Abstract
Germline-specific granules of unknown function are found in a wide variety of organisms, including C. elegans, where they are called P granules. P granules are cytoplasmic bodies in oocytes and early embryos. Throughout most of the C. elegans life cycle, however, P granules are associated with clusters of nuclear pore complexes (NPCs) on germ cell nuclei. We show that perinuclear P granules differ from cytoplasmic P granules in many respects, including structure, stability and response to metabolic changes. Our results suggest that nuclear-associated P granules provide a perinuclear compartment where newly exported mRNAs are collected prior to their release to the general cytoplasm. First, we show that mRNA export factors are highly enriched at the NPCs associated with P granules. Second, we discovered that the expression of high-copy transgenes could be induced in a subset of germ cells, and used this system to demonstrate that nascent mRNA traffics directly to P granules. P granules appear to sequester large amounts of mRNA in quiescent germ cells, presumably preventing translation of that mRNA. However, we did not find evidence that P granules normally sequester aberrant mRNAs, or mRNAs targeted for destruction by the RNAi pathway.
Keywords: P granules, Export, mRNA, C. elegans
INTRODUCTION
Germ cells or germ cell precursors in animals ranging from nematodes to mammals have distinctive cytoplasm, called germ plasm, with unique organelles called P granules in C. elegans (reviewed by Seydoux and Braun, 2006). Genetic and transplantation studies in Drosophila showed that germ plasm is both necessary and sufficient for germline development (reviewed by Extavour and Akam, 2003). Although similar transplantation studies have not been possible in C. elegans, P granules contain many proteins that are essential for germline development (reviewed by Strome, 2005). Moreover, loss of P granules in mex-3;gld-1 mutants is associated with a germline teratoma phenotype, where germ cells differentiate inappropriately as muscles and neurons (Ciosk et al., 2006).
P granules are best known as cytoplasmic organelles in oocytes and in early embryos, where they localize asymmetrically to germ cell precursors (Strome, 2005). Once the germ lineage is established, however, P granules associate with nuclei and remain perinuclear throughout development (Strome, 2005). Cytoplasmic P granules are highly dynamic, with rapid growth, fusion, and shrinkage behaviors reminiscent of liquid droplets; the dynamics of perinuclear P granules have not been determined (Brangwynne et al., 2009). Diverse proteins have been shown to be enriched in perinuclear and/or cytoplasmic P granules, such as components of the RNAi pathway (DRH-3, EGO-1, CSR-1, PRG-1 and WAGO-1); components of the spliceosome (Sm proteins); components of processing bodies (P-bodies) and stress granules; PGL-1, a protein that binds an isoform of the mRNA cap-binding protein eIF4E; the poly(A) binding protein PAB-1; the DEAD box RNA helicase GLH-1; and PIE-1, a protein involved in transcriptional repression during early embryogenesis (Amiri et al., 2001; reviewed by Anderson and Kedersha, 2009; Mello et al., 1996; reviewed by Updike and Strome, 2009). The diversity of P granule components and the phenotypes of mutants defective in individual components do not provide a simple, single model for P granule function. Moreover, most P granule components are localized to additional nuclear or cytoplasmic compartments, and it is not known whether P granule localization is crucial for the function of any component.
The finding that most P granule proteins have RNA binding motifs suggests a role in RNA metabolism (Updike and Strome, 2009). Previous studies showed that perinuclear P granules in quiescent, non-ovulating gonads contain high levels of mRNA, although it is not known how this stored mRNA traffics to P granules (Schisa et al., 2001). Yeast P-bodies can provide temporary storage for non-translated mRNA, in addition to serving as sites for mRNA degradation, and P granules contain the P-body-associated proteins CGH-1 and DCAP-2 (reviewed by Rajyaguru and Parker, 2009). However, P granules lack additional proteins that are found in P-bodies, and germ cells contain other granules that more closely resemble conventional P-bodies (Anderson and Kedersha, 2009).
Although perinuclear P granules might store RNA in quiescent gonads, their relationship to nascent mRNA in normal gonads is not clear. Perinuclear P granules are localized at clusters of nuclear pores, where they might interact with newly exported RNAs (Pitt et al., 2000). However, normal gonads incubated with [3H]-uridine show no enrichment of label in perinuclear P granules. Second, specific mRNAs examined by in situ hybridization show little or no enrichment in perinuclear P granules (Schisa et al., 2001). Thus, nascent mRNA might either transit through perinuclear P granules very rapidly upon export or be exported through nuclear pore complexes (NPCs) that are not associated with perinuclear P granules. The present study provides direct evidence that nascent mRNAs are exported predominantly through the NPCs associated with perinuclear P granules and that the nascent mRNA transits through perinuclear P granules before entering the general cytoplasm. Moreover, we show that perinuclear P granules, which are normally found in actively transcribing adult germ cells, differ in several respects from cytoplasmic P granules, which are found in transcriptionally quiescent oocytes and early embryos.
MATERIALS AND METHODS
C. elegans strains
Nematodes were cultured and manipulated genetically as previously described (Brenner, 1974). Unless otherwise indicated, experiments were performed on the N2 strain (var. Bristol), maintained at 20°C and analyzed as 1-day-old adults. The following strains and alleles were used: TJ375 [hsp-16-2::GFP::unc-54 3′UTR]; BS3426 [rme-2(b1005) unc-24(e138)/unc-5(e53) lin-45(dx19) (IV)]; JJ569 [mex-3(zu155)/hT1, him-5 (e1490)V]; RB912 [ddx-19(ok783)II]; CB3203 [ced-1(e1735)I]; MT5811 [ced-1(e1735)I; ced-3(n717)IV]; JJ2101 [zuIs242(nmy-2::PGL-1::GFP); unc-119]; and JJ2072 [zuIs237 (f58g11.2::F58G11.2::GFP); unc-119]. For extrachromosomal transgenic strains, wild-type worms were injected with either zuEx218 [pUS24 (hsp16.2::GFP::tbb-2 3′UTR)], zuEx215 [pUS22 (hsp16.2::GFP::pos-1 3′UTR)] or zuEx224 [pCL25 (hsp16.2::GFP::unc-54 3′UTR)] to obtain strains JJ1944, JJ1940 and JJ1959, respectively. For extrachromosomal transgenic arrays, wild-type worms were injected with plasmid plus a dominant rol-6 cotransformation marker (Mello and Fire, 1995). Strain JJ2072 with an integrated transgene zuIs237 was created by microparticle bombardment of unc-119 worms (Praitis et al., 2001).
Time-lapse imaging and photobleaching experiment
Confocal, live imaging was performed as previously described (Tenlen et al., 2008; Wolke et al., 2007). The following settings were used for acquisition: exposure, 400 milliseconds; laser intensity, 82%; gain, 1; step size, 1 μm; acquisition interval, 15 seconds. Particles were tracked using Imaris Image Analysis (5.5.3) software (Lammermann et al., 2008).
Photobleaching experiments were performed and analyzed as previously described (Tenlen et al., 2008). Twenty images (0.5 second intervals) were acquired before photobleaching; recovery was measured with 67 images (40 images at 0.5 second intervals, 20 images at 5 second intervals and 7 images at 20 second intervals).
Immunocytochemistry and microscopy
The following peptides were used to generate antibodies as previously described (Wayner and Carter, 1987): NPP-9 (YEPEVEFKPVIPLPDLVEVKT), NXF-1 (MERDGCFGNCWLRRWEKSD) and DDX-19 (QFVKPDSKYKFTKPATEE). P granules were immunostained as previously described with αPGL-1 (Kawasaki et al., 1998), K76 (Strome and Wood, 1983) or GLH-1 (Gruidl et al., 1996). The following antibodies were used: for H14, RDI-RNAP2-H14 (Research Diagnostics Inc.); for FISH, Fluorescent Antibody Enhancer Set for DIG Detection (Roche, 11768506910).
For immunostaining, adult hermaphrodites were dissected on microscope slides in M9 buffer (Brenner, 1974), to which was added an equal volume of 2× Formaldehyde Fix (6% paraformaldehyde in 40 mM NaCl, 5 mM KCl, 2 mM MgCl2 and 10 mM EGTA) to make a final concentration 1× for 5 minutes. The slides were frozen on dry ice, the coverslips removed and the slides immersed in –20°C MeOH (5 minutes) before rinsing in three changes of PBS for 5 minutes each. Slides were incubated with antibody either at 37°C for 1 hour, or overnight at 4°C. Tissues prepared for αNXF-1 staining were fixed with –20°C MeOH (5 minutes), then –20°C acetone (5 minutes), briefly drained and the gonads fixed for 5 minutes in 2× Formaldehyde Fix (double strength). High magnification images were acquired with a DeltaVision microscope and processed using deconvolution software (Applied Precision). Electron microscopy was performed as previously described (Pitt et al., 2000).
Inhibitor studies and RNA interference
α-amanitin (50 μg/ml in water; Sigma A2263) or cycloheximide (50 μg/ml in water; Sigma C7698) were injected into worms that were allowed to develop for the times indicated in the text before analysis. L4 worms were used for RNAi experiments and analyzed as 1-day-old adults as follows; the Ahringer library was used as source for dsRNA feeding strains unless otherwise indicated (Kamath et al., 2003). nxf-1: worms were soaked with C15H11.3 dsRNA (Tan et al., 2000). ddx-19: strain RB912 (Arur et al., 2009) was fed with T07D4.4 dsRNA for one generation at 25°C. npp-9: worms were fed F59A2.1 dsRNA. glh-1: worms were fed T21G5.3 dsRNA from the Vidal feeding library (Rual et al., 2004). smg-2: worms were fed Y48G8AL.6 dsRNA from the Vidal feeding library (Rual et al., 2004). pos-1 3′UTR: primers CAGAAGAGCTCTTTCTCTCGTCGAAATTTCTGA and ATCGAACTAGTGAGCGCTTTAAATATTGGAAAC were used to amplify the pos-1 3′UTR from genomic DNA. The PCR product was cloned into feeding vector pPD129.36 (Timmons and Fire, 1998).
Heat shock, in situ hybridization and FISH
Worms were heat-shocked at 34°C for 30 minutes and processed for in situ hybridization or FISH at various time points. The in situ procedure was modified from the protocol of G. Broitman-Maduro and M. Maduro (http://www.faculty.ucr.edu/~mmaduro/) as follows: 20-25 worms were cut in culture media (Wolke et al., 2007) to extrude gonads. An equal volume of 2× Formaldehyde Fix was added for 1.5 minutes to make final concentration to 1×, then removed and replaced with two washes of PBS plus 0.025% triton, followed by three washes of PBS. The gonads were affixed to a polylysine-glutaraldehyde slide prepared as follows: a small drop of 5% glutaraldehyde in PBS was placed on a freshly coated polylysine (Sigma) slide for 5 minutes and then removed. The gonads were immersed in –20°C MeOH (5 minutes), briefly drained and the gonads fixed for an additional 5 minutes in 2× Formaldehyde Fix before rinsing in three changes of PBS.
RESULTS
Perinuclear P granules are asymmetric structures with dynamic components
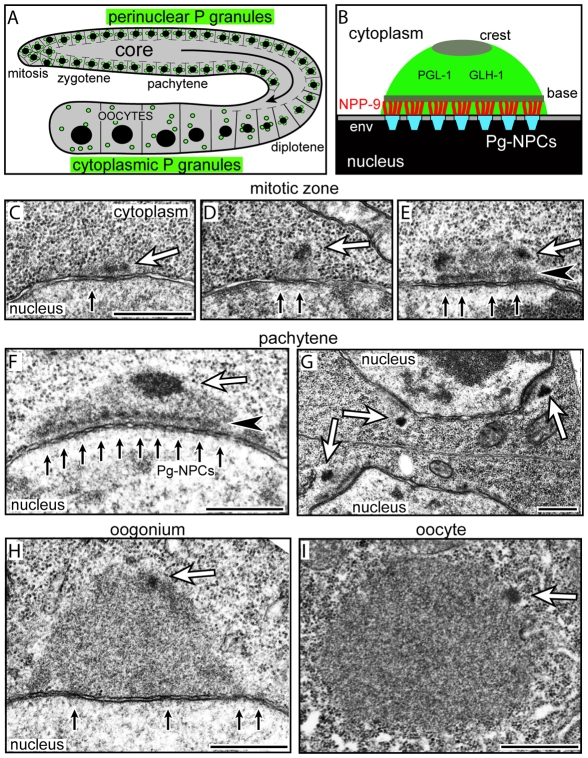
Each of the two arms of the C. elegans hermaphrodite gonad is a cylindrical array of germ cells progressing from mitosis through meiosis (Fig. 1A). Each germ cell has an opening connecting it with the shared, cytoplasmic core of the gonad; materials flow along the core toward enlarging oocytes at the end of the gonad. P granules are perinuclear in mitotic and meiotic germ cells until oogenesis, at which time they detach and become cytoplasmic (Fig. 1A) (Strome, 2005). Cytoplasmic P granules in oocytes and early embryos appear as homogenous, fibrillar granular bodies by electron microscopy, whereas perinuclear P granules can contain a distinct electron-dense base approximately 70-100 nm from the nuclear rim (Fig. 1E-G) (Pitt et al., 2000; Wolf et al., 1983). We examined the ultrastructure of perinuclear P granules in fourth larval stage (L4) germ cells and in adult germ cells in mitosis and at all stages of meiosis. In addition to the electron-dense base, we found that P granules have an electron-dense ovoid structure that we term the crest at their periphery (Fig. 1D-H; see also Fig. S1A in the supplementary material). Germ nuclei in the mitotic zone often contain small P granules with a crest but no obvious base; we presume that these are newly formed P granules (Fig. 1C,D). The crest appears to decrease in size and the base becomes less evident or disappears in late diplotene and diakinetic oogonia when P granules detach from the envelope (Fig. 1H,I).
Fig. 1.
Perinuclear P granules are asymmetric. (A) One arm of the adult hermaphrodite gonad showing nuclei (black) and P granules (green). (B) A single P granule as described in the text; compare with F. env, nuclear envelope; Pg-NPCs, P granule-associated nuclear pore complexes. (C-E) Examples of small, possibly newly formed, P granules in the mitotic zone showing nuclear pores (small black arrows), presumptive crests (white arrows) and the base (black arrowhead). (F-H) Perinuclear P granules in the pachytene zone (F,G) and in a late oogonium (H); note that NPCs are not clustered in late oogonia and oocytes. (I) Detached, cytoplasmic P granule in a young oocyte. Arrows and arrowheads are the same as in C-E. Scale bars: 500 nm.
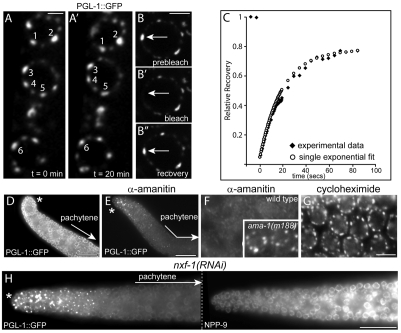
We wanted to determine whether perinuclear P granules exhibited the same dynamic behaviors of rapid growth, fusion and shrinkage described for cytoplasmic P granules (Brangwynne et al., 2009). Spinning disk confocal microscopy was used to generate movies of live pachytene-stage germ cells expressing the P granule component PGL-1 fused to Green Fluorescent Protein (GFP). Fifty perinuclear P granules that were selected at random for analysis could be tracked for 15-40 minutes before nuclear rotations or occasional body movements of the anesthetized animals shifted the P granule out of the visual field. During the period of observation, however, none of the selected P granules appeared to fuse or show marked changes in size (Fig. 2A,A′). We next asked whether PGL-1 itself was a stable component of perinuclear P granules by FRAP (fluorescence recovery after photobleaching) experiments. Individual photobleached P granules showed very rapid recovery of PGL-1::GFP fluorescence (t1/2 recovery=19±7 seconds, n=9; Fig. 2B-2B′,C). Thus, perinuclear P granules are asymmetric, relatively stable bodies that contain at least some highly dynamic components such as PGL-1.
Fig. 2.
Perinuclear P granules are persistent structures with dynamic components. (A,A′) P granules (numbered) on three pachytene germ nuclei filmed for 20 minutes. (B-B′) Photobleaching of a single perinuclear P granule (arrow); the elapsed time shown is 79 seconds. (C) Quantified fluorescence recovery curve for the P granule photobleached in B (black diamonds) compared with a single-exponential best-fit curve (white circles). (D) Dissected, normal gonad showing PGL-1::GFP in P granules and in the cytoplasm. (E) Gonad 5 hours after injection with α-amanitin showing PGL-1::GFP; clumps of PGL-1 persist in the distal mitotic germ cells but PGL-1 is lost from pachytene P granules. (F) High magnification of germ nuclei from the gonad in panel E; inset shows the same magnification of pachytene germ nuclei in an α-amanitin-injected ama-1(m188) control gonad. (G) Pachytene nuclei 5 hours after injection of cycloheximide. (H) The left panel shows pachytene-specific loss of PGL-1::GFP in an nxf-1(RNAi) gonad; the right panel shows the nuclear envelope staining (NPP-9) in the same gonad. Asterisk indicates the distal tip of the gonad. Scale bars: 2.5 μm in A,B; 25 μm in D,E,H; 5 μm in F,G.
PGL-1 is present throughout the germ cell cytoplasm but is enriched in P granules under normal growth conditions. However, PGL-1 disappears from perinuclear P granules in late stages of spermatogenesis and in pachytene germ cells of quiescent, non-ovulating gonads (Amiri et al., 2001; Schisa et al., 2001). To ask whether decreased transcriptional activity might trigger the loss of PGL-1 from perinuclear P granules, we injected young adult gonads with the transcriptional inhibitor α-amanitin. PGL-1::GFP disappeared progressively from P granules in pachytene germ cells beginning at 30 minutes (Fig. 2D,E; see also Fig. S2A in the supplementary material; n=10 gonads). Similar to observations of quiescent gonads, PGL-1::GFP persisted on P granules in diplotene germ cells and oocytes for at least 5 hours post-injection (see Fig. S2A in the supplementary material; data not shown). α-amanitin injection caused a pachytene-specific loss of the P granule component GLH-2 that was very similar to the loss of PGL-1 (see Fig. S2A in the supplementary material). Importantly, α-amanitin did not simply cause the loss of P granules; perinuclear P granules remained visible in gonads prepared for electron microscopy at 30 minutes and 3 hours after α-amanitin injection (see Fig. S2B-G in the supplementary material). The loss of perinuclear PGL-1 was not observed in (1) mock injected animals (data not shown), (2) ama-1(m188) mutant animals with a mutation in RNA polymerase II that confers resistance to α-amanitin (Fig. 2F) (Sanford et al., 1983) or (3) wild-type animals injected with a concentration of cycloheximide sufficient to block or markedly reduce translation of mex-3 mRNA in the gonad (Fig. 2G; see also Fig. S2H-K in the supplementary material; n=5 gonads). Animals depleted in NXF-1, an essential mRNA export factor in C. elegans (Tan et al., 2000), showed a similar, pachytene-specific, loss of PGL-1 (Fig. 2H).
We found that the loss of perinuclear PGL-1 in spermatogonia was associated with a marked decrease of immunostaining with H14 (see Fig. S3A,B in the supplementary material), an antibody that recognizes the phosphorylated carboxy-terminus of active RNA polymerase (Seydoux and Dunn, 1997). Similarly, we found that germ cells at early stages of apoptosis lost PGL-1 from perinuclear P granules and that this loss was associated with decreased staining with H14 (see Fig. S3C-F in the supplementary material). Thus, although PGL-1 is a prominent component of cytoplasmic P granules in oocytes and early embryos that are transcriptionally quiescent, transcriptional activity is required for PGL-1 to associate with perinuclear P granules in multiple examples of germ cells.
mRNA export factors are concentrated at P granule-associated NPCs
To examine the relationship between perinuclear P granules and nascent mRNA, we wanted markers to visualize both the cytoplasmic face of NPCs and the location of mRNA export factors. Previous studies visualized presumptive NPCs with the antibody mAb414, which recognizes several yeast and mammalian nucleoporins with phenylalanine glycine (FG) repeats (Davis and Blobel, 1986; Radu et al., 1995; Shah et al., 1998). However, mAb414 stains multiple proteins of unknown identity in C. elegans immunoblots (Geles and Adam, 2001), and some C. elegans proteins that are not nucleoporins contain FG repeats, notably the P granule protein GLH-1 (see below). Therefore, we generated monoclonal antibodies that recognize the C. elegans nucleoporin NPP-9, a predicted export factor DDX-19 and a known mRNA export factor NXF-1 (see Fig. S4A-G in the supplementary material).
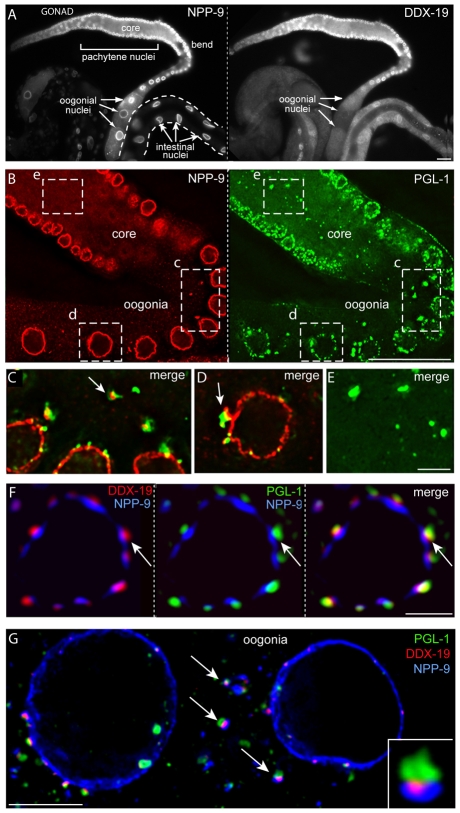
NPP-9 is an FG repeat-containing nucleoporin homologous to vertebrate Nup358/RanBP2, a major component of the eight fibers that extend from the cytoplasmic face of NPCs (Wu et al., 1995; Yokoyama et al., 1995). αNPP-9 stained the rim of all somatic and germ cell nuclei examined (Fig. 3A,B; see also Fig. S4A in the supplementary material). As P granules detach from the nuclear envelope during oogenesis, they appear to retain material stained by αNPP-9 (Fig. 3C,D) (see also Pitt et al., 2000). Cytoplasmic foci of PGL-1 were observed infrequently in the gonad core prior to P granule detachment (box e in Fig. 3B); however, these foci were not associated with NPP-9 (Fig. 3E). These foci might form de novo in the core or result from P granule fragmentation during germ cell apoptosis. NPP-9 was concentrated in large patches on germ nuclei that we presume are clustered NPCs, and was present in much smaller punctae that might be individual, or small groups of, NPCs (Fig. 4A). Co-staining for PGL-1 showed that a P granule was present on essentially all of the large patches of NPP-9: in a data set of 100 large patches (NPP-9 surface area=1.5±0.7 μm2), 99% were associated with a P granule. Small NPP-9 punctae (<0.8 μm2) were not associated with typically sized P granules (Fig. 4B). We hereafter refer to the large clusters of NPCs associated with P granules as Pg-NPCs (P granule-associated NPCs) to distinguish these from other NPCs. Pg-NPCs appeared to be distributed randomly on the surface of most germ nuclei. In L4 germ nuclei and in the zygotene/early pachytene adult nuclei, however, only NPCs were present where a prominent lobe from the nucleolus contacted the envelope (0/20 and 0/53 lobes were adjacent to Pg-NPCs in L4s and adults, respectively; see Fig. S1, open arrows, in the supplementary material).
Fig. 3.
DDX-19 is enriched at the base of P granules. (A) Gonad and intestine co-stained for NPP-9 and DDX-19 as indicated. (B) Eight-plane projection of gonad co-stained for NPP-9 and PGL-1 as indicated. (C-E) Single-plane merged images of dashed boxes in c, d and e are shown at high magnification in C (rotated), D and E, respectively. (F) Germ nucleus from a fourth larval stage (L4) gonad co-stained for DDX-19, PGL-1 and NPP-9 as indicated. (G) Adult oogonia showing detached P granules (green), NPP-9 (blue) and DDX-19 (red); inset shows a detached P granule at high magnification. Arrows indicate examples of detached P granules. Scale bars: 25 μm in A,B; 5 μm in C-E,G; 2.5 μm in F.
Fig. 4.
DDX-19 is concentrated at Pg-NPCs. (A) Surface view of germ nuclei in a gonad stained for NPP-9, PGL-1 and DDX-19 as indicated. (B) Merged images as indicated of a single germ nucleus (outlined) from A, stained as indicated. In all panels, arrows indicate large patches of NPP-9 associated with P granules (Pg-NPCs) and arrowheads indicate small foci of NPP-9 (NPCs). Scale bars: 2.5 μm.
DDX-19 is a predicted DEAD-box helicase related to the mRNA export factors DDX19 in mammals and Dbp5p in yeast (Arur et al., 2009; Hodge et al., 1999; Schmitt et al., 1999; Tseng et al., 1998). αDDX-19 showed low levels of staining at the nuclear rim and in the cytoplasm of somatic cells but much stronger staining on germ nuclei; staining diminished or disappeared on late oogonial nuclei that cease transcription (Fig. 3A). At high magnification, DDX-19 appeared concentrated in patches on both adult and larval germ nuclei (Fig. 3F; Fig. 4A). Co-staining with αNPP-9 and αPGL-1 showed that the vast majority of DDX-19 was localized at Pg-NPCs: in the data set of 100 large patches of NPP-9 described above, 94% of the P granule-positive patches colocalized with a patch of DDX-19 (Fig. 3F; Fig. 4A,B). The profile of each DDX-19 patch typically was smaller than the associated patch of NPP-9 but similar to that of PGL-1 (Fig. 4A,B). In cross-sections of nuclei, DDX-19 appeared concentrated between the zones of PGL-1 and NPP-9, forming a tripartite sandwich (Fig. 3F); remarkably, P granules initially maintained a similar, tripartite appearance after detaching from oogonia nuclei (Fig. 3G and inset).
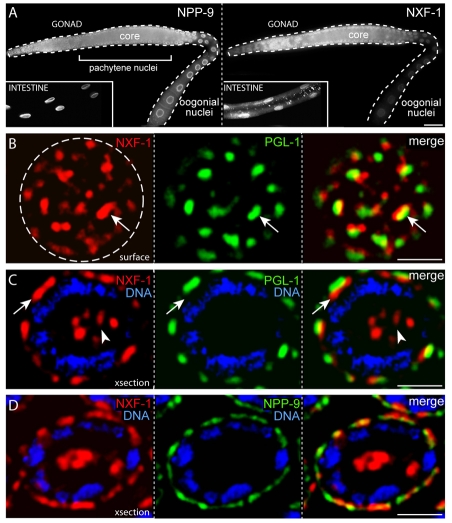
NXF-1 is the ortholog of the mRNA export factors NXF1/TAP in humans and Mex67p in yeast, and depletion of NXF-1 causes poly(A)-containing mRNA to be retained in C. elegans germ nuclei (Tan et al., 2000). αNXF-1 showed strong staining at the nuclear rim of somatic cells and germ cells (Fig. 5A). Co-staining experiments with αPGL-1 and αNXF-1 showed that nearly all the NXF-1 at the rim of germ nuclei is present at Pg-NPCs: surface views of nuclei showed a P granule in close association with the prominent patches of NXF-1 (Fig. 5B, arrow) and cross-sections showed that NXF-1 was concentrated at the bases of P granules (Fig. 5C, arrow). In addition to staining at the nuclear rim, αNXF-1 stained foci at the center of most germ nuclei, a region occupied by the nucleolus (Fig. 5C, arrowhead). NXF-1 localization at the nuclear rim was very similar to NPP-9 localization (Fig. 5D). Indeed, the αNXF-1 and αNPP-9 antibodies showed strong competition for staining on formaldehyde-fixed nuclei, necessitating the use of a denaturing fixative.
Fig. 5.
NXF-1 is concentrated at Pg-NPCs. (A) Gonad and intestine (inset) co-stained for NPP-9 and NXF-1 as indicated. (B) Surface view of a single pachytene germ nucleus (outlined) stained for NXF-1 and PGL-1 as indicated; note extensive association of the two proteins (arrow). (C) Optical cross-section of a single pachytene nucleus stained for NXF-1, PGL-1 and DNA as indicated. Note NXF-1 localization to the base of P granules (arrow) and in the nucleolus (arrowhead). (D) Optical cross-section of a single pachytene nucleus stained for NXF-1, NPP-9 and DNA as indicated. Scale bars: 25 μm in A; 2.5 μm in B-D.
Nascent mRNA is exported primarily from Pg-NPCs and enters P granules
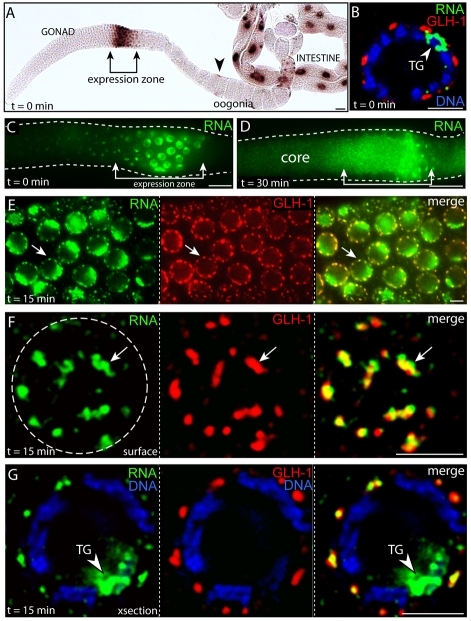
The above results show that two factors associated with mRNA export are localized predominantly at Pg-NPCs, strongly suggesting that Pg-NPCs are the principal sites of mRNA export under normal growth conditions. To examine nascent mRNA directly, we developed a technique for inducing and visualizing transgene expression in germ cells. Promoters for heat shock genes such as hsp-16.2 are used to induce expression in somatic cells (Fire et al., 1990), but endogenous hsp-16.2 is poorly expressed in germ cells (our unpublished data). Although high-copy transgenic arrays can greatly amplify somatic expression, such arrays are subject to repeat-induced silencing in germ cells (Kelly et al., 1997). Consistent with this view, we found that a high-copy transgene containing the hsp-16.2 promoter, the coding region for GFP and an unc-54 3′UTR [hsp-16.2::gfp::unc-54 (3′UTR)] (Link et al., 1999) showed no detectable mRNA expression in most germ cells following heat shock. Surprisingly, however, mRNA from this transgene was expressed at very high levels in late pachytene/diplotene germ cells; we refer to this region of the gonad as the expression zone (Fig. 6A,C).
Fig. 6.
Newly exported mRNA traffics through P granules. (A-G) Panels show in situ hybridization detection with either alkaline phosphatase (A) or fluorescence (all others) of tissues/nuclei after recovery from heat shock for the times (t) indicated. Worms contained a high-copy array of a hsp16.2::gfp::unc-54 3′UTR transgene; nascent mRNA was visualized with an in situ probe against gfp mRNA. (A) Gonad and intestine. The transgene is expressed in all intestinal nuclei, somatic nuclei in the gonad (arrowhead) and germ nuclei within the expression zone (bracket). (B) High magnification of a pachytene nucleus in the expression zone showing P granules, mRNA and DNA as indicated; the arrowhead indicates the presumptive site of transgene (TG) expression. (C) Surface view of the gonad immediately after heat shock. (D) Optical section through the gonad core 30 minutes after heat shock; transgene-derived mRNA is abundant in the core underlying the expression zone. (E) Germ nuclei 15 minutes after heat shock showing nascent mRNA and the P granule marker GLH-1 as indicated. The arrow points to one example of perinuclear foci of mRNA coinciding with P granules. (F) High-magnification surface view of a single pachytene nucleus stained for the induced mRNA and GLH-1 as indicated. Arrows indicate the same as in E. (G) Optical cross-section through a pachytene nucleus showing mRNA (green) in P granules (red). Merge is in yellow. Scale bars: 25 μm in A,C,D; 2.5 μm in B,E-G.
Several experiments were performed to characterize the induction of transgene-derived mRNA in the expression zone; in brief, these results suggest that the expression zone contains primarily sense, full-length mRNA (see Fig. S5 in the supplementary material). To examine nascent mRNA traffic, transgenic animals were heat-shocked at 34°C for 30 minutes, allowed to recover for 0 to 60 minutes, then processed for FISH (fluorescence in situ hybridization) and immunocytochemistry. Immediately following heat shock (t=0 minutes), transgenic mRNA appeared at high levels in two intranuclear foci that probably represent positions of the integrated transgene on the paired chromosomes (see legend to Fig. S5 in the supplementary material). Lower levels of mRNA were present throughout the nucleoplasm but not detected in the cytoplasm (Fig. 6B,C; see also summary in Table S1 in the supplementary material). By 10-15 minutes, the nascent mRNA showed additional localization to numerous perinuclear foci that colocalized with the P granule marker GLH-1 (Fig. 6E,F). Optical cross-sections showed that the nascent mRNA extended throughout P granules (Fig. 6G) and was largely outside the zone of NPP-9 (Fig. 7B). Between 15 and 30 minutes, the level of nascent mRNA decreased in P granules and increased in the cytoplasm and gonad core (Fig. 6D; data not shown). By 45 minutes, some mRNA appeared in late oogonia and oocytes, presumably through cytoplasmic flow (see below; data not shown). For comparison, measured rates of cytoplasmic flow in the core (∼7 μm/minute) (Wolke et al., 2007) would be expected to transport materials the distance from the expression zone to the oocytes in about 25-30 minutes. Finally, mRNA levels in all regions of the gonad diminished markedly by 60-90 minutes (see Fig. S5 in the supplementary material). We observed essentially identical temporal and spatial patterns of mRNA localization for transgenes encoding GFP with 3′UTRs from mRNAs that are either (1) transcribed and translated in the gonad, (2) transcribed but not translated in the gonad or (3) not normally expressed in the gonad (see Table S2 in the supplementary material). Thus, nascent mRNAs in general appear to localize transiently to P granules before dispersing in the cytoplasm.
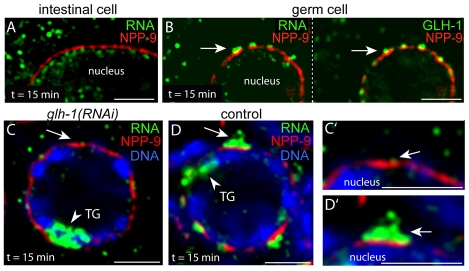
Fig. 7.
Perinuclear mRNA localization requires P granules. (A) Intestinal cell 15 minutes after heat shock, showing little or no mRNA (green) adjacent to the NPP-9 zone (red). (B) Germ cell in the same animals showing perinuclear foci of RNA (green, left panel) adjacent to the NPP-9 zone (red) and coincident with P granules (green, right panel). (C,C′) Single pachytene germ cell from a heat-shocked, glh-1(RNAi) animal. Staining as indicated for transgenic mRNA, NPP-9 and DNA. Note the very low level of perinuclear mRNA (arrow; shown at high magnification in C′). (D,D′) Germ cell from mock-treated control animal stained as indicated and showing a high level of perinuclear mRNA (arrow; shown at high magnification in D′). Scale bars: 25 μm in A,C,D; 2.5 μm in B,E-G.
Perinuclear localization of nascent mRNA depends on P granule proteins
Transient, perinuclear localization might result from specific mechanisms moving newly exported mRNA into and through P granules. Alternatively, localization might result simply from pulsed mRNA moving through clustered NPCs; if so, cells lacking P granules might show analogous localization. We examined the localization of nascent mRNA in intestinal cells; these polyploid cells produce much more transgene-derived mRNA than germ cells and have a high density of NPCs comparable to NPC clusters on germ nuclei (Fig. 6A; data not shown) (see also Pitt et al., 2000). Nascent mRNA was detected in the nucleoplasm and intestinal cytoplasm by 15 minutes post-heat shock but showed no obvious enrichment near the zone of NPP-9 (Fig. 7A). By contrast, germ cells in the same animals showed mRNA concentrated near the NPP-9 zone, within P granules (Fig. 7B). We next examined mRNA localization in germ cells depleted of GLH-1 by glh-1(RNAi); this treatment results in small, abnormal P granules that lack multiple P granule components (Gruidl et al., 1996; Kawasaki et al., 1998; Schisa et al., 2001). Similar to intestinal cells, the glh-1(RNAi) germ cells showed little mRNA accumulation near the zone of NPP-9, in contrast to mock-treated controls (Fig. 7C-D′; see Table S1 in the supplementary material).
P granules do not sequester mRNAs that are targeted for degradation by the RNAi or NMD pathways
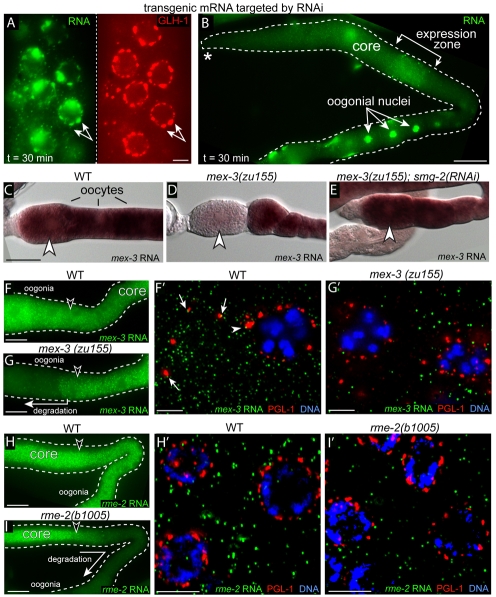
Because the double-stranded RNA-specific endonuclease Dicer is a component of nuclear-associated chromatoid bodies in mouse germ cells (Kotaja et al., 2006), we wanted to determine if mRNA destined for degradation by the RNAi pathway is sequestered or degraded in P granules. We used the pos-1 gene to analyze the RNAi pathway, as pos-1 is abundant in germ cells and highly sensitive to RNAi (Tabara et al., 1998). We first established that dsRNA targeting the pos-1 3′UTR was effective in depleting endogenous pos-1 mRNA within 24 hours (data not shown). Next, worms expressing a hsp-16.2::GFP transgene with the pos-1 3′UTR were treated with the same dsRNA for 24 hours, then heat shocked to induce mRNA expression. As observed under non-RNAi conditions, the mRNA passed through P granules into the cytoplasm by 30 minutes (Fig. 8A,B; see Table S1 in the supplementary material).
Fig. 8.
mRNAs targeted for degradation are not enriched in P granules. (A,B) FISH analysis of hsp16.2::gfp::pos-1 3′UTR expression on worms exposed to dsRNA for the pos-1 3′UTR. (A) Pachytene nuclei; double-headed arrow indicates an example of colocalized nascent mRNA (green) and P granules (red). (B) Low magnification view of the same gonad in A, showing that the induced mRNA has entered the gonad core and moved downstream to oogonia. (C-E) Panels show wild-type (WT) or mutant mex-3 mRNA detected by in situ hybridization in the strains indicated. mex-3(zu155) mRNA with a premature stop codon is degraded in the most mature oocytes, where it normally is translated (D, arrowhead), but persists when the NMD pathway is inhibited [smg-2(RNAi)] (E). (F-I′) FISH of the indicated mRNA in WT or mutant gonads (F-I); high magnification of the respective regions indicated by arrowheads are shown in F′-I′. Adjacent mRNA (green) and PGL-1 (red) signals are indicated in F′ for perinuclear P granules (arrowhead) and cytoplasmic P granules (arrows). Scale bars: 2.5 μm in A; 25 μm in B-I; 5 μm in F′-I′.
P granules contain some proteins found in P-bodies (see Introduction), and yeast mRNAs with premature stop codons accumulate in P-bodies (Sheth and Parker, 2006). To determine whether nonsense-containing mRNAs accumulate in P granules, we analyzed mex-3 and rme-2 mRNAs that are normally translated in oocytes (with cytoplasmic P granules) and late pachytene germ cells (perinuclear P granules), respectively. mex-3(zu155) and rme-2(b1005) mutants have mRNAs with premature stop codons and can be suppressed by inhibiting the NMD (nonsense-mediated decay) pathway (Fig. 8C-E) (Lee and Schedl, 2004). As expected for NMD, the mex-3 and rme-2 mutant mRNAs were present initially at wild-type levels but disappeared from oocytes and the pachytene zone, respectively, where translation normally ensues (compare Fig. 8F with 8G and Fig. 8H with 8I). We found that the mutant mRNAs showed no obvious enrichment in perinuclear or cytoplasmic P granules either before, or within, the degradation zones (Fig. 8F′-I′). Indeed, overlapping or adjacent foci of mRNA and PGL-1 staining were observed much more frequently for the abundant wild-type mRNAs than for the mutant mRNAs, presumably reflecting their relative abundance (compare Fig. 8F′ with 8G′).
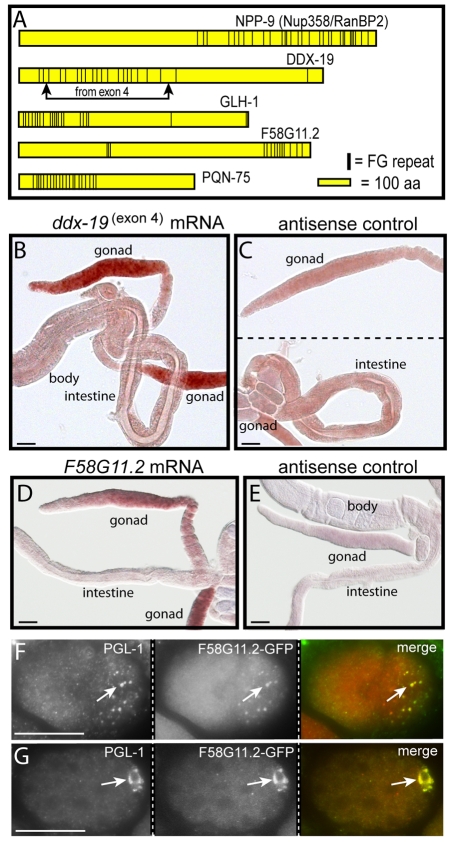
Multiple P granule proteins contain FG repeats
mRNA export through NPCs involves export factors such as NXF1 that bind clustered FG repeats on nucleoporins such as Nup358/RanBP2 (reviewed in Tran and Wente, 2006), and we noted previously that the GLH family of P granule proteins also contain clustered FG repeats (Fig. 9A) (Schisa et al., 2001). We noticed that one of the four predicted isoforms of C. elegans DDX-19 has a cluster of 20 FG-repeats, including 16 repeats of a GxFG motif found in a subset of nucleoporins (Fig. 9A). By contrast, human DDX19 has only a single FG sequence, and yeast Dbp5p has only two, widely separated FG sequences (GenBank accession numbers BC003626 and U28135). Nearly all of the FG repeats in the C. elegans DDX-19 isoform are encoded by a single, alternatively spliced fourth exon (Fig. 9A). We found that ddx-19 mRNA containing the fourth exon is abundant in germ cells but not detected above background in somatic cells (Fig. 9B,C).
Fig. 9.
FG-repeat proteins in P granules. (A) Diagram showing FG repeats (vertical bars) in the nucleoporin NPP-9 and in four additional C. elegans proteins encoded by gonad-enriched mRNAs. (B,C) In situ hybridization for sense (B) and antisense (C) ddx-19(exon4) mRNA. (D,E) In situ hybridization for sense (D) and antisense (E) f58g11.2 mRNA. (F)Two-cell embryo showing colocalization of F58G11.2::GFP with cytoplasmic P granules (arrow). (G) Fifty-cell embryo showing colocalization of F58G11.2::GFP with P granules (arrow). Scale bars: 25 μm.
Proteomic analyses in yeast and mammals have provided lists of all putative nucleoporins and major NPC-associated proteins (Cronshaw et al., 2002; Rout et al., 2000). We searched the C. elegans proteome for examples of predicted proteins with clustered GxFG repeats that are not known nucleoporins. In addition to the GLH proteins and the DDX-19 isoform, a cluster of GxFG repeats were present in PQN-75, a novel protein, and in F58G11.2, a predicted RNA helicase with no clear mammalian ortholog (Fig. 9A). A genomic in situ hybridization database indicated that both of the latter genes are highly enriched in gonads (NEXTDB Ver.4.0 at http://nematode.lab.nig.ac.jp/db2), and we confirmed this result for F58G11.2 (Fig. 9D,E). Despite several attempts, we failed to recover viable strains expressing GFP fused to PQN-75. However, worms expressing GFP fused to F58G11.2 showed variable localization to P granules in adult gonads and consistent localization to cytoplasmic P granules in early embryos (Fig. 9F) and to the nuclear-associated P granules in later embryos (Fig. 9G). We conclude that P granules contain at least three components with clustered GxFG repeats – the GLH family, DDX-19 and F58G11.2.
DISCUSSION
Pg-NPCs are principal sites of mRNA export in germ cells
We have shown that perinuclear P granules and cytoplasmic P granules differ in both morphology and dynamics. Although cytoplasmic P granules exist in transcriptionally quiescent oocytes, our study demonstrates that perinuclear P granules and their associated NPCs (Pg-NPCs) are the major sites of mRNA export in germ cells. We showed here that known and predicted mRNA export factors are localized at Pg-NPCs in both larval and adult germ cells under normal growth conditions (Figs 3, 5). Further, we showed that heat shock can induce expression of high-copy, transgenic arrays in a subset of germ cells, and that most of this nascent mRNA appears to be exported through Pg-NPCs (Fig. 6). We do not yet know why the transgenes are expressed only in these germ cells. However, the spatial, temporal, and genetic parameters of expression parallel the normal pattern of X chromosome-specific expression, suggesting a shared mechanism of activation or derepression (Kelly et al., 2002).
If most nascent mRNA is exported through Pg-NPCs, other NPCs might have distinct functions. For example, NPCs that are adjacent to the asymmetrically positioned nucleolus in yeast nuclei lack proteins such as Mlp1p and Mlp2p that prevent the export of non-spliced mRNAs (Galy et al., 2004; Strambio-de-Castillia et al., 1999); this functional asymmetry suggests that the nucleolar-associated NPCs might be specialized for transport in ribosome biogenesis (Tran and Wente, 2006). Interestingly, we found that NPCs, but not Pg-NPCs, were present where a lobe of the nucleolus contacts the nuclear envelope in some germ cells (see Fig. S1 in the supplementary material). Moreover, [3H]-uridine-labeled C. elegans gonads do not show an enrichment of label on perinuclear P granules (Schisa et al., 2001), and analogous labeling studies on C. bergerac gonads showed that most of this label is incorporated in rRNA rather than mRNA (Starck and Brun, 1977; Starck et al., 1983). If rRNA is not exported through Pg-NPCs, this might explain why perinuclear P granules in old, quiescent gonads accumulate large quantities of mRNA but lack detectable rRNA (Schisa et al., 2001).
Newly exported mRNA traffics through P granules
As viewed by electron microscopy, the cytoplasmic fibrils of NPCs are approximately 50 nm in length (Stoffler et al., 1999), whereas the electron-dense base of the P granule begins approximately 70 nm from the nuclear rim. As observed by immunocytochemistry, the mRNA export factor NXF-1 is concentrated below the P granules and is essentially coincident with NPP-9, a presumptive component of the NPC fibrils. Thus, mRNA released from an NPC should be at or near the electron-dense base of a P granule. P granules extend 300-400 nm from the nuclear rim into the cytoplasm, well beyond the predicted terminus of NPC fibrils, and our immunostaining experiments show that the P granule proteins GLH-1 and PGL-1 localize predominantly outside the zone of NPP-9 (Figs 6, 7). Thus, our FISH analysis allows us to conclude that (1) the perinuclear foci of newly exported RNA are clearly outside of NPCs (Fig. 7) and (2) that these foci overlap extensively with P granules (Fig. 6). Thus, most mRNAs enter a distinct compartment, the P granule, after their export from germ nuclei. We argue that perinuclear localization does not result simply from the pulsed generation of heat-shock-induced mRNA because this localization does not occur in intestinal cells lacking P granules or in germ cells lacking a subset of P granule proteins (Fig. 7). Instead, we propose that perinuclear localization results because nascent mRNAs move through P granules, and that this movement is significantly slower than diffusion in the surrounding cytoplasm.
Movement of mRNA through P granules might be similar to transport through the NPC. Free diffusion through the NPC is restricted because FG repeat nucleoporins form a hydrophobic meshwork in the central channel (reviewed by D’Angelo and Hetzer, 2008). The P granule protein GLH-1 contains a cluster of FG repeats, and we showed here that two additional P granule-enriched proteins (F58G11.2 and a germline-specific isoform of DDX-19) contain similar repeats. The presence of multiple FG repeat proteins in P granules raises the possibility that P granules confront newly exported mRNA with a second hydrophobic barrier to free diffusion. Indeed, we speculate that exposed hydrophobic domains of P granule proteins might underlie the unusual fusion behaviors of cytoplasmic P granules after detachment from the nuclear envelope (Brangwynne et al., 2009).
Transport through the NPC involves factors such as NXF1 that bind specifically to FG repeats, possibly dissolving the local hydrophobic meshwork (Ribbeck and Gorlich, 2001; Rout et al., 2000). In future studies, it will be interesting to determine whether mRNA movement through P granules involves analogous, dedicated transport factors or a sequence of random bind-and-release interactions with RNA-binding components of P granules. The finding that P granules have asymmetric morphology raises the possibility some of these components have ordered positions that contribute to mRNA movement or release.
P granules: assembly sites versus disposal centers
Materials diffusing randomly in germ cell cytoplasm would quickly exit into the gonad core, where they would be swept away toward distant oogonia (Wolke et al., 2007). Thus, perinuclear P granules might provide a crucial compartment to assemble complexes between nascent mRNA and regulatory small RNAs or proteins before the mRNA is diluted in the core cytoplasm. The germline teratoma phenotype of mex-3; gld-1 double mutants that lose P granules appears to result in part from the premature expression of somatic-specific transcription factors that normally function in early embryogenesis (see Introduction; Ciosk et al., 2006). Thus, the mutant germ cells might express the appropriate regulatory molecules but be inefficient in assembling complexes with target mRNAs in the absence of P granules. Totipotent germ cells could be particularly sensitive to the inappropriate expression of these transcription factors, as the misexpression of such factors can readily respecify the fates of multipotent early embryonic cells (Zhu et al., 1998).
We did not find evidence that P granules sequester or degrade mRNAs targeted by the RNAi pathway. Instead, the targeted mRNA moved through P granules into the general cytoplasm, similar to all other transgenic mRNAs examined in our study (Fig. 6). Conversely, nonsense-containing mutant mRNAs did not appear to relocate from the cytoplasm into P granules prior to or during degradation. However, we cannot exclude the possibility that degradation occurs so rapidly in P granules that relocation could not be detected. For example, although some yeast mRNAs targeted for NMD become visibly enriched in P-bodies, others do not (Sheth and Parker, 2006).
Although P granules do not appear to sequester mRNAs targeted for degradation, they store large amounts of mRNA in gonads that are not ovulating and can release this mRNA if ovulation resumes (Schisa et al., 2001). We favor the hypothesis that P granules are sequestering nascent mRNA in the quiescent gonads but could not test this directly with our heat shock system as these conditions prevented transgene expression (our unpublished observations). However, we showed that the localization of at least two P granule components, PGL-1 and GLH-2, is dependent on active transcription in pachytene germ cells, indicating that the composition, and possibly function(s), of P granules can be regulated by physiological state.
In conclusion, our results draw several basic distinctions between perinuclear P granules and cytoplasmic P granules. We suggest that perinuclear P granules are compartments where newly exported mRNAs find appropriate regulatory molecules before being released to the general cytoplasm. In this view, P granules might not have a unitary function in mRNA metabolism, but rather contribute to multiple facets of mRNA regulation. Clearly, much remains to be learned in future studies about the structure, composition and physiology of these interesting organelles.
Supplementary Material
Acknowledgments
We thank Elizabeth Wayner, Karen Bennett, Susan Strome, Christopher Link and Barbara Felder for reagents, Elizabeth Wayner, Kathryn English and Jacqualyn Burnson for technical assistance and Jessica Feldman and members of the Priess laboratory for critical reading of the manuscript. We thank Jeff Molk, Julio Vazquez and Dave McDonald for advice on imaging and analysis. Some of the nematode strains used in this work were provided by the Caenorhabditis Genetic Center, which is funded by the NIH National Center for Research Resources (NCRR). U.S. was supported by a fellowship from the Helen Hay Whitney Foundation and Howard Hughes Medical Institute (HHMI). S.D. was supported by a Developmental Biology Predoctoral Training Grant (T32HD007183) from the National Institute of Child Health and Human Development and HHMI. J.R.P. and J.P. were supported by the HHMI. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.044255/-/DC1
References
- Amiri A., Keiper B. D., Kawasaki I., Fan Y., Kohara Y., Rhoads R. E., Strome S. (2001). An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans. Development 128, 3899-3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. (2009). RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 10, 430-436 [DOI] [PubMed] [Google Scholar]
- Arur S., Ohmachi M., Nayak S., Hayes M., Miranda A., Hay A., Golden A., Schedl T. (2009). Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. Proc. Natl. Acad. Sci. USA 106, 4776-4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., Gharakhani J., Julicher F., Hyman A. A. (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729-1732 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The Genetics of Caenorhabditis elegans. Genetics 77, 71-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciosk R., DePalma M., Priess J. R. (2006). Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science 311, 851-853 [DOI] [PubMed] [Google Scholar]
- Cronshaw J. M., Krutchinsky A. N., Zhang W., Chait B. T., Matunis M. J. (2002). Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 158, 915-927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo M. A., Hetzer M. W. (2008). Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 18, 456-466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. (1986). Identification and characterization of a nuclear pore complex protein. Cell 45, 699-709 [DOI] [PubMed] [Google Scholar]
- Extavour C. G., Akam M. (2003). Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130, 5869-5884 [DOI] [PubMed] [Google Scholar]
- Fire A., Harrison S. W., Dixon D. (1990). A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene 93, 189-190 [DOI] [PubMed] [Google Scholar]
- Galy V., Gadal O., Fromont-Racine M., Romano A., Jacquier A., Nehrbass U. (2004). Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell 116, 63-73 [DOI] [PubMed] [Google Scholar]
- Geles K. G., Adam S. A. (2001). Germline and developmental roles of the nuclear transport factor importin alpha3 in C. elegans. Development 128, 1817-1830 [DOI] [PubMed] [Google Scholar]
- Gruidl M. E., Smith P. A., Kuznicki K. A., McCrone J. S., Kirchner J., Roussell D. L., Strome S., Bennett K. L. (1996). Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93, 13837-13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge C. A., Colot H. V., Stafford P., Cole C. N. (1999). Rat8p/Dbp5p is a shuttling transport factor that interacts with Rat7p/Nup159p and Gle1p and suppresses the mRNA export defect of xpo1-1 cells. EMBO J. 18, 5778-5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., et al. (2003). Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231-237 [DOI] [PubMed] [Google Scholar]
- Kawasaki I., Shim Y. H., Kirchner J., Kaminker J., Wood W. B., Strome S. (1998). PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell 94, 635-645 [DOI] [PubMed] [Google Scholar]
- Kelly W. G., Xu S., Montgomery M. K., Fire A. (1997). Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146, 227-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Schaner C. E., Dernburg A. F., Lee M.-H., Kim S. K., Villeneuve A. M., Reinke V. (2002). X-chromosome silencing in the germline of C. elegans. Development 129, 479-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaja N., Bhattacharyya S. N., Jaskiewicz L., Kimmins S., Parvinen M., Filipowicz W., Sassone-Corsi P. (2006). The chromatoid body of male germ cells: Similarity with processing bodies and presence of Dicer and microRNA pathway components. Proc. Natl. Acad. Sci. USA 103, 2647-2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann T., Bader B. L., Monkley S. J., Worbs T., Wedlich-Soldner R., Hirsch K., Keller M., Forster R., Critchley D. R., Fassler R., et al. (2008). Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51-55 [DOI] [PubMed] [Google Scholar]
- Lee M. H., Schedl T. (2004). Translation repression by GLD-1 protects its mRNA targets from nonsense-mediated mRNA decay in C. elegans. Genes Dev. 18, 1047-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link C. D., Cypser J. R., Johnson C. J., Johnson T. E. (1999). Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones 4, 235-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C., Fire A. (1995). DNA transformation. Methods Cell Biol. 48, 451-82 [PubMed] [Google Scholar]
- Mello C. C., Schubert C., Draper B., Zhang W., Lobel R., Priess J. R. (1996). The PIE-1 protein and germline specification in C. elegans embryos. Nature 382, 710-712 [DOI] [PubMed] [Google Scholar]
- Pitt J. N., Schisa J. A., Priess J. R. (2000). P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev. Biol. 219, 315-333 [DOI] [PubMed] [Google Scholar]
- Praitis V., Casey E., Collar D., Austin J. (2001). Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157, 1217-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A., Moore M. S., Blobel G. (1995). The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell 81, 215-222 [DOI] [PubMed] [Google Scholar]
- Rajyaguru P., Parker R. (2009). CGH-1 and the control of maternal mRNAs. Trends Cell Biol. 19, 24-28 [DOI] [PubMed] [Google Scholar]
- Ribbeck K., Gorlich D. (2001). Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 20, 1320-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout M. P., Aitchison J. D., Suprapto A., Hjertaas K., Zhao Y., Chait B. T. (2000). The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 148, 635-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual J. F., Ceron J., Koreth J., Hao T., Nicot A. S., Hirozane-Kishikawa T., Vandenhaute J., Orkin S. H., Hill D. E., van den Heuvel S., et al. (2004). Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14, 2162-2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford T., Golomb M., Riddle D. L. (1983). RNA polymerase II from wild type and alpha-amanitin-resistant strains of Caenorhabditis elegans. J. Biol. Chem. 258, 12804-12809 [PubMed] [Google Scholar]
- Schisa J. A., Pitt J. N., Priess J. R. (2001). Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development 128, 1287-1298 [DOI] [PubMed] [Google Scholar]
- Schmitt C., von Kobbe C., Bachi A., Pante N., Rodrigues J. P., Boscheron C., Rigaut G., Wilm M., Seraphin B., Carmo-Fonseca M., et al. (1999). Dbp5, a DEAD-box protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pore complex via a conserved interaction with CAN/Nup159p. EMBO J. 18, 4332-4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G., Dunn M. A. (1997). Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development 124, 2191-2201 [DOI] [PubMed] [Google Scholar]
- Seydoux G., Braun R. E. (2006). Pathway to totipotency: lessons from germ cells. Cell 127, 891-904 [DOI] [PubMed] [Google Scholar]
- Shah S., Tugendreich S., Forbes D. (1998). Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr. J. Cell Biol. 141, 31-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U., Parker R. (2006). Targeting of aberrant mRNAs to cytoplasmic processing bodies. Cell 125, 1095-1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck J., Brun J. (1977). Autoradiographic localization of RNA synthesis in vitro during oogenesis in Parascaris equorum. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 284, 1341-1344 [PubMed] [Google Scholar]
- Starck J., Gibert M.-A., Brun J., Bosch C. (1983). Ribosomal RNA synthesis and processing during oogenesis of the free living nematode Caenorhabditis elegans. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 75, 575-580 [Google Scholar]
- Stoffler D., Goldie K. N., Feja B., Aebi U. (1999). Calcium-mediated structural changes of native nuclear pore complexes monitored by time-lapse atomic force microscopy. J. Mol. Biol. 287, 741-752 [DOI] [PubMed] [Google Scholar]
- Strambio-de-Castillia C., Blobel G., Rout M. P. (1999). Proteins connecting the nuclear pore complex with the nuclear Interior. J. Cell Biol. 144, 839-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S. (2005). Specification of the germ line. WormBook, 1-10 doi/ 10.1895/wormbook.1.9.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S., Wood W. B. (1983). Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 35, 15-25 [DOI] [PubMed] [Google Scholar]
- Tabara H., Grishok A., Mello C. C. (1998). RNAi in C. elegans: soaking in the genome sequence. Science 282, 430-431 [DOI] [PubMed] [Google Scholar]
- Tan W., Zolotukhin A. S., Bear J., Patenaude D. J., Felber B. K. (2000). The mRNA export in Caenorhabditis elegans is mediated by Ce-NXF-1, an ortholog of human TAP/NXF and Saccharomyces cerevisiae Mex67p. RNA 6, 1762-1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenlen J. R., Molk J. N., London N., Page B. D., Priess J. R. (2008). MEX-5 asymmetry in one-cell C. elegans embryos requires PAR-4- and PAR-1-dependent phosphorylation. Development 135, 3665-3675 [DOI] [PubMed] [Google Scholar]
- Timmons L., Fire A. (1998). Specific interference by ingested dsRNA. Nature 395, 854 [DOI] [PubMed] [Google Scholar]
- Tran E. J., Wente S. R. (2006). Dynamic nuclear pore complexes: life on the edge. Cell 125, 1041-1053 [DOI] [PubMed] [Google Scholar]
- Tseng S. S., Weaver P. L., Liu Y., Hitomi M., Tartakoff A. M., Chang T.-H. (1998). Dbp5p, a cytosolic RNA helicase, is required for poly(A)+ RNA export. EMBO J. 17, 2651-2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D. L., Strome S. (2009). P granule assembly and function in C. elegans germ cells. J. Androl. (in press) [Google Scholar]
- Wayner E. A., Carter W. G. (1987). Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J. Cell Biol. 105, 1873-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N., Priess J., Hirsh D. (1983). Segregation of germline granules in early embryos of Caenorhabditis elegans: an electron microscopic analysis. J. Embryol. Exp. Morphol. 73, 297-306 [PubMed] [Google Scholar]
- Wolke U., Jezuit E. A., Priess J. R. (2007). Actin-dependent cytoplasmic streaming in C. elegans oogenesis. Development 134, 2227-2236 [DOI] [PubMed] [Google Scholar]
- Wu J., Matunis M. J., Kraemer D., Blobel G., Coutavas E. (1995). Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin, a homologous domain, and a leucine-rich region. J. Biol. Chem. 270, 14209-14213 [DOI] [PubMed] [Google Scholar]
- Yokoyama N., Hayashi N., Seki T., Pante N., Ohba T., Nishii K., Kuma K., Hayashida T., Miyata T., Aebi U., et al. (1995). A giant nucleopore protein that binds Ran/TC4. Nature 376, 184-188 [DOI] [PubMed] [Google Scholar]
- Zhu J., Fukushige T., McGhee J. D., Rothman J. H. (1998). Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev. 12, 3809-3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.