Abstract
Certain Drosophila embryonic epidermal cells construct actin-based protrusions, called denticles, which exhibit stereotyped, column-specific differences in size, density and hook orientation. This precise denticle pattern is conserved throughout all drosophilids yet studied, and screening for mutations that affect this pattern has been used to identify genes involved in development and signaling. However, how column-specific differences are specified and the mechanism(s) involved have remained elusive. Here, we show that the transcription factor Stripe is required for multiple aspects of this column-specific denticle pattern, including denticle hook orientation. The induction of stripe expression in certain denticle field cells appears to be the primary mechanism by which developmental pathways assign denticle hook orientation. Furthermore, we show that the cytoskeletal linker protein Short stop (Shot) functions both cell-autonomously and non-autonomously to specify denticle hook orientation via interaction with the microtubule cytoskeleton. We propose that stripe mediates its effect on hook orientation, in part, via upregulation of shot.
Keywords: Drosophila, Denticle, Short stop, Spectraplakin, Stripe
INTRODUCTION
During development, groups of equivalent cells differentiate to form a highly patterned body plan through the use of signals secreted by organizer regions. The process of differentiation therefore requires a cell to have the capacity to receive a signal, and to then translate that signal into some cellular response, such as a change in genetic program, adhesion properties or morphogenetic movements. Historically, developmental biology has focused primarily on the signal cascades that are required for differentiation, rather than on the vital next step of translating a developmental signal into a cellular event. However, the gap between developmental signaling cues and cell biological responses is being bridged, and certain model systems have emerged in recent years that can link organizing signals to cellular morphogenesis.
One such system is the ventral epidermis of the Drosophila embryo. In late embryogenesis, these epidermal cells adopt one of two cell fates: either they remain apically smooth or they produce an elaborately shaped actin-based protrusion called a denticle. Later, these latter cells secrete a hardened cuticle that conforms to the shape of the plasma membrane, molding the actin-based protrusions in the cuticle (Hillman and Lesnik, 1970; Martinez-Arias and Bate, 1993; Payre, 2004). Importantly, denticle characteristics differ from column to column, giving rise to a highly reproducible pattern that has served as the basis for several screens to identify patterning genes (Nüsslein-Volhard and Wieschaus, 1980).
It is not known how the details of this intricate pattern first arise, nor how they are achieved by cytoskeletal components. Although cytoskeletal coordination has been studied extensively in migrating and cultured cells, less is known about cytoskeletal coordination in epithelia in vivo. In fact, many epithelia produce actin-based protrusions that display elaborate shapes, such as vertebrate stereocilia and Drosophila sensory bristles and ventral denticles. However, how cytoskeletal networks are influenced to create these shapes is not well understood.
The denticle field within most abdominal parasegments consists of seven columns of epidermal cells that can each produce several denticles. Although the posterior-most two columns of cells produce small, non-descript denticles, cell columns 1-5 produce denticles that are uniquely patterned from column to column. These cell columns differ from one another in the size, shape, number and hook orientation of the denticles they produce, as well as in the expression of certain cell fate markers and signaling components (Lohs-Schardin et al., 1979; Martinez-Arias and Bate, 1993). Of note, cell columns 1 and 4 are the only columns that produce denticles that hook towards the anterior (see Fig. 1A). This anterior hooking is unique, in the wild-type denticle field as well as in situations in which the field is artificially expanded (O’Keefe, 1997; Szüts, 1997). This prompted us to examine what it is that is unique about columns 1 and 4 that allows them to produce denticles that have a reversed hook orientation. To date, no specific cell fate determinant has been found to be common to these two cell columns that might provide a clue to this process.
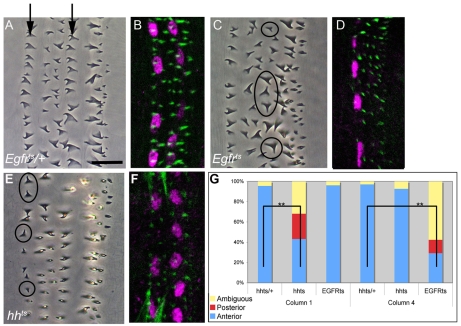
Fig. 1.
hedgehog and spitz are required for anterior denticle hooking. (A-F) Face-on view of Drosophila cuticle preparations (A,C,E) and staining with anti-Stripe (magenta) and phalloidin (green) (B,D,F). Circles indicate denticles with incorrect hook orientation. (A,B) Egfrts/+ embryo. Note the anterior hooking specifically in columns 1 and 4 (arrows). (C,D) Egfrts embryo, upshifted at 9 hours. Note the loss of Stripe in column 5. (E,F) hhts2 embryo, upshifted at 10 hours. Note the reduction of Stripe in column 2 (see text). Anterior is to the left in all figures. (G) Quantification of hook orientation in wild type (hhts2/+), hhts2 and Egfrts. **, P<0.01. Scale bar: 10 μm in A,C,E; 20 μm in B,D,F.
As the epidermal cells form denticles apically, a subset of these epidermal cells (called tendon cells) forms junctions with myotubes at their basal surface. This process is known as epidermal-muscle attachment (EMA). Upon muscle contraction, mechanical information is communicated from the EMA junction to the larval cuticle, which allows for efficient larval crawling. Interestingly, reversed hook orientation (towards the anterior) is found adjacent to muscle attachment sites, suggesting that the tendon cells might play some role in anterior hooking (see Fig. 2A). The early growth response (EGR)-like transcription factor Stripe is necessary for the specification and maturation of tendon cells, the recruitment of myotubes and the stabilization of EMA junctions (Becker et al., 1997; Frommer et al., 1996; Strumpf and Volk, 1998; Subramanian et al., 2003; Yarnitzky et al., 1997). Here, we describe a novel function of stripe in the tendon cells: assigning anterior denticle hooking to neighboring cells.
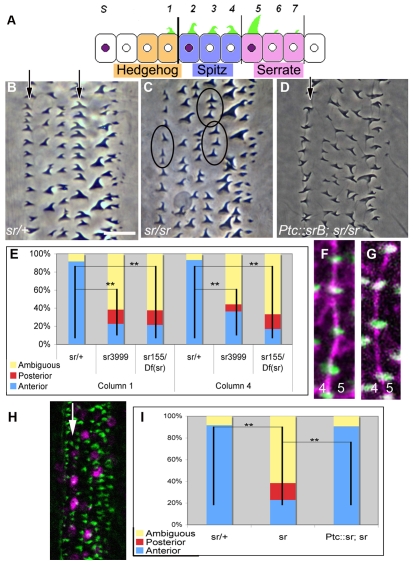
Fig. 2.
stripe is required for denticle hook orientation. (A) Schematic of one parasegment of ventral epidermis in cross-section, showing nuclear accumulation of Stripe (purple) in cells corresponding to denticle columns 2 and 5, and in one smooth field cell column (S). Note that denticle hook orientation reverses at stripe-expressing sites. (B) Wild-type cuticle preparation. Note the anterior hooking specifically in columns 1 and 4 (arrows). (C) sr3999 cuticle. Hooking defects are observed in both column 1 and 4 (circles). (D) ptc::sr; sr3999 cuticle. Note the restoration of anterior hooking in column 1 (arrow). (E) Quantification of denticle hook orientation in column 1 and column 4 in sr3999/+, sr3999 and sr155/Df(3)DG4 (the deficiency uncovering stripe) embryos. (F,G) Denticles arising from column 4 cells of wild-type (F; sr3999/+) or stripe mutant (G; sr3999) embryos showing phalloidin staining (green), with anti-phospho-tyrosine (magenta) to label cell outlines. Note that the actin-based protrusions arise from the posterior of the cell and elongate towards column 5 cells. (H) ptc::sr; sr3999 embryo. Note the restoration of column 2 Stripe expression (arrow). (I) Quantification of column 1 hook orientation in sr3999/+, sr3999 and ptc::sr; sr3999 embryos. **, P<0.01. Scale bar: 10 μm in B,C,D; 4 μm in F,G; 20 μm in H.
Although Stripe is presumed to have many targets, relatively few have been studied in detail. One such target is the spectraplakin short stop (shot). Spectraplakins are extremely large cytoskeletal proteins with characteristics of both the spectrin and plakin families, and are generally thought to function by linking cytoskeletal filaments to one another or to the membrane (Roper et al., 2002). shot, the only known Drosophila spectraplakin, contains at least four transcriptional start sites and exhibits extensive alternative splicing, producing a highly modular protein with diverse functions (Gregory and Brown, 1998; Lee et al., 2003; Lee et al., 2000; Roper and Brown, 2003; van Vactor et al., 1993). MACF and BPAG1 (dystonin), vertebrate homologs of Shot, are some of the few proteins capable of binding actin filaments and microtubules simultaneously (Karakesisoglou et al., 2000; Lee and Kolodziej, 2002a; Leung et al., 1999). Shot can also associate with junctional proteins, integrins and E-cadherin (Gregory and Brown, 1998; Lee et al., 2003; Lee and Kolodziej, 2002b; Roper and Brown, 2003; Subramanian et al., 2003).
Multiple isoforms of Shot are present in the Drosophila embryonic epidermis and these are distinctly regulated. Full-length and other long Shot isoforms are ubiquitously expressed in the epidermis from an early embryonic stage and are required to maintain epithelial integrity (Roper and Brown, 2003). However, a shorter isoform of Shot is upregulated in response to stripe specifically in the tendon cells (Becker et al., 1997; Roper and Brown, 2003). In these cells, Shot acts downstream of stripe, and is speculated to link the actin and microtubule cytoskeletons, preventing the tendon cell from rupturing upon muscle contraction, although alternative models remain possible (Bottenberg, 2009; Lee et al., 2007; Subramanian et al., 2003).
In this work, we link developmental patterning signals to the morphogenetic event of denticle shaping via stripe and shot. First, we demonstrate that stripe is required, non-cell-autonomously, for the unique anterior hook orientation observed in the column 1 and 4 denticles. Secondly, our data strongly suggest that stripe specifies anterior denticle hooking, in part via upregulation of shot. The involvement of shot reveals novel roles for spectraplakins and the microtubule cytoskeleton in the shaping of actin-based protrusions.
MATERIALS AND METHODS
Fly strains
We analyzed the null mutations for sr and mys, sr155 (FBal0032779) and mys1 (FBal0012678), as well as the strong sr hypomorphs sr3999 (FBal0009498) and sr461 (FBal0032784). Deficiencies uncovering shot were Df(2R)Exel7128 (FBab0038035) and Df(2R)383BSC (FBab0045182); the deficiency uncovering stripe was Df(3)DG4 (FBab0002521). All stocks were balanced over TM6 Hu Ubi-GFP, CyO Kr-Gal4 UAS-GFP, or FM7 B Kr-Gal4 UAS-GFP for cuticle analysis. ptc-Gal4, en-Gal4, hhts2, UAS-dicer2 and the deficiencies uncovering shot were from the Bloomington Stock Center; Egfrtsla (FBal0083481) was from Kevin Moses (Rodrigues et al., 2005) and Egfr24 (FBal0003552) was from Trudi Schüpbach (Clifford and Schüpbach, 1989); UAS-EFhand-GAS2-GFP, UAS-srB, UAS-srA and UAS-dsshot were from Talila Volk (Dorfman et al., 2002; Volohonsky et al., 2007); UAS-spastin was from Kai Zinn (Sherwood et al., 2004); and UAS-shotL(A) was from Peter Kolodziej (Lee and Kolodziej, 2002a).
Cuticle preparation and immunohistochemistry
Embryos were collected on apple agar plates, aged, and either processed to visualize cuticle pattern by phase-contrast microscopy (van der Meer, 1977) or fixed and processed for immunofluorescence. In most cases, embryos were genotyped using a fluorescence stereomicroscope. In most cases, embryos were processed for immunofluorescence using a high concentration-short fixation protocol, in which dechorionated embryos were rocked in vials containing a 1:1 mixture of heptane and 37% formaldehyde for 5-7 minutes (Teodoro and O’Farrell, 2003). Embryos were then pipetted onto a glass slide, picked up on double-sided sticky tape fixed to a cover slip, placed in a watch glass and covered with PBS. To preserve phalloidin binding to F-actin, a needle was used to poke the embryos out of their vitelline membranes. Fixation for anti-Shot and anti-Armadillo (Arm) involved a short heating step in which dechorionated embryos were immersed in a hot Triton X-100 (0.03%)-NaCl (0.4%) solution (E-Wash) and then quickly cooled by addition of excess chilled E-Wash. Vitelline membranes were removed by shaking the embryos vigorously in a 1:1 mixture of heptane and methanol. This fixation procedure appears to highlight the localization of proteins such as Arm at the zonula adherens at the expense of other cellular pools (Müller et al., 1999).
The following antibodies (and dilutions) were used for 2 hours at room temperature or overnight at 4°C: guinea pig anti-Stripe and rat anti-Stripe A (1:500, gifts from Talila Volk), mouse anti-Shot [1:10, Developmental Studies Hybridoma Bank (DSHB)], mouse anti-phosphorylated tyrosine (1:500, Molecular Probes), rat anti-Filamin [N- and C-terminal, 1:4000, a gift from Lynn Cooley (Sokol and Cooley, 2003)], rabbit anti-Arm (1:200, a gift from Eric Wieschaus, Princeton University), rat anti-DE-cadherin (Shotgun – FlyBase) (1:20, DSHB), mouse anti-Coracle (1:500, DSHB) or mouse anti-β-tubulin (1:10, DSHB). Secondary antibodies, used at 1:400 for 1 hour, were conjugated to Alexa Fluor (Molecular Probes) or Cy3 or Cy5 dyes (Jackson Labs). Alexa 488-, Alexa 568- or Rhodamine-coupled phalloidin was used at 1:200 (Molecular Probes). Stained embryos were mounted in Prolong Gold (Molecular Probes) and images were obtained using structured illumination (Zeiss Apotome) and assembled in Adobe Photoshop.
Temperature-shift experiments
Embryos were collected for 2 hours at room temperature, aged at 18°C for the indicated time, and then shifted to 29°C. The length of time at 18°C was determined by trial and error such that a denticle hooking phenotype was observed without cell fate change or cell column loss. For cuticle preparations, embryos were aged at 29°C until just prior to hatching (∼20-21 hours after egg laying), genotyped and processed. For immunofluorescence, embryos were aged at 29°C until stage 15-16, genotyped, then fixed and processed. For Egfrts experiments, embryos containing a heteroallelic combination of Egfrtsla over the amorphic Egfrf24 allele were used.
Quantification
To quantify denticle hook orientation, cuticles were prepared and visualized using standard phase-contrast microscopy. Each scorable denticle in the column of interest was manually tallied as either hooking towards the anterior or posterior, or as being ambiguous/unhooked. To quantify denticle density, we stained embryos with phalloidin to label the actin-based protrusions and for phosphorylated tyrosine to label cell outlines. We then manually tallied the number of protrusions per cell in each column. Whenever possible, segments A3-A7 were scored in each embryo. Data were gathered from multiple segments of multiple animals and compiled in Excel (Microsoft). For all genotypes, more than 100 individual denticles from at least five embryos were scored for quantification.
To quantify stripe-expressing nuclei (as in Fig. 1), embryos were sorted for genotype, stained with anti-Stripe and visualized by immunofluorescence. stripe-expressing nuclei were counted manually, and the ratio of stripe-expressing nuclei in column 2 versus column 5 was calculated and compared between genotypes. As a control, the ratio of stripe-expressing nuclei in column 5 versus the smooth field was also calculated and compared between genotypes.
shot dsRNA
Females expressing both UAS-shot-dsRNA and UAS-dicer2 (to strengthen the RNAi phenotype) were crossed to males expressing either Act5C-Gal4 or ptc-Gal4. As UAS-shot-dsRNA is on the X chromosome, half of the progeny should express shot-dsRNA in the ectopic pattern. Although we could not unambiguously genotype these embryos, roughly half (18 out of 46) of the embryos produced from this cross exhibited a pattern that deviated from the wild type and we assumed these to be the mutant class and scored these embryos for hook orientation as described above. In embryos stained in parallel, column-selective knockdown of Shot was observed (see Fig. 6).
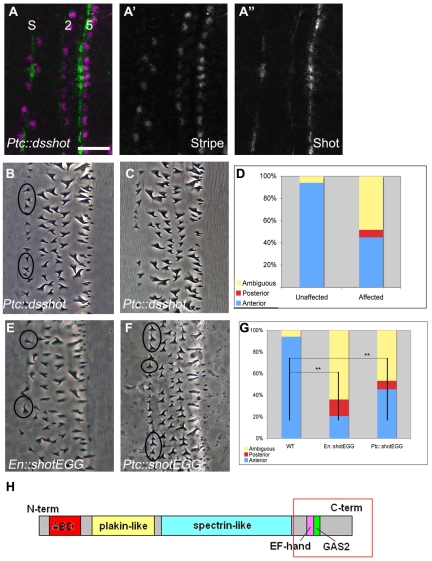
Fig. 6.
shot is required non-cell-autonomously. (A-A′) ptc::dsshot Drosophila embryo, showing Stripe (magenta; single channel in A′) and Shot (green; single channel in A′). Note the strong reduction of Shot in column 2, but not column 5 (compare with Fig. 5C). Ptc also drives expression in the smooth field, and is likely to account for the slight decrease of Shot in the ‘S’ column. (B) ptc::dsshot cuticle, showing hooking defects in column 1 (circles). (C) ptc::dsshot cuticle, showing hooking defects and a gap in column 1. (D) Quantification of column 1 hook orientation in ptc::dsshot. ‘Affected’ refers to those embryos assumed to carry the dsRNA transgene, whereas ‘unaffected’ refers to presumed sibling controls (see Materials and methods). (E) en::shot-EGG cuticle, showing hooking defects in column 1 (circles). (F) ptc::shot-EGG cuticle, showing hooking defects in column 1 (circles). Some hooking defects are also observed in column 4 and this is likely to be due to Ptc driving shot-EGG in column 4 cells. (G) Quantification of column 1 hook orientation in en::shot-EGG and ptc::shot-EGG. **, P<0.01. (H) Structure of Shot. The boxed regions are included in the shot-EGG construct. Scale bar: 20 μm in A-A′; 10 μm in B,C,E,F.
RESULTS
hedgehog and spitz are required for anterior denticle hooking
Once the denticle field is established, short-range signals subdivide the field into progressively smaller territories (Alexandre et al., 1999). It has been observed that the orientation of denticle hooks reverses across certain territorial boundaries, specifically at the boundaries between columns 1 and 2 and columns 4 and 5 (Alexandre et al., 1999). As we (and others) had previously shown that the Hedgehog (Hh)/Smoothened and Spitz (Spi)/Egfr pathways have specific effects across these respective boundaries (Hatini and DiNardo, 2001), we tested whether these signals were involved in denticle hooking. We bypassed earlier requirements for Hedgehog and Egfr by using temperature-sensitive alleles (hhts2, Egfrtsla) (Ma et al., 1993; Rodrigues et al., 2005). We raised embryos at the permissive temperature until the actin-based protrusions began to form, then we shifted to the restrictive temperature and scored denticle hook orientation after cuticle deposition.
Cell columns 1 and 4 are the only columns that produce denticles that hook towards the anterior (Fig. 1A). Anterior hook orientation is consistent in wild type, with ∼95% of column 1 and 4 denticles hooking towards the anterior and only ∼5% failing to hook (Fig. 1G, see Materials and methods for scoring criteria). When we inactivated Egfr, we found that 70% of column 4 denticles exhibited incorrect hook orientation (Fig. 1C,G), whereas all other columns were unchanged. Similarly, when we inactivated hh, 57% of column 1 denticles failed to hook towards the anterior (Fig. 1E,G), whereas all other columns remained wild-type. These hooking phenotypes were not due to changes in denticle field specification, as all denticle columns were present. From these data, we conclude that the hh and Egfr pathways are required for anterior denticle hooking in cell columns 1 and 4, respectively.
stripe is required for anterior denticle hook orientation
We next sought to identify a target(s) of these signaling pathways involved in denticle hook orientation. We and others have previously characterized a target gene common to both the Hh and Egfr pathways: that of the transcription factor stripe (Hatini and DiNardo, 2001). stripe is expressed in columns 2 and 5 in the prospective denticle field and in one additional column in the smooth field (Fig. 1B; Fig. 2A). Each column of stripe expression is dependent on distinct signals: Hh signaling is required in column 2 and Spi/Egfr signaling is required in column 5 (Hatini and DiNardo, 2001; Piepenburg et al., 2000; Wiellette and McGinnis, 1999).
First, we verified that Stripe protein was selectively depleted from the proper columns in embryos compromised for Hh or Egfr pathway activation. In Egfrts embryos, Stripe protein was lost selectively from cell column 5 and retained in column 2 (Fig. 1D) (Hatini and DiNardo, 2001). Although hhts embryos maintained Stripe expression in some column 2 cells (Fig. 1F), their number was significantly reduced from 11.5 to 6.2 Stripe-positive nuclei per column. The effect was selective for column 2, as column 5 remained unchanged compared with controls (14.5 per column; see Materials and methods).
To determine whether stripe is required for denticle hook orientation, we examined stripe mutant embryos. Compared with wild type, mutant embryos exhibited a dramatic decrease in anterior hooking in both cell columns 1 and 4 (Fig. 2C). In stripe mutant embryos, 88% of column 1 denticles and 63% of column 4 denticles exhibited a loss of anterior hooking (Fig. 2E).
These hooking defects did not appear to be associated with additional defects in planar cell polarity. In stripe mutant embryos, as in wild type, the denticles were arranged in columns (Fig. 2B,C) (Dickinson, 1997). Also, the actin-based protrusions remained positioned along the posterior edges of cells and exhibited posterior outgrowth (Fig. 2F,G) (Price et al., 2006; Walters et al., 2006). As stripe mutant embryos exhibited these characteristics of polarity, we conclude that the primary defect in stripe mutants is a loss of anterior denticle hooking orientation, rather than a defect in tissue polarity.
Previous work has suggested that the cuticle pattern arises as a result of local signaling interfaces within the epidermis (Alexandre et al., 1999). As stripe is induced asymmetrically across these interfaces via hh and spi, we propose that signaling interfaces could influence denticle diversity by initiating stripe expression in the neighboring cell (Fig. 2A) (Hatini and DiNardo, 2001).
stripe is required non-cell-autonomously for anterior denticle hook orientation
To test directly whether non-autonomous stripe expression is sufficient for anterior hooking, we attempted to rescue the column 1 hooking defect in the stripe mutant by adding back stripe to column 2 using the patched (ptc)-Gal4 driver. ptc is highly expressed in column 2 (and more weakly to the posterior), but not in column 1 (Fig. 2H). Restoring stripe expression using ptc-Gal4 indeed restored proper anterior hooking in cell column 1 (Fig. 2D,I, compare with 2C). We conclude that the requirement for stripe is strictly non-autonomous, and that the expression of stripe in cell column 2 is necessary and sufficient for the anterior hooking of cell column 1. Currently, no Gal4 drivers exist that can restore stripe selectively to column 5 cells, but based on our Egfrts data, we presume that column 5 expression of stripe similarly acts non-autonomously on column 4 hooking.
stripe specifies denticle density
Previously, cells of all columns were thought to produce two to three denticles each (Gergen and Wieschaus, 1985; Price et al., 2006; Szabad et al., 1979), but it was recently appreciated that denticle density varies across the field (Price et al., 2006). We found that cells can produce anywhere from a single denticle to as many as five denticles per cell (see Fig. S1 in the supplementary material). The stripe-expressing columns averaged the fewest denticles per cell (1.2), whereas column 1 cells averaged 2.4 denticles per cell (see Fig. S1 in the supplementary material; see Materials and methods). However, embryos null for stripe showed an increased denticle density for both columns 2 and 5 without affecting the other cell columns (see Fig. S1 in the supplementary material). Reciprocally, ectopic expression of stripe using engrailed (en)-Gal4, which is highly expressed in denticle column 1, significantly reduced average denticle density from 2.4 to 1.3 denticles per cell. These data indicate that stripe function plays a key role in specifying denticle density.
Muscle attachment is not required for denticle hook orientation
Since stripe is necessary for muscle attachment, we wondered whether the tension exerted by body wall muscles on column 2 and 5 cells was responsible for the anterior denticle hooking observed in neighboring cells. To test this, we examined myospheroid (mys) mutant embryos, which lack β-integrin, an essential component of EMA junctions required for late tendon cell maturation (Martin-Bermudo, 2000).
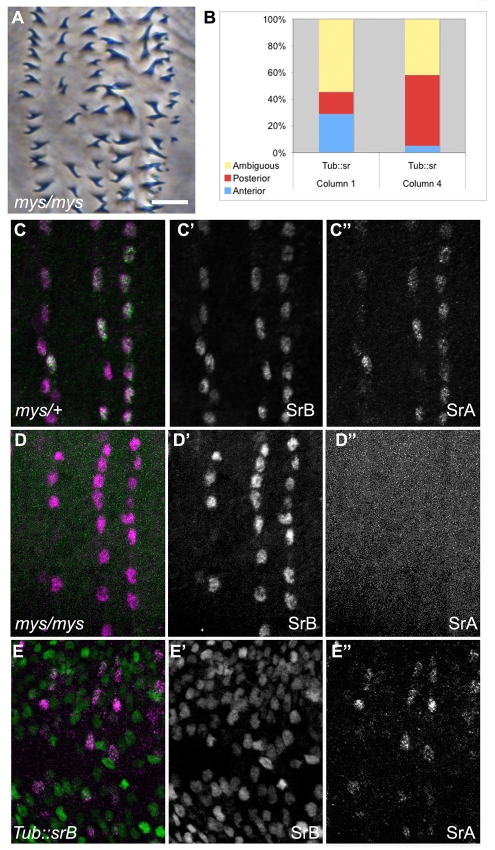
To confirm that EMA junctions are not formed in mys embryos, we stained mys mutant embryos with antibodies specific to each of the two stripe isoforms (Volohonsky et al., 2007). The early isoform, stripe-B, is induced via hh and spi (Hatini and DiNardo, 2001; Volohonsky et al., 2007). Stripe-B initiates tendon cell differentiation and attracts approaching myotubes (Volohonsky et al., 2007). When muscles attach, stripe gene expression switches to the late isoform, stripe-A (Nabel-Rosen et al., 1999; Volohonsky et al., 2007). Formation of proper EMA junctions is required for this switch, so the presence of Stripe-A within a cell indicates stably attached muscles (Becker et al., 1997). When we stained mys embryos with isoform-specific antibodies, we found that Stripe-B levels appeared comparable to those of wild type, but mys mutant embryos lacked detectable Stripe-A (Fig. 3D, compare with 3C). Therefore, we conclude that mys embryos lack stable EMA junctions, as previously reported (Martin-Bermudo, 2000). Although mys embryos fail to attach muscles, the denticle hooking pattern of mys embryos was indistinguishable from that of wild type (Fig. 3A). These data suggest that neither muscle tension nor complete EMA specification is required for anterior denticle hook orientation.
Fig. 3.
Muscle attachment is not required for denticle hook orientation. (A) mys1 cuticle, exhibiting proper hook orientation (compare with Fig. 1A). (B) Quantification of denticle hook orientation in Tubulin::stripe (Tub::sr) Drosophila embryos. (C-C′) Wild-type (mys/+) stage 16 embryo, showing Stripe-B (SrB, magenta; single channel in C′) and Stripe-A (SrA, green; single channel in C′). Both Stripe isoforms are localized to epidermal-muscle attachment (EMA) cells. (D-D′) mys1 embryo, showing Stripe-B (magenta; single channel in D′) and Stripe-A (green; single channel in D′). Note the loss of Stripe-A. (E-E′) Tub::srB, showing Stripe-B (green; single channel in E′) and Stripe-A (magenta; single channel in E′). Although the Stripe-B pattern is drastically altered, Stripe-A remains as in wild type. Scale bar: 10 μm in A; 20 μm in C-E′.
Furthermore, whereas broad ectopic expression of ectopic Stripe-B using Tubulin (Tub)-Gal4 led to hooking defects in both column 1 and column 4 (Fig. 3B), it did little to alter the location of muscle attachment sites or Stripe-A expression (Fig. 3E) (Becker et al., 1997). The lack of correlation between denticle phenotype and EMA specification indicates that stripe functions in denticle hooking independently of its role in muscle attachment.
shot is required for denticle hook orientation
As Stripe is a transcription factor, we wondered whether any known Stripe-induced genes might function in denticle hooking. One such target, shot, was of interest owing to its ability to organize cytoskeletal networks. To test its involvement in denticle hook orientation, we examined embryos deficient for shot. The shot3 allele has been suggested to be amorphic with respect to axon extension, although a small percentage of characterized axons exhibit a normal trajectory (Lee et al., 2000). However, in the epidermis, we detected residual Shot protein in tendon cells from shot3 embryos (see Fig. S2 in the supplementary material) (Roper and Brown, 2003). Thus, to definitively deplete Shot, we produced embryos containing a heteroallelic combination of mapped deletions that uncover the shot locus (see Fig. S2 in the supplementary material). We could not detect Shot protein in the tendon cells of these embryos (see Fig. S2 in the supplementary material). Such embryos exhibited a range of denticle phenotypes, from general shape and organization defects (Fig. 4C) to a more specific hooking defect in which 62% of column 1 denticles and 75% of column 4 denticles exhibited incorrect hook orientation (Fig. 4B,D), which is reminiscent of the stripe mutant phenotype.
Fig. 4.
shot is required for anterior denticle hooking. (A) Df/+ cuticle. (B) Df(2R)383BSC/Df(2R)Exel7128 (deficiencies uncovering shot) cuticle. Hooking defects are indicated by circles. (C) Df(2R)383BSC/Df(2R)Exel7128 cuticle. Note the disorganization and hooking defects in all columns. (D) Quantification of hook orientation in Df/+ and Df/Df embryos. **, P<0.01. (E) Act5c::dsshot cuticle, exhibiting global disorganization and hooking defects. Scale bar: 10 μm.
As shot is not the only gene uncovered by the heteroallelic deficiency, we wished to confirm that the phenotype observed was in fact due to the loss of Shot. We expressed shot double-stranded (ds) RNA using the general driver Actin (Act5c)-Gal4. In these embryos, we observed strong phenotypes that were very reminiscent of the deficiency phenotype, including defects in epithelial organization and hook orientation (Fig. 4E). We conclude that shot has a general role in epithelial organization (Roper and Brown, 2003) and acts in hooking orientation as well. The variable expressivity of the shot phenotype has been observed previously (Gregory and Brown, 1998; Lee et al., 2000) and suggests the existence of other proteins partially redundant with shot. Consistent with this, restoring the stripe-dependent isoform of shot [shotL(A)-GFP] (Lee and Kolodziej, 2002a) to column 2 was not sufficient to rescue the hooking defects of stripe mutants (see Fig. S3 in the supplementary material). This suggests the existence of other Stripe targets relevant to denticle hook orientation.
Shot is enriched in denticles and across certain cell interfaces
To further understand the denticle phenotypes, we reinvestigated the accumulation of Shot protein within denticle field cells, focusing apically, where the actin-based protrusions arise. As previously shown, in stage 14-15 embryos, Shot was detectable in all epidermal cells and enriched in tendon cells (Fig. 5A) (Gregory and Brown, 1998; Roper and Brown, 2003; Strumpf and Volk, 1998). We also observed Shot puncta that colocalized with Filamin (Cheerio – FlyBase), an actin-associated protein that is enriched in protrusions (Fig. 5A).
Fig. 5.
shot is enriched in denticles and across certain cell interfaces. (A-A′′) Stage 15 wild-type Drosophila embryo, showing colocalization of Shot (green; single channel in A′) with Filamin (magenta; single channel in A′), a marker of actin-based protrusions. Colocalization was detectable from the onset of actin-based protrusion formation. (A′′) Magnification of the boxed area in A. (B-B′′) Stage 16 wild-type embryo, showing Shot (magenta; single channel in B′) and Arm (green; single channel in B′). White line marks the anterior (left) boundary of tendon cells. Note the Shot accumulation on the anterior side (left) of the cell boundary. (B′′) Magnification of the boxed area in B. (C-C′′) Stage 17 wild-type embryo, showing Shot (green; single channel in C′) and Stripe (magenta; single channel in C′). Note the Shot protein enrichment anterior to tendon cell nuclei in the denticle field, but not in the smooth field (arrow). (C′′) Magnification of the boxed area in C. Scale bar: 20 μm, except 5 μm in A′′,B′′,C′′.
Although it was heavily enriched within the tendon cells, as expected, Shot exhibited an additional component not previously appreciated. Apically, at stage 15, using anti-Armadillo (Arm) to label adherens junctions, we observed Shot protein not only in column 2 cells, but also along the posterior of column 1 cells, leading to its apparent accumulation along both sides of the column 1-2 cell interface (Fig. 5B). We observed identical accumulation at the posterior of column 4 cells (Fig. 5B). Artifacts of imaging or cell architecture were ruled out, as expression of lacZ in the cytoplasm of column 2 cells was imaged solely within those cells (see Fig. S4A in the supplementary material). Thus, Shot specifically accumulated along the posterior interface within column 1 and 4 cells, abutting the Stripe-expressing column 2 and 5 cells. Interestingly, these are the same two interfaces along which denticles reverse their hook orientation, suggesting a correlation between the two events.
In older embryos, Shot protein became anteriorly enriched in column 2 and 5 cells. Although cuticle deposition prevents the visualization of cell outline markers at this stage, Shot was enriched anterior to tendon cell nuclei (Fig. 5C), which themselves are centrally positioned within the cells (see Fig. S4B,C in the supplementary material). This anterior bias was specific to the denticle field cells, as Shot accumulated on either side of nuclei within smooth field cells (Fig. 5C). As MACF can localize, stabilize and bundle microtubules, it is possible that this enrichment reflects a specialized cytoskeletal complex at the anterior interface of column 2 and 5 cells (Leung et al., 1999; Roper et al., 2002; Sanchez-Soriano et al., 2009).
shot functions non-cell-autonomously in denticle hooking
Although Shot is upregulated in the tendon cells, Shot is also present at lower levels throughout the epidermis and accumulates within the denticle itself (Fig. 5A) (Gregory and Brown, 1998; Roper and Brown, 2003), in addition to along the 1-2 and 4-5 boundaries. To assess which pool(s) of Shot is required for denticle hooking orientation, we asked whether interfering with Shot function in cell column 2 could cause a non-autonomous phenotype in column 1. First, we expressed shot dsRNA under the control of ptc-Gal4, which significantly reduced Shot protein levels in cell column 2 (Fig. 6A). We also interfered with Shot using a dominant-negative construct, UAS-shot-EGG, which contains its EF-hand domain and microtubule-binding GAS2 domain (Fig. 6H, boxed region) fused to GFP (Gregory and Brown, 1998; Subramanian et al., 2003). UAS-shot-EGG localizes to microtubules in tendon cells and presumably functions by blocking the interactions of all Shot isoforms with microtubules (Subramanian et al., 2003).
Remarkably, both ptc::dsshot and ptc::shot-EGG embryos displayed non-autonomous denticle hook orientation defects in column 1 (Fig. 6B,D,F,G). In addition, embryos depleted for Shot also exhibited gaps among column 1 denticles (Fig. 6C; see Materials and methods). Taken together, the stripe-dependent upregulation and accumulation of Shot across the column 1-2 (and 4-5) boundaries is suggestive of the non-autonomous requirement for Shot in denticle hook orientation. Furthermore, these results are consistent with a role for stripe-dependent shot expression in non-autonomous anterior hooking.
We turned next to the ubiquitously expressed pool of Shot, which accumulates along the actin-based protrusions and at the posterior boundary of column 1, and asked whether Shot might also play an intrinsic role in denticle shaping. To test this, we expressed UAS-shot-EGG under the control of en-Gal4, which is strongly expressed in cell column 1. In these embryos, ∼65% of column 1 denticles exhibited a defect in anterior hooking and appeared poorly shaped and aligned (Fig. 6E,G). Thus, we conclude that Shot is intrinsically required for denticles to hook properly, and we hypothesize that this reflects a role for the ubiquitously expressed pool of Shot. Since the dominant-negative construct, which is thought to block the interaction of Shot with the microtubule cytoskeleton, generated hooking defects when expressed in either column 2 or column 1 cells, we next tested whether microtubules acted in either, or both, of the non-autonomous and intrinsic facets of denticle hooking.
Shot functions with the microtubule cytoskeleton
As globally disrupting microtubules would be likely to cause a severe phenotype, we disrupted microtubules in a column-specific manner by ectopically expressing the microtubule-severing protein Spastin (Sherwood et al., 2004; Trotta et al., 2004).
First, we expressed UAS-spastin under the control of en-Gal4, severely depleting microtubules specifically in En-expressing cells, including column 1 (Fig. 7A). This correlated with a loss of column 1 denticle hooking, with ∼60% of column 1 denticles failing to hook (Fig. 7B,E). These data are consistent with the observation that late-stage protrusions contain microtubules at their base (Price et al., 2006).
Fig. 7.
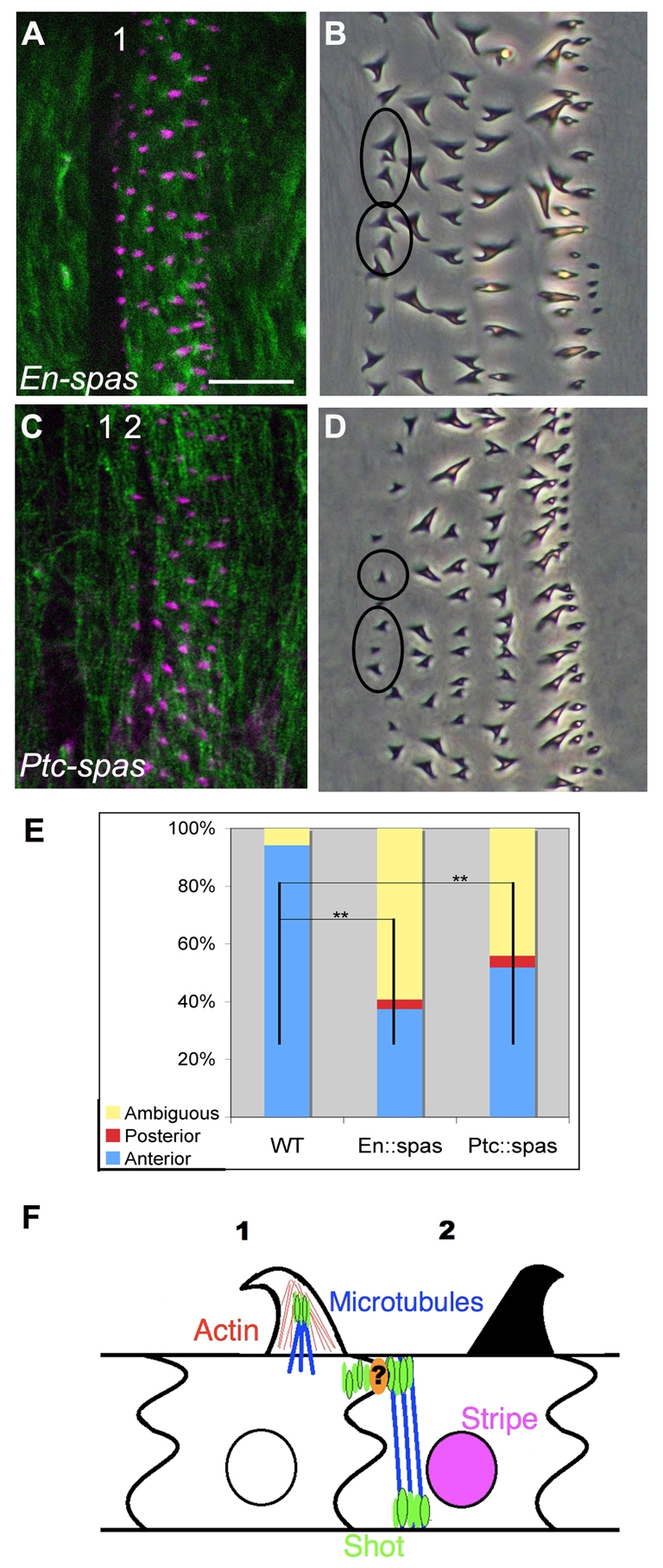
shot functions with the microtubule cytoskeleton. (A) en:spastin Drosophila embryo, showing phalloidin (magenta; to label actin) and β-tubulin (green) staining. Note the depletion of microtubules in column 1. (B) en-Gal4; UAS-spastin cuticle. Note the column 1 hooking defect. (C) ptc-Gal4; UAS-spastin embryo, showing phalloidin and β-tubulin staining. Note the depletion of microtubules in column 2. (D) ptc-Gal4; UAS-spastin. Note the column 1 hooking defect. It appeared that column 2 was also affected in these embryos, but this was difficult to score as column 2 exhibits only weak hooks. (E) Quantification of column 1 hook orientation in en-Gal4; UAS-spastin and ptc-Gal4; UAS-spastin embryos. **, P<0.01. (F) Model of stripe-dependent denticle hook orientation (see text). Attachments to muscle and exoskeleton are not depicted. Scale bar: 10 μm in B,D; 20 μm in A,C.
Next, we expressed UAS-spastin under control of ptc-Gal4, depleting microtubules in column 2 (Fig. 7C). ptc::spastin mutant cuticles exhibited a non-autonomous denticle orientation phenotype in column 1 (Fig. 7D), with ∼44% of column 1 denticles affected (Fig. 7E). Therefore, we conclude that microtubules are required both intrinsically for denticle hooking and in the non-autonomous specification of anterior hooking. Furthermore, these data are consistent with our hypothesis that Shot acts via the microtubule cytoskeleton to properly orient denticle hooks.
DISCUSSION
Screening for mutations that affect the cuticle pattern has been a powerful approach to identify genes involved in developmental decisions (Luschnig et al., 2004; Mayer and Nüsslein-Volhard, 1988; Nüsslein-Volhard and Wieschaus, 1980). Some years ago, it became clear that the column-specific differences in denticle shape and hook orientation occurred as a result of differential activation of the signaling pathways that pattern the epidermis (Alexandre et al., 1999). However, since then, no downstream targets have been identified that selectively affect column-specific denticle diversity, and the mechanisms involved have remained elusive. Here, we show that stripe integrates signaling information and positional cues to specify denticle density and anterior hook orientation. Furthermore, we show that stripe governs hook orientation, in part via the spectraplakin shot. Thus, the stripe-shot circuit has the potential to link the unpatterned blastoderm to the fully patterned cuticle.
Stripe links patterning signals to denticle diversity
stripe is a genetic target of both the Hh and Egfr pathways, and is regulated by Hh (and Wingless) directly via distinct cis-regulatory elements (Hatini and DiNardo, 2001; Piepenburg et al., 2000). Thus, our data suggest that the signals responsible for the elaborate denticle pattern (Alexandre et al., 1999) act by determining stripe expression in the appropriate cell columns.
Our work significantly advances the current understanding of how pattern diversity is produced within the cuticle. The allocation of cells into the smooth and denticle fields occurs when competitive signaling interactions between the Wingless and Egfr pathways result in restricted expression of the transcription factor Shavenbaby (Svb; Ovo – FlyBase) (Mevel-Ninio et al., 1991; O’Keefe et al., 1997; Payre et al., 1999; Szüts et al., 1997). Svb then regulates target genes that participate in actin filament, extracellular matrix and membrane morphogenesis to construct denticles (Chanut-Delalande et al., 2006). However, whereas svb is sufficient for denticle production, stripe is required for denticle diversity by regulating target genes that modulate denticle morphogenesis. How the Stripe targets interact with, and modify, the output of Svb targets in specific denticle columns will be interesting to explore.
Taking our data together with previously published work, stripe has at least three functions in this epidermis: the specification of the muscle attachment sites (Frommer et al., 1996; Volk and VijayRaghavan, 1994; Volohonsky et al., 2007), the reversal of denticle hooking orientation, and the determination of denticle density. At least some of these functions are separable, as we show that muscle attachment is not required for hook orientation. However, the ventral denticle pattern is conserved throughout all the drosophilids studied so far, which suggests that these three biological characteristics are functionally coupled (McGregor et al., 2007). This, in turn, suggests an explanation for why select denticle columns are anteriorly hooked. As muscle attachments are required for larval movement, perhaps a reversal in hook orientation at the attachment site provides for more efficient traction. Similarly, the denticles produced by tendon cells, although fewer, are often more robust and might be better able to transmit tension. We suggest that stripe could integrate patterning information to correlate muscle attachment with denticle number and shape, the latter via cytoskeletal remodeling or modulation of cuticle secretion components. It should be possible to design biophysical approaches to test these inferences.
Spectraplakins are required for denticle hooking
One Stripe target that we implicated is the spectraplakin, shot. We found that Shot acts intrinsically in denticle hooking, but is also important for the non-autonomous influence that one cell column exerts on another. The phenotypic similarities between stripe and its target shot strongly suggest that shot acts as part of a non-autonomous, stripe-controlled circuit, rather than in some parallel pathway. In turn, this suggests that the smaller, stripe-dependent Shot isoform functions in the non-autonomous circuit. In addition, although we found that muscle tension is not required, the involvement of Shot suggests that the non-autonomous signal from tendon cells to the responding cells might still be mechanical in nature. To our knowledge, this is the first work to demonstrate a functional role for either spectraplakins or microtubules in the shaping of actin-based protrusions.
shot is required intrinsically
Although Shot is required intrinsically for columns 1 and 4 to construct properly hooked denticles, there are two pools of Shot to consider here. Thus, we cannot differentiate whether Shot is required intrinsically to respond to stripe-dependent cues or for a separate structural purpose. Of course, these possibilities are not mutually exclusive, and it seems likely that Shot is intrinsically required at multiple steps.
Shot, actin filaments and microtubules are all found within the protrusion (Price et al., 2006). Since Shot has the ability to bind both actin and microtubules simultaneously (Karakesisoglou et al., 2000; Lee and Kolodziej, 2002a; Leung et al., 1999), the intrinsic role of Shot might be to physically link these cytoskeletal networks within the protrusion itself (Fig. 7F). In addition, it was recently demonstrated that MACF possesses actin-induced ATPase activity, which is hypothesized to move microtubules along actin filaments (Wu et al., 2008). Thus, Shot could function within the protrusion by maintaining physical tension between the cytoskeletal networks.
Conversely, as Shot is also enriched at the posterior cortex of the responding cells, this pool of Shot sits in close proximity to the denticles it influences (Fig. 7F). Thus, it is tempting to speculate that this pool of Shot is required to respond to the hooking cues given by the tendon cell. One unresolved issue is how Shot in column 1 becomes enriched along the 1-2 boundary. As Shot protein fails to localize to the posterior cortex of columns 1 and 4 in the absence of stripe (see Fig. S5 in the supplementary material), it is tempting to speculate that stripe-dependent Shot stabilizes intrinsic Shot at select column boundaries (Fig. 7F).
Shot is required non-cell-autonomously
Although we demonstrate that Shot is required in tendon cells for denticles in the adjacent column to hook properly (Fig. 6), the specific molecular requirement for Shot in the tendon cell remains to be determined.
The column 1-2 and 4-5 interfaces are enriched for a number of cytoskeletal components (Walters et al., 2006; Simone and DiNardo, 2010), and late stage embryos show dramatic enrichment of Shot protein at these interfaces (Fig. 5C). stripe does not control the special nature of these interfaces (R. Simone and S.D., unpublished observation), and raises the wider question of what initially differentiates these interfaces from other column boundaries. We speculate that the same signaling cues that initiate stripe expression (Hh and Spi) might also be responsible for the specialization of the 1-2 and 4-5 interfaces, and serve to coordinate cytoskeletal enrichment, muscle attachment and reversal of denticle hook orientation. As previously mentioned, we speculate that stripe-dependent Shot could be required to stabilize this enrichment along the 1-2 and 4-5 column boundaries.
Notably, in response to Stripe, a specialized microtubule array is formed within the tendon cell that runs from the basal to the apical cell surface (Becker et al., 1997; Gregory and Brown, 1998; Subramanian et al., 2003). Shot localizes along this array in two places – the basal cell surface, where the EMA junction will form, and the apical portion of the cell, where the microtubule array links to the cortical actin cytoskeleton (Gregory and Brown, 1998; Volk and VijayRaghavan, 1994). We do not know whether the apical-basal microtubule array is responsible for the localization of Shot, or whether this array is required for denticle hooking, although this would be consistent with the requirement we observed for microtubules.
Summary
It appears that the non-autonomous stripe-shot circuit culminates in the localization of Shot protein across the boundaries where denticle hooks reverse. As spectraplakins can stabilize, localize and bundle microtubule arrays, as well as create specialized membrane domains via membrane protein clustering (Leung et al., 1999; Roper et al., 2002; Sanchez-Soriano et al., 2009), a likely hypothesis is that Shot organizes a specialized microtubule array or other cytoskeletal complex at these interfaces.
As denticle hooks manifest themselves at the level of the cuticle and not in bending of the actin filaments themselves (S.A.D. and S.D., unpublished observation) (Dickinson, 1997; Price et al., 2006), we speculate that this complex could control polarized secretion, extracellular matrix deposition or membrane-matrix attachment. Alternatively, as we also discovered a role for microtubules in denticle hooking, perhaps Shot remodels the cytoskeleton via membrane-microtubule attachment, as it does in tracheal cells (Lee and Kolodziej, 2002b).
Supplementary Material
Acknowledgments
We thank the fly community, especially Talila Volk, and the Bloomington Stock Center for stocks and antibodies; Talila Volk and Nick Brown for their insightful input; the Ghabrial and DiNardo laboratories for discussions; and Mitra Eghbal for work on denticle density. This work was supported by an NIH Training Program in Genetics T32GM008216 to S.A.D. and NIH RO1 GM45747 to S.D. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.045450/-/DC1
References
- Alexandre C., Lecourtois M., Vincent J. (1999). Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development 126, 5689-5698 [DOI] [PubMed] [Google Scholar]
- Becker S., Pasca G., Strumpf D., Min L., Volk T. (1997). Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development 124, 2615-2622 [DOI] [PubMed] [Google Scholar]
- Bottenberg W., Sanchez-Soriano N., Alves-Silva J., Hahn I., Mende M., Prokop A. (2009). Context-specific requirements of functional domains of the Spectraplakin Short stop in vivo. Mech. Dev. 126, 489-502 [DOI] [PubMed] [Google Scholar]
- Chanut-Delalande H., Fernandes I., Roch F., Payre F., Plaza S. (2006). Shavenbaby couples patterning to epidermal cell shape control. PLoS Biol. 4, e290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford R. J., Schüpbach T. (1989). Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics 123, 771-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson W. J., Thatcher J. W. (1997). Morphogenesis of denticles and hairs in drosophila embryos: involvement of actin-associated proteins that also affect adult structures. Cell Motil. Cytoskeleton 38, 9-21 [DOI] [PubMed] [Google Scholar]
- Dorfman R., Shilo B. Z., Volk T. (2002). Stripe provides cues synergizing with branchless to direct tracheal cell migration. Dev. Biol. 252, 119-126 [DOI] [PubMed] [Google Scholar]
- Frommer G., Vorbruggen G., Pasca G., Jackle H., Volk T. (1996). Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 15, 1642-1649 [PMC free article] [PubMed] [Google Scholar]
- Gergen J. P., Wieschaus E. F. (1985). The localized requirements for a gene affecting segmentation in Drosophila: analysis of larvae mosaic for runt. Dev. Biol. 109, 321-335 [DOI] [PubMed] [Google Scholar]
- Gregory S. L., Brown N. H. (1998). Kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 143, 1271-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V., DiNardo S. (2001). Distinct signals generate repeating striped pattern in the embryonic parasegment. Mol. Cell 7, 151-160 [DOI] [PubMed] [Google Scholar]
- Hillman R., Lesnik L. H. (1970). Cuticle formation in the embryo of Drosophila melanogaster. J. Morphol. 131, 383-395 [Google Scholar]
- Karakesisoglou I., Yang Y., Fuchs E. (2000). An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J. Cell Biol. 149, 195-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Lee S., Zadeh A. D., Kolodziej P. A. (2003). Distinct sites in E-cadherin regulate different steps in Drosophila tracheal tube fusion. Development 130, 5989-5999 [DOI] [PubMed] [Google Scholar]
- Lee S., Kolodziej P. A. (2002a). Short Stop provides an essential link between F-actin and microtubules during axon extension. Development 129, 1195-1204 [DOI] [PubMed] [Google Scholar]
- Lee S., Kolodziej P. A. (2002b). The plakin Short Stop and the RhoA GTPase are required for E-cadherin-dependent apical surface remodeling during tracheal tube fusion. Development 129, 1509-1520 [DOI] [PubMed] [Google Scholar]
- Lee S., Harris K. L., Whitington P. M., Kolodziej P. A. (2000). short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. J. Neurosci. 20, 1096-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Nahm M., Lee M., Kwon M., Kim E., Zadeh A. D., Cao H., Kim H. J., Lee Z. H., Oh S. B., et al. (2007). The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development 134, 1767-1777 [DOI] [PubMed] [Google Scholar]
- Leung C. L., Sun D., Zheng M., Knowles D. R., Liem R. K. (1999). Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J. Cell Biol. 147, 1275-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohs-Schardin M., Cremer C., Nüsslein-Volhard C. (1979). A fate map for the larval epidermis of Drosophila melanogaster: localized cuticle defects following irradiation of the blastoderm with an ultraviolet laser microbeam. Dev. Biol. 73, 239-255 [DOI] [PubMed] [Google Scholar]
- Luschnig S., Moussian B., Krauss J., Desjeux I., Perkovic J., Nüsslein-Volhard C. (2004). An F1 genetic screen for maternal-effect mutations affecting embryonic pattern formation in Drosophila melanogaster. Genetics 167, 325-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Zhou Y., Beachy P. A., Moses K. (1993). The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell 75, 927-938 [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M. D. (2000). Integrins modulate the Egfr signaling pathway to regulate tendon cell differentiation in the Drosophila embryo. Development 127, 2607-2615 [DOI] [PubMed] [Google Scholar]
- Martinez-Arias A., Bate M. (1993). The Development of Drosophila melanogaster Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Mayer U., Nüsslein-Volhard C. (1988). A group of genes required for pattern formation in the ventral ectoderm of the Drosophila embryo. Genes Dev. 2, 1496-1511 [DOI] [PubMed] [Google Scholar]
- McGregor A. P., Orgogozo V., Delon I., Zanet J., Srinivasan D. G., Payre F., Stern D. L. (2007). Morphological evolution through multiple cis-regulatory mutations at a single gene. Nature 448, 587-590 [DOI] [PubMed] [Google Scholar]
- Mevel-Ninio M., Terracol R., Kafatos F. C. (1991). The ovo gene of Drosophila encodes a zinc finger protein required for female germ line development. EMBO J. 10, 2259-2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. A., Samanta R., Wieschaus E. (1999). Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development 126, 577-586 [DOI] [PubMed] [Google Scholar]
- Nabel-Rosen H., Dorevitch N., Reuveny A., Volk T. (1999). The balance between two isoforms of the Drosophila RNA-binding protein how controls tendon cell differentiation. Mol. Cell 4, 573-584 [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795-801 [DOI] [PubMed] [Google Scholar]
- O’Keefe L., Dougan S. T., Gabay L., Raz E., Shilo B. Z., DiNardo S. (1997). Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development 124, 4837-4845 [DOI] [PubMed] [Google Scholar]
- Payre F. (2004). Genetic control of epidermis differentiation in Drosophila. Int. J. Dev. Biol. 48, 207-215 [DOI] [PubMed] [Google Scholar]
- Payre F., Vincent A., Carreno S. (1999). ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature 400, 271-275 [DOI] [PubMed] [Google Scholar]
- Piepenburg O., Vorbruggen G., Jackle H. (2000). Drosophila segment borders result from unilateral repression of hedgehog activity by wingless signaling. Mol. Cell 6, 203-209 [PubMed] [Google Scholar]
- Price M. H., Roberts D. M., McCartney B. M., Jezuit E., Peifer M. (2006). Cytoskeletal dynamics and cell signaling during planar polarity establishment in the Drosophila embryonic denticle. J. Cell Sci. 119, 403-415 [DOI] [PubMed] [Google Scholar]
- Rodrigues A. B., Werner E., Moses K. (2005). Genetic and biochemical analysis of the role of Egfr in the morphogenetic furrow of the developing Drosophila eye. Development 132, 4697-4707 [DOI] [PubMed] [Google Scholar]
- Roper K., Brown N. H. (2003). Maintaining epithelial integrity: a function for gigantic spectraplakin isoforms in adherens junctions. J. Cell Biol. 162, 1305-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper K., Gregory S. L., Brown N. H. (2002). The ‘spectraplakins’: cytoskeletal giants with characteristics of both spectrin and plakin families. J. Cell Sci. 115, 4215-4225 [DOI] [PubMed] [Google Scholar]
- Sanchez-Soriano N., Travis M., Dajas-Bailador F., Goncalves-Pimentel C., Whitmarsh A. J., Prokop A. (2009). Mouse ACF7 and Drosophila Short stop modulate filopodia formation and microtubule organisation during neuronal growth. J. Cell Sci. 122, 2534-2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood N. T., Sun Q., Xue M., Zhang B., Zinn K. (2004). Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2, e429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone R. P., DiNardo S. (2010). Actomyosin contractility and Discs large contribute to junctional conversion in guiding cell alignment within the Drosophila embryonic epithelium. Development 137, 1385-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol N. S., Cooley L. (2003). Drosophila filamin is required for follicle cell motility during oogenesis. Dev. Biol. 260, 260-272 [DOI] [PubMed] [Google Scholar]
- Strumpf D., Volk T. (1998). Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, vein, at the muscle-tendon junction site. J. Cell Biol. 143, 1259-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Prokop A., Yamamoto M., Sugimura K., Uemura T., Betschinger J., Knoblich J. A., Volk T. (2003). Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr. Biol. 13, 1086-1095 [DOI] [PubMed] [Google Scholar]
- Szabad J., Schüpbach T., Wieschaus E. (1979). Cell lineage and development in the larval epidermis of Drosophila melanogaster. Dev. Biol. 73, 256-271 [DOI] [PubMed] [Google Scholar]
- Szüts D., Freeman M., Bienz M. (1997). Antagonism between EGFR and Wingless signaling in the larval cuticle of Drosophila. Development 124, 2309-3219 [DOI] [PubMed] [Google Scholar]
- Teodoro R. O., O’Farrell P. H. (2003). Nitric oxide-induced suspended animation promotes survival during hypoxia. EMBO J. 22, 580-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta N., Orso G., Rossetto M. G., Daga A., Broadie K. (2004). The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr. Biol. 14, 1135-1147 [DOI] [PubMed] [Google Scholar]
- van der Meer S. (1977). Optical clean and permanent whole mount preparation for phase contrast microscopy of cuticular structures of insect larvae. Drosoph. Inf. Serv. 52, 160-161 [Google Scholar]
- van Vactor D., Sink H., Fambrough D., Tsoo R., Goodman C. S. (1993). Genes that control neuromuscular specificity in Drosophila. Cell 73, 1137-1153 [DOI] [PubMed] [Google Scholar]
- Volk T., VijayRaghavan K. (1994). A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development 120, 59-70 [DOI] [PubMed] [Google Scholar]
- Volohonsky G., Edenfeld G., Klambt C., Volk T. (2007). Muscle-dependent maturation of tendon cells is induced by post-transcriptional regulation of stripeA. Development 134, 347-356 [DOI] [PubMed] [Google Scholar]
- Walters J. W., Dilks S. A., DiNardo S. (2006). Planar polarization of the denticle field in the Drosophila embryo: roles for Myosin II (zipper) and fringe. Dev. Biol. 297, 323-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiellette E. L., McGinnis W. (1999). Hox genes differentially regulate Serrate to generate segment-specific structures. Development 126, 1985-1995 [DOI] [PubMed] [Google Scholar]
- Wu X., Kodama A., Fuchs E. (2008). ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell 135, 137-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitzky T., Min L., Volk T. (1997). The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes Dev. 11, 2691-1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.