Abstract
The vertebrate cranial base is a complex structure composed of bone, cartilage and other connective tissues underlying the brain; it is intimately connected with development of the face and cranial vault. Despite its central importance in craniofacial development, morphogenesis and tissue origins of the cranial base have not been studied in detail in the mouse, an important model organism. We describe here the location and time of appearance of the cartilages of the chondrocranium. We also examine the tissue origins of the mouse cranial base using a neural crest cell lineage cell marker, Wnt1-Cre/R26R, and a mesoderm lineage cell marker, Mesp1-Cre/R26R. The chondrocranium develops between E11 and E16 in the mouse, beginning with development of the caudal (occipital) chondrocranium, followed by chondrogenesis rostrally to form the nasal capsule, and finally fusion of these two parts via the midline central stem and the lateral struts of the vault cartilages. X-Gal staining of transgenic mice from E8.0 to 10 days post-natal showed that neural crest cells contribute to all of the cartilages that form the ethmoid, presphenoid, and basisphenoid bones with the exception of the hypochiasmatic cartilages. The basioccipital bone and non-squamous parts of the temporal bones are mesoderm derived. Therefore the prechordal head is mostly composed of neural crest-derived tissues, as predicted by the New Head Hypothesis. However, the anterior location of the mesoderm-derived hypochiasmatic cartilages, which are closely linked with the extra-ocular muscles, suggests that some tissues associated with the visual apparatus may have evolved independently of the rest of the “New Head”.
Keywords: cranial base, chondrocranium, neural crest, mesoderm, Wnt1, Mesp1, New Head Hypothesis, tissue origins, cranial development
INTRODUCTION
The skull is divided into two compartments: the viscerocranium, which comprises the facial skeleton (and some anterior neck structures), and the neurocranium, which together encases the brain and cranial sense organs. The neurocranium is formed from the endochondral bones of the cranial base and the intramembranous bones of the vault. The viscerocranium and the vault of the neurocranium are relatively well studied; however, development of the cranial base has not been extensively investigated in the mouse. Because the cranial base spans almost the entire rostro-caudal length of the head, from the foramen magnum to the tip of the cartilaginous nasal septum, its proper development is crucial for subsequent normal development of the cranial vault and facial bones. In this paper we analyze the development of the mouse cranial base and determine the relative contributions of neural crest- and mesoderm-derived cells by means of in vivo permanent cell markers.
In the adult mouse, the cranial base is composed of the ethmoid, presphenoid, basisphenoid, and basioccipital bones along with the auditory capsules of the temporal bones. Early in development, the cranial base first appears as a sheet of undifferentiated mesenchymal cells that condense and chondrify to form the chondrocranium, a solid cartilaginous structure that spans the entire length of the skull and is formed by the fusion of numerous individual cartilages that appear at distinct locations and times. The bones of the cranial base then undergo endochondral ossification to form the chondrocranium. Thus the structural origin of each bone of the cranial base can be traced from a number of chondrocranial cartilages. Understanding chondrocranial development is a critical first step to understanding formation and growth of the mature cranial base.
In his seminal book, The Development of the Vertebrate Skull (1937), de Beer provided an in-depth analysis of chondrocranial development in a number of mammals. However, he notes that “no systematic study has been made of the development of the skull in either rats or mice”. Since that time, the mouse has become a popular model organism for studies of craniofacial development, and a detailed understanding of the normal development of all components of the skull is essential for underpinning experimental and pathogenesis approaches. Most published studies that include the cranial base have examined late embryonic and postnatal endochondral ossification processes, particularly development and growth of the synchondroses (for example Isii-Suzuki et al., 1999; Chen et al., 1999; Gakunga et al., 2000; Eswarakumar et al., 2002; Shum et al., 2003; Olsen et al., 2005; Young et al., 2006; Koyama et al., 2007; Nagayama et al., 2008) or altered cranial base morphology in mutant, knock-in, knock-out, or transgenic mice (for example Jones & Roberts, 1988; Rintala et al., 1993; Lozanoff et al., 1994; Kaufman et al., 1995; Ma & Lozanoff, 1996; Chung et al., 1997; Belo et al., 1998; McBratney et al., 2003; Hallgrimsson et al., 2006). A minority of studies have focused on early developmental aspects, from its origin as undifferentiated mesenchyme through chondrogenesis of the chondrocranium (Wood et al., 1991; Savontaus et al., 2004; Nie et al., 2005a; Nie et al., 2005b; Kettunen et al., 2006; Nie, 2006a; Nie, 2006b), yet understanding the early developmental processes that create the cranial base will show us how the adult morphology arises. This insight is relevant not just to the cranial base itself but to the whole skull, since growth and form of the cranial base plays a central role in the covariation of other craniofacial components (Lieberman et al., 2008).
Defining the tissue boundary between neural crest-derived and mesoderm-derived components of the cranial base may be a valuable endeavor as such a boundary has been shown to have clear developmental significance in the vault (Jiang et al., 2002; Evans & Noden, 2006; Merrill et al., 2006; Yoshida et al., 2008; reviewed in Gross and Hanken, 2008). Although the tissue origins of the murine cranial base have been neglected, the rest of the cranium has been investigated. The skeleton of the mouse viscerocranium is derived solely from neural crest cells (Jiang et al., 2002). Most studies in the chicken and mouse report that the vault of the neurocranium is derived from both neural crest cells and mesoderm (Le Lièvre, 1978; Noden, 1978, 1984, 1988; Jiang et al., 2002; Evans & Noden, 2006), although one study reports that the chicken cranial vault is entirely neural crest-derived (Couly et al., 1993). Studies on the tissue origins of the cranial base in the chicken have defined its relative neural crest- and mesoderm-derived components (Le Lievre, 1978; Noden, 1988; Couly et al., 1993; Le Douarin et al., 1993; Lengele et al., 1996; Le Douarin & Kalcheim, 1999). An in vivo analysis of tissue origins of the mouse cranial base is required for a determination of which cartilages of the chondrocranium are derived from neural crest versus mesoderm in this mammal.
In this paper, we examine the development of the mouse cranial base from E11 to E16. We show that the cartilages of the chondrocranium appear at specific locations and times, with chondrification beginning in the posterior cranial base, followed by the anterior (rostral) cranial base, leading ultimately to fusion of the two parts via a midline stem and lateral struts by E16. We also investigate the tissue origins of the mouse cranial base using in vivo permanent cell markers in Wnt1-Cre/R26R and Mesp1-R26R transgenic mice (Jiang et al., 2000, 2002; Yoshida et al., 2008). According to the New Head Hypothesis (NHH) proposed by Gans and Northcutt (1983; Northcutt & Gans, 1983), the neural crest-mesoderm boundary should correlate with the rostral-most tip of the notochord, thereby creating a coincident boundary with the prechordal-chordal boundary in the cranium. In the mouse, the prechordal-chordal boundary is between the basisphenoid and basioccipital bones (Barteczko & Jacob, 1999), and our observations confirm that the major neural crest-mesoderm boundary is located between the cartilages that contribute to these two bones. However, the mesoderm-derived hypochiasmatic cartilages are an exception to this generalization, as they are located among neural crest-derived cartilages in the anterior cranial base. Furthermore, although the hypophyseal cartilage is derived from neural crest cells, the bone that replaces it (the basisphenoid bone) has some mesoderm cell contributions in its caudal portion. The broader implications of these results to general bone development and evolution of the mammalian cranium are discussed.
MATERIALS AND METHODS
Inbred Mice and Staging Criteria
Chondrocranial development was examined in detail in C57BL/6J embryos from timed pregnant females obtained from Jackson Laboratories. All embryos were first staged using criteria established by Theiler (1989) and Miyake et al. (1996) so that chondrocranial development could be observed relative to developmental stage rather than conception date. After being designated a specific stage (T18 to T24), embryos were labeled according to the general corresponding conception age (E11 to E16). Only C57BL/6J embryos were used for results in Figure 1 and Figure 3. Figure 2 includes embryos from A/J (Fig. 2A) and DBA/2J (Fig. 2B & C) inbred lines obtained from timed pregnant females from Jackson Laboratories.
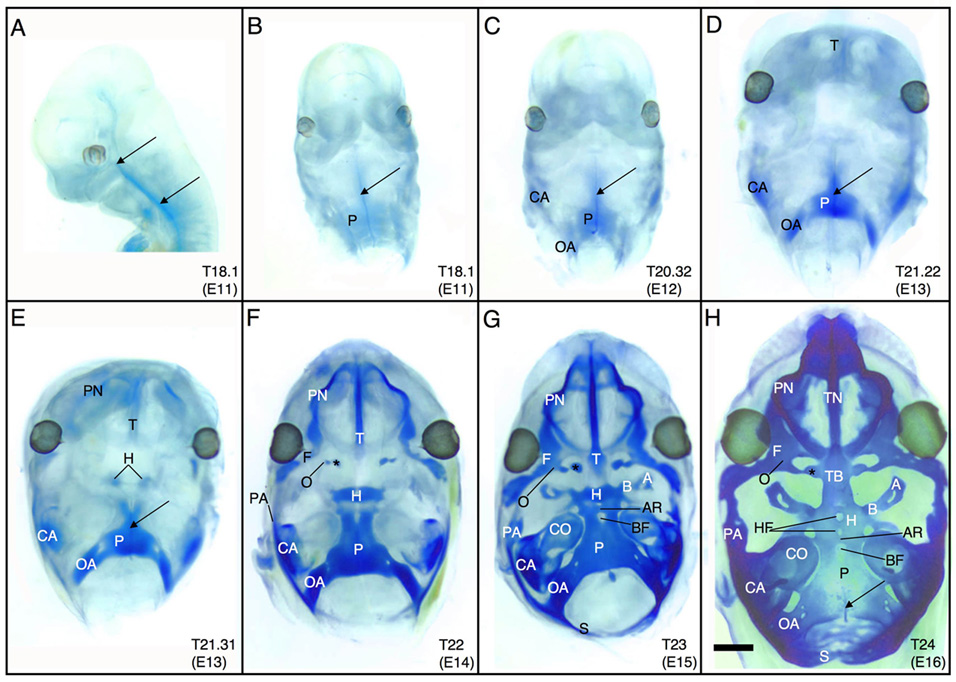
Figure 1.
Morphogenesis of the chondrocranium as shown by Alcian blue stained and cleared whole mount C57BL/6J embryos from Theiler Stages18 to 24 (E11 to E16). All images show the chondrocranium from a dorsal aspect (norma basalis interna) except for A, which shows a lateral perspective. (A,B) The parachordal cartilage is the first to appear caudally in the cranium at E11 in close association with the notochord (arrows). (C–G) Between E12 and E15, individual cartilages chondrify. (H) At E16, the chondrocranium is fully formed in the mouse; all cartilages have fused and ossification has just begun in the posterior chondrocranium. *=hypochiasmatic cartilage. Scale bar in H=1 mm. See key for abbreviations.
Figure 3.
In situ hybridization of Col2 on sagittal sections of C57BL/6J embryos from E12 through E16. (A) At E12, Col2 expression can be seen in the parachordal cartilage, notochord (at tip of arrow), and some mesenchyme of the anterior cranial base. (B–D) Expression of Col2 increases in cartilages of the cranial base as they continue to chondrify from E13 to E15. (E) At E16, detection of Col2 decreases in the parachordal and hypophyseal anlagen where endochondral ossification is beginning. Normal fenestrae disrupt the continuity of cartilages in some sections (C & E); these are not present in the adult cranial base. See key for abbreviations.
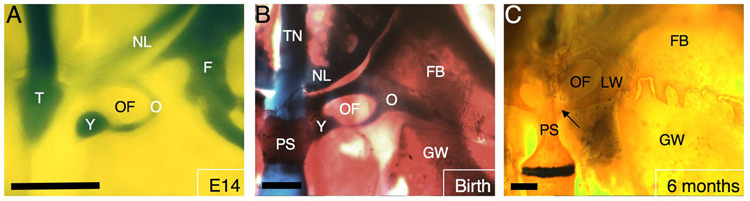
Figure 2.
Dorsal view of endocranium in whole mounts showing development of cartilages and bones in the optic region. (A) At E14, the orbital, frontal, hypochiasmatic, and trabecular cartilages are present in the optic region of the chondrocranium. (B) At 1-day after birth (P1), ossification has begun in the body of the presphenoid bone, the frontal bone, and the greater wing (ala temporalis) of the basisphenoid bone. (C) By at least 6 months after birth, extensive ossification and remodeling has occurred around the optic foramen, with the hypochiasmatic and orbital cartilages forming the lesser wing of the prepshenoid bone. The arrow indicates the portion of the postoptic root of the lesser wing that derives from the hypochiasmatic cartilage. Laterally, the frontal bone and greater wing of the basisphenoid bone have formed interdigitations where they meet. Scale bars in A–C=0.5 mm. See key for abbreviations.
Whole-Mount Staining
Some embryos were stained with Alcian blue alone in order to focus on chondrocranial development, making a stain for bone (such as alizarin red) unnecessary for this portion of the study. Whole embryos from E11 to E16 were dissected from the uterus in PBS and stored in 70% EtOH. After being fixed in Bouin’s fixative overnight, embryos were washed in 0.1% NH4OH, 70% EtOH until all traces of the yellow fixative were gone and embryos appeared bleached. Embryos were then equilibrated in 5% acetic acid twice for 1 hour each time and left overnight in 0.05% Alcian blue 8GX (Fisher) in fresh 5% acetic acid. This was followed by washing twice for 1 hour each in 5% acetic acid, soaking in methanol twice for 1 hour each and clearing in 1:2 benzyl alcohol:benzyl benzoate (BABB). Crania were then removed from the body and heads dissected to reveal the cranial base. Meckel’s cartilage was removed for photographs.
For combined alizarin red and Alcian blue staining of postnatal mouse crania, the vault and brain was removed prior to fixing overnight in 100% EtOH. Cartilage was then stained overnight in 80ml 95%EtOH, 20ml glacial acetic acid, 15 mg Alcian blue (Sigma). After washing twice in 95% EtOH and soaking in 2% KOH in water for 3 hours, bone was stained in 1% KOH, 7.5mg/ml alizarin red S (Sigma) in water overnight. Specimens were destained in 20% glycerol, 1% KOH in water for 2 days, and then put in 20% glycerol in water, changed daily for 5 days. Finally, crania were placed in 20% glycerol, 20% EtOH overnight and then stored and photographed in 50% glycerol, 50% EtOH.
Microscopy
Whole embryos at E14 were dissected from the uterus in PBS and stored in 70% EtOH. Specimens were dehydrated, cleared in xylene, and mounted in paraffin. Wax blocks were sectioned at 10 µm. Slides were then cleared in xylene, rehydrated, soaked in 1% Alcian blue (Sigma) in 3% acetic acid for 1 hour, followed by dehydration and a final clearing in xylene. Slides were mounted with coverslips and viewed on a microscope.
In situ hybridization
C57BL/6J embryos from E12 to E16 obtained from timed pregnant females from Jackson Laboratories were fixed in 4% paraformaldehyde at 4°C overnight. Specimens were then dehydrated, soaked in xylene at room temperature and placed in melted paraffin up to 65°C for no more than 2 hours. Sections were cut at 10µm.
Immediately prior to hybridization, slides were deparaffinized in xylene and rehydrated. Slides were then incubated in 0.2M HCL for 15 minutes and 10mg/ml of Proteinase K in 10mM Tris pH7.5/1mM CaCl2 at 37°C for 15 minutes, being washed in PBS at room temperature before and after each of these steps. Slides were then further fixed in 4% paraformaldehyde for 5 minutes at room temperature, washed in PBS, and then acetylated in 0.25% acetic acid for 15 minutes. After washing in PBS, slides were dehydrated in an ascending alcohol series and left to air dry for 30 minutes. Col2a1 antisense probes synthesized to cover 405bp of cDNA were labeled with digoxigenin-11-UTP as described in Gong et al. (2001). Sox9 antisense probe was also used as described by Huang et al. (1997). Hybridization was carried out as described by Bohme at al. (1995) and detected, counterstained, and mounted as described by Gong et al. (2001).
Transgenic Mice and X-Gal Staining
The protocol for construction of Wnt1-Cre/R26R transgenic mice has previously been described by Jiang et al. (2000) and for Mesp1-Cre/R26R by Saga et al. (1999). All cells and their progeny with an active Wnt1 or Mesp1 promoter stably maintain expression of the recombined R26R allele, thus driving the LacZ expression allele, allowing us to examine neural crest or mesodermal contributions to structures in both pre- and postnatal mice by X-Gal staining (Jiang et al., 2002; Yoshida et al., 2008). Staining with X-Gal was carried out by conventional methods on whole embryos from E8.5 to E10.5 and on heads with the superior vault and brain removed prior to staining for E15.5 fetuses and postnatal mice. Sections of X-Gal stained E10.5 embryos were dehydrated and embedded in paraffin prior to sectioning at 10 µm. E14 and E17.5 mice were frozen in OCT compound, cut at 10 µm on a cryostat, and then X-gal stained. Some sections were counter-stained with eosin, nuclear-fast red, or hematoxylin. Between 5 and 15 embryos or crania were examined at each stage for Wnt1-Cre/R26R mice. The cranial base of 1 to 2 Mesp1-Cre/R26R mice from a larger sample was examined at each stage for this study. X-Gal-positive tissues and cells were consistent for all samples, with Wnt1-Cre/R26R samples staining positively in a complimentary pattern to that of Mesp1-Cre/R26R samples.
RESULTS
Chondrocranial Development in the Mouse
The general theme of chondrocranial development in the mouse is the following sequence: development of the caudal chondrocranium, followed by rostral chondrogenesis in the nasal capsule, and finally fusion of these two parts via the midline, central stem and the lateral struts of the vault cartilages (Fig. 1). Although the complete chondrocranium is a continuous cartilaginous structure, it develops from a number of distinct cartilages that appear at specific locations and times. Morphogenesis of the chondrocranium begins with the appearance of the parachordal cartilage at T18.1 (E11) (Fig. 1A, B). Like many midline cartilages in vertebrates, the parachordal cartilages likely first appear as a pair that quickly fuse (De Beer, 1937), but this fusion likely occurred prior to our observations at T18.1 (E11) and may even occur at the prechondrogenic mesenchymal condensation stage. At T18.1, the notochord rests on the superior surface of the parachordal cartilage and extends rostrally as far as Rathke’s pouch. By T20.32 (E12), the occipital arch cartilages develop just caudal to the parachordal and the canicular part of the auditory capsules develops laterally (Fig. 1C). At T21.22 (early E13), chondrogenesis of the trabecular cartilage begins in the presumptive nasal capsule (Fig. 1D). By the T21.31 stage (late E13), the hypophyseal cartilages appear in the center of the chondrocranium (just inferior to the developing pituitary gland), and the paranasal cartilages begin to form the lateral walls of the nasal capsule (Fig. 1E). T22 (E14) is characterized by the appearance of 3 distinct pairs of cartilages in the optic region: the orbital, frontal, and hypochiasmatic cartilages (Fig. 1F). The hypophyseal cartilages have also fused in the midline by this stage, while the trabecular cartilage has continued chondrifying caudally. Laterally, the parietal cartilages have just begun to chondrify at the junction of the cranial base and presumptive vault. At stage T23 (E15), the basitrabecular and ala temporalis cartilages have formed lateral to the hypophyseal cartilages, while the acrochordal cartilages have fused to create a bar at the rostral end of the parachordal cartilages, forming the rostral border of the basicranial fenestra (Fig. 1G). The cochlear part of the auditory capsules has also chondrified, and the supraoccipital cartilages have developed caudally forming the superior border of the foramen magnum.
At stage T24 (E16), the chondrocranium is fully formed in the mouse (Fig. 1H) as determined by the appearance and fusion of all of the cartilages that contribute to the chondrocranium. Key aspects of the mature chondrocranium are fusion of the central stem (trabecular plate) in the midline at the junction of the trabecular and hypophyseal cartilages and fusion of the frontal and parietal cartilages laterally, thereby linking the rostral nasal capsule to the caudal cranial base. Remnants of the notochord are still visible superior to the parachordal cartilage. Also, the mouse chondrocranium can have a number of midline fenestrae, as clearly seen in the specimen in Figure 1H. The hypophyseal cartilage can have up to two hypophyseal fenestrae, while the rostral end of the parachordal cartilage often has one basicranial fenestra. These fenestrae are not sites where nerves and blood vessels traverse the chondrocranium, and they disappear as a normal mouse matures. Finally, although the trabecular cartilage has developed as a single cartilaginous unit from the tip of the nasal capsule to the center of the chondrocranium, it is worth distinguishing the nasal portion from the basal portion of the trabecular plate at this stage. Later in development, the nasal portion will remain cartilaginous as the nasal septum, while the more caudal basal portion will form the body of the presphenoid bone. Also, the pterygoid cartilages appear on the ventral surface of the hypophyseal cartilage at this stage (not shown).
The optic region of the chondrocranium deserves special attention for a number of reasons (Fig. 2). First the hypochiasmatic cartilages (ala hypochiasmatica) have been described by de Beer (1937, p.389) as “paired cartilages of independent origin”, which may develop in a variety of locations in the optic region of different mammals. For example, they develop next to the preoptic root of the orbital cartilage in rabbits, attached to the postoptic root (pila metoptica) of the orbital cartilage in the armadillo, and attached to both roots in humans (de Beer, 1937). In the mouse, the hypochiasmatic cartilages appear independently at E14 (Fig. 1F) and then further develop in close association with the postoptic roots of the orbital cartilages (Fig. 2A). Although the hypochiasmatic cartilages form a robust bridge linking the postoptic roots with the body of the presphenoid bone just after birth (Fig. 2B), extensive remodeling during ossification eventually diminishes them so that by 6 months post-natal they merely form the most medial portions of the postoptic roots of the lesser (orbital) wings of the sphenoid bone (Fig. 2C).
There are also two distinct cartilages lateral to the optic foramen, the orbital cartilage and the frontal cartilage (Fig. 2A). DeBeer (1937) identified cartilage immediately laterally to the optic foramen in mammals as simply the orbital cartilage and did not note a distinct cartilage in relation to the frontal bone. However, in the mouse, there are two distinct centers of chondrification that contribute to two different bones in the adult, thus we suggest giving each a distinct name (Fig. 2). The orbital cartilage first appears in a semi-circle around the optic foramen (Fig. 2A) and then chondrifies further laterally (Fig. 2B). The orbital cartilage will eventually fuse with the hypochiasmatic cartilage, forming the lesser wing of the sphenoid, which is fused to the central body of the presphenoid bone (Fig. 2C). Meanwhile, the frontal cartilage forms from a separate chondrification center lateral to the orbital cartilage and later ossifies as part of the frontal bone (Fig. 2). Although it can be difficult to discern separate orbital and frontal cartilages in some preparations (Fig. 1H), other preparations show a clear border between these two cartilages that is maintained during ossification (Fig. 2).
Chondrocranial development can be observed in sections using classic Alcian blue staining or in situ hybridization with Col2a1 (Col2) and Sox9. All cartilages of the cranial base begin to express Col2a1 and Sox9 and are positively stained by Alcian blue as they develop between E12 and E16 (data not shown). Midline Col2 labeled sections are shown here to illustrate key features of chondrocranial development from E12 to E16 (Figure 3). Col2 expression can be observed in the parachordal cartilage at E11 (data not shown) and E12 (Fig. 3A). Where the notochord is present in sections, it is Col2 positive, along with some mesenchyme in the anterior cranial base (Fig. 3A). At E13, Col2 expression is seen in the chondrifying hypophyseal and trabecular cartilages and is more intense in the parachordal cartilage (Fig. 3B). As chondrification continues at E14, expression is also seen where the acrochordal cartilage will develop, which creates the rostral border of the basicranial fenestra (Fig. 3C). Both hypophyseal and basicranial fenestrae divide the midline cartilages into separate pieces in some sections (Fig. 3C & E). Expression in the chondrocranium is strongest at E15 (Fig. 3D). By E16, Col2 expression decreases in the hypophyseal and parachordal cartilages, where endochondral ossification has just begun (Fig. 3E).
The Col2 sections further reveal some unique features of the mouse cranial base (Fig. 3). The pituitary gland develops directly above the hypophyseal cartilage, which will eventually develop into the basisphenoid bone and spheno-occipital synchondrosis. A sella turcica, or depression in the superior surface of the cranial base, is not prominent in the mouse. Instead, the pituitary is encased in connective tissues that suspends the gland above the dorsal surface of the cranial base. An acrochordal cartilage is present but, unlike other mammals, is flush with the floor of the cranial base; this cartilage is homologous with the dorsum sellae, which in many other mammals forms a vertical projection that the pituitary rests against. Hypophyseal and basicranial fenestrae are normally present in the mouse chondrocranium (Fig. 3), but the exact number of fenestrae is variable (0–2 hypophyseal fenestrae and 1 basicranial fenestra). The fenestrae normally disappear prior to birth but may persist postnatally in some mutant mice (B. McBratney-Owen, unpublished observations).
Tissue Origins of the Cranial Base
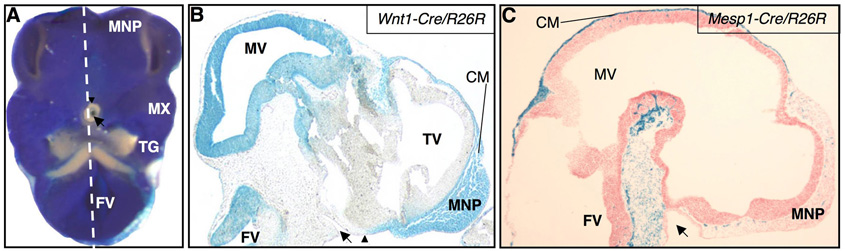
Fate mapping of the cranial base was accomplished with Wnt1-Cre/R262R and Mesp1-Cre/R26R transgenic mice. Previous research in mice has shown that neural crest cells migrate from the dorsal neural tube to the frontonasal process between somite stages 5 and 23 (E8 to E9.5) (Jiang et al, 2002). We show here that by E10.5, neural crest cells have migrated ventrally under the forebrain, populating the presumptive cranial base rostral to Rathke’s pouch (Fig. 4A,B). A ventral view of the cranium reveals neural crest-derived tissues in the nasal processes, the maxillary process, and the trigeminal ganglion (Fig. 4A). In contrast, Rathke’s pouch can be distinctly identified as an X-Gal-negative invagination surrounded rostrally and laterally, but not caudally, by X-Gal-positive tissue (Fig. 4A). Undifferentiated cranial mesenchyme rostral to Rathke’s pouch is positive for the neural crest lineage marker (Fig. 4B), while cranial mesenchyme caudal to Rathke’s pouch, and caudal to an equivalent level of the diencephalon dorsally, is mesoderm-derived (Fig. 4C). Scattered X-Gal-positive cells within the neural crest-derived mesenchyme of Mesp1-Cre-R26R embryos have been identified as angioblasts and endothelial cells of developing blood vessels (Yoshida et al., 2008).
Figure 4.
Neural crest and mesodermal contributions to the E10.5 cranial base of X-Gal stained crania. (A) Ventral view of a Wnt1-Cre/R26R cranium cut transversely just inferior to the maxillary processes and through the fourth ventricle showing cells of the neural crest lineage inhabiting the ventral face. Neural crest cells are not present in the ectodermal invagination of Rathke’s pouch (arrow) or in tissue just caudal to it. (B) A section of a Wnt1-Cre/R26R embryo reveals neural crest-derived cells in the undifferentiated cranial mesenchyme of the developing anterior cranial base and face; an arrowhead indicates the caudal border of neural crest cell migration in the midline at this age, just rostral to Rathke’s pouch (arrow). (C) A Mesp1-Cre/R26R sagitally sectioned cranium shows mesoderm-derived cranial mesenchyme caudal to Rathke’s pouch; there is also a LacZ positive layer of undifferentiated cranial mesenchyme deep to a layer of LacZ negative ectoderm in the caudal presumptive vault. Dashed line in A indicates placement of section in B. See key for abbreviations.
By E14, the relationship between the forming cartilages of the chondrocranium and their tissue origins can begin to be observed in more detail in sectioned material (Figure 5). The midline border of neural crest-derived tissue is between the hypophyseal cartilages and cartilages of the posterior cranial base, directly below the forming pituitary gland (Fig. 5B). In the posterior cranial base, only the parachordal cartilage has chondrified by E14, but the acrochordal cartilage will appear rostrally by the next day, creating the cartilaginous neural crest-mesoderm interface with the hypophyseal cartilage. The mesenchyme rostral to the hypophyseal cartilage is also derived from neural crest cells and will form the trabecular cartilage (Fig. 5B). More laterally, however, not all cartilages rostral to the hypophyseal cartilage are formed from neural crest cells (Fig. 5C,D). The hypochiasmatic cartilages (Y) are X-Gal negative, suggesting a mesodermal origin.
Figure 5.
Neural crest cell contributions to the rostral chondrocranium at E14 shown with Alcian blue stained C57BL/6J mouse sections and X-Gal/Hematoxylin stained Wnt1-Cre/R26 mouse sections. The whole mount insert identifies plane of section for A&B (midsagittal) and C&D (parasagittal). (A & B) The caudal border of neural crest cells is between the hypophyseal and parachordal cartilages. The future location of the acrochordal cartilage will form by E15 in X-Gal negative mesenchyme at the location of the asterisk (*). The arrow in (A) identifies a portion of the notochord superior to the parachordal cartilage. (B) In the midline, neural crest cells populate the hypophyseal cartilage and all rostral mesenchyme that will form the trabecular cartilage. (C & D) Laterally, the hypophyseal cartilage is still X-Gal positive, but the hypochiasmatic cartilage is negative, suggesting it has a mesodermal origin. See key for abbreviations.
At E17.5, most of the anterior cranial base appears to be derived from neural crest cells except for the hypochiasmatic cartilages, which are derived from mesoderm (Figure 6). The trabecular plate is derived from neural crest cells and has not yet begun to ossify into the presphenoid bone at this stage (Fig. 6A, B). The basisphenoid bone has begun to ossify from the hypophyseal cartilage; interestingly, these sections show a mixture of mesoderm- and neural crest cell-derived osteoblasts in the region of mineralizing matrix in the maturing body of this bone (Fig. 6C, D). Also, the periosteum of the basisphenoid bone is derived from both tissue sources; there appears to be a clear rostro-caudal boundary on both the ventral and dorsal surfaces of the bone, with the dorsal boundary being more rostrally placed than the ventral boundary (Fig. 6C, D). Both the fibrous and cellular layers of the periosteum are derived from the same tissue source. Recent reports have shown that there is endogenous ß-gal activity in bone, specifically in osteoclasts (Odgren et al., 2006; Kopp et al., 2007), so we used TRAP staining to identify osteoclasts in the cranial base of mice at E17.5 (data not shown). Less than 10% of observed cells in the periosteum and developing marrow cavity were TRAP positive. We can therefore conclude that the majority of LacZ positive cells observed in our sections are not osteoclasts and are positive due to lineage-specific, promoter-driven ß-gal expression. While the neural crest cell-derived osteoblasts in the marrow cavity of the basisphenoid bone must originate from the neural crest cell-derived periosteum, the mesoderm-derived osteoblasts could originate from the adjacent mesodermal periosteum or via invading blood vessels bringing cells from a distant mesodermal source.
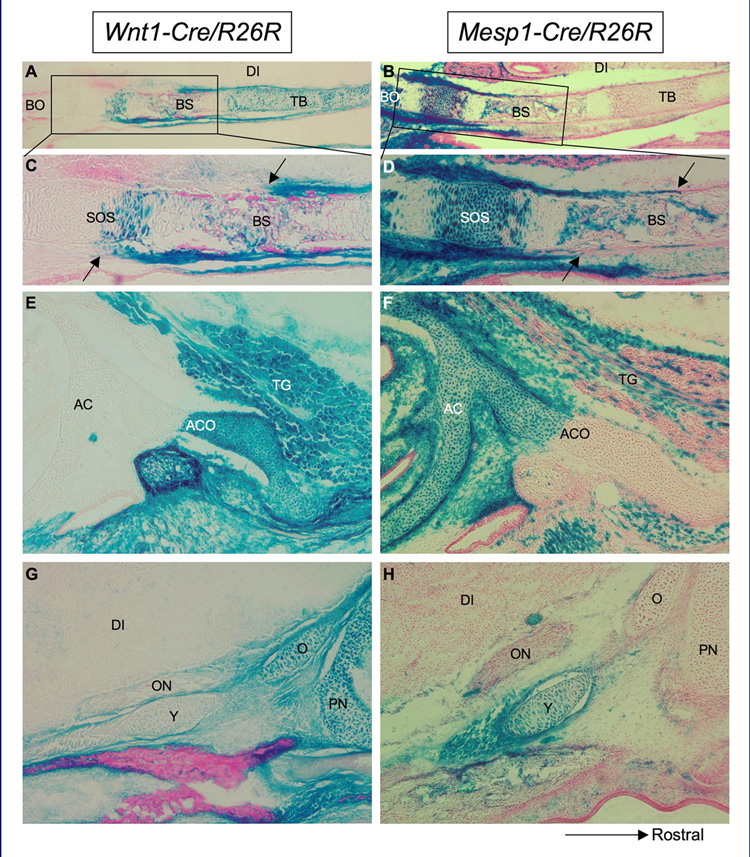
Figure 6.
X-Gal-stained sagittal sections of Wnt1-Cre/R26R (A,C,E, G) and Mesp1- Cre/R26R (B,D,F, H) mice at E17.5 showing the tissue origins of cranial base structures. A–D are midsagittal; E–H are parasagittal; rostral is to the right. Magnification of A & B is ×5; all others at ×10. See note in results section for this figure regarding endogenous ß-gal activity in bone (A,B). In the midline, the basal portion of the trabecular cartilage is neural crest cell-derived while the basioccipital bone shows only mesodermal contributions. (C,D) Although the basisphenoid bone develops from the completely neural crest-derived hypophyseal cartilage, its periosteum has both neural crest and mesodermal origins, with a clear boundary between the two components (arrows). Note that, due to glycogen deposition in the cytoplasm of hypertrophic chondrocytes (which is lost during tissue preparation) (Ross & Pawlina, 2006), the cytoplasm of hypertrophic chondrocytes was consistently LacZ-negative in both Wnt1-Cre/R26R and Mesp1-Cre/R26R mice, but nuclear staining indicating tissue lineage can still be observed. (E,F) The lateral neural crest-mesoderm boundary of the cranial base is found between the neural crest-derived alicochlear commissure of the basisphenoid bone and the mesoderm-derived auditory capsule. (G,H) The hypochiasmatic cartilage is entirely mesoderm-derived. See key for abbreviations.
The spheno-occipital synchondrosis also has a unique tissue origin, being partially neural crest and partially mesoderm derived (Fig. 6A–D); there are some neural crest-derived cells in the rostral portion of this cartilaginous structure, but none in the caudal portion (Fig. 6C). Mesoderm-derived cells contribute to all parts of the spheno-occipital synchondrosis and basioccipital bone (which has by this stage ossified from the parachordal and acrochordal cartilages). More laterally, the neural crest-mesodermal boundary lies between the alicochlear commissures of the basisphenoid bone and auditory capsules (Fig. 6E & F). The hypochiasmatic cartilage is entirely mesoderm-derived, in contrast with the orbital and paranasal cartilages (Fig. 6G & H).
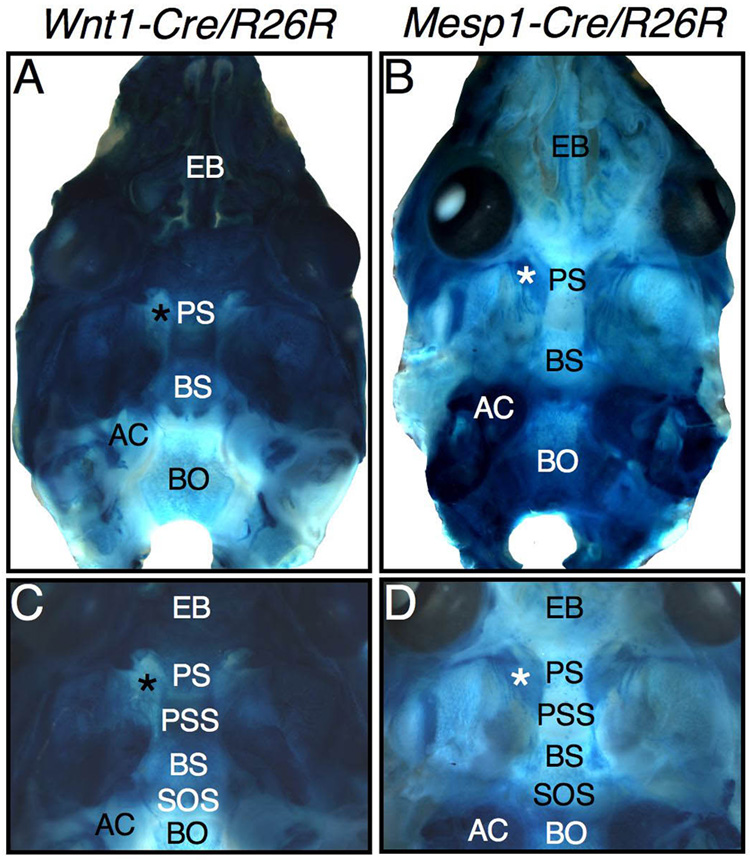
At birth, whole mount cranial bases clearly illustrate the largely neural crest-derived anterior cranial base relative to the mesoderm-derived posterior cranial base (Figure 7). The nasal capsule and bones associated with the trabecular cartilage (the body of the presphenoid bone and the ethmoid bone) are neural crest-derived (Fig. 7A). The basisphenoid bone and its associated greater wings (ala temporalis) appear to be largely neural crest derived, forming a border with the mesoderm-derived basioccipital bone and auditory capsules (Fig. 7A–D). However, the E17.5 sections reveal that the caudal portion of the basisphenoid bone has a mesoderm-derived periosteum and some cells of mesodermal origin within the developing bone (Fig. 6B & D), making this midline neural crest-mesoderm border less discrete histologically in the bony cranial base than in the chondrocranium of the fetus (Fig. 5). Altogether, development of the mammalian cranial base can be viewed as a dual dynamic process, with migration of neural crest-derived mesenchyme around the frontonasal process, eventually forming most of the anterior cranial base, integrated with the building of the posterior cranial base from mesoderm-derived mesenchyme.
Figure 7.
Neural crest and mesoderm contributions to the cranial base at birth (P0) shown with X-Gal-stained crania of a Wnt1-Cre/R26R mouse (A & C) and a Mesp1-Cre/R26R mouse (B & D). In general, the anterior cranial base is neural crest-derived whereas the posterior cranial base is mesoderm-derived. The exception to this is the mesoderm-derived hypochiasmatic cartilages, which contribute to the postoptic root of the presphenoid bone. *hypochiasmatic cartilage. See key for abbreviations.
Development of the Synchondroses
Synchondroses are similar to the epiphyseal growth plates of long bones except that they are double-faced, with a resting zone in the middle sandwiched between pairs of proliferating and hypertrophic chondrocyte zones. Postnatally, they are important longitudinal growth centers of the cranial base. In the mouse, there are two synchondroses in the midline cranial base: the spheno-occipital and presphenoidal synchondroses. The mouse does not have a sphenoethmoidal synchondrosis. Recent work by Wealthal and Herring (2006) found that endochondral ossification occurs at the junction between the developing ethmoid and presphenoid bones via a briefly existing, single growth plate which disappears by E15, leaving interstitial growth as the major contributor to elongation of the septum at the end of the fetal period and postnatally.
As described above, we showed that neural crest cells migrate around the frontonasal process and into the cranial base as far caudally as Rathke’s pouch (Figure 4). Rathke’s pouch itself is formed from an invagination of ectoderm and is eventually separated from the oral cavity by the cranial base. At E10.5, neural crest cells have migrated back to and partially around Rathke’s pouch, creating an ectodermal keyhole-shaped invagination at the caudal border of neural crest-derived cranial base tissues (Fig. 8A). By E15, this same keyhole shape can be observed in the mesenchyme of the developing spheno-occipital synchondrosis between the hypophyseal and parachordal cartilages (Fig. 8B). This suggests that as Rathke’s pouch is sealed off from the oral cavity, the opening of the pouch itself is filled in with mesenchyme from a non-neural crest source (possibly from the adjacent mesoderm-derived mesenchyme that forms the acrochordal cartilage), conserving the keyhole-shaped pattern in an X-Gal stained Wnt1-Cre/R26R fetus (Fig. 8B). Subsequently, the spheno-occipital synchondrosis will develop in that location from the both neural crest and mesoderm-derived mesenchyme. The boundary between neural crest and mesoderm-derived chondrocytes observed in midsagittal and parasagittal sections of this synchondrosis (Figure 6A–D) indicates that the embryonic relationship is maintained during late fetal development.
Figure 8.
The developing spheno-occipital synchondrosis shows a mixed tissue origin in X-Gal stained whole mount crania of Wnt1-Cre/R26R (A,B,D,E) and Mesp1-Cre/R26R (C) mice. (A) At E10.5, a whole mount endocranium (dorsal view) shows that neural crest-derived mesenchyme has migrated as far caudally as Rathke’s pouch in the midline, which forms a keyhole shaped invagination (white arrow) surrounded by X-Gal positive tissue. (B) At E15, a whole mount endocranium (dorsal view) shows that the presumptive spheno-occipital synchondrosis (dashed outline) has two X-Gal positive projections that surround the same keyhole shape (white arrow), which is now filled with LacZ-negative, mesoderm-derived tissue. (C–E) Sagitally cut and X-Gal-stained whole mount transgenic newborn crania reveal that the dual tissue origin of the spheno-occipital synchondrosis changes postnatally. (C & D) The rostral half of the spheno-occipital synchondrosis has a dual origin whereas the caudal half appears to be wholly mesoderm-derived. (E) By P10, the spheno-occipital synchondrosis appears to have lost its neural crest contribution. (C–E ) The presphenoidal synchondrosis is completely neural crest-derived and remains so through P10. See key for abbreviations.
However, the spheno-occipital synchondrosis does not retain its dual tissue contributions as development proceeds postnatally (Fig. 8C–E). At birth, part of this synchondrosis is mesoderm-derived (Fig. 8C), while the remainder consists of cells derived from the neural crest (Fig. 8D). By 10 days after birth, the spheno-occipital synchondrosis no longer has any neural crest cell-derived chondrocytes (Fig. 8E). Since chondrocytes in the proliferative zone eventually move through the hypertrophic zone, followed by apoptosis (Shapiro et al., 2005), neural crest cells populating the rostral zones of the spheno-occipital synchodrosis may disappear from this synchondrosis by following this normal developmental route. In contrast, the presphenoidal synchondrosis develops from neural crest cell-derived chondrocytes at the caudal edge of the trabecular plate (Fig. 6A, B), remaining entirely composed of neural crest-derived cells at birth and up to at least 10 postnatal days (Fig. 8D, E).
DISCUSSION
Mosaic Development of the Cranial Base
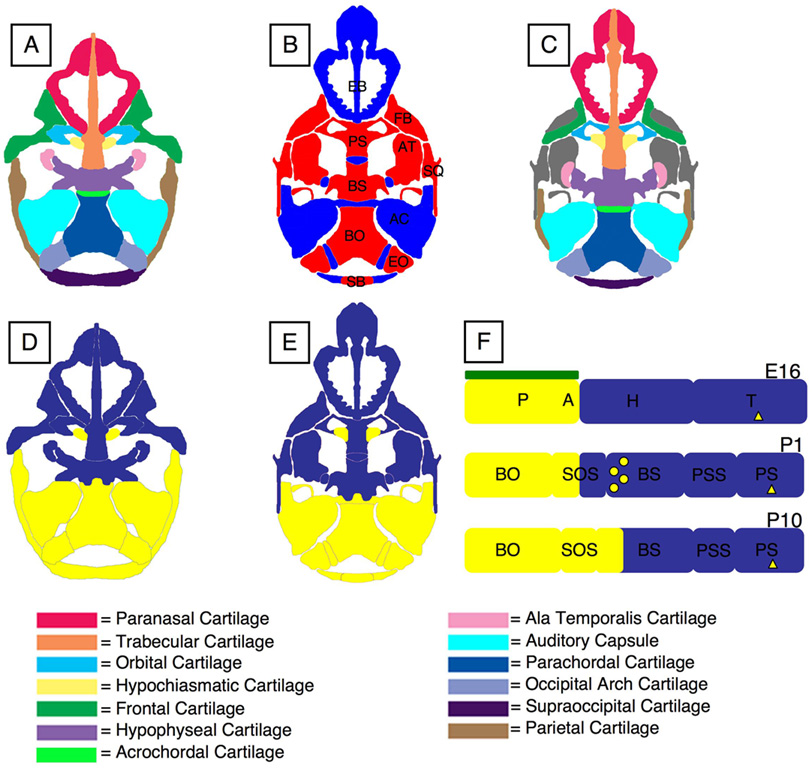
The cranial base of the mouse develops from 14 pairs of cartilages that appear at distinct locations and times and are derived from either mesoderm or neural crest cells. Fig. 9 summarizes our findings. Neural crest cells destined for the anterior cranial base leave the lateral edges of the neural folds during cranial neurulation, beginning early on E8.5 (Jiang et al., 2002). They migrate over the forebrain and around the eyes to form the frontonasal process, forming a boundary over the rostral diencephalon and just caudal to the eyes. Between E11 and E14, these neural crest-derived cells chondrify as the trabecular, paranasal, frontal, orbital, hypophyseal, and pterygoid cartilages (Fig. 9A & D). The mesoderm-derived hypochiasmatic cartilages also chondrify by E14 within the anterior cranial base. Meanwhile, the posterior cranial base develops wholly from mesoderm, with the auditory capsules and parachordal, acrochordal, exoccipital, and supraoccipital cartilages chondrifying between E11 and E15 (Fig. 9A & D). Formation of the mature chondrocranium is complete by E16, with a largely neural crest cell-derived anterior cranial base and mesoderm-derived posterior cranial base (Fig. 9A & D).
Figure 9.
Schematic Summary of Results. (A) The individual cartilages of the mature chondrocranium (E16) are shown in different colors (the pterygoid cartilages appear on the ventral surface of the hypophyseal cartilage and are therefore not shown). (B) A newborn cranial base is shown here with bone in red, cartilage in blue. (C) The newborn cranial base is shown with colors corresponding to the cartilages from which each bone matures. Bones shown in grey ossify intramembranously and do not develop from the chondrocranium. (D) The tissue origins of the mature chondrocranium at E16 and (E) the newborn cranial base are shown with cartilages and bones derived from neural crest cells in blue and those derived from mesoderm in yellow. (F) A schematic drawing of the sagittal neural crest-mesoderm boundary in the cranial base at E16, P1, and P10. The notochord is shown in green above the parachordal cartilage at E16. The presence of non-neural crest-derived osteoblasts in the caudal portion of the basisphenoid bone at birth is indicated by circles. The mesoderm-derived hypochiasmatic cartilage is shown as a yellow triangle on the trabecular cartilage prior to birth and on the presphenoid bone after birth. Notice that the neural crest-mesoderm boundary moves rostrally out of the spheno-occipital synchondrosis and into the basisphenoid bone as development proceeds postnatally. See key for abbreviations.
The bones and cartilages that form the mature cranial base are identifiable at birth (Fig. 9B). Each bone is derived from a number of cartilages, revealing their mosaic origins (Fig. 9C & E). The ethmoid bone develops from the neural crest cell-derived paranasal and trabecular cartilages. The presphenoid bone develops from the neural crest cell-derived orbital and trabecular cartilages and from the mesoderm-derived hypochiasmatic cartilages. The basisphenoid bone develops from the entirely neural crest-derived hypophyseal, ala temporalis, and pterygoid cartilages, but endochondral ossification also introduces osteoblasts from a mesodermal source in the caudal portion of this bone. Inferior portions of the frontal and parietal bones ossify from the neural crest cell-derived frontal and mesoderm-derived parietal cartilages via a unique ossification process that is distinct from normal endochondral or intramembranous ossification (Holmbeck et al., 2003). Finally, although the basioccipital, exoccipital, and supraoccipital bones develop from distinct acrochordal and parachordal, occipital arch, and supraoccipital cartilages, respectively, these bones eventually fuse to form the single, mature mesoderm-derived occipital bone.
Evolution of the Vertebrate Cranial Base
In their New Head Hypothesis (NHH), Gans & Northcutt (1983; Northcutt & Gans, 1983) proposed that vertebrates evolved by adding a ‘new head’ rostral to the notochord, made of ectodermally derived sense organs and nervous structures, to aid in predatory behavior. Subsequent work described this building of a new head as including, among other things, an “anterior extension of the connective tissues providing a connection among the sensory capsules” (Gans, 1993) and specifically posited that these rostral connective tissues are neomorphic, developing from neural-crest derived mesenchyme (Northcutt, 2005). The NHH predicts that the prechordal-chordal boundary should be coincident with the neural crest-mesoderm boundary and that connective tissues of the prechordal head (including bone and cartilage) should be derived from the neural crest. So far, the skeletal and connective tissues of the rostral cranium of zebrafish, lungfish, axolotls, amphibians, and birds have been shown to be derived from the neural crest while cranial muscles are derived from prechordal and cephalic paraxial mesoderm (Schilling and Kimmel, 1994; Noden et al., 1999; Olsson et al., 2001; Ericsson et al., 2004; Hanken and Gross, 2005; Noden and Francis-West, 2006; Ericsson et al., 2008).
In this paper we show that there are coincident neural crest-mesoderm and prechordal-chordal boundaries between the hypophyseal and acrochordal cartilages in mice (Fig. 9F), providing support for the NHH. However, these results do not completely fulfill the predictions of the NHH due to the presence of the mesoderm-derived hypochiasmatic cartilages rostral to the notochord. In other words, a small portion of connective tissue in the prechordal cranial base of the “New Head” in the mouse is not derived from neural crest. The presence of the mesoderm-derived hypochiasmatic cartilages among neural crest cell-derived tissues suggests that they may have an evolutionary origin independent of the origin of the “New Head”.
The hypochiasmatic cartilages have so far only been documented in mammalian taxa (humans, monkeys, hedgehogs, rabbits, dogs, cats: DeBeer, 1937) and, in this paper, mice; they appear as independent cartilages in variable places around the optic foramina, eventually contributing to bones in the sphenoid region (DeBeer, 1937). These cartilages provide an attachment point for the extra-ocular muscles (Dierbach, 1985), and, as these muscles are derived from prechordal and cephalic paraxial mesoderm (Wachtler et al., 1984; Noden et al, 1999; Noden & Francis-West, 2006), it is not unreasonable to hypothesize that the morphologically associated hypochiasmatic cartilages evolved neomorphically in situ from the same mesodermal source. In vertebrates, the central nervous system or developing sensory organs can provide spatial programming cues to cranial connective tissue precursors that, in turn, provide positional information to developing muscles, blood vessels, and neurons (Noden and Trainor, 2005; Noden and Francis-West, 2006). Using that model, the developing optic epithelium may provide spatial programming cues to the mesenchymal cells of the presumptive hypochiasmatic cartilages, which then provide positional information to the extra-ocular muscles. Since the spatial position of the hypochiasmatic cartilages appears to be variable in mammals, the optic epithelium could direct their location to a position that would be optimal for eye function specific to each organism. Having a closely associated mesenchymal cell source that is variable both anatomically and in tissue origin may provide an evolutionary avenue for functional adaptation of the visual apparatus.
The hypochiasmatic cartilages may have evolved in the ancestor to all mammals, independently and repeatedly in different mammalian lineages, or in the ancestor of all tetrapods. Early chondrocranial development needs to documented in other mammalian taxa to see if these small and transient cartilages are present and common to all mammals. If so, they may have evolved at the origin of the mammalian clade. However, if other mammalian taxa do not have these cartilages, either they evolved in the ancestor of all mammals but were subsequently lost in some lineages or they evolved independently a number of times in only some mammalian clades.
A tetrapod origin for the hypochiasmatic cartilages may also be supported if cranial base element homology can be resolved more completely among mammals, birds, and reptiles. Kuratani (1989) suggests that the supratrabecular cartilages of reptiles may be homologous with the hypochiasmatic cartilages of mammals. In chickens, the supratrabecular cartilages serve as the origins of the rectus muscles of the eyes (DeBeer, 1937), supporting at least a functional equivalence with the hypochiasmatic cartilages. A mesoderm-derived supratrabecular cartilage in the chicken (or other amniote) is consistent with a homology with the mammalian hypochiasmatic cartilages. Unfortunately, the tissue origin of the supratrabecular cartilages of birds is unclear since they fuse with the trabecular and polar cartilages at the point where the neural crest-mesoderm boundary has been reported in the chicken chondrocranium (Couly et al., 1993). Examination of the tissue origin of the supratrabecular cartilages of the chicken while they are still independent of other chondrocranial cartilages at the 7-day stage (DeBeer, 1937) is needed.
The tissue origin of the rest of the cranial base of the chicken is well studied, with the neural crest-mesoderm boundary formed at the prechordal-chordal boundary between the basi-presphenoid and basi-postsphenoid (Le Lievre, 1978; Noden, 1988; Couly et al., 1993; Le Douarin et al., 1993; Lengele et al., 1996; Le Douarin & Kalcheim, 1999). However, it is difficult to compare these results with the mouse due to the confusing use of similar terms to describe structures that probably non-homologous. For example, the basi-presphenoid identified in the chicken chondrocranium by Couly et al. (1993) consists of the caudal end of the trabecular cartilage fused with the supratrabecular and polar cartilages (DeBeer, 1937). While the trabecular cartilage is homologous with the structure of the same name in mice (and does develop into a portion of the presphenoid bone), the polar cartilages of chickens have been described as homologous to the alicochlear commissures of the mouse (DeBeer, 1937). Although there is also a neural crest-mesoderm boundary in the alicochlear commissures of the mouse, these are lateral structures not connected to the presphenoid bone, suggesting that the basi-presphenoid of the chicken is not homologous to the presphenoid bone of the mouse. Regardless of the difficulties presented by homology of the cranial base, the NHH is supported by the observations in the chicken and mouse, since the neural crest-mesoderm boundary is coincident with the rostral tip of the notochord in both species.
Morphogenesis and the Role of Tissue Origins
Although the tissue origin of each cranial base cartilage has been shown to be discrete, being derived either from neural crest or mesoderm cells, some of the bones that develop from these cartilages show dual tissue origins. The presphenoid bone has a dual tissue origin, with the orbital and trabecular cartilages being derived from neural crest cells whereas the hypochiasmatic cartilages (which eventually form just the postoptic roots of the presphenoid bone) are derived from mesoderm. The basisphenoid bone also has a dual tissue origin, but via a different mechanism. The hypophyseal cartilage, which develops into the body of the basisphenoid bone, is derived solely from neural crest cells, but cells of both neural crest and mesodermal origin are found in the periosteum and early marrow cavity of the developing basisphenoid bone. While the neural crest cell-derived osteoblasts likely came from neural crest cell-derived periosteum, the mesoderm-derived osteoblasts may develop from the periosteum or an external osteoprogenitor source that invades via blood vessels.
That some bones, but not the cartilages they develop from, have dual tissue contributions should draw our attention to an important distinction: although a mature bone may have osteogenic contributions from cells of different tissue origins, the cartilages from which the bone develops are each derived from one tissue type, and it is the cartilages (and the mesenchyme from which they condense) that first establish the morphological pattern. Rather than being potentially distracted by the source of osteogenic cells that arrive on the scene after much of the morphological pattern is well established, it may be more interesting to focus on the fact that most of the mesenchyme that forms the anterior cranial base cartilages originates from frontonasal and post-optic streams of migrating neural crest cells. After entering the area beneath the telencephalon and rostral diencephalon, these mesenchyme cells condense and chondrify in a specific spatiotemporal pattern to form cartilages that provide a framework for the characteristic adult morphology. It is with this in mind that we can ask: what are the signals that establish the pattern in the mesenchyme, and does it matter whether the cells are derived from neural crest or mesoderm in order to respond to specific patterning signals?
Research suggests that the notochord is a major signaling center for patterning the parachordal cartilage (Nie et al., 2005b; Young et al., 2006), but less is known about what provides the patterning cues for the anterior cranial base. In gastrulating embryos, the mesendoderm-derived prechordal plate is rostral to the notochord and directly below the presumptive prosencephalon (Tam & Behringer, 1997). Anterior midline tissues in this region have distinct and segmented molecular expression, which appears to be involved in regionalizing the brain (Camus et al., 2000). Later, neural crest cells migrate to the presumptive cranial base between the neuroepithelium and facial ectoderm, and, by E10.5, neural crest-derived cells are the only mesenchymal cells that populate the presumptive anterior cranial base, although the prechordal plate mesodermal cells will later give rise to the extrinsic ocular muscles and possibly the hypochiasmatic cartilages. It is interesting to consider the patterning of the connective tissues of the anterior cranial base in conjunction with the major innovations of the ‘New Head’, the expanded forebrain and sense organs. There is evidence that ectodermal signaling plays a role in morphogenesis of the underlying mesenchyme. In zebrafish, morphogenesis of the trabecular cartilage and ethmoid plate relies on hedgehog signaling from the brain and facial ectoderm (Wada et al., 2005). In the chicken, Shh and Fgf8 signaling in the neural and facial ectoderm is important for facial outgrowth (Schneider et al., 2001; Abzhanov and Tabin, 2004; Abzhanov et al., 2007). Future research should focus on the possible roles of the neuroepithelium and surface ectoderm in determining where neural crest cell-derived cells migrate to and condense in the presumptive anterior cranial base and whether they are restricted from entering the chordal region due to inhibitory interactions with regionally expressed proteins (such as from the brain, surface ectoderm, mesoderm-derived mesenchyme and/or the notochord itself). Once the influential signaling centers are known, it will be interesting to determine whether all mesenchyme, regardless of tissue origin, has the ability to respond to the ectodermal signals or whether there are different responses to permissive and inhibitory signals due to intrinsic capabilities born from their tissue history.
ACKNOWLEDGEMENTS
We are grateful to Dr. Jim Hanken for providing helpful comments on a draft of this manuscript. B. M.-O. was generously supported by NIH grants AR 36819 and T32 DE017544 (to B.R. Olsen) and a graduate student fellowship from the Department of Anthropology at Harvard University.
Abbreviations Key
- A
ala temporalis cartilage
- AC
auditory capsule
- ACO
alicochlear commissure
- AR
acrochordal cartilage
- AT
ala temporalis (greater wing) of basisphenoid bone
- B
basitrabecular process
- BF
basicranial fenestra
- BO
basioccipital bone
- BS
basisphenoid bone
- CA
canicular part of auditory capsule
- CM
cranial mesenchyme
- CO
cochlear part of auditory capsule
- DI
diencephalon
- EB
ethmoid bone
- EO
exoccipital bone (will eventually fuse to basioccipital bone)
- F
frontal cartilage
- FB
frontal bone
- FV
fourth ventricle of hindbrain
- GW
greater wing (ala temporalis) of the basisphenoid bone
- H
hypophyseal cartilage
- HF
hypophyseal fenestra
- LW
lesser wing of the presphenoid bone
- MNP
medial nasal process
- MV
mesencephalic vesicle of midbrain
- MX
maxillary process
- NL
orbitonasal lamina of the paranasal cartilage
- O
orbital cartilage
- OA
occipital arch cartilage
- OF
optic foramen
- ON
optic nerve
- OR
optic recess
- P
parachordal cartilage
- PA
parietal cartilage
- PI
developing pituitary gland
- PN
paranasal cartilage
- PS
presphenoid bone
- PSS
presphenoidal synchondrosis
- S
supraoccipital cartilage
- SB
supraoccipital bone (will eventually fuse to basioccipital bone)
- SOS
spheno-occipital synchondrosis
- SQ
squamosal bone
- T
trabecular cartilage (trabecular plate)
- TB
basal portion of trabecular plate
- TG
trigeminal nerve
- TN
nasal portion of trabecular plate
- To
Tongue
- TV
third ventricle of forebrain
- Y
hypochiasmatic cartilage
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abzhanov A, Tabin CJ. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Devel. Biol. 2004;273(1):134–148. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Cordero DR, Sen J, Tabin CJ, Helms JA. Cross-regulatory interactions between Fgf8 and Shh in the avian frontonasal prominence. Congenit.Anom. 2007;47(4):136–148. doi: 10.1111/j.1741-4520.2007.00162.x. [DOI] [PubMed] [Google Scholar]
- Barteczko K, Jacob M. Comparative study of shape, course, and disintegration of the rostral notochord in some vertebrates, especially humans. Anat. Embryol. 1999;200:345–366. doi: 10.1007/s004290050285. [DOI] [PubMed] [Google Scholar]
- Belo JA, Leyns L, Yamada G, De Robertis EM. The prechordal midline of the chondrocranium is defective in Goosecoid-1 mouse mutants. Mech Dev. 1998;71(1–2):15–25. doi: 10.1016/s0925-4773(97)00204-9. [DOI] [PubMed] [Google Scholar]
- Bohme K, Li Y, Oh PS, Olsen BR. Primary structure of the long and short splice variants of mouse collagen XII and their tissue-specific expression during embryonic development. Dev Dyn. 1995;204(4):432–445. doi: 10.1002/aja.1002040409. [DOI] [PubMed] [Google Scholar]
- Camus A, Davidson BP, Billiards S, Khoo P, Rivera-Perez JA, Wakamiya M, Behringer RR, Tam PP. The morphogenetic role of the midline mesendoderm and ectoderm in the development of the forebrain and the midbrain of the mouse mebryo. Development. 2000;2000:1799–1813. doi: 10.1242/dev.127.9.1799. [DOI] [PubMed] [Google Scholar]
- Chen L, Adar R, Yang X, Monsonego EO, Li C, Hauschka PV, Yayon A, Deng CX. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J Clin Invest. 1999;104(11):1517–1525. doi: 10.1172/JCI6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KS, Jacenko O, Boyle P, Olsen BR, Nishimura I. Craniofacial abnormalities in mice carrying a dominant interference mutation in type X collagen. Dev Dyn. 1997;208(4):544–552. doi: 10.1002/(SICI)1097-0177(199704)208:4<544::AID-AJA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin N. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- De Beer G. The Development of the Vertebarate Skull. Oxford: Clarendon Press; 1937. [Google Scholar]
- Dierbach AR. Morphogenesis of the cranium of Cavia porcellus L. II: Comparative part and literature Gegenbaurs Morphol Jahrb. 1985;131(5):617–642. [PubMed] [Google Scholar]
- Ericsson R, Cerny R, Falck P, Olsson L. Role of cranial neural crest cells in visceral arch muscle positioning and morphogenesis in the Mexican axolotl, Ambystoma mexicanum. Dev Dyn. 2004;231(2):237–247. doi: 10.1002/dvdy.20127. [DOI] [PubMed] [Google Scholar]
- Ericsson R, Joss J, Olsson L. The fate of cranial neural crest cells in the Australian lungfish, Neoceratodus forsteri. J Exp Zoolog B Mol Dev Evol. 2008;310(4):345–354. doi: 10.1002/jez.b.21178. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Monsonego-Ornan E, Pines M, Antonopoulou I, Morriss-Kay GM, Lonai P. The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development. 2002;129(16):3783–3793. doi: 10.1242/dev.129.16.3783. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Noden DM. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dyn. 2006;235(5):1310–1325. doi: 10.1002/dvdy.20663. [DOI] [PubMed] [Google Scholar]
- Gakunga PT, Kuboki Y, Opperman LA. Hyaluronan is essential for the expansion of the cranial base growth plates. J Craniofac Genet Dev Biol. 2000;20(2):53–63. [PubMed] [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–274. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Gans C. Evolutionary origin of the vertebrate skull. In: Hanken J, Hall BK, editors. The skull. Vol. 2. Chicago: University of Chicago Press; 1993. pp. 1–35. [Google Scholar]
- Gong SG. Phenotypic and molecular analyses of A/WySn mice. Cleft Palate Craniofac J. 2001;38(5):486–491. doi: 10.1597/1545-1569_2001_038_0486_pamaoa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Gross JB, Hanken J. Review of fate-mapping studies of osteogenic cranial neural crest in vertebrates. Dev Biol. 2008;317(2):389–400. doi: 10.1016/j.ydbio.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Hallgrimsson B, Brown JJ, Ford-Hutchinson AF, Sheets HD, Zelditch ML, Jirik FR. The brachymorph mouse and the developmental-genetic basis for canalization and morphological integration. Evol Dev. 2006;8(1):61–73. doi: 10.1111/j.1525-142X.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- Hanken J, Gross JB. Evolution of cranial development and the role of neural crest: insights form amphibians. J Anat. 2005;207(5):437–446. doi: 10.1111/j.1469-7580.2005.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H. MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J Cell Biol. 2003;163(3):661–671. doi: 10.1083/jcb.200307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LF, Fukai N, Selby PB, Olsen BR, Mundlos S. Mouse clavicular development: analysis of wild-type and cleidocranial dysplasia mutant mice. Dev.Dyn. 1997;210(1):33–40. doi: 10.1002/(SICI)1097-0177(199709)210:1<33::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Isii-Suzuki M, Suda N, Yamazaki K, Kuroda T, Senior PV, Beck F, Hammond VE. Differential responses to parathyroid hormone-related protein (PTHrP) deficiency in the carious craniofacial cartilages. Anat Rec. 1999;255(4):452–457. doi: 10.1002/(SICI)1097-0185(19990801)255:4<452::AID-AR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowditch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson R, Sucov H, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Developmental Biology. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Jones E, Roberts GJ. The midline cartilages of the cranial base of the hypopituitary dwarf mouse (dw/dw). A histological and autoradiographic study. J Anat. 1988;159:137–145. [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH, Chang HH, Shaw JP. Craniofacial abnormalities in homozygous Small eye (Sey/Sey) embryos and newborn mice. J Anat. 1995;186:607–617. [PMC free article] [PubMed] [Google Scholar]
- Kettunen P, Nie X, Kvinnsland IH, Luukko K. Histological development and dynamic expression of Bmo2-6 mRNAs in the embryonic and postnatal mouse cranial base. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(12):1250–1258. doi: 10.1002/ar.a.20402. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Hooper AT, Shmelkov SV, Rafii S. Beta-galactosidase staining on bone marrow. The osteoclast pitfall. Histol Histopathol. 2007;22(9):971–976. doi: 10.14670/HH-22.971. [DOI] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra R, Pacifici M. Conditional Kif3a ablation causes abnormal hedgehog signalling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134:2159–2169. doi: 10.1242/dev.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S. Development of the orbital region in the chondrocranium of Caretta caretta. Reconsideration of the vertebrate neurocranium configuration. Anat Anz. 1989;169(5):335–349. [PubMed] [Google Scholar]
- Le Douarin NM, Ziller C, Couly GF. Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Devel. Biol. 1993;159:24–49. doi: 10.1006/dbio.1993.1219. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Le Lievre CS. Participation of neural crest-derived cells in the genesis of the skull in birds. J. Embryol. Exp. Morphol. 1978;47:17–37. [PubMed] [Google Scholar]
- Lengele B, Schowing J, Dhem A. Embryonic origin and fate of chondroid tissue and secondary cartilages in the avian skull. Anat. Rec. 1996;246:377–393. doi: 10.1002/(SICI)1097-0185(199611)246:3<377::AID-AR9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Hallgrímsson B, Liu W, Parsons TE, Jamniczky HA. Spatial packing, cranial base angulation, and craniofacial shape variation in the mammalian skull: testing a new model using mice. J Anat. 2008;212:720–735. doi: 10.1111/j.1469-7580.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozanoff S, Jureczek S, Feng T, Padwal R. Anterior cranial base morphology in mice with midfacial retrusion. Cleft Palate Craniofac J. 1994;31(6):417–428. doi: 10.1597/1545-1569_1994_031_0417_acbmim_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Ma W, Lozanoff S. Morphological deficiency in the prenatal anterior cranial base of midfacially retrognathic mice. J Anat. 1996;188:547–555. [PMC free article] [PubMed] [Google Scholar]
- McBratney BM, Margaryan E, Ma W, Urban Z, Lozanoff S. Frontonasal dysplasia in 3H1 Br/Br mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;271(2):291–302. doi: 10.1002/ar.a.10034. [DOI] [PubMed] [Google Scholar]
- Merrill AE, Bochukova EG, Brugger SM, Ishii M, Pilz DT, Walls SA, Lyons KM, Wilkie AO, Maxson RE., Jr Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Hum Mol Genet. 2006;15(8):1319–1328. doi: 10.1093/hmg/ddl052. [DOI] [PubMed] [Google Scholar]
- Miyake T, Cameron AM, Hall BK. Detailed staging of inbred C57BL/6 mice between Theiler’s (1972) stages 18 and 21 (11–13 days of gestation) based on craniofacial development. J Craniofac. Genet. Dev Biol. 1996;16(1):1–31. [PubMed] [Google Scholar]
- Nagayama M, Iwamoto M, Hargett A, Kamiya N, Tamamura Y, Young B, Morrison T, Takeuchi H, Pacifici M, Enomoto-Iwamoto M, Koyama E. Wnt/beta-catenin signaling regulates cranial base development and growth. J. Dent. Res. 2008;87:244–249. doi: 10.1177/154405910808700309. [DOI] [PubMed] [Google Scholar]
- Nie X. Sox9 mRNA expression in the developing palate and craniofacial muscles and skeletons. Acta Odontol Scand. 2006a;64(2):97–103. doi: 10.1080/00016350500420089. [DOI] [PubMed] [Google Scholar]
- Nie X. Developmentally regulated expression of MSX1, MSX2, and Fgfs in the developing mouse cranial base. Angle Orthod. 2006b;76(6):990–995. doi: 10.2319/082305-298. [DOI] [PubMed] [Google Scholar]
- Nie X, Luukko K, Fjeld K, Kvinnsland IH, Kettunen P. Developmental expression of Dkk1-3 and Mmp9 and apoptosis in the cranial base of mice. J. Mol. Histol. 2005a;36:419–426. doi: 10.1007/s10735-005-9014-5. [DOI] [PubMed] [Google Scholar]
- Nie X, Luukko K, Kvinnsland IH, Kettunen P. Developmentally regulated expression of Shh and Ihh in the developing mouse cranial base: comparison with Sox9 expression. Anat Rec A Discov Mol Cell Ecol Biol. 2005b;286(2):891–898. doi: 10.1002/ar.a.20231. [DOI] [PubMed] [Google Scholar]
- Noden DM. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev. Biol. 1978;67:296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- Noden DM. Craniofacial development: New views on old problems. Anat. Rec. 1984;208:1–13. doi: 10.1002/ar.1092080103. [DOI] [PubMed] [Google Scholar]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103 suppl.:121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Noden DM, Marcucio R, Borycki AG, Emerson CP., Jr Differentiation of avian craniofacial muscles: I. Pattern of early regulatory gene expression and myosin heavy chain synthesis. Dev Dyn. 1999;216(2):96–112. doi: 10.1002/(SICI)1097-0177(199910)216:2<96::AID-DVDY2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235(5):1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. The New Head Hypothesis revisited. J Exp Zool (Mol Dev Evol) 2005;304B:274–297. doi: 10.1002/jez.b.21063. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol. 1983;58(1):1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Odgren PR, MacKay CA, Mason-Savas A, Yang M, Mailhot G, Birnbaum MJ. False-positive beta-galactosidase staining in osteoclasts by endogenous enzyme: studies in neonatal and month-old wild-type mice. Connect Tissue Res. 2006;47(4):229–234. doi: 10.1080/03008200600860086. [DOI] [PubMed] [Google Scholar]
- Olsen BR, Kolpakova E, McBratney-Owen B, Li X, Zhou J, Fukai N. Genetic and Epigenetic Determinants of Skeletal Morphogenesis-Role of Cellular Polarity and Ciliary Function in Skeletal Development and Growth. Oral Biosci. Med. 2005;2:57–65. [Google Scholar]
- Olsson L, Falck P, Lopez K, Cobb J, Hanken J. Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Dev Biol. 2001;237(2):354–367. doi: 10.1006/dbio.2001.0377. [DOI] [PubMed] [Google Scholar]
- Rintala M, Metsaranta M, Garofalo S, de Crombrugghe B, Vuorio E, Ronning O. Abnormal craniofacial morphology and cartilage structure in transgenic mice harboring Gly→Cys mutation in the cartilage-specific type II collagen gene. J Craniofac Genet Dev Biol. 1993;13(3):137–146. [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and requirted for the formation of a single heart tube. Development. 1999;126(15):3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Savontaus M, Rintala-Jamsa M, Morko J, Ronning O, Metsaranta M, Vuorio E. Abnormal craniofacial development and expression patterns of extracellular matrix components in transgenic Del1 mice harboring a deletion mutation in the type II collagen gene. Orthod Craniofac Res. 2004;7(4):216–226. doi: 10.1111/j.1601-6343.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120(3):483–494. doi: 10.1242/dev.120.3.483. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Hu D, Rubenstein JL, Maden M, Helms JA. Local retinoid signalling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128(14):2755–2767. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- Shapiro IM, Adams CS, Freeman T, Srinivas V. Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res C Embryo Today. 2005;75(4):330–339. doi: 10.1002/bdrc.20057. [DOI] [PubMed] [Google Scholar]
- Shum L, Wang X, Kane AA, Nuckolls GH. BMP4 promotes chondrocyte proliferation and hypertrophy in the endochondral cranial base. Int J Dev Biol. 2003;47(6):423–431. [PubMed] [Google Scholar]
- Tam P, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mech. Devel. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Theiler K. The house mouse: atlas of embryonic development. New York: Springer-Verlag; 1989. [Google Scholar]
- Wachtler F, Jacob HJ, Jacob M, Christ B. The extrinsic ocular muscles in birds are derived from the prechordal plate. Naturwissenschaften. 1984;71(7):379–380. doi: 10.1007/BF00410750. [DOI] [PubMed] [Google Scholar]
- Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- Wealthal RJ, Herring SW. Endochondral Ossification of the Mouse Nasal Septum. Anat. Rec. 2006;288A:1163–1172. doi: 10.1002/ar.a.20385. [DOI] [PubMed] [Google Scholar]
- Wood A, Ashhurst DE, Corbett A, Thorogood P. The transient expression of type II collagen at tissue interfaces during mammalian craniofacial development. Development. 1991;111(4):955–968. doi: 10.1242/dev.111.4.955. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008 doi: 10.1016/j.mod.2008.06.007. In press. [DOI] [PubMed] [Google Scholar]
- Young B, Minugh-Purvis N, Shimo T, St-Jacques B, Iwamoto M, Enomoto-Iwamoto M, Koyama E, Pacifici M. Indian and sonic hedgehogs regulate syhnchondrosis growth plate and cranial base development and function. Dev Biol. 2006;299(1):272–282. doi: 10.1016/j.ydbio.2006.07.028. [DOI] [PubMed] [Google Scholar]