Abstract
The NG2 proteoglycan has been shown to promote proliferation and motility in a variety of cell types. The presence of NG2 on oligodendrocyte progenitor cells (OPCs) suggests that the proteoglycan may be a factor in expansion of the OPC pool to fill the entire central nervous system prior to OPC differentiation to form myelinating oligodendrocytes. Comparisons of postnatal cerebellar myelination in wild type and NG2 null mice reveal reduced numbers of OPCs in developing white matter of the NG2 null mouse. Quantification of BrdU incorporation shows that reduced proliferation is a key reason for this OPC shortage, with the peak of OPC proliferation delayed by 4-5 days in the absence of NG2. As a result of the subnormal pool of OPCs, there is also a delay in production of mature oligodendrocytes and myelinating processes in the NG2 null cerebellum. NG2 may promote OPC proliferation via enhancement of growth factor signaling or mediation of OPC interaction with unmyelinated axons.
Keywords: oligodendrocyte progenitors, cell proliferation, differentiation, myelination, NG2 proteoglycan, cerebellum
INTRODUCTION
In contrast to astrocytes, whose support functions and relationships with neurons remain poorly understood in many instances, the function of oligodendrocytes is relatively clear. This class of glia is responsible for myelination and trophic support of axons in the central nervous system (Foran and Peterson, 1992, Relvas et al., 2001, Miller, 2002, Fox et al., 2003, Lappe-Siefke et al., 2003, Bartzokis, 2005, Rosenberg et al., 2007). Myelination is critical for providing the membranous insulation that allows for rapid conduction of nerve impulses in large diameter axons. The structure of myelinated axons, including the architecture of nodes of Ranvier at which saltatory conduction occurs, is well-described, as are the ionic mechanisms underlying impulse conduction itself. However, the molecular signals that control myelination are not known in this type of detail (Gudz et al., 2002, Fox et al., 2003, Papay et al., 2004, Rosenberg et al., 2007). This is also true for the process of remyelination of axons that have lost their myelin sheath due to pathologies such as multiple sclerosis (Franklin et al., 1992, Mathis et al., 2000, Franklin, 2002). Because defects in myelination and re-myelination are not compatible with normal function of the nervous system, the identification of molecules and signaling pathways that regulate myelin development, maintenance, and repair is important from both mechanistic and clinical standpoints.
The NG2 chondroitin sulfate proteoglycan has proved to be one of the most reliable and widely-used markers for oligodendrocyte progenitor cells (OPCs) in the central nervous system (CNS) (Nishiyama et al., 1996b, a, Keirstead et al., 1998, Dawson et al., 2000, Stallcup, 2002). These progenitors are critical for generating the pool of cells that give rise to myelinating oligodendrocytes. Use of OPC markers such as NG2, PDGFRα, and Olig-1/2 has led to the realization that oligodendrocyte progenitors not only generate oligodendrocytes during CNS development, but also persist as the largest population of cycling cells in the mature CNS (Gensert and Goldman, 1997, Dawson et al., 2000, Horner et al., 2000, Arnett et al., 2004). These so-called “adult” OPCs serve as a source of cells for myelin repair (Gensert and Goldman, 1997, Keirstead and Blakemore, 1997), but may also have other poorly-understood functions of mature glia (Nishiyama et al., 2002), including structural contributions to nodes of Ranvier (Butt et al., 1999, Ong and Levine, 1999, Butt et al., 2002) and reception of synaptic input from neurons (Lin and Bergles, 2004, Paukert and Bergles, 2006).
Understandably, studies in these areas have focused almost exclusively on the properties of “NG2 glia” themselves rather than on the importance of the NG2 molecule in development of the oligodendrocyte lineage. Nevertheless, there are good reasons to suspect that NG2 might be an important player in the biology of OPCs. Like many other proteoglycans, NG2 has important co-receptor and/or modulatory roles in a variety of signaling mechanisms. Early work was suggestive of a cooperative relationship between NG2 and PDGFRα, the receptor responsible for mediating OPC responses to the key growth factor PDGF (Nishiyama et al., 1996b, a, Murtie et al., 2005b). Subsequently, NG2 has been shown to contribute to both proliferation and motility in numerous normal and neoplastic cell types (Burg et al., 1997, Burg et al., 1998, Grako et al., 1999), affecting not only growth factor signaling, but also beta-1 integrin activation (Fukushi et al., 2004, Makagiansar et al., 2007, Chekenya et al., 2008). Since cell motility and proliferation are two key factors necessary for generating sufficient numbers of progenitors throughout the CNS, the role of NG2 in progenitor function might well be an important one.
Development of an NG2 null mouse (Grako et al., 1999) has been extremely important in allowing us to investigate the functional importance of the proteoglycan in vivo (Ozerdem and Stallcup, 2004, de Castro et al., 2005, Hossain-Ibrahim et al., 2007, Kadoya et al., 2008). Although subtle in nature, changes resulting from NG2 ablation add to our knowledge of the important accessory roles played by proteoglycans in modulating cellular responses to the microenvironment. Of importance to the current work is the finding that ablation of NG2 leads to delays in neovascularization and skin development due to reduced proliferation of pericytes and keratinocytes, respectively (Ozerdem and Stallcup, 2004, Kadoya et al., 2008). We are now able to demonstrate an important functional role for NG2 in timely development of the pool of OPCs in the early postnatal cerebellum. Ablation of NG2 results in delayed expansion of the OPC pool, with a subsequent delay in oligodendrocyte production and the development of myelinating processes.
EXPERIMENTAL PROCEDURES
Animals
All experimental work was conducted according to guidelines issued by the National Institutes of Health and the Society for Neuroscience, following procedures approved by the Office of Laboratory Animal Welfare. Animal work was performed subsequent to approval by the Burnham Institutional Animal Care and Use Committee. The Burnham vivarium has been an AAALAC accredited facility since 1989. NG2 null mice were generated by a homologous recombination strategy and backcrossed for 10 generations onto the C57Bl/6 background (Grako et al., 1999). The current experiments utilized wildtype (NG2+/+) and knockout (NG2−/−) littermates derived from matings of heterozygous (NG2+/−) parents. Genotyping was done by pcr analysis using the following set of primers. NG2(f): CAG GTC AGA CTT GCC CTG. NG2(r): GCT GCC CGT CAG CCA CAG GC. Neo(r): GCC GCC CCG ACT GCA TCT. In this system, wild type and NG2 null alleles yield products of 600 and 570 bp, respectively.
Tissue preparation and immunohistochemistry
Wild type and NG2-null male littermates were used at postnatal days 1, 4, 6, 7, 9, 14, and 21. For determination of mitotic indices, animals received three consecutive intraperitoneal doses of 5-bromo-2-deoxyuridine (BrdU, 80mg/kg) at 6, 4, and 2 hours prior to euthanasia. For examination of cell cycle progression, a single 80 mg/kg dose of BrdU was given at postnatal day 4, followed by euthanasia after 10 and 20 hour survival periods. The animals were deeply anesthetized with ice and/or Ketamine/Xylazine (100/10 mg/kg) and transcardially perfused with 0.1 M PBS, followed by 4% paraformaldehyde (pH 7.4). The cerebellum was removed and post-fixed in buffered 4% paraformaldehyde for 24 hours at 4°C. The tissue was then cryoprotected for 24 hours at 4°C in 0.1M phosphate buffer containing 20% sucrose. Thereafter, 20 μm transverse sections were cut on a cryostat microtome (Cryocut, 1800) at −16 °C and collected free-floating in 0.1 M PBS containing 0.02% sodium azide.
For immunostaining, free-floating sections were first incubated for 60 min at room temperature in 0.1M PBS containing 5% normal goat serum and 0.8% Triton X-100. Sections were then incubated overnight at 4°C with primary antibodies diluted in PBS containing 0.8% Triton X-100, 0.02% sodium azide, and 5% normal goat serum. The following primary antibodies were used: 1) guinea pig anti-NG2 (1:25; (Ozerdem et al., 2001); 2) rabbit anti-PDGFRα (1:100; (de Castro et al., 2005)); 3) rat anti-BrdU (OBT0030G, Serotec, 1:50); 4) mouse anti-Pan-Axonal Neurofilament (smi-312R, Sternberger, 1:500); 5) mouse anti-Adenomatous Polyposis Coli (Clone CC1, OP80, Calbiochem, 1:50) 6) mouse or rabbit anti-myelin basic protein (MBP, Sternberger MSMI 94, 1:500 or Chemicon, AB980 1:100). After washing three times for 10 min each with PBS, the sections were incubated in appropriate combinations of secondary antibodies: goat anti-mouse (Alexa 488; A11029, Invitrogen), anti-rabbit (Alexa 568; A11036 or Alexa 647; A21245, Invitrogen), donkey anti-guinea pig (Cy2 or Cy3; 706-225-148 or 706-165-148, Jackson ImmunoResearch), and/or goat anti-rat (Alexa 488; A11006, Invitrogen). Secondary antibodies were diluted 1:250 in the same solution as the primary antisera. In the case of BrdU, sections were incubated in 2N HCl for 30 min at 37 °C, followed by boric acid neutralization (pH 8.5) for 10 min, and then processed via the immunostaining protocol described above. 4′-6-diamidino-2-phenylindole (DAPI, 4 g/mL, D3571, Invitrogen) was used for general nuclear staining of all sections. After washing three times for 10 min with PBS, the sections were mounted on slides, air-dried, and then cover-slipped with Vectashield (H-1000, Vector lab).
Quantitative assessment and image processing
For quantitative analyses of various aspects of myelination, wild type and NG2 null littermates from at least 3 separate litters were examined at each time point. For each animal, 3 parasagital sections (located between the superior cerebellar peduncles) were used to scan the DWM region using confocal microscopy (FV 1000 and FV10-ASW Ver. 2.0, Olympus). From each scan a z-stack was captured with 10 optical sections, each separated by 1 μm. The numbers of PDGFR OPCs), APC (oligodendrocytes), and BrdU-immunoreactive (IR) cells were counted in the developing white matter (DWM; see Fig. 2G) of each animal and were normalized to the same volume (106 μm3) using Image Pro Plus 5.1 (MediaCybernetics). The average density of OPCs and oligodendrocytes in animals of the same age and genotype could then be expressed as a mean value ± SD.
Figure 2.
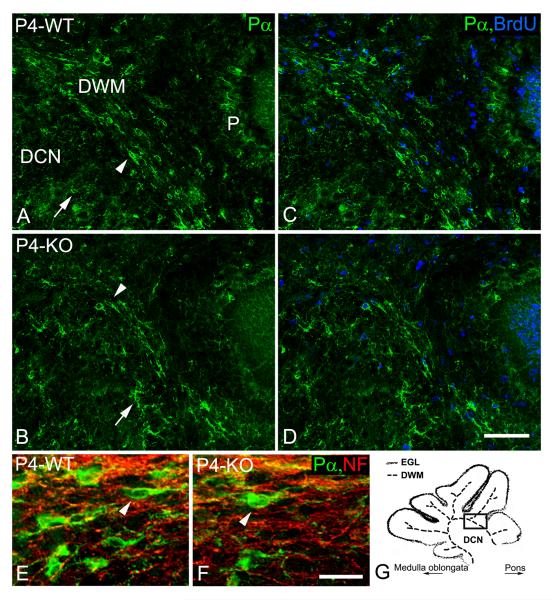
Distribution and proliferation of oligodendrocyte progenitors at the 4th postnatal day. In contrast to stellate-shaped (arrows) PDGFRα-immunoreactive (Pα) OPCs, which were observed mainly in deep cerebellar nuclei (DCN), longitudinally-oriented OPCs (arrowheads) were seen mainly in the developing white matter (DWM) of wild type (A, C, E; WT) and NG2-null (B, D, F; KO) pups at the 4th postnatal day (P4). Fewer longitudinally-oriented OPCs were distributed between neurofilament-positive cerebellar axons (NF, red; D - dapi, blue) in the DWM of NG2-null animals (F). In contrast to the DCN, BrdU/PDGFRα-positive OPCs were more abundant in the DWM of wild type (C) and NG2-null (D) pups at the 4th postnatal day. Some BrdU/PDGFRα-positive OPCs were also observed in the Purkinje cell layer (P). The schematic drawing (G) depicts the developing white matter (DWM), deep cerebellar nuclei (DCN) and the external granular layer (EGL) at the level of the superior cerebellar peduncle. The solid rectangle (G) indicates the region shown in other images (A-D). Scale bars: 20 μm (E, F), 50 μm (A-D).
Semi-quantitative evaluation of pan-neurofilament (NF)-stained axon tracts in developing white matter was performed at postnatal day 4 within the area delineated by the box in Fig. 2G. At this site, a 100 μm line was drawn orthogonal to the long axis of the tract, and the number of axons crossing the line was determined in more than 20 sections for each genotype. Axon diameters and the width of the tract were also quantified in this same location.
Mitotic indices for OPCs were calculated as the percentage of BrdU-positive cells in the PDGFRα-positive population. Myelin basic protein (MBP)-immunoreactive areas were measured in 10 similar parasagital sections by scanning with a fluorescence microscope (10x lens, Nikon), CoolSnap Pro monochrome camera and QCapture Pro program (MediaCybernetics). Average MBP-immunoreactive areas were determined for animals of the same age and genotype, and were expressed as mean values ± SD. All images were processed with Adobe Photoshop CS3 Ver. 10.0 (Adobe Systems) to standardize brightness and contrast. All data were analyzed statistically using ANOVA and un-paired t-tests. P-values less than 0.05 were considered statistically significant.
RESULTS
Identification of oligodendrocyte progenitors and oligodendrocytes
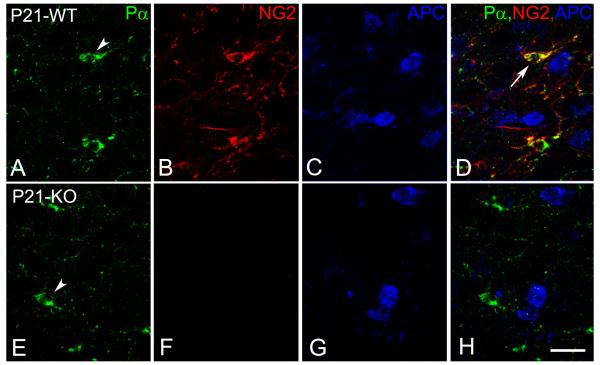
In the context of the developing central nervous system, NG2 is expressed in wild type mice not only by OPCs, but also by pericytes associated with the microvasculature. We were able to distinguish between these cell types on the basis of both their morphologies and their expression of two different species of PDGF receptors: PDGFRα in the case of oligodendrocyte progenitors (Fig. 1A, E) and PDGFRβ in the case of pericytes (not shown). While NG2 expression is absent in NG2 null mice (Fig. 1F), the morphology and size of PDGFRα-positive OPCs are comparable to those seen in wild type mice (Fig. 1E). We are therefore able to use PDGFRα as an NG2-independent marker for OPCs in both wild type and NG2 null mice. As OPCs differentiate along the oligodendrocyte lineage pathway, both NG2 and PDGFRα are down-regulated, as noted previously (Nishiyama et al., 1996a). Mature oligodendrocytes expressing APC (Figs. 1C, G) are thus distinguishable from OPCs, and co-exist alongside the NG2-positive, PDGFRα progenitors (Fig. 1 D, H).
Figure 1.
Expression of PDGFRα (A,E; Pα) and NG2 (B,F) on oligodendrocyte progenitors (OPC, arrowhead) and APC (visualized with the CC1 antibody) on mature oligodendrocytes (C, G) of wild type (WT) and NG2-null (KO) pups at the 21st postnatal day (P21). PDGFRα and NG2 (arrow) were down-regulated on mature APC oligodendrocytes (D; blue). Scale bar: 20 μm.
Distribution of PDGFRα-positive OPCs in the NG2 null and wild type cerebellum
The following discussion deals with OPC distribution and proliferation in the developing cerebellar white matter (DWM) around the periphery of the deep cerebellar nuclei (DCN) and in the interior of folia in the cerebellar cortex (Fig. 2G). Throughout the developmental time period studied, PDGFRα-expressing cells are not observed in the germinal zone lining the fourth ventricle or in the external granular layer (EGL) of the cerebellum itself, consistent with the idea that OPC markers are not expressed by germinal cells. At postnatal day 1 (P1), stellate-shaped PDGFRα-positive OPCs with multiple branched processes are widely distributed within the developing cerebellum in oth wild type and NG2 null mice. Stellate-shaped PDGFRα-positive OPCs are still observed in the DCN at the 4th postnatal day (P4) in both wild type and NG2-null pups (Figs. 2A, B). However in the DWM, OPCs become more bipolar and are oriented longitudinally along axonal tracts. In comparison to wild type animals, substantially fewer longitudinally-oriented PDGRα-immunoreactive OPCs are observed in the DWM of the NG2-null cerebellum at P4 (Figs. 2C, D, and E, F).
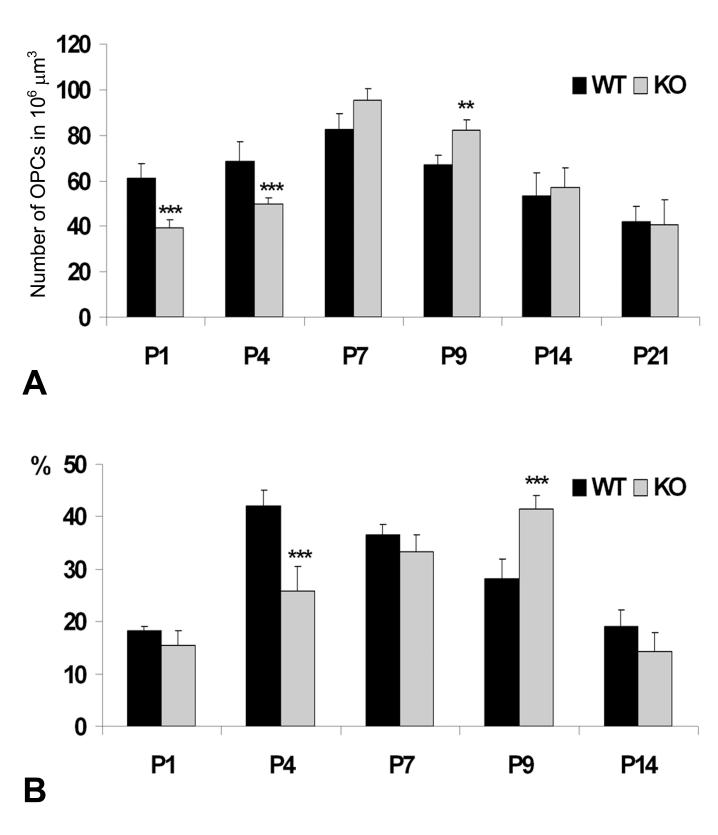
Since the width and fiber content of DWM axonal tracts are indistinguishable in wild type and NG2 null mice (Fig. 2E, F; Table I), the NG2-dependent deficit in OPCs in this location must reside in the OPCs themselves rather than in the axons. Quantifying the numbers of PDGFRα-positive cells in the DWM reveals significant reductions of OPCs in NG2 null mice at P1 and P4 (Fig. 3A). By P7, the distribution pattern and density of PDGFRα-positive OPCs have become similar in the wild type and NG2 null cerebellum, possibly even shifting in favor of the NG2 null mouse at P9.
Table I.
Characterization of DWM axon tracts at P4 in wild type and NG2 null cerebellum.
| WT (μm) | KO (μm) | |
|---|---|---|
| Tract width | 56.65 ± 5.3 | 57.89 ± 6.39 |
| Axon density | 43.5 ± 4.18 | 44.72 ± 5.05 |
| Axon diameter | 0.63 ± 0.18 | 0.62 ± 0.19 |
As described in Materials and Methods, axon tract width, axon density, and axon diameter were analyzed at postnatal day 4 in DWM of wild type (WT) and NG2 null (KO) cerebellum. More than 24 sections were examined for each genotype. Results represent the mean ± S.D
Figure 3.
Quantitative analyses of PDGFRα-immunoreactive OPCs in the DWM of wild type (WT) and NG2-null (KO) cerebella from P1 to P21 (A). OPCs were counted in the standard volume (106 μm3) in all animals. The mitotic labeling index of OPCs (B) is presented as a percentage of PDGFRα-positive cells labeled with BrdU in the DWM. The results are expressed as mean ± SD. Statistical significance is indicated by **p < 0.01; ***p < 0.001.
Proliferation of PDGFRα-positive OPCs in the NG2 null and wild type cerebellum
Proliferation of OPCs was evaluated by visualizing incorporation of BrdU into the DNA of PDGFRα-positive cells (Figs. 2C, D). In the DWM at P4, the mitotic labeling index of OPCs is reduced by 40% in the absence of NG2 (Fig. 3B), providing a likely explanation for the reduced OPC density seen in the DWM of null mice at this age (Fig. 3A). At P7 and at P9, the NG2 null OPC labeling index has caught and surpassed, respectively, the index of wild type OPCs, consistent with the OPC densities observed in the DWM (Fig. 3A). In essence, the peak of OPC proliferation in DWM is shifted from P4 to P9 by the ablation of NG2 (Fig. 3B). The effect of NG2 ablation on cell cycle progression was examined more closely in a second type of experiment in which BrdU was administered to P4 pups via a single injection, followed by survival times of 10 and 20 hours (Table II). At 10 hours, the majority of BrdU-positive OPC nuclei are heavily labeled in both wild type and NG2 null animals. At 20 hours, nuclear BrdU labeling has weakened in the majority of OPC nuclei in wild type animals, reflecting dilution of the label by mitosis. In contrast, the majority of NG2 null OPC nuclei are still heavily labeled after 20 hours, consistent with retarded progression through the cell cycle in the absence of NG2.
Table II.
Evaluation of cell cycle progression
| WT |
NG2-null |
|||||
|---|---|---|---|---|---|---|
| Intensity | ++ | + | − | ++ | + | − |
| BrdU inj. | ||||||
| 10H | 43.2 ± 2.3 | 21.4 ± 1.6 | 35.4 ± 3.3 | 39.3 ± 3.7 | 15.3 ± 5.8 | 45.5 ± 3.5* |
| 20H | 34.2 ± 1.3 | 40.1 ± 2.7 | 25.7 ± 5 | 40 ± 4.8* | 27.2 ± 3.8* | 32.7 ± 3.3* |
Six wild type and 6 NG2 null pups were given a single pulse of BrdU at postnatal day 4, and were then euthanized after 10 hour (3 mice of each genotype) and 20 hour (3 mice of each genotype) survival periods. BrdU incorporation by PDGFRα-positive OPCs was grouped into three categories: heavily and completely labeled nuclei (++), weakly and partially labeled nuclei (+), and unlabeled nuclei (−). At 10 hours in both genotypes, the majority of labeled cells exhibit heavily labeled nuclei. After 20 hours, this is still true of NG2 null OPCs. However, the majority of wild type OPCs now have weakly labeled nuclei, indicative of dilution of the label by mitosis. Results represent the mean ± S.D.
Statistical significance: p < 0.001 compared to values obtained in WT mice.
Interestingly, in both wild type and NG2-null pups between P4 and P9, OPC mitotic indices are 2-fold lower in the DCN than in the DWM. In addition, significant differences in mitotic labeling index between wild type and NG2-null mice are not seen in the DCN at any time point (data not shown). Since the DWM is rich in axon tracts, compared to the DCN, these observations may be indicative of an important role for NG2-dependent OPC-axon interactions in stimulating OPC proliferation.
Maturation of PDGFRα-positive OPCs in the NG2-null and wild type cerebellum
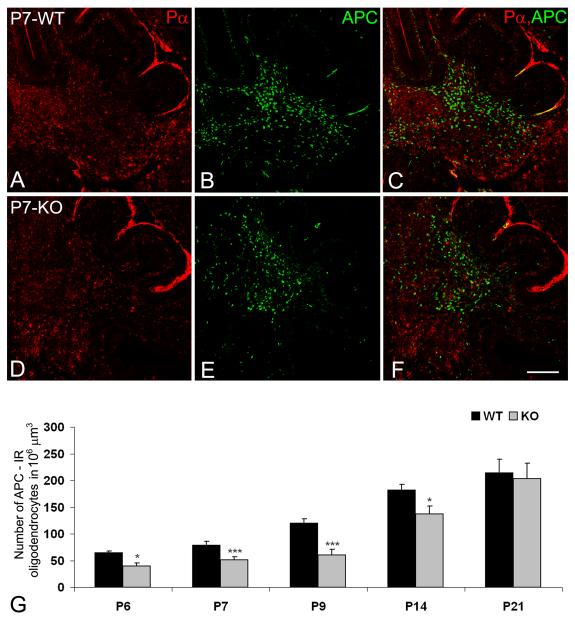
The first mature oligodendrocytes, identified by expression of APC and MBP, are seen in both wild type and NG2 null mice at postnatal day 6 (P6). In the ventral part of the DWM, MBP expression is detectable in both oligodendrocyte somata and processes. One day later, MBP-immunoreactive processes are also seen in the DWM of the cerebellar cortex. Compared to wild type animals, fewer APC-positive oligodendrocyte somata are observed in the DWM of the NG2 null cerebellum (Figs. 4A-F) throughout the first two weeks postnatally (Fig. 4G). Consistent with this reduction in oligodendrocyte number, we also find reduced areas occupied by MBP-positive processes in the NG2-null DWM during the second postnatal week (Fig. 5). Differences between MBP-immunoreactive areas and the abundance of APC-positive oligodendrocytes in wild type and NG2-null mice are no longer evident by the 21st postnatal day (Fig. 5E). By presenting the ratio of PDGFRα-positive OPCs to APC-positive oligodendrocytes over the first 3 postnatal weeks of cerebellar development, Table III further emphasizes the delayed production of mature oligodendrocytes in the NG2 null DWM.
Figure 4.
The distribution of PDGFRα-immunoreactive OPCs (Pα, red) and APC-immunoreactive (green) mature oligodendrocytes in wild type (A-C) and NG2-null (D-F) cerebella at P7. The abundance of APC-positive cells (G) was determined in parasagital sections cut between the left and right superior cerebellar peduncles. Results are expressed as mean values in a standard volume (106 μm3) ± SD. Statistical significance is indicated by **p < 0.01; ***p < 0.001. Scale bar: 200 μm (A-F).
Figure 5.
MBP-immunoreactive areas (green) in the P7 and P9 cerebella of wild type (A, C) and NG2-null (B, D) mice at level of the superior cerebellar peduncles. Larger MBP-positive areas were detected in wild type animals during the first two postnatal weeks (E). The results are expressed as mean area between the left and right superior cerebellar peduncles ± SD. Statistical significance is indicated by *p < 0.05; ***p < 0.001. Scale bar: 60 μm (A, B); 500 μm (C, D).
Table III.
Ratio between OPCs and mature oligodendrocytes in the cerebellar DWM
| P6 Pα : APC |
P7 Pα : APC |
P9 Pα : APC |
P14 Pα : APC |
P21 Pα : APC |
|
|---|---|---|---|---|---|
| WT | 1:0.61 | 1 : 0.95 | 1 : 1.78 | 1 : 3.39 | 1 : 5.08 |
| KO | 1 : 0.51 | 1 : 0.54 | 1 : 0.98 | 1 : 2.4 | 1 : 4.97 |
Average numbers of PDGFRα-IR OPCs (Pα) and APC-IR mature oligodendrocytes were determined in a standard tissue volume (106 μm3). Delayed oligodendrocyte maturation in the absence of NG2 is indicated by the increased Pα:APC ratio in KO mice between postnatal days 6 and 14.
DISCUSSION
Both cell culture and in vivo studies have contributed to the identification of a population of oligodendrocyte progenitor cells that initially arise in ventral regions of the neural tube (Raff et al., 1983, Raff et al., 1984, Noble et al., 1988, Raff et al., 1988, Noll and Miller, 1993, Pringle and Richardson, 1993, Dawson et al., 2000, Rowitch, 2004, Ligon et al., 2006). During subsequent development, these OPCs proliferate and migrate to fill the entire CNS, and then give rise to myelinating oligodendrocytes according to well-defined timetables in each of the respective white matter tracts (Foran and Peterson, 1992). Since neurons originate, migrate, differentiate, and interact according to precise schedules, the events associated with oligodendrocyte generation, migration, and maturation must also be carefully orchestrated in order to optimize interactions between the two populations of cells.
Our evidence indicates that the NG2 proteoglycan is an important factor in promoting effective expansion of this OPC pool, a function that is critical for the timely generation of mature oligodendrocytes in sufficient numbers to ensure efficient axonal myelination. For example, we show that while PDGFRα-immunoreactive OPCs are evenly distributed in the postnatal day 1 cerebellum in both wild type and NG2 null mice, these progenitors are present in reduced numbers in the absence of NG2. During the next few days of cerebellar development, the number of OPCs in developing white matter tracts of the NG2 null mouse continues to be reduced compared to what is seen in wild type mice. Our data suggest that generation of a full pool of OPCs is delayed by as much as 4-5 days in DWM of the NG2 null cerebellum. Subsequent generation of the complement of APC-positive oligodendrocytes and MBP-positive oligodendrocyte processes is correspondingly delayed in these areas. A primary cause for this myelination deficit appears to be reduced proliferation of OPCs in the absence of NG2, as judged by BrdU incorporation. Retarded cell cycle progression in NG2 null OPCs leads to reduced mitotic indices for these cells at P1 and P4. We have also observed this type of cell cycle slowing in dermal keratinocytes in the NG2 null mouse (Kadoya et al, 2008), suggesting that this may be a consistent result of NG2 ablation.
We have previously shown in cell culture experiments that NG2 can promote cell proliferation both by activation of β1 integrin signaling (Makagiansar et al., 2007) and by activation of growth factor receptor signaling (Nishiyama et al., 1996b, Goretzki et al., 1999, Grako et al., 1999). In vivo, utilizing comparisons of wild type and NG2 null mice, the effects of NG2 on cell proliferation have previously been demonstrated for microvascular pericytes (Ozerdem and Stallcup, 2004) and immature keratinocytes (Kadoya et al., 2008). In our current myelination studies, although we have not yet identified specific mechanisms responsible for the stimulatory effects of NG2 on OPC proliferation, we can nevertheless make some educated guesses based on known functions of the proteoglycan. An attractive hypothesis for explaining the decrease in OPC proliferation in the NG2 null mouse is the impairment of PDGF-AA and FGF2 signaling. These two growth factors are essential for delaying differentiation of OPCs and mediating adequate expansion of the OPC pool (Noble et al., 1988, Richardson et al., 1988, Hart et al., 1989, Bogler et al., 1990, McKinnon et al., 1990, Wolswijk and Noble, 1992, Calver et al., 1998, van Heyningen et al., 2001, Woodruff et al., 2004, Murtie et al., 2005a). Defective oligodendrocyte development and severe hypomyelination have been demonstrated in PDGF-A knockout mice (Fruttiger et al., 1999). Similarly, alterations in FGF2 signaling have deleterious effects on oligodendrocyte differentiation and subsequent myelination (Murtie et al., 2005a, Zhou et al., 2006, Zhou and Armstrong, 2007). Via a novel mechanism that does not involve chondroitin sulfate chains, NG2 can bind with relatively high affinity to PDGF-AA and FGF-2 (Goretzki et al., 1999), and is effective in sequestration and presentation of these growth factors to their cognate receptors. Using aortic smooth muscle cells derived from the NG2 null mouse, we have demonstrated the loss of PDGF-AA (Grako et al., 1999) and FGF-2 (W.B. Stallcup, unpublished observation) signaling capabilities in the absence of NG2. We have also shown that NG2 null OPCs in culture are insensitive to PDGF-AA and FGF-2 stimulation (Stallcup, 2002), consistent with the reduced proliferation seen in vivo.
OPC proliferation is also influenced by interaction with other OPCs (Kirby et al., 2006) and with axons (Barres and Raff, 1993, 1994, 1999, Gao and Miller, 2006). The sensitivity of OPCs to electrical activity in axons may well be based on the presence of functional synapses between neurons and OPCs (Bergles et al., 2000, Belachew and Gallo, 2004, Lin and Bergles, 2004, Paukert and Bergles, 2006, Karadottir et al., 2008, Kukley et al., 2008). The establishment and maintenance of these synaptic contacts from early time points in OPC development (Kukley et al., 2008) suggests that axonally-derived signals represent an important mechanism by which progenitors coordinate their proliferation and differentiation with the functional needs of neuronal circuits. In addition to its involvement in growth factor-mediated OPC proliferation, NG2 may also be involved in these progenitor-axon interactions. Several studies have established the importance of beta-1 integrin signaling in the process of OPC-axon interaction and myelination (Buttery and ffrench-Constant, 1999, Relvas et al., 2001, Baron et al., 2005, Lee et al., 2006). The ability of NG2 to interact physically with beta-1 integrins and to promote their activation (Fukushi et al., 2004, Makagiansar et al., 2007, Chekenya et al., 2008) suggests a role for the proteoglycan in integrin-dependent OPC-axon communication. In addition, a physical interaction between NG2 on the OPC surface and N-cadherin on axonal surfaces (W.B. Stallcup, unpublished observation) provides another means by which the proteoglycan may mediate OPC-axon crosstalk. We have shown that wild type mice have higher numbers of OPCs than NG2 null mice in axon-rich areas of the DWM, while OPC numbers in wild type versus NG2 null mice are similar in the DCN, where axons are less abundant. This may be indicative of a significant role for NG2 in axon-dependent OPC proliferation. NG2-dependent interaction between OPCs and axons in the wild type mouse might be expected to result in patterns of OPC process extension or arborization that are different from those found in the NG2 null mouse. In our current experiments, we have not been able to detect reproducible differences between OPC processes in wild type and NG2 null mice. In both cases, process numbers range between 2-6 per cell (data not shown). However, the details of process morphology and branching are not clearly quantifiable at the current level of resolution. Future studies will incorporate ultrastructural methodologies capable of detecting such differences, as well as possible differences in myelin structure that exist between wild type and NG2 null mice.
Reduced OPC recruitment or increased OPC apoptosis in the NG2 null mouse would also appear to be important issues in light of previous information linking NG2 to increased cell motility (Burg et al., 1997, Burg et al., 1998, Grako et al., 1999, Fukushi et al., 2004, Makagiansar et al., 2004, Makagiansar et al., 2007) and decreased apoptosis (Chekenya et al, 2008). For example, decreased OPC motility or increased OPC apoptosis might contribute to the reduced number of OPCs seen in the NG2 null cerebellum at P1, a time at which differences in OPC proliferation are not yet apparent (Fig. 3A and B). In the case of apoptosis, immunolabeling for activated caspase-3 in either the wild type or NG2 null cerebellum reveals only small numbers of pre-apoptotic cells, insufficient to explain the observed differences in OPC abundance (data not shown). In the case of cell motility, it has not been straightforward to distinguish recruitment from proliferation in the current experimental system. Initial results from spinal cord demyelination/remyelination studies appear more promising in this regard. Re-population of small, well-defined areas demyelinated by lysolecithin microinjection may allow more clear-cut identification of differences based on deficits in NG2-dependent cell motility. Since remyelination is mediated by so-called “adult” OPCs that are also characterized by expression of NG2 and PDGFRα, comparisons of wild type and NG2 null mice are likely to be effective in defining the role of the proteoglycan in the repair process. Based on experience with other experimental systems (vascularization, tumor progression, wound healing) in which the importance of NG2 is magnified in adulthood relative to early development, we are hopeful that the effects of NG2 ablation will be even more dramatic in demyelination/remyelination than we have seen during developmental myelination.
Acknowledgements
This work was supported by Postdoctoral Fellowship 82922 from the Craig H. Neilsen Foundation (KK) and by NIH grant PO1 HD 25938 (WBS)
Abbreviations
- APC
Adenomatous Polyposis Coli (Clone CC1)
- BrdU
5-bromo-2-deoxyuridine
- DAPI
4′-6-diamidino-2-phenylindole
- DCN
deep cerebellar nuclei
- DWM
developing white matter
- EGL
external granular layer
- KO
NG2 knockout
- MBP
myelin basic protein
- NF
pan neurofilament
- P
Purkinje cell layer
- Pα
platelet-derived growth factor receptor alpha
- P1 to P21
postnatal days 1 - 21
- OPCs
oligodendrocyte progenitor cells
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49:467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Axonal control of oligodendrocyte development. J Cell Biol. 1999;147:1123–1128. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Brain Myelination in Prevalent Neuropsychiatric Developmental Disorders: Primary and Comorbid Addiction. Adolesc Psychiatry. 2005;29:55–96. [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Gallo V. Synaptic and extrasynaptic neurotransmitter receptors in glial precursors’ quest for identity. Glia. 2004;48:185–196. doi: 10.1002/glia.20077. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bogler O, Wren D, Barnett SC, Land H, Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci U S A. 1990;87:6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MA, Grako KA, Stallcup WB. Expression of the NG2 proteoglycan enhances the growth and metastatic properties of melanoma cells. J Cell Physiol. 1998;177:299–312. doi: 10.1002/(SICI)1097-4652(199811)177:2<299::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Burg MA, Nishiyama A, Stallcup WB. A central segment of the NG2 proteoglycan is critical for the ability of glioma cells to bind and migrate toward type VI collagen. Exp Cell Res. 1997;235:254–264. doi: 10.1006/excr.1997.3674. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Chekenya M, Krakstad C, Svendsen A, Netland IA, Staalesen V, Tysnes BB, Selheim F, Wang J, Sakariassen PO, Sandal T, Lonning PE, Flatmark T, Enger PO, Bjerkvig R, Sioud M, Stallcup WB. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182–5194. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- de Castro R, Jr., Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol. 2005;192:299–309. doi: 10.1016/j.expneurol.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Foran DR, Peterson AC. Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J Neurosci. 1992;12:4890–4897. doi: 10.1523/JNEUROSCI.12-12-04890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Colello RJ, Macklin WB, Fuss B. Phosphodiesterase-Ialpha/autotaxin: a counteradhesive protein expressed by oligodendrocytes during onset of myelination. Mol Cell Neurosci. 2003;23:507–519. doi: 10.1016/s1044-7431(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. Type 1 astrocytes fail to inhibit Schwann cell remyelination of CNS axons in the absence of cells of the O-2A lineage. Dev Neurosci. 1992;14:85–92. doi: 10.1159/000111651. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, Richardson WD. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Miller RH. Specification of optic nerve oligodendrocyte precursors by retinal ganglion cell axons. J Neurosci. 2006;26:7619–7628. doi: 10.1523/JNEUROSCI.0855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem. 1999;274:16831–16837. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- Grako KA, Ochiya T, Barritt D, Nishiyama A, Stallcup WB. PDGF (alpha)-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci. 1999;112(Pt 6):905–915. doi: 10.1242/jcs.112.6.905. [DOI] [PubMed] [Google Scholar]
- Gudz TI, Schneider TE, Haas TA, Macklin WB. Myelin proteolipid protein forms a complex with integrins and may participate in integrin receptor signaling in oligodendrocytes. J Neurosci. 2002;22:7398–7407. doi: 10.1523/JNEUROSCI.22-17-07398.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Bolsover SR, Raff MC. PDGF and intracellular signaling in the timing of oligodendrocyte differentiation. J Cell Biol. 1989;109:3411–3417. doi: 10.1083/jcb.109.6.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain-Ibrahim MK, Rezajooi K, Stallcup WB, Lieberman AR, Anderson PN. Analysis of axonal regeneration in the central and peripheral nervous systems of the NG2-deficient mouse. BMC Neurosci. 2007;8:80. doi: 10.1186/1471-2202-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya K, Fukushi J, Matsumoto Y, Yamaguchi Y, Stallcup WB. NG2 proteoglycan expression in mouse skin: altered postnatal skin development in the NG2 null mouse. J Histochem Cytochem. 2008;56:295–303. doi: 10.1369/jhc.7A7349.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D. Glial cells are born with synapses. FASEB J. 2008;22:2957–2969. doi: 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lee KK, de Repentigny Y, Saulnier R, Rippstein P, Macklin WB, Kothary R. Dominant-negative beta1 integrin mice have region-specific myelin defects accompanied by alterations in MAPK activity. Glia. 2006;53:836–844. doi: 10.1002/glia.20343. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004;47:290–298. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- Makagiansar IT, Williams S, Dahlin-Huppe K, Fukushi J, Mustelin T, Stallcup WB. Phosphorylation of NG2 proteoglycan by protein kinase C-alpha regulates polarized membrane distribution and cell motility. J Biol Chem. 2004;279:55262–55270. doi: 10.1074/jbc.M411045200. [DOI] [PubMed] [Google Scholar]
- Makagiansar IT, Williams S, Mustelin T, Stallcup WB. Differential phosphorylation of NG2 proteoglycan by ERK and PKCalpha helps balance cell proliferation and migration. J Cell Biol. 2007;178:155–165. doi: 10.1083/jcb.200612084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C, Hindelang C, LeMeur M, Borrelli E. A transgenic mouse model for inducible and reversible dysmyelination. J Neurosci. 2000;20:7698–7705. doi: 10.1523/JNEUROSCI.20-20-07698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronson SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Murtie JC, Zhou YX, Le TQ, Armstrong RC. In vivo analysis of oligodendrocyte lineage development in postnatal FGF2 null mice. Glia. 2005a;49:542–554. doi: 10.1002/glia.20142. [DOI] [PubMed] [Google Scholar]
- Murtie JC, Zhou YX, Le TQ, Vana AC, Armstrong RC. PDGF and FGF2 pathways regulate distinct oligodendrocyte lineage responses in experimental demyelination with spontaneous remyelination. Neurobiol Dis. 2005b;19:171–182. doi: 10.1016/j.nbd.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996a;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996b;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Noll E, Miller RH. Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development. 1993;118:563–573. doi: 10.1242/dev.118.2.563. [DOI] [PubMed] [Google Scholar]
- Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papay R, Gaivin R, McCune DF, Rorabaugh BR, Macklin WB, McGrath JC, Perez DM. Mouse alpha1B-adrenergic receptor is expressed in neurons and NG2 oligodendrocytes. J Comp Neurol. 2004;478:1–10. doi: 10.1002/cne.20215. [DOI] [PubMed] [Google Scholar]
- Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515–521. doi: 10.1016/j.conb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff MC, Williams BP, Miller RH. The in vitro differentiation of a bipotential glial progenitor cell. EMBO J. 1984;3:1857–1864. doi: 10.1002/j.1460-2075.1984.tb02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relvas JB, Setzu A, Baron W, Buttery PC, LaFlamme SE, Franklin RJ, ffrench-Constant C. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Curr Biol. 2001;11:1039–1043. doi: 10.1016/s0960-9822(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg SS, Powell BL, Chan JR. Receiving mixed signals: uncoupling oligodendrocyte differentiation and myelination. Cell Mol Life Sci. 2007;64:3059–3068. doi: 10.1007/s00018-007-7265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- van Heyningen P, Calver AR, Richardson WD. Control of progenitor cell number by mitogen supply and demand. Curr Biol. 2001;11:232–241. doi: 10.1016/s0960-9822(01)00075-6. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–262. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Zhou YX, Armstrong RC. Interaction of fibroblast growth factor 2 (FGF2) and notch signaling components in inhibition of oligodendrocyte progenitor (OP) differentiation. Neurosci Lett. 2007;421:27–32. doi: 10.1016/j.neulet.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YX, Flint NC, Murtie JC, Le TQ, Armstrong RC. Retroviral lineage analysis of fibroblast growth factor receptor signaling in FGF2 inhibition of oligodendrocyte progenitor differentiation. Glia. 2006;54:578–590. doi: 10.1002/glia.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]