Abstract
Src family kinases (SFKs) are modular signaling proteins possessing SH3, SH2, and tyrosine kinase domains. The SH3 and SH2 domains of SFKs have dual roles: they regulate the activity of the kinases, and they also target SFKs to their cellular substrates. We generated a series of novel SFKs by replacing the SH2 and SH3 domains of Hck with the syntrophin PDZ domain. In some constructs, the negative regulatory tyrosine in the C-terminal tail was also replaced with a PDZ ligand sequence. When expressed in mammalian cells, the substrate specificity of the PDZ-kinases was directed to a different group of proteins than wild-type Hck. The PDZ-kinases phosphorylate neuronal nitric oxide synthase (nNOS), a known binding partner of the syntrophin PDZ domain. We also introduced a PDZ ligand at the C-terminus of the adaptor protein Cas. PDZ-Hck kinases phosphorylate the engineered Cas protein in Cas–/– cells and restore the migration defect of these cells. A PDZ-kinase was also functional in rewiring MAPK signaling via an engineered ErbB2 construct containing a PDZ ligand sequence. Several of the PDZ-kinases show autoregulatory properties similar to natural SFKs. Thus, the PDZ–ligand interaction is able to functionally replace the normal SH2–pY527 interaction that regulates SFKs. Our data highlight the modularity and evolvability of signaling proteins.
Like many signaling proteins, Src family kinases (SFKs)1 are molecular switches composed of physically and functionally separable modular domains (1). Their modular structure enables SFKs to participate in diverse signaling pathways (2–4). From N- to C-terminus, SFKs possess unique, SH3, SH2, and tyrosine kinase domains. In the basal state, SFK catalytic domains are inhibited by intramolecular interactions involving the SH2 and SH3 domains. The SH2 domain binds a sequence in the C-terminal tail that requires phosphorylation of Tyr527, and the SH3 domain binds to a linker region between the SH2 and catalytic domains (5, 6). Disruption of these interactions leads to SFK activation and cell transformation (5–8). The SH2 and SH3 domains of SFKs also play an important role in substrate recognition, because many substrates possess ligands for the domains (2, 9). Activated mutants of Src (such as v-Src, the transforming protein from Rous sarcoma virus) use SH3/SH2 interactions to phosphorylate substrates and transform cells, while point mutations and deletions within the SH3/SH2 domains interfere with transformation (2). The domain architecture of SFKs is conserved across all species, including choanoflagellates, the most primitive organisms known to possess SFKs (10). The dual functionality of the SH2 and SH3 domains (substrate targeting and autoregulation) raises the question of which role emerged first in evolution. Studies on choanoflagellate SFKs suggest that substrate targeting evolved earlier and that the complex modes of autoregulation seen in nonreceptor tyrosine kinases arose more recently in evolution (11, 12).
There are a finite number of modular domains, and they recombined to generate novel signaling proteins during the course of evolution (1). Domains can also be recombined experimentally to create novel switch-like proteins (13). The actin polymerization domain of N-WASP was joined with combinations of PDZ and SH3 domains and their respective ligands (14). This led to a variety of novel modular proteins which activated actin polymerization in the presence of exogeneous PDZ/SH3 ligands. The synthetic proteins also displayed different modes of regulation: some were activated by both inputs (PDZ and SH3), while others were activated by a single input, analogous to logic gates (14). A similar study achieved control of Rho-family guanine nucleotide exchange factors (GEFs) using a heterologous regulatory module (15). These studies on WASP and GEFs focused on rewiring the input control of these activities. The dual functionality of SFK SH3 and SH2 domains (regulation and substrate targeting), and the fine-tuning of the intramolecular interactions (16), has suggested that it would be difficult to reengineer them (17).
In this study, we generated novel tyrosine kinases which recapitulated the signaling properties of natural SFKs. In one construct, we replaced the SH2 domain of the SFK Hck with a PDZ domain to redirect the enzyme's substrate specificity. In additional constructs, we replaced the entire regulatory apparatus of Hck with a PDZ domain and C-terminal PDZ ligand sequences. The resulting artificial PDZ-Hck kinases displayed three salient features of modular signaling proteins: (i) their substrate specificity was governed by the PDZ domain; (ii) they displayed autoregulatory properties similar to natural SFKs; and (iii) they were versatile and could be used to rewire two separate signaling pathways. Our data highlight the modularity and evolvability of signaling proteins and suggest that the targeting function of modular domains is most amenable to manipulation.
MATERIALS AND METHODS
Reagents and Antibodies
DMEM, trypsin-EDTA, penicillin, streptomycin, and amphotericin B were purchased from GIBCO (Cellgro). FBS, Polybrene, anti-Flag HRP, and anti-tubulin antibodies were from Sigma. Cas C-20 antibody was from Santa Cruz Biotech (Santa Cruz, CA), and Cas monoclonal antibody was from BD Biosciences (San Jose, CA). Antiphosphotyrosine 4G10 mouse monoclonal and ErbB2 antibody was from Millipore, and anti-pY416 antibody was from Biosource. The ErbB2 hybridoma 4D10 was a kind gift from Dr. Deborah Brown (Stony Brook University). Erk and p-Erk antibodies were from Cell Signaling Technology. HRP conjugated secondary antimouse and antirabbit antibodies, and ECL and ECL+ kits were from Amersham.
Plasmid Construction
For mammalian expression, all constructs were subcloned into pCMV-Tag2B (Stratagene) between the EcoRI and XhoI sites. The neuronal nitric oxide synthase (nNOS) construct was subcloned into pXJ-HA between the BamHI and XhoI sites. For retroviral expression, PDZ-kinase constructs were subcloned into the EcoRI and XhoI sites of pMSCV-IRES-GFP (a kind gift from Dr. Nicholas Carpino, Stony Brook University). To generate Cas-VKESLV, site-directed mutagenesis was performed on wild-type Cas cloned into pCDNA6. Cas-VKESLV was further subjected to site-directed mutagenesis to delete the Src binding sequence of Cas. To produce the kinase-dead ErbB2 construct with a C-terminal PDZ ligand, pCDNA3-kdErbB2 (Dr. Len Neckers, NIH) was subcloned into pBS-SK(±) and site-directed mutagenesis was performed. The kdErbB2-VKESLV was subcloned back into pCDNA3 for mammalian expression. All constructs were confirmed by restriction digestion and sequencing.
Cell Culture and Transient Transfection
SYF cells were purchased from ATCC (Manassas, VA) and gradually adapted for maintenance in DMEM containing 10% FBS and 1× antibiotic/antimycotic at 37 °C in a humidified 5% CO2 incubator. Cells were transiently transfected using TransIT LT1 (Mirus) transfection reagent following the manufacturer's recommendations. Typically, 1–10 mg of DNA was transfected using a DNA:TransIT ratio of 1:2.
Western Blotting and Immunoprecipitation
Cells were transiently transfected and harvested 40 h post transfection in RIPA buffer containing 1 mM sodium orthovanadate and 10 mM sodium fluoride. Protein concentration was estimated using the Bradford assay (Bio-Rad). For analysis of whole cell lysates, lysates (10–50 μg) were separated on 10% SDS–PAGE and transferred onto PVDF membranes. The membranes were probed with anti-Flag, anti-pY416, anti-tubulin and 4G10 antibodies. For immunoprecipitation, lysates (500 μg) were precleared for 1 h at 4 °C, followed by incubation with antibody (1–2 μg) for 2 h or overnight at 4 °C on a rotator. Immunoprecipitates were washed three times with buffer, and proteins were eluted in Laemmli buffer, separated by SDS–PAGE, transferred to PVDF, and analyzed by Western blotting as shown in the figures. Quantification was carried out with the LI-COR Odyssey Quantification software package (version 2.0). All Western blot experiments were performed at least three times, and typical results are shown.
Retrovirus Generation and Infection
Retroviruses were derived essentially as described (18, 19). Briefly, Phoenix retrovirus producer cell lines were obtained from Dr. Nicholas Carpino (Stony Brook) and were maintained in DMEM containing 10% FBS and 1× antibiotic/antimycotic solution. Cells were transiently transfected with 10 μg of the retroviral constructs along with 10 μg helper plasmid. Retroviruses were collected at 32 °C for 2 days at 12 h intervals. Aliquots were frozen at –80 °C. Cas–/– cells were transiently transfected with Cas-VKESLV and selected with 5 μg/mL blasticidin (Sigma) for one week. For infection, 2 × 105 Cas–/– cells stably expressing Cas-VKESLV were plated in a 6 well plate and spin infected with the retroviruses along with Polybrene (4 μg/mL). Two days postinfection >90% cells were GFP positive. The cells were harvested and used for wound healing assays.
Migration and Wound Healing Assays
For migration assays, Cas–/– cells were transiently transfected with Cas-VKESLV along with PDZ-kinase or Hck. The cells were harvested 24 h post transfection and 2 × 105 cells were placed in a 12 well transwell migration assay plate. Cells were allowed to migrate for 12 h in a 5% CO2, humidified incubator maintained at 37 °C. Cells were washed with PBS, harvested from the bottom of the transwell insert and the bottom of the well in a final volume of 100 μL of DMEM, and counted using a hemocytometer. Each condition was processed in triplicate, and the percentage migration was calculated using the formula:
Wound healing assays were performed essentially as described (20). Retrovirally infected Cas–/– cells were plated on 60 mm dishes and grown to confluency. The plates were washed with 1× PBS and multiple wounds were scratched using a P200 pipet tip across the plate. Phase contrast images of wounds were imaged at 10 min intervals for 10–12 h using a Carl Zeiss inverted microscope with 20× objective.
RESULTS
Domain Architecture of Novel Kinases
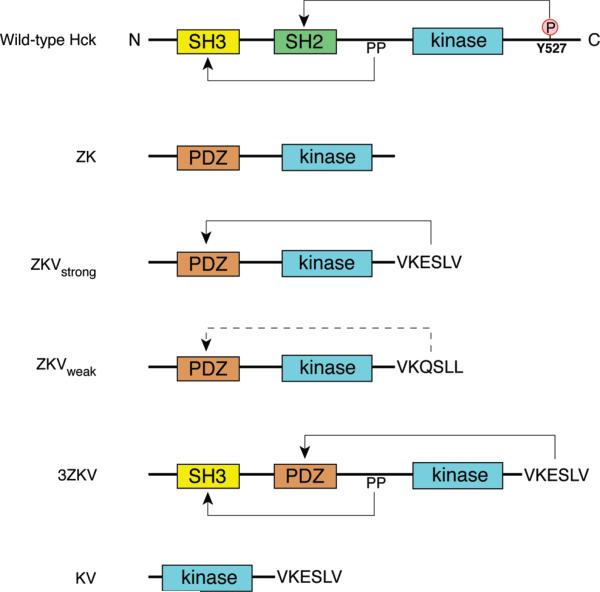
We generated a panel of novel kinases in which the SH2 and SH3 domains of the SFK Hck were replaced by the syntrophin PDZ domain. The PDZ domain was chosen because it is similar in size to the SH2 domain and because it binds to ligands at the C-termini of target proteins. Thus, it could potentially recapitulate both functions of the SH2 domain (substrate targeting and pY527 tail binding). Construct ZK represents the most basic PDZ-kinase, with only the substrate targeting function intact. In constructs ZKVweak and ZKVstrong, the SH2 domain of Hck was replaced by the PDZ domain, and the C-terminal pTyr sequence that normally interacts with the SH2 domain of Hck was replaced by a PDZ ligand (Figure 1). Construct ZKVstrong contains the high-affinity PDZ ligand sequence VKESLV, which binds to the syntrophin PDZ domain with a Kd of 8 μM (21). ZKVweak contains the sequence VKQSLL, which binds with a Kd of 100 μM (14). These values are comparable to the Kd for the Src SH2 domain binding to the relatively low-affinity pY527 tail sequence (29 μM) (22). The length and sequence of the linker between PDZ and kinase domains were designed to provide sufficient flexibility for an intramolecular interaction between PDZ and ligand to occur. In construct 3ZKV, the SH3 domain was retained, while the SH2–pY527 interaction was replaced by the nonnative PDZ–ligand pair. Because 3ZKV contained the polyproline linker sequence of WT Hck between the PDZ and catalytic domains, this kinase potentially has two autoinhibitory interactions, one natural (via the SH3 domain) and one artificial (via the PDZ domain). We also generated the control construct KV, which possesses a kinase domain and C-terminal PDZ ligand sequence but lacks the PDZ domain (Figure 1). All kinase constructs contained an N-terminal FLAG tag, and as a consequence they lacked the normal SFK N-terminal fatty acylation motif. Thus, the targeting signal for membrane-associated substrates was also removed in the PDZ-kinases.
Figure 1.
Domain architecture of Hck and PDZ-kinases. The PDZ-kinases are named according to the position of the domains from N- to C-terminus. The PDZ domain is denoted by Z, the SH3 domain by 3, kinase domain by K, and C-terminal PDZ ligand by V. The polyproline type II helix that binds to the SH3 domain is denoted by PP. ZKVstrong has the high-affinity PDZ ligand sequence VKESLV (Kd = 8 μM) at the C-terminus, while ZKVweak has the low-affinity sequence VKQSLL (Kd = 100 μM). For wild-type Hck, arrows indicate intramolecular autoinhibitory interactions, and for PDZ-kinases, arrows indicate predicted interactions. The dashed lines in ZKVweak denote a weaker affinity interaction between the PDZ domain and the C-terminal PDZ ligand.
PDZ Domain Redirects Specificity of Hck
We first tested whether the presence of the PDZ domain altered Hck substrate specificity. To examine phosphorylation of cellular proteins, we expressed the PDZ-kinases ZKVstrong and ZK in Src/Yes/Fyn triple-knockout (SYF) cells (23) and measured global tyrosine phosphorylation by antiphosphotyrosine immunoblotting of lysates. The pattern of cellular phosphoproteins was markedly different when the PDZ domain was attached to the catalytic domain, as compared to WT Hck or the KV control (Figure 2). A smaller number of proteins are phosphorylated by the KV control than the PDZ-containing kinases ZKVstrong and ZK, consistent with the role of associated domains in directing SFK substrate recognition. We also compared the PDZ-containing kinases to an activated (tail tyrosine mutated) form of Hck (Supplemental Figure 1, Supporting Information). Some cellular proteins appeared to be phosphorylated both by YF-Hck and the PDZ-kinases, but there were clear differences in the lower molecular weight range. The data suggest that the PDZ domain retargeted Hck to an alternative group of substrates. We also observed that the ZKVstrong and 3ZKV constructs, which possessed C-terminal PDZ ligands, were less active than the ZK construct lacking the ligand (Figure 2 and Supplemental Figure 1, Supporting Information), raising the possibility that the engineered PDZ–ligand interaction was functioning to repress kinase activity (see below).
Figure 2.
PDZ domain redirects specificity of the kinase. SYF cells were transiently transfected with wild-type Hck or the PDZ-kinases. The cells were harvested 40 h post-transfection, and whole cell lysates (50 μg) were separated on a 10% SDS–PAGE and transferred onto PVDF membranes. The membrane was probed with anti-pTyr antibody, 4G10. To measure expression, the membrane was probed with anti-Flag antibody. The larger band in the KV lane is a nonspecific band present in anti-FLAG immunoblots. Anti-tubulin blotting was performed as a loading control.
Syntrophin PDZ Domain Directs Phosphorylation of nNOS
One known binding partner of the syntrophin PDZ domain is the PDZ domain of nNOS; the two domains heterodimerize in a linear head-to-tail interaction (24). To test whether the substrate specificity of the PDZ-Hck constructs mirrored the specificity of the associated PDZ domain, we expressed the PDZ-kinases along with nNOS in SYF cells and immunoprecipitated nNOS, and examined its phosphorylation using anti-pTyr Western blotting. All kinase constructs containing a PDZ domain promoted nNOS phosphorylation, while the kinase domain alone (KV) did not (Figure 3), although KV was catalytically active (Figure 2). We found that WT Hck phosphorylates nNOS (Supplemental Figure 2, Supporting Information), presumably due to an interaction with the SH2 or N-terminal unique domain, which are missing in the PDZ-kinases. In the context of the engineered kinases, recognition of nNOS is strictly dependent on the presence of the associated PDZ domain.
Figure 3.
Syntrophin PDZ domain directs phosphorylation of nNOS. SYF cells were transiently transfected with the PDZ-kinases along with HA-tagged nNOS PDZ domain. The cells were harvested 40 h post-transfection and lysates were subjected to immunoprecipitation using anti-HA antibody. The immunoprecipitates were separated by 10% SDS–PAGE, and Western blotting was carried out with anti-pTyr antibody. The membrane were stripped and reprobed with anti-HA antibody to ensure equivalent nNOS PDZ immunoprecipitation. The intensity of the pTyr signal was quantified relative to ZKVstrong, after normalization to the nNOS signal. Kinase expression was measured in whole cell lysates using anti-Flag antibody.
Autoregulation of PDZ-Kinases
Next, we tested for inhibition of kinase activity by the PDZ–ligand interaction. We immunoprecipitated PDZ-kinases from SYF cell lysates and probed for autophosphorylation on the activation loop (pY416) as a measure of kinase activity. Construct ZK (lacking a C-terminal PDZ ligand) displayed strong activity, and ZKVweak was comparable to ZK (Figure 4). ZKVstrong and 3ZKV, with high-affinity PDZ ligands at the C-terminus, were inhibited relative to ZK. Thus, the degree of regulation correlated with the strength of the autoinhibitory interaction. The autophosphorylation of 3ZKV was comparable to WT Hck (Figure 4), suggesting that transplantation of the PDZ ligand can yield a kinase with similar autoregulatory properties as WT Hck.
Figure 4.
Activation state of the PDZ-kinases. SYF cells were transiently transfected with empty vector, ZKVstrong, ZKVweak, ZK, WT Hck, or 3ZKV. Cells were harvested 40 h post transfection and immunoprecipitation reactions were carried out with anti-Flag antibody. Immunoprecipitates were separated on a 10% SDS–PAGE and transferred onto a PVDF membrane. The membrane was probed with anti-pTyr416 antibody and with anti-Flag antibody. In the anti-Flag blot, a nonspecific band that comigrated with ZKVstrong, ZKVweak, and ZK was present in all lanes; this band was consistently observed in anti-Flag blots of SYF cell lysates.
PDZ-Kinase Leads to Phosphorylation of Engineered Cas
Next, we tested whether the PDZ-kinases could be directed toward Cas, a known SFK substrate. Cas is a large, multidomain scaffolding protein that resides in focal adhesions. Cas undergoes rapid phosphorylation following mitogenic stimulation and in response to fibronectin attachment via integrin receptors (25, 26). Phosphorylation of Cas plays a critical role in cell adhesion, migration, and proliferation. Cas contains a C-terminal Src binding site (SBS) with ligands for the SH2 and SH3 domains of Src (27, 28). We added the PDZ ligand VKESLV to the C-terminus of Cas and mutated the SH3 ligand sequence of Cas, which is the most important feature of Src binding (29). The mutated SH3 ligand is designated PPX. We coexpressed Cas-VKESLV-PPX and the PDZ-kinases in Cas–/– cells (cells lacking endogenous Cas) and immunoprecipitated Cas-VKESLV-PPX from the lysates. Constructs containing a PDZ domain target Cas-VKESLV-PPX for phosphorylation, while the kinase domain alone (KV) does not (Figure 5). Thus, PDZ-mediated targeting can position the catalytic domain for phosphorylation of a substrate that is usually driven by SH3/SH2 interactions. Wild-type Hck phosphorylated Cas-VKESLV-PPX weakly, probably due to residual binding between the SH2 domain of Hck and Y668 in the Src binding sequence of Cas-VKESLV-PPX. The order of activity of the synthetic constructs toward Cas-VKESLV-PPX (ZK > ZKVweak >ZKVstrong > 3ZKV) is consistent with the existence of autoregulatory properties in the constructs.
Figure 5.
Reengineered Cas is phosphorylated by PDZ-kinase. (A) A schematic diagram depicting the domain architecture of wild-type Cas and a mutant Cas construct containing a PDZ ligand. The domains of Cas include the SH3 domain, the substrate region with 15 potential tyrosine phosphorylation sites, and the Src binding sequence (SBS). The SBS has a polyproline rich region for Src SH3 domain binding and a tyrosine (Y668) which, when phosphorylated, binds to the Src SH2 domain. In Cas-VKESLV-PPX, the PDZ ligand sequence VKESLV was added to the C-terminus of Cas, and the polyproline region was mutated in order to block interactions with endogenous SFKs. (B) Cas–/– cells were transiently cotransfected with the kinases and Cas-VKESLV-PPX. The cells were harvested 40 h post-transfection and lysates were subjected to immunoprecipitation using anti-Cas antibody. The immunoprecipitates were separated on a 7.5% SDS–PAGE and immunoblotted with anti-pTyr antibody 4G10. The same membrane was stripped and reprobed with anti-Cas antibody to ensure equivalent Cas pull-down. The intensity of the pTyr signal was quantified relative to WT Hck, after normalization to the anti-Cas signal. Kinase expression was assessed by anti-Flag Western blotting of cell lysates.
Reengineered Cas and PDZ-Kinase Restore Migration Defect
Cas–/– cells are defective in cell migration (25, 26, 30). Expression of SFKs alone in these cells is not sufficient to overcome this defect (31, 32). We tested whether the synthetic connection between Cas-VKESLV-PPX and PDZ-kinase could restore migration signaling in these cells. Coexpression of Cas-VKESLV-PPX and PDZ-kinase (ZK), but not wild-type Hck, potentiated cell migration (Figure 6). Cas-VKESLV-PPX alone gave some migration activity, probably due to its phosphorylation via SH2 ligand binding by endogenous SFKs present in Cas–/– cells (Figure 6). The migration activity for ZK plus Cas-VKESLV-PPX was comparable to the activity of WT Hck plus a form of Cas with an intact polyproline sequence (Cas-VKESLV, Figure 6). We confirmed these findings by wound healing assays of Cas–/– cells alone (Video 1) or expressing ZK and Cas-VKESLV-PPX (Video 2). The activity of ZK was also higher than an activated mutant of Hck (YF-Hck), in which the inhibitory tyrosine at the C-terminus was mutated to Phe (Supplemental Figure 3, Supporting Information). These results show that a functional connection between SFKs and Cas can be produced using non-native interactions. The results are consistent with a previous study in which Src was targeted to Cas when the two proteins were fused to amphipathic helices that dimerized to form a coiled-coil (33).
Figure 6.
PDZ-kinase and reengineered Cas restore migration defect of Cas-deficient cells. Migration assays on Cas–/– cells expressing the indicated constructs were carried out in a 96-well transwell migration assay chamber. Expression of kinases and Cas variants was confirmed by Western blotting (Supplemental Figure 4, Supporting Information). Migration experiments were also carried out with Cas-VKESLV, a Cas construct that contains a Src binding sequence with an intact polyproline sequence plus the PDZ ligand at the C-terminus.
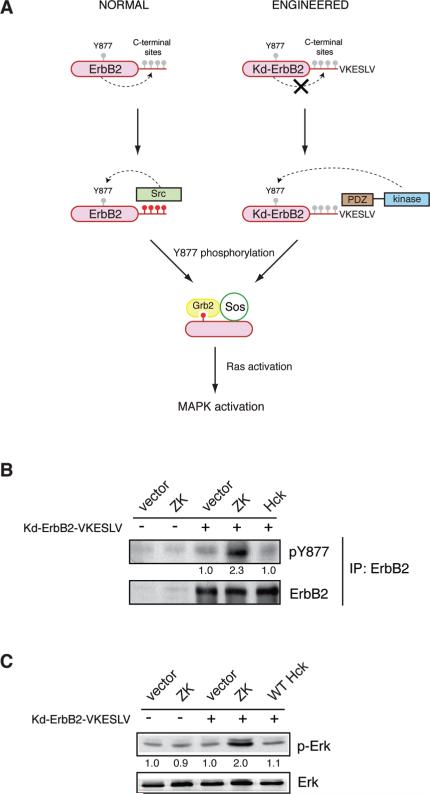
PDZ-Hck and Engineered ErbB2 Restores MAPK Signaling
To test the generality of these observations, we studied PDZ-driven substrate targeting in another system. Autophosphorylation of the RTK ErbB2 leads to the recruitment of SFKs to form a signaling complex. SFKs then phosphorylate Y877 in the activation loop of ErbB2, which creates a binding site for Grb2 and promotes downstream Ras-MAPK signaling (34). We eliminated the normal mode of SFK binding by producing a kinase-dead form of ErbB2. We placed a PDZ ligand (VKESLV) at the C-terminus of kinase-dead ErbB2 (Figure 7A). We co-expressed Kd-ErbB2-VKESLV and PDZ-kinase (ZK) in COS7 cells, immunoprecipitated Kd-ErbB2-VKESLV, and examined phosphorylation of Tyr877 by Western blotting. Whereas WT Hck was unable to phosphorylate Y877 of Kd-ErbB2-VSELV, the PDZ-kinase promoted phosphorylation (Figure 7). Coexpression of Kd-ErbB2-VKESLV and PDZ-kinase also led to an increase in MAPK (Erk) phosphorylation, while the phosphorylation of Erk in cells expressing Kd-ErbB2-VKESLV alone or Kd-ErbB2-VKESLV plus Hck was comparable to COS7 cells (Figure 7). The results point to the flexibility of the targeting function in the associated domains of SFKs and show that cell signaling can be rewired by engineering protein–protein interactions.
Figure 7.
Rewiring of Ras-MAPK signaling. (A) Model for rewired signaling via engineered ErbB2 and PDZ-kinase. Wild-type ErbB2 autophosphorylates sites in the C-terminus, leading to recruitment of Src. Src phosphorylates Y877 of ErbB2, recruiting the Grb2/Sos complex and promoting downstream Ras/MAPK signaling. Kd-ErbB2-VKESLV is not phosphorylated on the C-terminus, but PDZ-kinase binds to the PDZ ligand and phosphorylates Y877. Gray circles represent unphosphorylated tyrosines, and red circles represent phosphorylated sites. (B) COS7 cells were transiently transfected with ZK or Hck in the presence or absence of Kd-ErbB2-VKESLV. The cells were harvested 40 h post-transfection and lysates were subjected to immunoprecipitation using anti-ErbB2 antibody. The immunoprecipitates were separated by 10% SDS–PAGE and Western blotting was carried out with anti-pTyr877 antibody. Gel quantification is given below the pY877 blot. The membranes were stripped and reprobed with anti-ErbB2 antibody to ensure equivalent ErbB2 immunoprecipitation. (C) COS7 cell lysates were separated by 10% SDS–PAGE and probed with anti-pErk and anti-Erk antibodies. Gel quantification is given below the pErk blot. Expression of ErbB2 and kinases was assessed by Western blotting (Supplemental Figure 5, Supporting Information).
DISCUSSION
Extensive domain shuffling occurred during the course of evolution of metazoans. This is readily apparent from the structures of modern signaling proteins, which are constructed with a modular architecture that makes them highly evolvable. The prevalence of domain shuffling is also apparent in choanoflagellates, unicellular organisms that are closely related to metazoans. The genome of the choanoflagellate Monosiga brevicollis contains a number of signaling proteins with domain combinations not found in higher eukaryotes (10, 35, 36).
During the course of evolution, it appears that there has been a gradual separation of catalytic and targeting functions in signaling enzymes (37). Many metabolic enzymes (e.g., phosphofructokinase and hexokinase) have catalytic and regulatory functions integrated in the same domain (38). Eukaryotic Ser/Thr kinases such as PDK1 and MAP kinases possess substrate docking sites in the catalytic domain (39–42). In tyrosine kinases, which arose more recently, the catalytic and specificity-determining modules are separated into independently folding domains. This arrangement makes tyrosine kinases more flexible in terms of evolution, and a variety of domain combinations are found in the families of tyrosine kinases (43–45). Adaptor and scaffolding proteins are among the most recently evolved signaling proteins, indicative of a complete separation of the targeting and catalytic functions on different polypeptides (1, 46).
The modular design of SFKs is crucial for substrate recognition, but it has an additional functional consequence: the SH3 and SH2 domains participate in autoinhibitory interactions that allow SFKs to act as complex switches (5, 6). Theneedto maintain the repressed state of SFKs has been fine-tuned through evolution for cell survival. In this study, we tested whether new functionality could be imparted to such a complex switch by replacing the conserved domains. We replaced the regulatory apparatus of a SFK with a heterologous PDZ domain and PDZ-binding ligand, and tested effects on substrate targeting and regulation.
We observed clear changes in the substrate specificity of the PDZ-kinases, with the PDZ domain acting as the dominant element in substrate recognition (Figures 2, 3, and 5). The phosphorylation of Cas-VKESLV by the PDZ-kinases was compatible with the normal cellular function of Cas in promoting migration (Figure 6). Thus, the normal connections in this signaling pathway can be replaced by heterologous connections. This result is comparable to earlier studies in which Src was retargeted to Cas using a coiled-coil dimerization strategy (33). We also show that the PDZ-kinase can be targeted to ErbB2-VKESLV and that phosphorylation occurs in a manner that enables downstream Ras-MAPK signaling (Figure 7). These findings are consistent with the role of domain shuffling in establishing new connectivities in signaling; these connectivities can be exploited experimentally. For example, using a chimeric adaptor protein consisting of the SH2 domain of Grb2 fused to the death effector domain of Fadd, normally proliferative EGFR signaling was redirected to trigger cell death (47).
Our results also suggest that it is possible to engineer a novel mode of allosteric regulation in a SFK. PDZ and SH2 domains are similar in size and shape, and the preference for PDZ domains binding to protein C-termini suggested that the natural interaction between the SH2 domain and pY-containing tail might be functionally replaced by a PDZ C-terminal tail interaction. Indeed, construct ZKVstrong had lower activity than ZK, which lacks the PDZ ligand (Figures 2–5). Construct ZKVweak, with a low-affinity tail, showed higher levels of activity than ZKVstrong. Although the repression is not as tight as that observed for natural SFKs, these results are consistent with studies on a SFK with a high-affinity SH2 ligand (pYEEI) engineered in the C-terminus; this SFK is more difficult to activate by SH2 ligands than wild-type SFKs, which possess a low-affinity tail sequence (16). The highest level of repression was observed in 3ZKV, which has two possible modes of regulation; in fact, its autoinhibition (as measured by autophosphorylation and Cas phosphorylation) is comparable to that observed for the SFK Hck (Figures 4 and 5). Our results suggest that novel signaling kinases with engineered targeting and regulation might be created. From an engineering standpoint, it appears to be more difficult to create intramolecular interactions which repress catalytic activity than interactions which regulate substrate access. In the latter case, the positioning of the interacting partners on the kinase is more flexible, while relatively precise interdomain interactions are necessary for autoinhibition.
Our studies shed light on how the substrate targeting and regulatory functions in modular signaling proteins might have evolved. Construct ZK, the most basic PDZ-kinase, could be viewed as a model for an early intermediate in SFK evolution. The altered substrate specificity of ZK (Figures 2–5) suggests that substrate targeting may have evolved first as a function of the accessory domains in tyrosine kinases. ZKVweak and ZKVstrong would represent successive stages in the development of allosteric control. Because these constructs lack the specific interdomain contacts observed in c-Src, they do not possess all of Src's regulatory properties (e.g., phosphodependent regulation, cooperativity). Further evolutionary fine-tuning would then be necessary to develop the mature allosteric regulation observed in modern SFKs. Consistent with this, a recent study from our laboratory on choanoflagellate SFKs shows that even though M. brevicollis Src is phosphorylated at the C-terminus regulatory Y527 by Csk, the regulation observed in mammalian SFKs has not yet evolved in this organism (11).
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Nick Carpino (Stony Brook) for providing the retroviral constructs and Phoenix cell line, and Deborah Brown (Stony Brook) for providing us with ErbB2 antibody. We thank Dr. Howard Crawford (Stony Brook) for assistance with luminometer measurements.
Footnotes
This work was supported by National Institutes of Health Grant CA 58530 to W.T.M.
Abbreviations: SH3, Src homology domain 3; SH2, Src homology domain 2; SFKs, Src family kinases; SYF, Src, Yes, Fyn knockout cells; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; PVDF, polyvinylene difluoride; PDZ, domain found in post synaptic density protein (PSD95), Drosophila disk large tumor suppressor (DlgA), and zonula occludens-1 protein (zo-1); NOS, nitric oxide synthase; MAPK, mitogen activated protein kinase; GEF, guanine nucleotide exchange factor; WASP, Wiskott-Aldrich syndrome protein; FBS, fetal bovine serum; HRP, horseradish peroxidase; ECL, enhanced chemiluminescence; DMEM, Dulbecco's modified Eagle medium.
SUPPORTING INFORMATION AVAILABLE
Additional Western blotting and migration experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Bhattacharyya RP, Remenyi A, Yeh BJ, Lim WA. Domains, motifs, and scaffolds: the role of modular interactions in the evolution and wiring of cell signaling circuits. Annu. Rev. Biochem. 2006;75:655–680. doi: 10.1146/annurev.biochem.75.103004.142710. [DOI] [PubMed] [Google Scholar]
- 2.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim. Biophys. Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 5.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr. Opin. Struct. Biol. 1997;7:777–785. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 7.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement [see comments]. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 8.Briggs SD, Sharkey M, Stevenson M, Smithgall TE. SH3-mediated Hck tyrosine kinase activation and fibroblast transformation by the Nef protein of HIV-1. J. Biol. Chem. 1997;272:17899–17902. doi: 10.1074/jbc.272.29.17899. [DOI] [PubMed] [Google Scholar]
- 9.Miller WT. Determinants of substrate recognition in non-receptor tyrosine kinases. Acc. Chem. Res. 2003;36:393–400. doi: 10.1021/ar020116v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Young SL, King N, Miller WT. Signaling properties of a non-metazoan Src kinase and the evolutionary history of Src negative regulation. J. Biol. Chem. 2008;283:15491–15501. doi: 10.1074/jbc.M800002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segawa Y, Suga H, Iwabe N, Oneyama C, Akagi T, Miyata T, Okada M. Functional development of Src tyrosine kinases during evolution from a unicellular ancestor to multicellular animals. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12021–12026. doi: 10.1073/pnas.0600021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dueber JE, Yeh BJ, Bhattacharyya RP, Lim WA. Rewiring cell signaling: the logic and plasticity of eukaryotic protein circuitry. Curr. Opin. Struct. Biol. 2004;14:690–699. doi: 10.1016/j.sbi.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Dueber JE, Yeh BJ, Chak K, Lim WA. Reprogramming control of an allosteric signaling switch through modular recombination. Science. 2003;301:1904–1908. doi: 10.1126/science.1085945. [DOI] [PubMed] [Google Scholar]
- 15.Yeh BJ, Rutigliano RJ, Deb A, Bar-Sagi D, Lim WA. Rewiring cellular morphology pathways with synthetic guanine nucleotide exchange factors. Nature. 2007;447:596–600. doi: 10.1038/nature05851. [DOI] [PubMed] [Google Scholar]
- 16.Porter M, Schindler T, Kuriyan J, Miller WT. Reciprocal regulation of Hck activity by phosphorylation of Tyr(527) and Tyr(416). Effect of introducing a high affinity intramolecular SH2 ligand. J. Biol. Chem. 2000;275:2721–2726. doi: 10.1074/jbc.275.4.2721. [DOI] [PubMed] [Google Scholar]
- 17.Harrison SC. Variation on an Src-like theme. Cell. 2003;112:737–740. doi: 10.1016/s0092-8674(03)00196-x. [DOI] [PubMed] [Google Scholar]
- 18.Mikhailik A, Ford B, Keller J, Chen Y, Nassar N, Carpino N. A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling. Mol. Cell. 2007;27:486–497. doi: 10.1016/j.molcel.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods (San Diego, Calif. 2005;37:208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Harris BZ, Hillier BJ, Lim WA. Energetic determinants of internal motif recognition by PDZ domains. Biochemistry. 2001;40:5921–5930. doi: 10.1021/bi010142l. [DOI] [PubMed] [Google Scholar]
- 22.Bradshaw JM, Grucza RA, Ladbury JE, Waksman G. Probing the “two-pronged plug two-holed socket” model for the mechanism of binding of the Src SH2 domain to phosphotyrosyl peptides: a thermodynamic study. Biochemistry. 1998;37:9083–9090. doi: 10.1021/bi973147k. [DOI] [PubMed] [Google Scholar]
- 23.Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science. 1999;284:812–815. [PubMed] [Google Scholar]
- 25.Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 26.O'Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 27.Nakamoto T, Sakai R, Ozawa K, Yazaki Y, Hirai H. Direct binding of C-terminal region of p130Cas to SH2 and SH3 domains of Src kinase. J. Biol. Chem. 1996;271:8959–8965. doi: 10.1074/jbc.271.15.8959. [DOI] [PubMed] [Google Scholar]
- 28.Burnham MR, Bruce-Staskal PJ, Harte MT, Weidow CL, Ma A, Weed SA, Bouton AH. Regulation of c-SRC activity and function by the adapter protein CAS. Mol. Cell. Biol. 2000;20:5865–5878. doi: 10.1128/mcb.20.16.5865-5878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellicena P, Miller WT. Processive phosphorylation of p130Cas by Src depends on SH3-polyproline interactions. J. Biol. Chem. 2001;276:28190–28196. doi: 10.1074/jbc.M100055200. [DOI] [PubMed] [Google Scholar]
- 30.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patwardhan P, Shen Y, Goldberg GS, Miller WT. Individual Cas phosphorylation sites are dispensable for processive phosphorylation by Src and anchorage-independent cell growth. J. Biol. Chem. 2006;281:20689–20697. doi: 10.1074/jbc.M602311200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg GS, Alexander DB, Pellicena P, Zhang ZY, Tsuda H, Miller WT. Src phosphorylates Cas on tyrosine 253 to promote migration of transformed cells. J. Biol. Chem. 2003;278:46533–46540. doi: 10.1074/jbc.M307526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A, Antoku S, Fujiwara K, Mayer BJ. Functional interaction trap: a strategy for validating the functional consequences of tyrosine phosphorylation of specific substrates in vivo. Mol Cell Proteomics. 2003;2:1217–1224. doi: 10.1074/mcp.M300078-MCP200. [DOI] [PubMed] [Google Scholar]
- 34.Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol. Cell. Biol. 2007;27:220–228. doi: 10.1128/MCB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning G, Young SL, Miller WT, Zhai Y. The protist, Monosiga brevicollis, has a tyrosine kinase signaling network more elaborate and diverse than found in any known metazoan. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9674–9679. doi: 10.1073/pnas.0801314105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pincus D, Letunic I, Bork P, Lim WA. Evolution of the phospho-tyrosine signaling machinery in premetazoan lineages. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9680–9684. doi: 10.1073/pnas.0803161105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim WA. The modular logic of signaling proteins: building allosteric switches from simple binding domains. Curr. Opin. Struct. Biol. 2002;12:61–68. doi: 10.1016/s0959-440x(02)00290-7. [DOI] [PubMed] [Google Scholar]
- 38.Traut T. Allosteric Regulatory Enzymes. SpringerLink; 2008. [Google Scholar]
- 39.Biondi RM, Cheung PC, Casamayor A, Deak M, Currie RA, Alessi DR. Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA. Embo J. 2000;19:979–988. doi: 10.1093/emboj/19.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem. J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frodin M, Jensen CJ, Merienne K, Gammeltoft S. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. Embo J. 2000;19:2924–2934. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holland PM, Cooper JA. Protein modification: docking sites for kinases. Curr. Biol. 1999;9:R329–331. doi: 10.1016/s0960-9822(99)80205-x. [DOI] [PubMed] [Google Scholar]
- 43.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 44.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 45.Krupa A, Srinivasan N. The repertoire of protein kinases encoded in the draft version of the human genome: atypical variations and uncommon domain combinations. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-12-research0066. RESEARCH0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 47.Howard PL, Chia MC, Del Rizzo S, Liu FF, Pawson T. Redirecting tyrosine kinase signaling to an apoptotic caspase pathway through chimeric adaptor proteins. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11267–11272. doi: 10.1073/pnas.1934711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.