Abstract
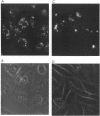
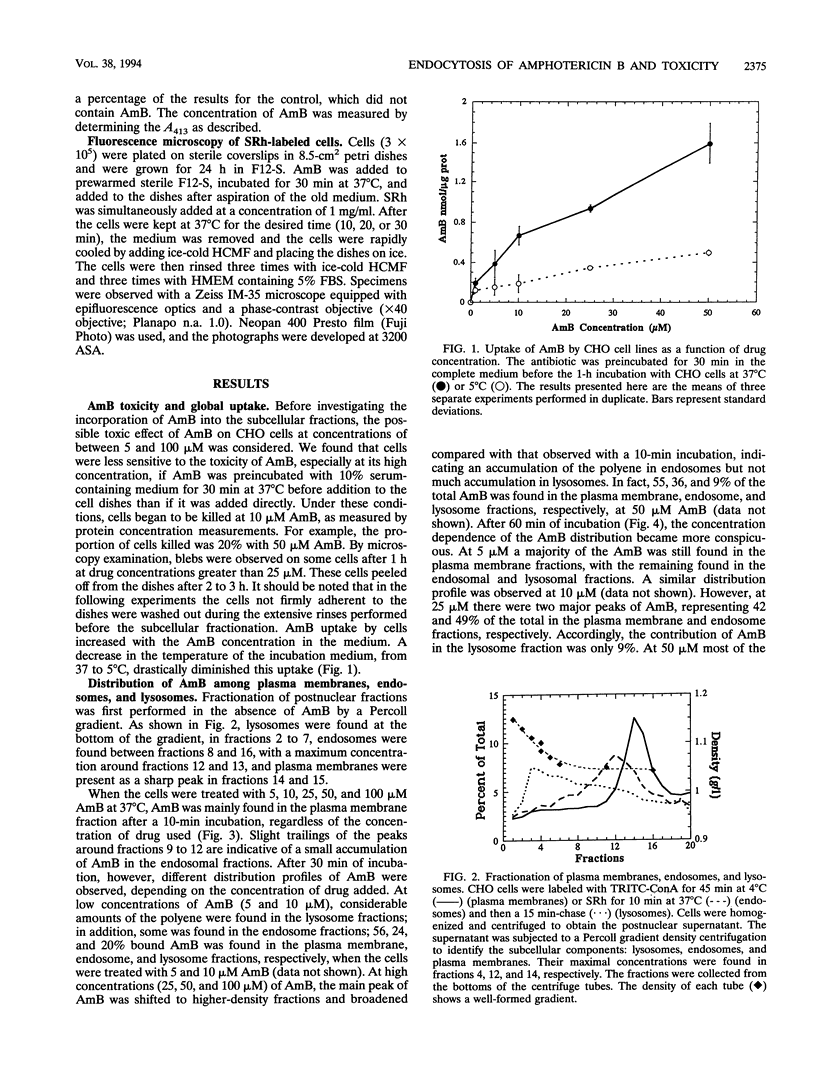
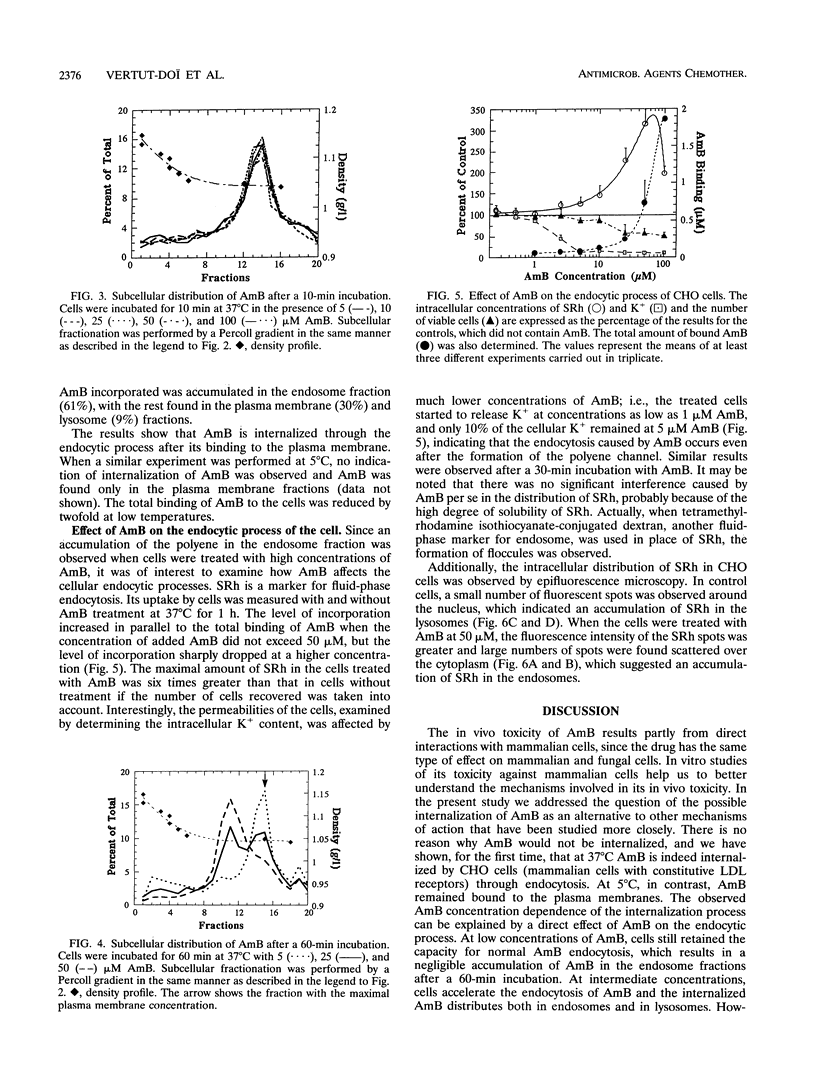
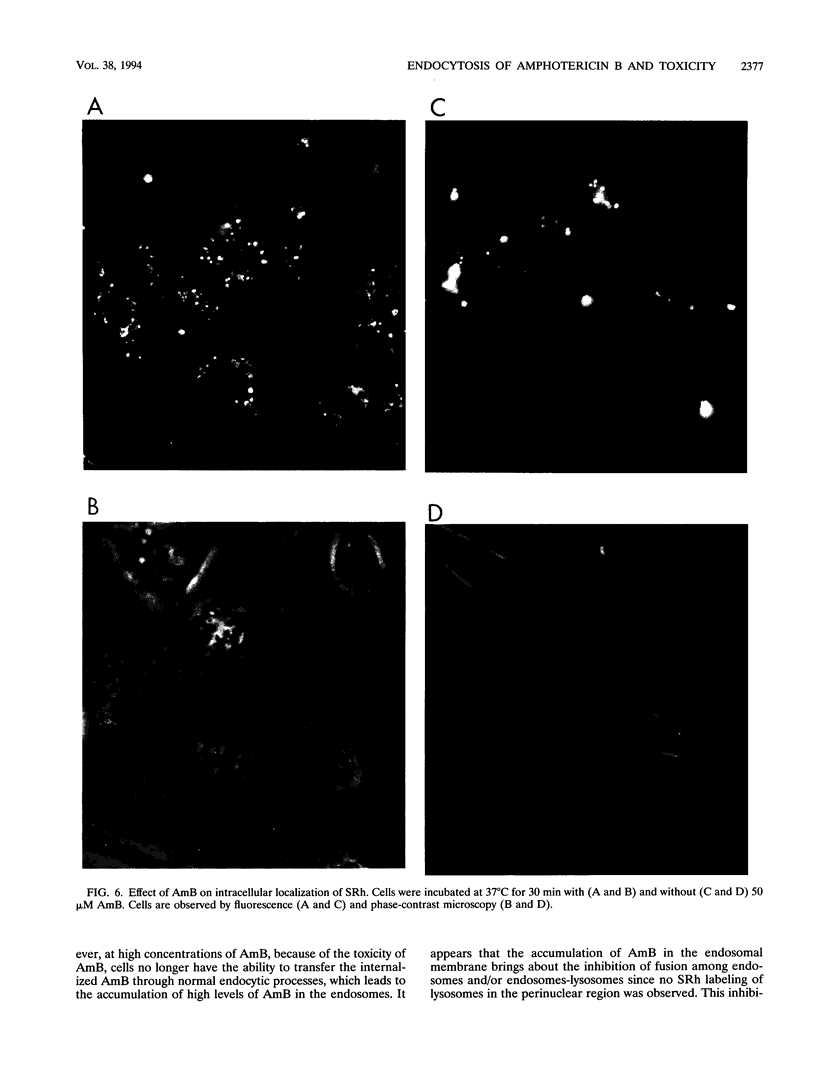
We describe the fate of the polyene antibiotic amphotericin B (AmB) after its interaction with Chinese hamster ovary (CHO) cells. The global uptake of AmB by these cells was measured at 37 degrees C after a 1-h incubation in the presence of 5% fetal bovine serum. It increased with the total concentration of drug and reached a plateau of approximately 1 nmol/mg of cell protein for an external concentration of 25 microM. The same experiment performed at 5 degrees C revealed a drastic decrease in uptake. The distribution of the drug among plasma membranes, endosomes, and lysosomes was then investigated after the separation of the postnuclear fractions by a Percoll gradient. After a 10-min incubation, AmB was found only in the plasma membrane fraction, regardless of the drug concentrations used (5 to 100 microM). After 60 min, at low drug concentrations (5 and 10 microM) AmB was found to be incorporated mainly in plasma and lysosomal fractions. At high concentrations (50 microM) AmB accumulated in endosomal fractions and plasma membranes. At intermediate concentrations (25 microM) AmB was distributed among the three fractions. When the same experiment was carried out at 5 degrees C, AmB was associated only with the plasma membrane even after 60 min, which was consistent with the absence of endocytotic process at low temperature. The effect of AmB on the endocytic process resulted in the increased uptake of sulforhodamine B, a fluid-phase marker of endocytosis, as well as by the accumulation of sulforhodamine in spots scattered in the cytoplasms of AmB-treated cells, in contrast to the accumulation around the nuclei observed in the control cells. These results are interpreted as indicating that AmB is internalized by the cells through endocytosis and that high concentrations of the drug block the fusion between endosomes and/or the fusion between endosomes and lysosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alston-Smith J., Pertoft H., Laurent T. C. Endocytosis of hyaluronan in rat Kupffer cells. Biochem J. 1992 Sep 1;286(Pt 2):519–526. doi: 10.1042/bj2860519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwicz J., Gareau R., Audet A., Morisset A., Villiard J., Gruda I. Inhibition of the interaction between lipoproteins and amphotericin B by some delivery systems. Biochem Biophys Res Commun. 1991 Dec 16;181(2):722–728. doi: 10.1016/0006-291x(91)91250-g. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Cruz J., Khan M. N., Posner B. I. Uptake of insulin and other ligands into receptor-rich endocytic components of target cells: the endosomal apparatus. Annu Rev Physiol. 1985;47:383–403. doi: 10.1146/annurev.ph.47.030185.002123. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Low R. B. Endocytosis: a review of mechanisms and plasma membrane dynamics. Biochem J. 1983 Jan 15;210(1):1–13. doi: 10.1042/bj2100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S. F., Shohet S. B., Nathan D. G., Gardner F. H. The effect of amphotericin B on erythrocyte membrane cation permeability: its relation to in vivo erythrocyte survival. J Lab Clin Med. 1969 Jun;73(6):980–987. [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986 Dec 22;864(3-4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Braell W. A. Fusion between endocytic vesicles in a cell-free system. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Bolard J., Kobayashi G. S., Levy R. A., Ostlund R. E., Jr, Schlessinger D., Medoff G. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis. 1984 Jun;149(6):986–997. doi: 10.1093/infdis/149.6.986. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Schwartz D. R., Vertut-Croquin A., Schlessinger D., Kobayashi G. S., Medoff G. Involvement of oxidative damage in erythrocyte lysis induced by amphotericin B. Antimicrob Agents Chemother. 1985 Feb;27(2):172–176. doi: 10.1128/aac.27.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Powderly W. G., Kobayashi G. S., Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother. 1990 Feb;34(2):183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. C., Sipe D. M., Murphy R. F. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Sawano F., Geiger D., Gorden P., Perrelet A., Orci L. Potassium depletion and hypertonic medium reduce "non-coated" and clathrin-coated pit formation, as well as endocytosis through these two gates. J Cell Physiol. 1989 Mar;138(3):519–526. doi: 10.1002/jcp.1041380311. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Mâle P., Mellman I. Acidification and ion permeabilities of highly purified rat liver endosomes. J Biol Chem. 1989 Feb 5;264(4):2212–2220. [PubMed] [Google Scholar]
- Fuchs R., Schmid S., Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc Natl Acad Sci U S A. 1989 Jan;86(2):539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Henry-Toulmé N., Seman M., Bolard J. Interaction of amphotericin B and its N-fructosyl derivative with murine thymocytes: a comparative study using fluorescent membrane probes. Biochim Biophys Acta. 1989 Jul 10;982(2):245–252. doi: 10.1016/0005-2736(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Koldin M. H., Kobayashi G. S., Brajtburg J., Medoff G. Effects of elevation of serum cholesterol and administration of amphotericin B complexed to lipoproteins on amphotericin B-induced toxicity in rabbits. Antimicrob Agents Chemother. 1985 Jul;28(1):144–145. doi: 10.1128/aac.28.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause H. J., Juliano R. L. Interactions of liposome-incorporated amphotericin B with kidney epithelial cell cultures. Mol Pharmacol. 1988 Sep;34(3):286–297. [PubMed] [Google Scholar]
- Kravchenko L. S. Issledovanie proteoliticheskoi aktivnosti lizosom pochek sobak pri deistvii amfoteritsina v in vitro. Biokhimiia. 1977 Sep;42(9):1655–1659. [PubMed] [Google Scholar]
- Kusumi A., Tsuji A., Murata M., Sako Y., Yoshizawa A. C., Kagiwada S., Hayakawa T., Ohnishi S. Development of a streak-camera-based time-resolved microscope fluorimeter and its application to studies of membrane fusion in single cells. Biochemistry. 1991 Jul 2;30(26):6517–6527. doi: 10.1021/bi00240a024. [DOI] [PubMed] [Google Scholar]
- Larkin J. M., Brown M. S., Goldstein J. L., Anderson R. G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983 May;33(1):273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Levy R. A., Ostlund R. E., Jr, Brajtburg J. The effects of amphotericin B on lipid metabolism in cultured human skin fibroblasts. In Vitro Cell Dev Biol. 1985 Jan;21(1):26–31. doi: 10.1007/BF02620910. [DOI] [PubMed] [Google Scholar]
- Ohashi M., Murata M., Ohnishi S. A novel fluorescence method to monitor the lysosomal disintegration of low density lipoprotein. Eur J Cell Biol. 1992 Oct;59(1):116–126. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rashid F., Horobin R. W., Williams M. A. Predicting the behaviour and selectivity of fluorescent probes for lysosomes and related structures by means of structure-activity models. Histochem J. 1991 Oct;23(10):450–459. doi: 10.1007/BF01041375. [DOI] [PubMed] [Google Scholar]
- Raub T. J., Koroly M. J., Roberts R. M. Endocytosis of wheat germ agglutinin binding sites from the cell surface into a tubular endosomal network. J Cell Physiol. 1990 Apr;143(1):1–12. doi: 10.1002/jcp.1041430102. [DOI] [PubMed] [Google Scholar]
- Salzman N. H., Maxfield F. R. Fusion accessibility of endocytic compartments along the recycling and lysosomal endocytic pathways in intact cells. J Cell Biol. 1989 Nov;109(5):2097–2104. doi: 10.1083/jcb.109.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Sundan A., Olsnes S. Effect of potassium depletion of cells on their sensitivity to diphtheria toxin and pseudomonas toxin. J Cell Physiol. 1985 Jul;124(1):54–60. doi: 10.1002/jcp.1041240110. [DOI] [PubMed] [Google Scholar]
- Surarit R., Shepherd M. G. The effects of azole and polyene antifungals on the plasma membrane enzymes of Candida albicans. J Med Vet Mycol. 1987 Dec;25(6):403–413. doi: 10.1080/02681218780000491. [DOI] [PubMed] [Google Scholar]
- Vertut-Croquin A., Brajtburg J., Medoff G. Two mechanisms of synergism when amphotericin B is used in combination with actinomycin D or 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea against the human promyelocytic leukemia cell line HL-60. Cancer Res. 1986 Dec;46(12 Pt 1):6054–6058. [PubMed] [Google Scholar]
- Vertut-Doi A., Hannaert P., Bolard J. The polyene antibiotic amphotericin B inhibits the Na+/K+ pump of human erythrocytes. Biochem Biophys Res Commun. 1988 Dec 15;157(2):692–697. doi: 10.1016/s0006-291x(88)80305-x. [DOI] [PubMed] [Google Scholar]
- Wasan K. M., Brazeau G. A., Keyhani A., Hayman A. C., Lopez-Berestein G. Roles of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993 Feb;37(2):246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]