiments were performed. At 3 days after injection, oocytes were equilibrated at room temperature in OR-2. To begin experiments, [14C] ascorbate (300 μM) was added for the times specified. After incubation at room temperature, oocytes were washed 4 times with ice-cold PBS. Individual oocytes per replicate were solubilized with 10% SDS, and internalized radioactivity was quantified by scintillation spectrometry as pmol/oocyte. Each data point represents the mean ± SD of 10 oocytes.
SNP modeling.
Ascorbate absorption, distribution, and renal excretion was studied previously in 22 young healthy volunteers at steady state for doses between 30 and 2,500 mg/d (10, 11). Based on the first data set from men, a multicompartment pharmacokinetic model was developed (52). Ascorbate pharmacokinetic values obtained from 16 normal young women (11) were used to refine this model, which was used to predict plasma ascorbate concentrations in women with SNPs in SLC23A1. Because renal reabsorption depicted in our model is dependent on ascorbate transport by SLC23A1, ascorbate transport into oocytes was used as a measure of SLC23A1 activity. The common type of SLC23A1 was considered as 100% active (equivalent to the mean value of renal reabsorptive parameters in normal volunteers), and a hypothetical 0% active SLC23A1 (sham-injected oocytes or deletion construct) was used as control. Ascorbate transport by SLC23A1 with 3 different SNPs and control were calculated as values between 0% and 100%, based on oocyte data. Subjects were assumed to be homozygous for each SNP shown.
Statistics.
All data are displayed as mean ± SD. When 3 or more groups were compared, 1-way ANOVA was used followed by Tukey’s multiple comparison test (Graphpad Prism version 5.01). When 2 groups were compared, 1-tailed Student’s t test was used (Excel 2002). A P value of 0.05 or less was considered significant.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of NIDDK and National Human Genome Research Institute (NHGRI), NIH. We thank Lisa Garrett and the members of the Transgenic Mouse Core of NHGRI as well as Lauren Brinster (Division of Veterinary Research, NIH) for their excellent technical assistance.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(4):1069–1083. doi:10.1172/JCI39191.
Robert Faulhaber-Walter’s present address is: Department of Nephrology, Medizinische Hochschule Hannover, Germany.
References
- 1.Nishikimi M, Yagi K. Biochemistry and molecular biology of ascorbic acid biosynthesis. Subcell Biochem. 1996;25:17–39. doi: 10.1007/978-1-4613-0325-1_2. [DOI] [PubMed] [Google Scholar]
- 2.[No authors listed]. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 39-1995. A 72-year-old man with exertional dyspnea, fatigue, and extensive ecchymoses and purpuric lesions. N Engl J Med. 1995;333(25):1695–1702. doi: 10.1056/NEJM199512213332508. [DOI] [PubMed] [Google Scholar]
- 3. Food and Nutrition Board (U.S.R.C.)Recommended Dietary Allowances . Washington, DC: National Academy Press; 1989. [Google Scholar]
- 4.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 5.Harper AE. The recommended dietary allowances for ascorbic acid. Ann N Y Acad Sci. 1975;258:491–497. doi: 10.1111/j.1749-6632.1975.tb29307.x. [DOI] [PubMed] [Google Scholar]
- 6.Levine M, Eck P. Vitamin C: working on the x-axis. Am J Clin Nutr. 2009;90(5):1121–1122. doi: 10.3945/ajcn.2009.28687. [DOI] [PubMed] [Google Scholar]
- 7.Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314(14):892–902. doi: 10.1056/NEJM198604033141407. [DOI] [PubMed] [Google Scholar]
- 8. Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds. Vitamin C. In:Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids . Washington, DC: National Academy Press. 2000:95–185. [Google Scholar]
- 9. Levine M, Katz A, Padayatty SJ. Vitamin C. In:Modern Nutrition in Health and Disease . Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Philadelphia, PA: Lippincott Williams and Wilkins. 2006:507–524. [Google Scholar]
- 10.Levine M, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a Recommended Dietary Allowance. Proc Natl Acad Sci U S A. 1996;93(8):3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001;98(17):9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padayatty SJ, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 13.Tsukaguchi H, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399(6731):70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 14.Sotiriou S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8(5):514–517. doi: 10.1038/nm0502-514. [DOI] [PubMed] [Google Scholar]
- 15.Washko PW, Wang Y, Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem. 1993;268(21):15531–15535. [PubMed] [Google Scholar]
- 16.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364(6432):79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 17.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997;272(30):18982–18989. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- 18. May JM, Asard H. Ascorbate recycling. In:Vitamin C: Functions and Biochemistry in Animals and Plants. Asard H, May JM, Smirnoff H, eds. London, United Kingdom: BIOS Scientific Publishers. 2004:139–157. [Google Scholar]
- 19.Qi Z, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286(3):F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 20.Faulhaber-Walter R, et al. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol. 2008;19(4):722–730. doi: 10.1681/ASN.2007060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89(3):457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Corpe CP, et al. 6-Bromo-6-deoxy-L-ascorbic acid: an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J Biol Chem. 2005;280(7):5211–5220. doi: 10.1074/jbc.M412925200. [DOI] [PubMed] [Google Scholar]
- 23.Casanueva E, Polo E, Tejero E, Meza C. Premature rupture of amniotic membranes as functional assessment of vitamin C status during pregnancy. Ann N Y Acad Sci. 1993;678:369–370. doi: 10.1111/j.1749-6632.1993.tb26150.x. [DOI] [PubMed] [Google Scholar]
- 24.Chappell LC, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810–816. doi: 10.1016/S0140-6736(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 25.Siega-Riz AM, Promislow JH, Savitz DA, Thorp JM, Jr, McDonald T. Vitamin C intake and the risk of preterm delivery. Am J Obstet Gynecol. 2003;189(2):519–525. doi: 10.1067/S0002-9378(03)00363-6. [DOI] [PubMed] [Google Scholar]
- 26.Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2005;192(2):520–521. doi: 10.1016/j.ajog.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354(17):1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 28.Padayatty SJ, Levine M. Vitamins C and E and the prevention of preeclampsia. N Engl J Med. 2006;355(10):1065. doi: 10.1056/NEJMc061414. ; author reply 1066. [DOI] [PubMed] [Google Scholar]
- 29.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A. 2000;97(2):841–846. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosso P, Norkus E. Prenatal aspects of ascorbic acid metabolism in the albino rat. J Nutr. 1976;106(6):767–770. doi: 10.1093/jn/106.6.767. [DOI] [PubMed] [Google Scholar]
- 31.Kratzing CC, Kelly JD. Ascorbic acid synthesis by the mammalian fetus. Int J Vitam Nutr Res. 1986;56(1):101–103. [PubMed] [Google Scholar]
- 32.Jenness R, Birney EC, Ayaz KL, Buzzell DM. Ontogenetic development of L-gulonolactone oxidase activity in several vertebrates. Comp Biochem Physiol B. 1984;78(1):167–173. doi: 10.1016/0305-0491(84)90162-7. [DOI] [PubMed] [Google Scholar]
- 33.Kondo Y, et al. Senescence marker protein 30 functions as gluconolactonase in L-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc Natl Acad Sci U S A. 2006;103(15):5723–5728. doi: 10.1073/pnas.0511225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhariwal KR, Hartzell WO, Levine M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am J Clin Nutr. 1991;54(4):712–716. doi: 10.1093/ajcn/54.4.712. [DOI] [PubMed] [Google Scholar]
- 35.Eck P, et al. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet. 2004;115(4):285–294. doi: 10.1007/s00439-004-1167-x. [DOI] [PubMed] [Google Scholar]
- 36.Eck P, Erichsen HC, Taylor JG, Corpe C, Chanock SJ, Levine M. Genomic and functional analysis of the sodium-dependent vitamin C transporter SLC23A1-SVCT1. Genes Nutr. 2007;2(1):143–145. doi: 10.1007/s12263-007-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furusawa H, et al. Vitamin C is not essential for carnitine biosynthesis in vivo: verification in vitamin C-depleted senescence marker protein-30/gluconolactonase knockout mice. Biol Pharm Bull. 2008;31(9):1673–1679. doi: 10.1248/bpb.31.1673. [DOI] [PubMed] [Google Scholar]
- 38. Lind J.Treatise on Scurvy: A Bicentenary Volume Containing a Reprint of the First Edition of A Treatise of the Scurvy. Edinburgh, United Kingdom: Edinburgh University Press; 1951. [Google Scholar]
- 39.Levine M, et al. Ascorbic acid and in situ kinetics: a new approach to vitamin requirements. Am J Clin Nutr. 1991;54(6 Suppl):1157S–1162S. doi: 10.1093/ajcn/54.6.1157s. [DOI] [PubMed] [Google Scholar]
- 40.Ames BN, Elson-Schwab I, Silver EA. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms. Am J Clin Nutr. 2002;75(4):616–658. doi: 10.1093/ajcn/75.4.616. [DOI] [PubMed] [Google Scholar]
- 41.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103(47):17589–17594. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burns JJ, Evans C, Trousof N. Stimulatory effect of barbital on urinary excretion of L-ascorbic acid and nonconjugated D-glucuronic acid. J Biol Chem. 1957;227(2):785–794. [PubMed] [Google Scholar]
- 43. Hogan B, Beddington R, Costantini F, and Lacy E.Manipulating the Mouse Embryo: A Laboratory Manual . Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 44.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzuchin A, Walton RJ, Thibert RJ. Determination of total and conjugated glucuronic acid in serum and urine employing a modified naphthoresorcinol reagent. Biochem Med. 1971;5(2):135–157. doi: 10.1016/0006-2944(71)90082-2. [DOI] [PubMed] [Google Scholar]
- 46.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89(5):397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 47.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 2004;65(5):1959–1967. doi: 10.1111/j.1523-1755.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 48.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol. 2004;286(6):F1116–F1119. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
- 49.Eisner C, et al. Major contribution of tubular secretion to creatinine clearance in mice [published online ahead of print December 21, 2009] . Kidney Int. doi:10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz ML, Rice LM, Gao CL. Dietary carnitine supplements slow disease progression in a putative mouse model for hereditary ceroid-lipofuscinosis. J Neurosci Res. 1997;50(1):123–132. doi: 10.1002/(SICI)1097-4547(19971001)50:1<123::AID-JNR13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 51.Chen L, et al. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292(1):F27–F37. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- 52.Graumlich JF, et al. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res. 1997;14(9):1133–1139. doi: 10.1023/A:1012186203165. [DOI] [PubMed] [Google Scholar]
- 53.Chabardes-Garonne D, et al. A panoramic view of gene expression in the human kidney. Proc Natl Acad Sci U S A. 2003;100(23):13710–13715. doi: 10.1073/pnas.2234604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lash AE, et al. SAGEmap: a public gene expression resource. Genome Res. 2000;10(7):1051–1060. doi: 10.1101/gr.10.7.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erichsen HC, et al. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol. 2006;163(3):245–254. doi: 10.1093/aje/kwj035. [DOI] [PubMed] [Google Scholar]
- 56.National Center for Biotechnology Information. Single Nucleotide Polymorphism database. http://www.ncbi.nlm.nih.gov/projects/SNP/. Updated December 30, 2008. Accessed January 28, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.