Abstract
The potent immunosuppressive action of rapamycin is commonly ascribed to inhibition of growth-factor-induced T-cell proliferation. However, it is now evident that the serine/threonine protein kinase mammalian target of rapamycin (mTOR) has an important role in the modulation of both innate and adaptive immunity. mTOR regulates diverse functions of professional antigen-presenting cells, such as dendritic cells (DCs), and has important roles in the activation of conventional T cells and the function and proliferation of regulatory T cells. Here, we review our current understanding of the mTOR pathway and the consequences of mTOR inhibition, both in DCs and T cells, including new data on the regulation of FOXP3 expression.
Rapamycin was isolated in the early 1970s from a soil sample obtained on Easter Island (Rapa Nui) and identified as a potent anti-fungal metabolite 1. This macrolide, which is produced by Streptomyces hygroscopicus, was found to inhibit cell proliferation and to have potent immunosuppressive activity. It is used for prevention of kidney transplant rejection2. Rapamycin and its derivatives are undergoing clinical testing for prophylaxis of graft rejection3 and graft-versus-host disease (GVHD) [G]4, chemotherapy of some cancers5 and the prevention of restenosis following angioplasty6.
Our understanding of the mechanisms that underlie the unique immunosuppressive profile of rapamycin continues to evolve. In line with this, the central and pervasive role of the serine/threonine kinase ‘mammalian target of rapamycin’ (mTOR) in innate and adaptive immunity is becoming apparent. Blockade of mTOR by rapamycin impairs dendritic cell (DC) maturation and function, and inhibits T-cell proliferation, a mechanism that underpins its immunosuppressive effect. There is now strong evidence that mTOR is crucial for the regulation of antigen responsiveness in CD4+ T cells. This effect seems to be mediated by an influence of mTOR inhibition on naturally-occurring regulatory T (TReg) cells, which have a key role in immunological tolerance. Exciting information has emerged regarding the role of the phosphatidylinositol-3-kinase (PI3K)–AKT–mTOR pathway in regulating DC and T-cell function, particularly in relation to the expression of forkhead box P3 (FOXP3) and the differentiation of TReg cells. Here, we review the remarkable recent progress in elucidation of the mechanisms by which mTOR inhibition affects intracellular signalling pathways in immune cells, particularly DCs and T cells, and how this influences immunity.
The mTOR signalling pathway
Discovery of mTOR
Genetic screening of Saccharomyces cerevisiae identified two targets of rapamycin (TOR) genes (TOR1 and TOR2) and gave an early indication of the importance of TOR in control of cell proliferation7. Rapamycin forms a complex with the immunophilin [G] FK506-binding protein 1A, 12 kDa (FKBP12) to inhibit TOR function8 (BOX 1). A single mTOR gene was subsequently identified in rat, mouse and human cells, with a high degree of homology to yeast TORs9-11. Subsequent work has identified mTOR as an atypical serine/threonine protein kinase (BOX 1) with a central role in mammalian development12, 13. It is a crucial regulator of cell growth and proliferation, and of physiological events such as transcription, mRNA turnover, translation, ribosomal biogenesis, vesicular trafficking, autophagy, cytoskeletal organization and cell size14.
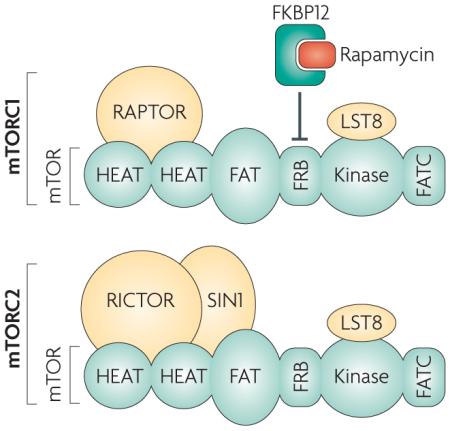
Box 1 | Two distinct mTOR-containing complexes and their interaction with rapamycin.
Mammalian target of rapamycin (mTOR) is a large (~289 kDa), atypical kinase, which, like other members of the phosphatidylinositol kinase-related kinase (PIKK) family, contains a carboxy-terminal serine/threonine protein kinase domain14, 18, 19. Also consistent with other PIKKs, mTOR contains a FRAP-ATM-TTRAP (FAT) domain, and a carboxy-terminal FAT domain (FATC) that might have a role in mTOR structure and stability134. Mammalian LST8 (mLST8, also known as GβL) associates with the kinase domain of mTOR and is thought to facilitate mTOR signalling, but its precise functional role is yet to be defined135, 136. In addition, the large mTOR protein with its multiple domains seems to be involved in various protein–protein interactions that determine the physiological functions of mTOR. It has been established that mTOR exists in at least two distinct complexes, defined as mammalian target of rapamycin complex 1 (mTORC1) and mTORC2. The mTOR kinase and mLST8, together with the regulatory associated protein of mTOR (Raptor) form mTORC1. Raptor is essential for mTORC1 activity and is proposed to interact with mTOR through shared HEAT (Huntington, Elongation Factor 3, PR65/A, TOR) domains19. Rapamycin binds to the immunophilin FK506-binding protein 1A, 12 kDA (FKBP12) to form a drug–receptor complex that specifically and effectively blocks mTORC1 activity. The FKBP12–rapamycin complex binds just amino-terminal to the kinase region of mTOR (in the FKBP12–rapamycin-binding domain, FRB)136 and disrupts the in vitro and in vivo activity of mTORC1, potentially by disrupting the interaction between Raptor and mTOR14. mTORC2 also contains mLST8, but instead of Raptor, associates with rapamycin-independent companion of mTOR (Rictor) and possibly stress-activated MAP kinase-interacting protein 1 (mSIN1; also known as MAPK-associated protein 1)19, 137. Unlike mTORC1, mTORC2 is resistant to direct inhibition by rapamycin. It is unknown what prevents the interaction between the FKBP12–rapamycin complex and the FRB on mTORC214.
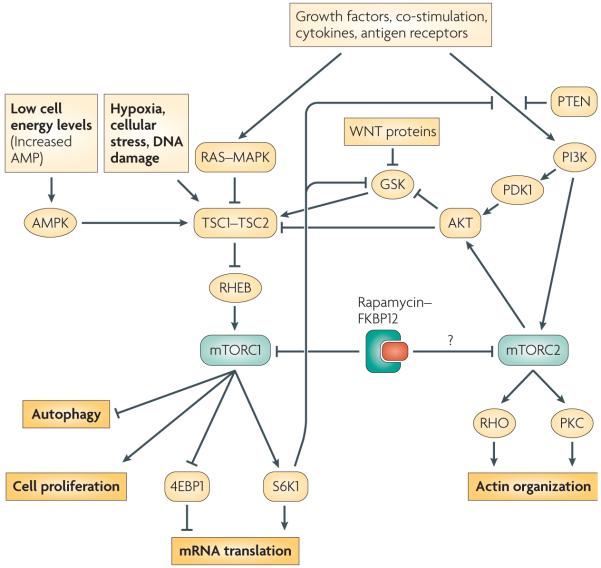
mTOR exists in at least two complexes, mTOR complex 1 (mTORC1) and mTORC2 (BOX 1), that have distinct relationships both to upstream and downstream effectors and to each other (FIG. 1). mTOR in mTORC1 is exquisitely sensitive to inhibition by rapamycin, whereas mTOR in mTORC2 is resistant to rapamycin, for an unknown reason. Notably, there are also rapamycin-insensitive functions of mTORC1 in mouse embryonic fibroblasts that have been identified recently using a new inhibitor (Torin 1) of both mTORC1 and mTORC215. Also prolonged exposure to rapamycin can impede the assembly of mTORC2 in some cells5, 16, 17.
Figure 1. mTORC1 (mammalian target of rapamycin complex 1) and mTORC2 signalling pathways.
mTORC1 is the direct target of the rapamycin–FK506-binding protein 1A, 12 kDa (FKBP12) complex and regulates cell growth and size by controlling translation, ribosome biogenesis and autophagy. Diverse signals, arising from growth factors, such as insulin and Fms-like tyrosine kinase 3 ligand (FLT3L), various cytokines, ligated costimulatory molecules and antigen receptors, WNT (Wingless and Integrase-1) proteins, and the relative cellular energy and oxygen levels, determine mTORC1 activity as a result of their effects on the tuberous sclerosis complex 1 (TSC1)–TSC2 complex, which is the main negative regulator of mTORC1. Activation of Ras–MAPK (mitogen-activated protein kinase) and phosphatidylinositol-3-kinase (PI3K)–AKT results in inhibitory phosphorylation of TSC2 and removes repression of Rheb (Ras homologue enriched in brain), which is the mTORC1 stimulator. Activation of AKT by PI3K is negatively regulated by phosphatase and tensin homologue (PTEN). Activated mTORC1 promotes translation through stimulating S6K1 (p70 ribosomal protein S6 kinase 1) and inhibiting 4EBP1 (eIF4E binding protein 1). Activated S6K1 can also feed back to negatively regulate input from PI3K-AKT by facilitating the degradation of signaling intermediates between surface receptors (such as the insulin receptor) and PI3K. Low energy and nutrient levels, as well as hypoxic conditions, increase TSC1–TSC2-mediated inhibition of mTORC1 through input from GSK3 (glycogen synthase kinase 3) and AMPK (AMP-activated protein kinase). mTORC2 is not inhibited directly by rapamycin, but long-term rapamycin administration disrupts its assembly in some cells. mTORC2, activated by PI3K, directly phosphorylates AKT. mTORC2 also regulates cytoskeleton dynamics. PDK1,= phosphoinositide-dependent kinase 1.
Signalling through mTORC1
In immune cells, mTORC1 is a regulator of cell growth and other processes downstream of PI3K–AKT, Wingless and Integrase-1 (WNT)–glycogen synthase kinase 3 (GSK3) and AMP-activated protein kinase (AMPK) signalling (FIG. 1). Details of mTORC1 signalling can be found in recent comprehensive reviews14, 18, 19. Tuberous sclerosis complex 1 (TSC1) and TSC2 together form a functional complex that acts as the upstream inhibitor of mTORC1. Growth factors, cytokines, co-stimulatory molecules and antigen receptors activate PI3K, which subsequently activates AKT. Fully activated AKT inhibits TSC2 by phosphorylation20, thereby negating its inhibitory effect on mTORC1. Activation of the RAS–mitogen-activated protein kinase (MAPK) pathway also leads to inhibition of the TSC1–TSC2 complex14. Alternatively, cellular stresses and DNA damage can inhibit mTORC1 by promoting the regulatory capacity of TSC1–TSC214, 19. The inhibitory activity of the TSC1–TSC2 complex is mediated by targeting RHEB (RAS homologue enriched in brain), a RAS-like guanosine triphosphatase (GTPase) and a positive regulator of mTORC118, 19.
Removal of mTORC1 inhibition results in its phosphorylation of S6 kinase 1 (S6K1) and the eukaryotic initiation factor 4EBP1 (eIF4E binding protein 1). Phosphorylated S6K1 promotes mRNA translation and cell growth by enhancing the biosynthesis of the translational apparatus in the cell14, 19. The phosphorylation of 4EBP1 prevents its inhibition of eIF4E, which also stimulates translation14. WNT proteins bind to the Frizzled family of receptors, which are involved in regulation of effector T-cell development, TReg-cell activation and DC maturation21. The WNT pathway influences the mTORC1 pathway by inhibiting GSK3, which in the absence of WNT signaling, is an additional negative regulator of mTORC122.
Signalling through mTORC2
Knowledge of the more recently defined mTORC2 is limited compared with mTORC1, given the lack of a mTORC2-specific inhibitor. Studies targeting Rictor (rapamycin-insensitive companion of mTOR; a defining component of mTORC2; BOX 1) through small interfering RNA (siRNA) [G]23, 24 and studies of Rictor knock-out mice13 have shown the importance of mTORC2 in mammalian development and several cellular processes. mTORC2-mediated phosphorylation of AKT is stimulated by insulin and can be blocked by PI3K inhibitors25. However, knockdown of Rictor does not decrease S6K1 activation24, indicating that mTORC2 does not activate mTORC124.
Targeting Rictor has also established that mTORC2 regulates the actin cytoskeleton through the small GTPase RHO and protein kinase C (PKC)14. TSC1–TSC2 has been shown to regulate cell adhesion and migration26, but it is not clear whether TSC1–TSC2 signals to and regulates mTORC2 directly. Regulation of cell movement and adhesion is an important feature of effective immune responses, so it is possible that mTORC2 might also be shown to modulate immune reactivity when specific inhibitors become available.
S6K1-mediated regulation of mTOR signalling
mTOR is a crucial coordinator of signalling pathways, so it is not unexpected that feedback inhibition is an important component of the pathway. Activated S6K1, the main effector of uninhibited mTORC1, negatively regulates input from PI3K–AKT to mTORC119, 27-29 by phosphorylating and initiating the degradation of insulin receptor substrate 1 (IRS1), which is the molecular intermediate between the insulin receptor and PI3K19, 27-31. Whether S6K1 can also negatively regulate input from other receptor systems that activate mTOR through PI3K has not been defined. Activated S6K1 can also positively regulate mTOR activation. GSK3, a unique kinase that is constitutively activated in the absence of growth factors, negatively regulates the mTOR pathway by stimulating the TSC1–TSC2 complex. Under certain conditions, activation of S6K1 can negatively regulate GSK332, 33, thus facilitating cell proliferation.
Overall, the mTOR kinase, functioning in mTORC1 and mTORC2, acts as a coordinator of signalling pathways that shape the response of cells to various stimuli. Immune cells, using receptors that signal through mTOR directly or indirectly, modulate host responses based on their “perception” of environmental danger. We focus here on the role of mTOR in DCs, macrophages and T cells, but it is worth noting that the inhibition of mTOR by rapamycin has effects on other immune cells (BOX 2) that are outside the scope of this Review.
Box 2 | Effect of mTOR inhibition on B cells, NK cells, neutrophils and mast cells.
In addition to its influence on antigen-presenting cells and T cells, rapamycin exerts direct effects on other immune cells that impair their proliferation and function. mTOR inhibition thus exerts a broad spectrum of inhibitory effects on immune effector cells.
B cells
Phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) are required for B-cell proliferation. Early and late B-cell receptor (BCR) signals are blocked by mTOR inhibition138. Thus, rapamycin inhibits BCR-induced S6K1 activation and DNA synthesis in mouse B cells139. B-cell responses to lipopolysaccharide are also sensitive to rapamycin140. mTOR inhibition also suppresses mouse splenic B-cell activation through CD40141, resulting in decreased proliferation and differentiation to IgMhiIgDlow cells. Rapamycin inhibits CD40-specific antibody-mediated prevention of apoptosis induced by BCR cross-linking and suppresses human B-cell proliferation after CD40 ligation in the presence of B-cell-activating cytokines, accompanied by increased apoptosis142. Moreover, rapamycin potently inhibits the number of IgM,- and to a lesser extent, IgG-producing B cells. When Staphylococcus aureus and CD40L are used as stimulants, rapamycin decreases IL-2-dependent and -independent human B cell proliferation, as well as IL-2-dependent differentiation to antibody-secreting cells143. Rapamycin also suppresses cytokine-induced proliferative responses and IgM production by pre-activated human B cells144.
NK cells
Rapamycin inhibits the proliferation of rat primary natural killer (NK) cells and of the RNK-16 NK-cell line, by blocking progression from the G1 to S phase of the cell cycle145, but it does not affect interferon-γ secretion by primary NK-cell lines. NK-cell-mediated killing of conventional YAC-1 target cells is modestly decreased by mTOR inhibition. In vivo, rapamycin administration decreases the number of circulating NK cells in rat liver allograft recipients145, but does not seem to influence NK-cell cytotoxic activity in rats receiving hamster skin xenografts146.
Neutrophils
Rapamycin potently suppresses human neutrophil chemotaxis and chemokinesis elicited by granulocyte/macrophage colony-stimulating factor (GM-CSF), and inhibits responses to IL-8147, 148. Rapamycin inhibits the increase in S6K1 activity induced by GM-CSF, and also inhibits GM-CSF–induced actin polymerization, a marker of leukocyte migration. mTOR complex 1 participates in Toll-like receptor 2- and 4-induced neutrophil activation and acute lung injury149.
Mast cells
Rapamycin has little effect on histamine release from lung mast cells, but antagonizes the inhibitory effect of FK506 on anti-IgE-induced histamine release from human mast cells150.
mTOR in DCs and macrophages
DCs and macrophages are phagocytic cells, resident in almost all tissues. Both cell types degrade pathogens and function as antigen-presenting cells. Whereas macrophages have a scavenging role and activate and recruit other immune cells, DCs are uniquely well-equipped to present antigen to T cells and initiate adaptive immune responses.
DC differentiation and maturation
The suppressive effects of mTOR inhibition on DC differentiation and maturation are well-documented in vitro. In addition, rapamycin inhibits the differentiation and mobilization of mouse DCs in vivo in response to administration of Fms-like tyrosine kinase 3 ligand (FLT3L) [G]34. Whereas an early study suggested that rapamycin did not affect the phenotypic differentiation or CD40L-induced maturation of human monocyte-derived DCs in vitro, but rather promoted the apoptosis of these DCs and CD34+-derived DCs35, studies of mouse bone marrow-derived DCs34 showed that mTOR inhibition suppressed IL-4-dependent maturation through the post-transcriptional downregulation of the IL-4R complex. In vitro and in vivo, rapamycin inhibited costimulatory molecule expression by DCs, and suppressed IL-4-induced IL-12 and TNF production and T-cell stimulatory function34. Further work has shown that mTOR inhibition during DC differentiation inhibits the upregulation of CD86 expression induced by Toll-like receptor (TLR) ligands (such as LPS or CpG DNA) or CD40-specific monoclonal antibody36. mTOR inhibition also decreases nitric oxide (NO) production by LPS-stimulated macrophages37, an effect attributed, in part, to impaired secretion of IFNβ, which is an autocrine co-factor for NO production. Furthermore, the inactivation of mTOR decreases IL-2 secretion upon stimulation of DCs through the C-type lectin receptor Dectin 138, indicating that cytokine production through C-type lectin receptor activation involves mTOR.
Immature DCs undergo differentiation in response to hypoxia and over-express hypoxia-inducible factor 1α (HIF1α) and its downstream target genes, including vascular endothelial growth factor and glucose transporter-139. mTOR inhibition attenuates these responses, and rapamycin can suppress hypoxia-induced inflammation through inhibition of the HIF-1α pathway39. Whereas these observations all relate to the effects of soluble rapamycin, there are recent reports that the delivery of rapamycin through biodegradable nano- or microparticles enhances its inhibitory effects on the maturation and T-cell stimulatory function of mouse and human DCs40-42.
Antigen uptake and presentation
DCs use several pathways for antigen uptake: phagocytosis, constitutive macropinocytosis [G] and mannose receptor-mediated endocytosis. Rapamycin impairs the macropinocytosis and endocytosis of antigens by cultured mouse immature bone marrow-derived DCs43. Rapamycin also inhibits endocytosis in vivo in mouse splenic DCs34. Human monocyte-derived DCs differentiated in the presence of rapamycin have decreased expression of antigen uptake receptors (such as CD205, CD32, CD46 and CD91) and decreased receptor-mediated and fluid-phase endocytosis and phagocytosis of bacteria and apoptotic cells44 (FIG. 2A). Although the mechanisms by which rapamycin inhibits endocytosis are unclear, the inhibition of mTOR by short-term exposure to rapamycin abolishes the translation of Rho-associated kinase 1 (ROCK1) in mouse macrophages, which results in the inhibition of phagocytosis and chemotaxis45.
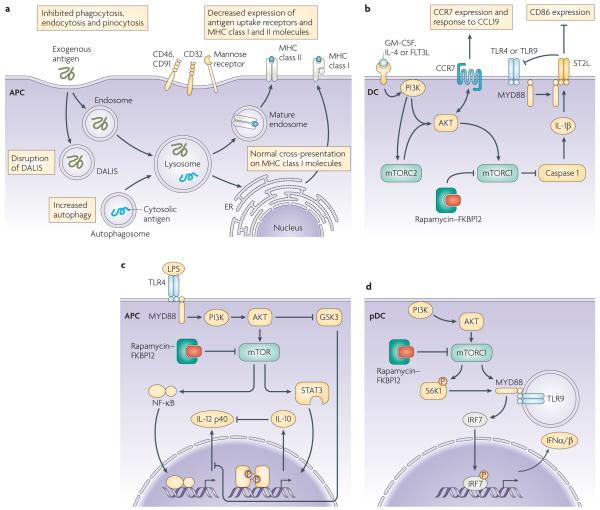
Figure 2. mTOR and rapamycin regulate APC function.
a | Several changes are observed in antigen acquisition and presentation by antigen–presenting cells (APCs) exposed to or differentiated in rapamycin. Rapamycin inhibits endocytosis and phagocytosis, and the expression of antigen uptake receptors and MHC class II molecules, and disrupts dendritic cell aggresome-like structures (DALIS), although cross-presentation of exogenous antigen on MHC class I molecules might not be affected. Rapamycin facilitates autophagy, an evolutionarily conserved process and could thereby influence self-antigen presentation. Thus, mTOR has a role in regulation of immune responses at an early stage, influencing how exogenous and endogenous antigen is acquired, processed and presented. b | Interruption of mTOR signalling in monocytes, macrophages and dendritic cells (DCs) during Toll-like receptor (TLR) ligation results in increased IL-12 production by decreasing IL-10 production and derepressing NF-κB. This indicates that mTOR is a crucial mediator of T-cell polarization and immune responses. c | In plasmacytoid DCs (pDCs), coordinated signalling through TLR9 and phosphatidylinositol-3-kinase (PI3)K–mTOR is needed to drive type-1 IFN-α/β production. S6K phosphorylation by mTORC1 promotes the interaction of MyD88–TLR9–IFN regulatory factor (IRF)7 and the subsequent translocation of phosphorylated IRF7 to the nucleus to initiate transcription of the genes encoding type-1 IFN. mTOR has been reported to interact with MyD88 and positively regulate cytokine production mediated by IRF5 and IRF7. d | Differentiation of DCs in rapamycin generates DCs with weak T-cell stimulatory capacity, but intact/improved homing to CCL21. mTOR is a negative regulator of caspase-1 and treatment with rapamycin therefore promotes IL-1β production by caspase 1. IL-1β subsequently induces expression of ST2, which sequesters MyD88 and negatively regulates TLR4 and TLR9 signalling. Failure of rapamycin to impede AKT signalling might allow/promote CCR7 activity and DC migration to CCL21.
Unlike calcineurin inhibitors [G] (such as cyclosporin A [CsA] and FK506), rapamycin does not inhibit the presentation of MHC-restricted antigens (such as ovalbumin [OVA]) or of minor histocompatibility antigens [G] to T cells by mouse bone marrow-derived DCs46-48. This might reflect the inhibitory effects of calcineurin inhibitors on early events (such as cytokine production) in DC-induced activation of naïve T cells, whereas rapamycin inhibits subsequent events. There is, however, recent evidence that mTOR inhibition can suppress the transient aggregation of ubiquitylated cytoplasmic proteins during lipopolysaccharide (LPS)-induced DC maturation49, 50. These ‘aggresomes’ (DC aggresome-like structures; DALIS [G]) (FIG. 2A) might function as depots of antigen that can be cross-presented by MHC class I molecules, and they might allow DCs to coordinate their maturation and antigen-presenting functions during migration from the periphery to secondary lymphoid tissue.
Cross presentation of antigen is essential for the generation of cytotoxic T-cell responses against certain tumour cells and viruses. The fact that calcineurin inhibitors, but not rapamycin, inhibit the cross presentation of an exogenous antigen by mouse DCs47 might explain why, in mice bearing an allograft and a tumour, CsA inhibits allograft rejection but promotes tumour growth (through inhibiting the cross-presentation of tumour-specific antigens), whereas rapamycin suppresses both graft rejection and tumour growth51.
Autophagy [G] is a constitutive process in DCs52 in which autophagosomes sequester intracellular contents (such as damaged organelles and macromolecules) and target them for lysosomal degradation. This process is thought to be involved in the MHC class II antigen presentation pathway leading to self tolerance, and in innate and adaptive immunity53, 54. TOR1 and TOR2 are involved in repression of autophagy in Saccharomyces cerevisiae55. Rapamycin induces autophagy in yeast56. and also in mouse macrophages and DCs, enhancing their Ag presenting ability57. Further studies are required to determine the extent to which autophagy influences the peptide–MHC class II repertoire, and how this might be affected by mTOR inhibition. Overall, rapamycin can interfere with antigen uptake in DCs and can modulate events associated with antigen presentation, although the mechanistic basis of these effects requires clarification.
DC survival
DC survival is important for the induction of immune responses, for example to viruses and tumours35, 58. Blocking of granulocyte/macrophage colony-stimulating factor (GM-CSF) signalling by rapamycin induced the apoptosis of both human monocyte-derived DCs and DCs derived from CD34+ precursor cells, but not of monocytes or macrophages. By contrast, the frequency of apoptotic or dead cells was consistently <10% in rapamycin-conditioned, bone marrow-derived mouse DC cultures, as well as for in vivo-generated DCs from rapamycin-treated mice34. As pointed out by these authors, pro- and anti-apoptotic effects of rapamycin have been reported for different cell types, possibly reflecting differential sensitivity of mTORC2 to disruption in these cells. Interestingly, no increase in cell death was observed following mTOR inhibition by rapamycin in virus-infected, FLT3L-derived DCs38.
DC migration
By increasing expression of the chemokine receptor CCR7, rapamycin increases the migration of human DCs in response to CCL19 in vitro and mouse DC migration to lymph nodes in vivo59. Similarly, functional expression of CCR7 is retained on mouse rapamycin-conditioned DCs36, 60, which promotes their homing to secondary lymphoid tissue during inflammatory responses following allogeneic haematopoietic cell transplantation60. Intact in vivo migration of syngeneic rapamycin-conditioned DCs to secondary lymphoid tissue has also been reported in a heart allograft model61. Retention of this in vivo migratory ability is probably crucial for the immune-regulatory properties ascribed to rapamycin-conditioned DCs, to allow these cells to reach appropriate T-cell areas in the lymphoid tissue.
Cytokine production by DCs and pDCs
The coordinated secretion of pro- and anti-inflammatory cytokines is essential for effective immunity62. An unexpected finding is that mTOR suppresses caspase-1 activation (the molecular basis of which is unclear) and therefore the production of bioactive IL-1β38. Both in vitro and in vivo, mTOR inhibition elicits de novo production of IL-1β by otherwise phenotypically immature, mouse bone marrow-derived DCs63. Moreover, IL-1β production by rapamycin-conditioned DCs promotes overexpression of the transmembrane form of the IL-1R family member, IL-1R-like 1 (also known as ST2)63 (FIG. 2B), the recently identified receptor for IL-33, which promotes T helper 2 (TH2)-cell responses64. ST2 has also been implicated as a potent negative regulator of TLR signalling in macrophages65. In keeping with this function, IL-1β-induced ST2 expression suppresses responses of rapamycin-conditioned mouse DCs to TLR and CD40 ligation, an effect that is absent in ST2−/− DCs63. So, by inducing IL-1β production, with consequent upregulation of ST2 expression, mTOR inhibition impedes DC maturation and their ability to stimulate effector T cell responses. By contrast, these rapamycin-conditioned DCs favour the differentiation of potent, alloantigen-specific CD4+CD25+FOXP3+ TReg cells (see later)36.
PI3K negatively regulates TLR-mediated IL-12 production by mouse DCs66, a feedback mechanism that might prevent excessive TH1-cell polarization. Recently, it has been shown that two other kinases, mTOR and GSK3, differentially regulate IL-12 production by mouse DCs67. Blocking mTOR increases IL-12 production by LPS-stimulated DCs, an effect that is mediated by inhibition of negative feedback through autocrine IL-10, and activation of mTOR (by transduction of a constitutively active form of Rheb) inhibits IL-12 production. By contrast, GSK inhibition attenuates IL-12 production, but increases IL-10 production by LPS-stimulated DCs. In related studies, the activation of mTOR in mononuclear phagocytes increased the activity of signal transducer and activator of transcription 3 (STAT3) and the production of IL-10, but decreased the production of pro-inflammatory molecules (such as IL-12) and nuclear factor-κB (NF-κB) activation; mTOR inhibition had reciprocal effects67. Similarly, rapamycin increased IL-12 production and decreased IL-10 production by human DCs stimulated with LPS or Staphylococcus aureus 68. Such findings imply that mTOR and GSK3 pathways might regulate the TH1–TH2-cell balance through modulation of IL-10 and IL-12 production by DCs (FIG. 2C). Rapamycin-exposed monocytes induce the polarization of TH1 cells and TH17 cells68, and rapamycin greatly increases the expression of IL-12/IL-23 p40 (p40) and IL-23 p19 (p19) mRNA and IL-23 protein in human macrophages induced by Mycobacterium tuberculosis69. Collectively, these observations identify the GSK3–mTOR pathway as a key regulator of innate immune homeostasis.
The mTOR pathway has recently been implicated in regulation of the production, by plasmacytoid (p)DCs70, of type-1 IFNs (IFN-α/β) that are crucial for anti-viral immunity. mTOR associates with the scaffold protein MyD88 to allow activation of IFN regulatory factor 5 (IRF5) and IRF7, which are master transcription factors for type-1 IFN genes38, 71. Thus, inhibition of mTOR signaling during pDC activation through TLR9 blocks the interaction between TLR9 and MyD88 and the subsequent phosphorylation and nuclear translocation of IRF7, resulting in impaired IFNα/β production72 (FIG. 2D). Decreased IFNα levels in serum and decreased production of IFNα by pDCs in response to stimulation with CpG or a viral vaccine, leads to impaired adaptive CD8+ T-cell-mediated immune responses72.
In summary, rapamycin exerts multiple effects on DC differentiation, maturation and function. It interferes with antigen uptake and might modulate antigen presentation; its differential effects on cytokine production and chemokine receptor expression regulate interactions between innate immunity and adaptive T-cell responses.
mTOR in effector and regulatory T cells
T cells have a fundamental role in host responses to invading pathogens and tumours. TReg cells exert control over the reactivity of effector T cells and their important role is emphasized by the severe pathological conditions associated with TReg-cell deficiency73. As our understanding of the fine balance between T cells and TReg cells becomes clearer, the influence of mTOR inhibition on their activity is providing surprising insights.
Thymocyte development
In rodents, blockade of mTOR activity causes profound thymic involution, associated with decreased T-cell output74. Recent reports have shown that prolonged rapamycin administration blocks the conversion of thymocytes from double-negative (CD4−CD8−) to double-positive T cells75. Although the absolute number of T cells is decreased, rapamycin administration does not alter the proportion of CD4+ single positive cells that up-regulate expression of FOXP3 in the thymus76. This indicates that the ontogeny of natural TReg cells is unaffected by mTOR blockade. This is surprising in view of recent evidence that FOXP3 expression is negatively regulated by the PI3K–AKT–mTOR axis; thymocytes expressing a constitutively active form of AKT were unable to up-regulate FOXP3 expression77. Thus, it might be expected that, by blocking mTOR (which lies downstream of AKT), a higher proportion of CD4+ cells would up-regulate FOXP3. Absence of such an effect in the thymus suggests that thymic TReg cell generation is based on the initiation of a genetic program78 controlled by AKT signaling but independent of mTOR activity.
T-cell activation and anergy
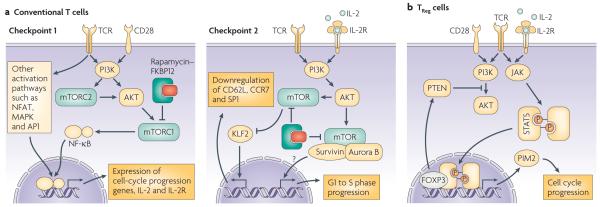
T-cell activation in the periphery is a complex process requiring progress through two major checkpoints (FIG. 3A) that limit entrance into the cell cycle79. Progression from G0 to G1 of the cell cycle is the first checkpoint. Detailed analysis of the molecular processes that underlie the two-signal T-cell activation model [G] has shown that, in addition to TCR-driven activation of NFAT and MAPK, integration of both TCR and CD28 signals is required for PI3K and AKT activation. As discussed earlier, mTOR is a target of AKT and, through its activity in the TORC1 and TORC2 complexes, can stimulate four pivotal processes that regulate progression from G0 to G1: increased mRNA translation, increased glycolysis and consequent ATP accumulation, degradation of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 and promotion, in combination with the transcriptional regulators NFAT and AP1, of IL-2 and IL-2 receptor α (IL-2Rα) expression. The increase in protein production as a result of mTOR activity allows the T cells to reach the critical mass required for successful division, a process that requires the energy made available through increased ATP. Initial progression from G0 to G1 is then regulated by the activity of CdK proteins, which drives the cell to the second checkpoint: the transition from the G1 to S phase of the cell cycle. Successful progression through this transition requires sustained stimulation through TCR and CD28, together with autocrine signalling by the IL-2 produced during progression through the first checkpoint. The PI3K–AKT–mTOR pathway is also involved in transmission of this IL-2R signal79. It now seems that mTOR activity at this phase of the cell cycle is in the form of a new complex with the proteins Survivin (an inhibitor of apoptosis family protein) and Aurora B (a serine/threonine kinase)80. The expression and activity of both Survivin and Aurora B depends on CD28 stimulation and IL-2 signalling. The mTOR–Survivin–Aurora B complex has target specificity similar to TORC1, with an additional Aurora-regulated capacity to control Rb phosphorylation, cyclin A expression, and Cdk1 and Cdk2 activity, all of which are involved in G1- to S-phase progression79. Interestingly, similar to TORC1, this new mTOR complex is susceptible to inhibition by rapamycin.
Figure 3. Proposed molecular mechanisms responsible for T-cell susceptibility or resistance to rapamycin.
a | In conventional T cells, mTOR integrates the TCR and CD28 signals that are necessary to pass two checkpoints of T-cell activation. The transition from G0 to G1 phase of the cell cycle (checkpoint 1) requires activation of NFAT, MAPKs and NF-κB. The activity of NF-κB is controlled by the PI3K–AKT–mTOR pathway. mTOR works as part of the complexes TORC1 and TORC2, of which the former is susceptible to inhibition by rapamycin. The coordinated activity of NFAT, AP1 and NF-κB regulates multiple genes involved in cell-cycle progression and the expression of IL-2 and its high-affinity receptor. IL-2R signals through the PI3K–AKT-mediated activation of mTOR complexed with survivin and aurora B, which regulates G1- to S-phase progression (checkpoint 2). In addition, mTOR activity is involved in rapamycin-sensitive down-regulation of the transcription factor Kruppel-like factor 2 (KLF2), which controls expression of lymphoid tissue-homing molecules to ensure that activated T cells can exit the lymph nodes. b | The ability of TReg cells to proliferate when stimulated in the presence of rapamycin seems to be connected to two effects. First, TReg cells do not down-regulate phosphatase and tensin homologue (PTEN) expression after TCR engagement, which impedes the activation of the rapamycin-susceptible PI3K–AKT–mTOR pathway. Second, FOXP3 drives expression of phosphatidylinositol mannoside (PIM)2, reinforced by IL-2- and TCR-mediated activation of signal transducer and activator of transcription (STAT)5, which compensates for AKT inactivity and promotes cell-cycle progression.
An additional immunosuppressive effect of mTOR blockade during T-cell activation has been recently identified. IL-2–IL2R signalling in differentiating effector T cells results in down-regulation of the transcription factor Kruppel-like factor 2 (KLF2), which controls expression of the homing molecules, SP1, CD62L, CCR7 and other chemokine receptors. This decrease in KLF2 levels depends on PI3K activation and involves mTOR activation81. The presence of rapamycin during IL-2 signalling prevents the down-regulation of KLF2 and consequently, the modulation of expression of the aforementioned homing receptors necessary for the egress of effector T cells from lymph nodes. So, in addition to blocking cell-cycle progression during T-cell activation, rapamycin-mediated mTOR inhibition could sequester activated T cells in lymphoid tissues and prevent them from reaching the target tissue.
mTOR activation is a key process in preventing T-cell anergy [G]. Treatment of antigen-stimulated T cells with rapamycin slows cell-cycle progression to the G1 phase and prevents the down-regulation of genes involved in the development of anergy82. In addition, it is now clear that IL-2–IL-2R signalling has a direct role in the avoidance of anergy83. An excess of IL-2 can reverse anergy through an mTOR-dependent, rapamycin-sensitive signalling pathway84, 85. These observations support the view that pro-anergy factors are induced early after TCR stimulation, and are subsequently degraded or suppressed in response to IL-2R signalling. This indicates that conventional mTORC1 activity is necessary for T-cell activation, but is insufficient to reverse the program of anergy induction. A separate, IL-2-specific signalling event is required during the G1 phase to preserve antigen responsiveness, and might involve the aforementioned Survivin–Aurora B–mTOR complex. The activity of this complex may underlie the recent observations regarding the involvement of the PI3K–AKT–mTOR axis in the induction of FOXP3 expression in the periphery (see below).
Rapamycin is most effective at preventing T-cell division under conditions of low IL-2 availability79. In the presence of optimal autocrine IL-2 secretion and/or exogenous IL-2, rapamycin delays but cannot prevent later cell division86. This alternative regulation of late cell-cycle progression by IL-2 seems to depend on the recently discovered STAT5-dependent expression and activity of the serine/threonine kinase phosphatidylinositol mannoside 2 (PIM2)87. PIM2 can maintain nutrient uptake and ATP synthesis at a high level and, similarly to AKT, regulates cell survival during blastogenesis. mTOR-induced signalling might regulate the rate of initial cell-cycle entry and the integration of sequential signals dictated by TCR–CD28 engagement and IL-2 production, but it becomes dispensable later, when significant quantities of IL-2 are available86.
TReg-cell homeostasis, function and proliferation
Rapamycin does not affect the function and homeostasis of TReg cells to the same extent as ‘conventional’ T cells, as recently reviewed88. In vitro exposure of mouse or human CD4+CD25+ TReg cells to rapamycin does not impair their ability to suppress effector T-cell proliferation89, an effect that is lost using the calcineurin inhibitor CsA90, 91. Additionally, prolonged in vivo rapamycin administration results in a pronounced, relative increase in the number of TReg cells compared with CD4+ T cells in all lymphoid organs, although the absolute numbers of all T cells are decreased, as in the thymus76, 92. A similar finding has been made in kidney transplant recipients92, 93; patients treated with rapamycin have a markedly increased frequency of CD4+CD25+FOXP3+ T cells over bulk CD4+ T cells. This effect is reversed in patients given calcineurin inhibitors. In a TCR-transgenic mouse model, rapamycin was used in vitro, in combination with IL-2, to selectively expand cells with regulatory activity from a starting population of bulk CD4+ T cells94. This observation was then confirmed for human TReg cells89, 95, 96, which could be expanded selectively in vitro from both healthy donors and patients with autoimmune disease.
The suppressive activity and proliferative capacity of TReg cells depend on TCR engagement and IL-2 availability. Differential susceptibility to rapamycin inhibition suggested that the TCR signalling pathway in TReg cells differed from that in conventional T cells. The observation that T-cell stimulation in the presence of rapamycin favored upregulation of FOXP3 expression and acquisition of a regulatory phenotype97, offered the alternative explanation that the observed increase in TReg-cell frequency after prolonged rapamycin administration could derive from peripheral conversion of T cells into TReg cells (see next section). However, our current understanding is that both processes can occur. Mouse TReg cells are defective in their ability to activate phospholipase C-γ and generate the downstream signals that result in NFAT, NF-κB and Ras–ERK–AP1 activation98. In parallel, TReg cells are defective in phosphorylation of AKT in response to stimulation, and restoration of AKT activity impairs their suppressor function99. This has been further clarified by a report that TReg cells express high levels of the negative PI3K regulator, phosphatase and tensin homolog (PTEN)100, 101. In contrast to conventional T cells, PTEN expression is maintained in TReg cells after TCR stimulation. Targeted depletion of PTEN does not affect the suppressive capacity of TReg cells, but increases their sensitivity to rapamycin. These data indicate that TReg cells do not rely on the conventional PI3K–AKT–mTOR activation pathway, and explain their lower susceptibility to rapamycin inhibition.
The above observations do not clarify the mechanism of TReg-cell-specific activation in the presence of rapamycin. The IL-2R–STAT5 pathway is essential for TReg-cell homeostasis and activity102, 103. It has been reported recently that FOXP3 expression is associated with the induction of PIM2 expression104. Notably, PIM kinases have been implicated in conferring T-cell resistance to rapamycin-mediated inhibition, when STAT5-signalling cytokines (including IL-2) are present in adequate amounts87. It is therefore possible that TReg cells can integrate TCR and IL-2 signalling in a PIM-dependent pathway that allows them to progress through the cell cycle despite mTOR inhibition (FIG. 3B).
Induction of FOXP3 expression
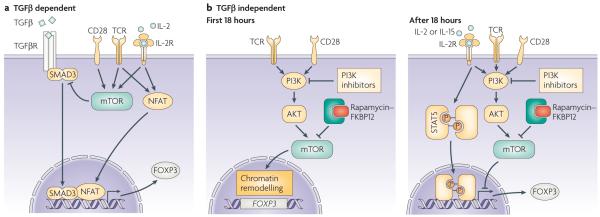
Natural TReg cells arise in the thymus, but CD4+FOXP3+ T cells with regulatory capacity can also be generated in the periphery (induced TReg cells) by antigen-driven conversion of naïve T cells under certain conditions105. In particular, in vitro TCR stimulation of CD4+CD25− T cells in the presence of transforming growth factor-β (TGFβ) and IL-2 induces FOXP3 expression. The observation that rapamycin favors this conversion has focussed much attention on the involvement of mTOR in regulation of FOXP3 expression (FIG. 4A). The induction of FOXP3 expression by TGFβ depends on activation of the transcription factor mothers against decapentaplegic homolog 3 (SMAD3)106 which, in combination with TCR-induced NFAT, binds to the enhancer region of the FOXP3 gene and promotes chromatin remodeling necessary for translation. Interestingly, signaling through the AKT–mTOR pathway can inhibit activation of SMAD3107, providing an explanation for the observation that mTOR inhibition through rapamycin favours FOXP3 up-regulation.
Figure 4. Mechanisms of FOXP3 induction in naïve T cells.
a | Stimulation of FOXP3− T cells through TCR and CD28 in the presence of TGFβ promotes expression of the FOXP3 gene through the cooperation of NFAT and mothers against decapentaplegic homologue 3 (SMAD3). This process is counteracted by mTOR activation, which explains the increased expression of FOXP3 when stimulation takes place in the presence of rapamycin (which inhibits mTOR activation). b | Limited TCR–CD28 stimulation (<18 hours) promotes a PI3K–AKT–mTOR-mediated re-organization of chromatin that includes increased accessibility to the FOXP3 gene. Prolonged TCR/CD28 stimulation prevents, again through activation of the PI3K–AKT–mTOR pathway, the expression of FOXP3 which would otherwise probably be induced by signal transducer and activator of transcription (STAT)5-activating cytokines generated during the initial stimulation. This two-step model rationalizes the opposing effects of rapamycin administration during T-cell activation that have been observed, which probably depend on the timing of mTOR inhibition.
T cells with constitutively active AKT have impaired up-regulation of FOXP3 in response to activation in the presence of TGFβ77. Interestingly, when rapamycin is present at the time of transduction of constitutively active AKT, the inhibitory effect is lost. This implies that a rapamycin-sensitive signal involving AKT activation (and consequently, mTOR) is responsible for the control of peripheral FOXP3 induction. This effect is not restricted to FOXP3, and extends to approximately 50% of the genes modulated in response to TGFβ77, which confirms that conversion to a regulatory phenotype involves complex genetic re-programming78. However, important differences remain between natural TReg cells and induced FOXP3+ T cells. Expression of a constitutively active form of AKT in thymocytes decreases thymic development of TReg cells, without affecting T-cell ontogeny77, confirming the ability of the AKT–mTOR pathway to prevent TReg-cell development. Expression of FOXP3 in already differentiated circulating TReg cells, however, is not affected by AKT transduction, supporting the proposed model that a specific genetic program is implemented in TReg cells during their development.
A process of FOXP3 up-regulation, independent of TGFβ signalling, has begun to be characterized (FIG. 4B). Premature interruption of in vitro TCR–CD28 stimulation (after 18h) is associated with the upregulation of FOXP3 expression in approximately 10% of T cells108. Addition of PI3K inhibitors or rapamycin after the interruption of TCR stimulation increased this fraction to up to 75% of T cells. The effect did not involve TGFβ signalling, as neutralizing antibodies specific for TGFβ and SMAD kinase inhibition did not affect FOXP3 induction promoted by inhibition of PI3K and mTOR. Interestingly, this effect was closely associated with the timing of interruption of TCR–CD28 stimulation and the addition of PI3K and mTOR inhibitors. Early addition of the inhibitors blocked the necessary T-cell activation, whereas prolongation of TCR–CD28 stimulation beyond 18 hours markedly decreased FOXP3 up-regulation. In addition, following initial TCR–CD28 stimulation, histone modifications (that grant genes accessibility to transcription factors) at the FOXP3 locus were observed and subsequently lost after continuous TCR–CD28 signalling. These findings suggest that rapamycin administration establishes conditions for the initiation of a two-step process of FOXP3 up-regulation, similar to that described for thymic TREG development109, 110. TCR–CD28 signalling causes chromatin remodeling that predisposes towards expression of the FOXP3 gene. This first step probably involves PI3K–AKT–mTOR signalling, as the addition of inhibitors at the same time as TCR–CD28 stimulation prevents the necessary T-cell activation. After prolonged TCR–CD28 stimulation, the accessibility of the FOXP3 gene is then restricted in a process that involves a second round (or continuation) of PI3K–AKT–mTOR activity. It is reasonable to imply that, by blocking this second round of signalling, the pre-activated cell can then respond differently to endogenous cytokine (such as IL-2 and IL-15) stimulation. This promotes activation of the STAT5 pathway, which is involved in both intrathymic TReg-cell development and the modulation of FOXP3 expression in peripheral T cells102, 103, 111. This model of FOXP3 induction might explain the recent observation that a combination of histone/protein deacetylase (HDAC) inhibition and rapamycin augments FOXP3+ TReg cells in vivo112.
On the whole, these observations delineate differences between T cells and TReg cells that shed light on previously unappreciated effects of mTOR inhibition. The observation that rapamycin causes a generalized increase in the frequency of FOXP3+ cells is now interpreted as the sum of two effects — the ability of TReg cells to proliferate in the presence of rapamycin, and the promotion of FOXP3 expression in peripheral T cells that are then converted into modulators of immune reactivity.
Implications of mTOR inhibition for therapeutic immunosuppression
The remarkable inhibitory action of rapamycin on DCs and effector T cells, but not Treg cells, singles out mTOR inhibition as a promising therapeutic strategy in transplantation and autoimmune disease.
Transplant tolerance
In renal transplantation, rapamycin is a powerful anti-rejection agent when used judiciously with other immunosuppressants. It has unique anti-atherogenic and anti-neoplastic properties that distinguish it from other anti-rejection drugs, and can promote tolerance and decrease the incidence of chronic allograft nephropathy113. Rapamycin was used initially with CsA, but it is also effective when combined with other immunosuppressants. However, the effects of calcineurin inhibitors (such as CsA) and rapamycin in transplantation seem to be markedly different. In rodents, rapamycin, but not CsA, permits activation-induced death of the large number of alloreactive effector T cells and favours tolerance induction 114, 115; in addition, rapamycin enhances, whereas CsA prevents, the tolerance-promoting ability of costimulation blockade116, 117. In transplant recipients, calcineurin inhibitors, but not rapamycin, decrease the proportion of CD4+CD25+FOXP3+ TReg cells, a presumed negative effect of the former agents93. Recently, use of FOXP3 reporter mice has shown that rapamycin promotes the de novo (TGFβ-dependent) conversion of alloantigen-specific CD4+ TReg cells under tolerizing conditions, whereas CsA abrogates this process118. Converted TReg cells were more resistant to apoptosis than conventional T cells, and adoptive transfer of the former potently suppressed the rejection of donor but not third-party skin grafts. The ability of rapamycin plus IL-10 to induce T regulatory type-1 (Tr1) cells [G] that mediate stable, alloantigen-specific tolerance in pancreatic islet cell transplantation has also been reported119. Collectively, these data provide further evidence that the differential effects of rapamycin on effector T cells and TReg cells (both naturally-occurring and induced), favour its ability to promote tolerance and support its use in tolerance-promoting protocols.
Recent reports suggest that mTOR inhibition, together with targeting of other key molecules involved in immune regulation, can promote transplant tolerance in mice. The chemokine receptor CXCR3 and its ligands (CXCL9, CXCL10 and CXCL11) constitute an important pathway for effector T-cell recruitment. When combined with a sub-therapeutic regimen of rapamycin, CXCR3-specific monoclonal antibody induces indefinite (>100 day) survival of heart or islet allografts120. A second finding concerns HDAC9 inhibition and consequent increases in FOXP3 expression, as well as the production and function of TReg cells112. When HDAC inhibition is combined with a short course of low-dose rapamycin, permanent, donor-specific, TReg-cell-dependent heart and pancreatic islet allograft survival is achieved112. TReg cells also seem to be important in mediating the tolerogenic effect of minimally effective rapamycin in combination with monoclonal antibodies specific for T-cell immunoglobulin domain and mucin 1 (TIM1), which is associated with a TH1- to TH2-type cytokine switch in experimental heart transplantation121.
Tolerogenic cell therapy in transplantation
The capacity of rapamycin to deplete effector T cells, but to spare the growth and function of TReg cells, can be exploited in the design of novel and safe protocols for cell therapy of allograft rejection and other T-cell-mediated disorders. Rapamycin-conditioned APCs are poor stimulators of allogeneic CD4+ effector T-cell proliferation, but enrich for potent FOXP3+ TReg cells36. Such rapamycin-conditioned murine DCs pulsed with donor alloantigen then adoptively transferred to organ allograft recipients, in combination with a short post-operative course of low-dose rapamycin, induce indefinite (>100 day) graft survival36 Also, rapamycin-conditioned DCs of recipient genotype inhibit GVHD after allogeneic haematopoietic cell transplantation, consistent with their tolerogenic phenotype60. CCR5, CCR7 and CD62L expression on rapamycin-conditioned DCs is not affected by mTOR inhibition, which allows them to traffick to secondary lymphoid tissues, where their immunoregulatory function is required59-61. Such observations might, in part, explain the recently reported beneficial effects of rapamycin on the incidence of GVHD after haematopoietic cell transplantation in the clinic122, 123. As an example of an alternative mechanism, alloantigen-specific CD4+CD25+ TReg cells expanded in vitro in response to immature allogeneic DCs, then infused into rapamycin-conditioned heart allograft recipients without use of a T-cell-depletion strategy, induce indefinite graft survival, in association with T-cell anergy124.
Autoimmune disease
There are numerous reports that mTOR inhibition can suppress experimental autoimmune diseases, in particular type-1 diabetes, lupus nephritis and adjuvant arthritis125. Rapamycin combined with IL-10 blocks the incidence of type-1 diabetes and induces long-term tolerance without chronic immunosuppression in diabetes-prone, non-obese diabetic (NOD) mice126. In this model, rapamycin mediates the accumulation of suppressive CD4+CD25+FOXP3+ TReg cells in the pancreas, preventing diabetes. By contrast, when rapamycin is combined with CD3-specific antibody, it exerts a detrimental effect on disease outcome in NOD mice127. As discussed by the authors, rapamycin may interfere with restoration of IL-2 production (defective in NOD mice) by anti-CD3, and thus prevent a crucial role for this cytokine in maintenance of tolerance in these diabetes-prone animals. In patients with type-1 diabetes, rapamycin promotes the ex vivo proliferation of functional TReg cells (CD4+CD25+FOXP3+)89. Moreover, naturally occurring TReg cells from patients with type-1 diabetes on rapamycin monotherapy have restored ability to suppress proliferation of CD4+CD25− effector T cells compared with TReg cells before treatment128. In NZB × NZW F1 female mice (a model of systemic lupus erythematosus), administration of rapamycin (from 12–37 weeks of age) decreases the production of autoantibodies, glomerular deposits of immunoglobulins and the development of proteinuria, and prolongs survival129. Also in this model, rapamycin attenuates the severity of established nephritis through reduced lymphoproliferation, decreased CCL5 expression and decreased lymphoinfiltration of the kidneys130. Rapamycin is also effective for the treatment of de novo autoimmune hepatitis after human liver transplantation131, and has recently been used to treat a case of refractory Crohn’s disease132. Interestingly, rapamycin might be a clinically effective and safe therapeutic option in IPEX (immune dysregulation, polyendocrinopathy, enteropathy and x-linked syndrome) [G] and IPEX-like patients133, in whom naturally occurring FOXP3+ TReg cells are absent, resulting in severe autoimmune disease.
Conclusions
In summary, whereas the importance of mTORC1 in regulation of innate and adaptive immunity is now well-recognized, the role of mTORC2 has yet to be clarified. New evidence that mTOR regulates cytokine production by APCs in response to inflammatory stimuli suggests a pivotal role for this molecule in determining the nature of T-cell responses. The mechanisms by which rapamycin suppresses immunity have been expanded from inhibition of T-cell proliferation, to blockade of DC maturation and support of TReg cells, including their de novo induction. Ongoing and future areas of enquiry, which are likely to further elucidate the role of mTOR in the regulation of immunity and tolerance, include investigation of the role of the newly-identified mTOR–Survivin–Aurora B complex in T-cell activation (and in other immune cells, including DCs), clarification of the role of PIM1 and PIM2 in determining TReg-cell resistance to mTOR inhibition, and in-depth understanding of TGFβ-dependent and -independent mechanisms of FOXP3 up-regulation and their physiological role (which will be relevant to therapeutic application, as they are both affected by mTOR modulation). Insight is also needed into the role of mTOR in memory T cells.
Acknowledgements
The authors’ work is supported by National Institutes of Health grants R01 AI060994, R01 AI067541 and U01 AI051698 and by the Roche Organ Transplantation Research Foundation 874279717 (to A.W.T.), by an American Heart Association Beginning Grant-in-Aid (to G.R.) and by NIH postdoctoral fellowship F32 AI072940 (to H.R.T.)
References
- 1.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 2.Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59:3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- 3.Eisen HJ, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N. Engl. .J Med. 2003;349:847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 4.Armand P, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J. Clin. Oncol. 2008;26:5767–5774. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nature Rev. Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 6.Roiron C, Sanchez P, Bouzamondo A, Lechat P, Montalescot G. Drug eluting stents: an updated meta-analysis of randomised controlled trials. Heart. 2006;92:641–649. doi: 10.1136/hrt.2005.061622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 8.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 9.Brown EJ, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 10.Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc. Natl. Acad. Sci. U S A. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 12.Gangloff YG, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guertin DA, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Thoreen CC, et al. An ATP-competitive mTOR inhibitor reveals rapamycin-insensitive functions of mTORC1. J. Biol. Chem. 2009 doi: 10.1074/jbc.M900301200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarbassov DD, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Z, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 20.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nature Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 21.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nature Rev. Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 22.Inoki K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 23.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 24.Jacinto E, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. References 23 and 24 established that mTOR exists in two functionally distinct complexes, - rapamycin-sensitive mTORC1, containing raptor, and rapamycin-resistant mTORC2, containing rictor.
- 25.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3 T3-L1 adipocytes. J. Biol. Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 26.Astrinidis A, et al. Tuberin, the tuberous sclerosis complex 2 tumor suppressor gene product, regulates Rho activation, cell adhesion and migration. Oncogene. 2002;21:8470–8476. doi: 10.1038/sj.onc.1205962. [DOI] [PubMed] [Google Scholar]
- 27.O’Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tremblay F, Gagnon A, Veilleux A, Sorisky A, Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes. Endocrinology. 2005;146:1328–1337. doi: 10.1210/en.2004-0777. [DOI] [PubMed] [Google Scholar]
- 29.Sun SY, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 30.Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by Serine 312 phosphorylation. J. Biol. Chem. 2003;278:8199–8211. doi: 10.1074/jbc.M209153200. [DOI] [PubMed] [Google Scholar]
- 31.Harrington LS, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J. Cell. Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong JL, Bonavaud SM, Toole BJ, Yeaman SJ. Regulation of glycogen synthesis by amino acids in cultured human muscle cells. J. Biol. Chem. 2001;276:952–956. doi: 10.1074/jbc.M004812200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J. Immunol. 2006;176:770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 34.Hackstein H, et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. The first demonstration that rapamycin administration impairs steady-state DC generation and inhibits their maturation in vivo.
- 35.Woltman AM, et al. Rapamycin induces apoptosis in monocyte- and CD34-derived dendritic cells but not in monocytes and macrophages. Blood. 2001;98:174–180. doi: 10.1182/blood.v98.1.174. [DOI] [PubMed] [Google Scholar]
- 36.Turnquist H, et al. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein SL, et al. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-beta. J. Leukoc. Biol. 2000;67:405–414. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz F, et al. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur. J. Immunol. 2008;38:2981–2992. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 39.Rama I, et al. Hypoxia stimulus: An adaptive immune response during dendritic cell maturation. Kidney Int. 2008;73:816–825. doi: 10.1038/sj.ki.5002792. [DOI] [PubMed] [Google Scholar]
- 40.Haddadi A, et al. Delivery of rapamycin by PLGA nanoparticles enhances its suppressive activity on dendritic cells. J. Biomed. Mater Res A. 2008;84:885–898. doi: 10.1002/jbm.a.31373. [DOI] [PubMed] [Google Scholar]
- 41.Das S, Haddadi A, Veniamin S, Samuel J. Delivery of rapamycin-loaded nanoparticle down regulates ICAM-1 expression and maintains an immunosuppressive profile in human CD34+ progenitor-derived dendritic cells. J. Biomed. Mater. Res A. 2008;85:983–992. doi: 10.1002/jbm.a.31557. [DOI] [PubMed] [Google Scholar]
- 42.Jhunjhunwala S, Raimondi G, Thomson AW, Little SR. Delivery of rapamycin to dendritic cells using degradable microparticles. J. Controlled Release. 2009;133:191–197. doi: 10.1016/j.jconrel.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100:1084–1087. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 44.Monti P, et al. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation. 2003;75:137–145. doi: 10.1097/00007890-200301150-00025. [DOI] [PubMed] [Google Scholar]
- 45.Fox R, et al. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. E.M.B.O. J. 2007;26:505–515. doi: 10.1038/sj.emboj.7601522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsue H, et al. Contrasting impacts of immunosuppressive agents (rapamycin, FK506, cyclosporin A, and dexamethasone) on bidirectional dendritic cell-T cell interaction during antigen presentation. J. Immunol. 2002;169:3555–3564. doi: 10.4049/jimmunol.169.7.3555. [DOI] [PubMed] [Google Scholar]
- 47.Lee YR, et al. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005;105:3951–3955. doi: 10.1182/blood-2004-10-3927. [DOI] [PubMed] [Google Scholar]
- 48.Imai A, et al. Inhibition of endogenous MHC class II-restricted antigen presentation by tacrolimus (FK506) via FKBP51. Eur. J. Immunol. 2007;37:1730–1738. doi: 10.1002/eji.200636392. [DOI] [PubMed] [Google Scholar]
- 49.Fassbender M, Herter S, Holtappels R, Schild H. Correlation of dendritic cell maturation and the formation of aggregates of poly-ubiquitinated proteins in the cytosol. Med. Microbiol. Immunol. 2008;197:185–189. doi: 10.1007/s00430-008-0091-4. [DOI] [PubMed] [Google Scholar]
- 50.Lelouard H, et al. Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature. 2002;417:177–182. doi: 10.1038/417177a. [DOI] [PubMed] [Google Scholar]
- 51.Koehl GE, et al. Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation. 2004;77:1319–1326. doi: 10.1097/00007890-200405150-00002. [DOI] [PubMed] [Google Scholar]
- 52.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nature Rev. Immunol. 2008;8:607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gannage M, Munz C. Macroautophagy in immunity and tolerance. Traffic. 2009 doi: 10.1111/j.1600-0854.2009.00883.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Kamada Y, et al. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell. Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 57.Jagannath C, et al. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nature Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 58.Woltman AM, et al. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27KIP1 expression. Blood. 2003;101:1439–1445. doi: 10.1182/blood-2002-06-1688. [DOI] [PubMed] [Google Scholar]
- 59.Sordi V, et al. Differential effects of immunosuppressive drugs on chemokine receptor CCR7 in human monocyte-derived dendritic cells: selective upregulation by rapamycin. Transplantation. 2006;82:826–834. doi: 10.1097/01.tp.0000235433.03554.4f. [DOI] [PubMed] [Google Scholar]
- 60.Reichardt W, et al. Impact of mammalian target of rapamycin inhibition on lymphoid homing and tolerogenic function of nanoparticle-labeled dendritic cells following allogeneic hematopoietic cell transplantation. J. Immunol. 2008;181:4770–4779. doi: 10.4049/jimmunol.181.7.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am. J. Transplant. 2005;5:228–236. doi: 10.1046/j.1600-6143.2004.00673.x. The first demonstration that recipient-derived myeloid DC, generated in rapamycin and pulsed with donor alloantigen, can promote indefinite organ allograft survival in the absence of any other therapy.
- 62.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 63.Turnquist HR, et al. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J. Immunol. 2008;181:62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Brint EK, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nature Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 66.Fukao T, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nature Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 67.Ohtani M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. References 67 and 68 revealed that mTOR acts downstream of TLR4, as a crucial regulator of pro- and anti-inflammatory cytokine production.
- 69.Yang CS, et al. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell. Microbiol. 2006;8:1158–1171. doi: 10.1111/j.1462-5822.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 70.Colina R, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 71.Honda K, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 72.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nature Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. In vitro and in vivo evidence that plasmacytoid DC require activation of mTOR to initiate IFN-α production downstream of TLR9 ligation by CpG, or in response to viral challenge.
- 73.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Luo H, Duguid W, Chen H, Maheu M, Wu J. The effect of rapamycin on T cell development in mice. Eur. J. Immunol. 1994;24:692–701. doi: 10.1002/eji.1830240331. [DOI] [PubMed] [Google Scholar]
- 75.Damoiseaux JG, Defresne MP, Reutelingsperger CP, Van Breda Vriesman PJ. Cyclosporin-A differentially affects apoptosis during in vivo rat thymocyte maturation. Scand. J. Immunol. 2002;56:353–360. doi: 10.1046/j.1365-3083.2002.01110.x. [DOI] [PubMed] [Google Scholar]
- 76.Coenen JJ, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 2007;39:537–545. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 77.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008;205:565–574. doi: 10.1084/jem.20071477. The authors demonstrate that the AKT-mTOR signaling pathway dominantly regulates TGFβ-dependent FOXP3 induction in peripheral CD4+ T cells.
- 78.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nature Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 79.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin. Immunol. 2007;19:162–172. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nature Immunol. 2007;8:64–73. doi: 10.1038/ni1413. This reference demonstrates mTOR involvement in a new complex, the activity of which is influenced by IL-2-mediated signaling, and that is necessary for the G1-S transition of the cell cycle.
- 81.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nature Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilbert KM, Weigle WO. Th1 cell anergy and blockade in G1a phase of the cell cycle. J. Immunol. 1993;151:1245–1254. [PubMed] [Google Scholar]
- 83.Boussiotis VA, et al. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 84.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int. Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 85.Allen A, et al. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J. Immunol. 2004;172:4797–4803. doi: 10.4049/jimmunol.172.8.4797. [DOI] [PubMed] [Google Scholar]
- 86.Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J. Immunol. 2006;176:2730–2738. doi: 10.4049/jimmunol.176.5.2730. [DOI] [PubMed] [Google Scholar]
- 87.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J. Exp. Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nature Rev. Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 89.Battaglia M, et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. The first report of the reduced susceptibility of TReg to the anti-proliferative effect of rapamycin.
- 90.Zeiser R, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018–1023. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 92.Noris M, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J. Am. Soc. Nephrol. 2007;18:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 93.Segundo DS, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82:550–557. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 94.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 95.Strauss L, et al. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J. Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 96.Keever-Taylor CA, et al. Rapamycin enriches for CD4+ CD25+ CD27+ Foxp3+ regulatory T cells in ex vivo-expanded CD25-enriched products from healthy donors and patients with multiple sclerosis. Cytotherapy. 2007;9:144–157. doi: 10.1080/14653240601145223. [DOI] [PubMed] [Google Scholar]
- 97.Valmori D, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 98.Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and Ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J. Immunol. 2006;177:2186–2194. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- 99.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 100.Zeiser R, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walsh PT, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett. 2007;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeiser R, Negrin RS. Interleukin-2 receptor downstream events in regulatory T cells: implications for the choice of immunosuppressive drug therapy. Cell Cycle. 2008;7:458–462. doi: 10.4161/cc.7.4.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J. Immunol. 2008;180:5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol. Rev. 2006;212:163–169. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 106.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 107.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. E.M.B.O. J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. These references (106 and 108) provide complementary insight into the molecular mechanisms by which TCR signaling regulates expression of FOXP3 in peripheral T cells.
- 109.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burchill MA, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Passerini L, et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int. Immunol. 2008;20:421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 112.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nature Med. 2007;13:1299–1307. doi: 10.1038/nm1652. This report demonstrates that combined pharmacologic interference with mTOR signalling and with the epigenetic control of FOXP3 expression (histones acetylation), promotes its up-regulation in peripheral T cells.
- 113.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 114.Li XC, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nature Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 115.Li Y, et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Zheng XX, Li XC, Zand MS, Strom TB. Combined costimulation blockade plus rapamycin but not cyclosporine produces permanent engraftment. Transplantation. 1998;66:1387–1388. doi: 10.1097/00007890-199811270-00021. [DOI] [PubMed] [Google Scholar]
- 117.Blaha P, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101:2886–2893. doi: 10.1182/blood-2002-10-3014. References 115 and 117 demonstrate how mTOR inhibition, unlike calcineurin inhibition, does not block the induction of experimental transplant tolerance.
- 118.Gao W, et al. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Battaglia M, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–49. [PubMed] [Google Scholar]
- 120.Uppaluri R, et al. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation. 2008;86:137–147. doi: 10.1097/TP.0b013e31817b8e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ueno T, et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J. Clin. Invest. 2008;118:742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Antin JH, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102:1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 123.Cutler C, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raimondi G, et al. Induction of heart transplant tolerance by post-transplant administration of allo-Ag apecific Treg and rapamycin. Am. J. Transplant. 2008;8:249. Abstract #266. [Google Scholar]
- 125.Carlson RP, Hartman DA, Ochalski SJ, Zimmerman JL, Glaser KB. Sirolimus (rapamycin, Rapamune) and combination therapy with cyclosporin A in the rat developing adjuvant arthritis model: correlation with blood levels and the effects of different oral formulations. Inflamm. Res. 1998;47:339–344. doi: 10.1007/s000110050339. [DOI] [PubMed] [Google Scholar]
- 126.Battaglia M, et al. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes. 2006;55:1571–1580. doi: 10.2337/db05-1576. [DOI] [PubMed] [Google Scholar]
- 127.Valle A, et al. Rapamycin prevents and breaks the anti-CD3-induced tolerance in NOD mice. Diabetes. 2009 doi: 10.2337/db08-1432. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Monti P, et al. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes. 2008;57:2341–2347. doi: 10.2337/db08-0138. This paper shows that rapamycin monotherapy in patients with autoimmune diabetes improves the function of naturally-occurring TReg cells
- 129.Ramos-Barron A, et al. Prevention of murine lupus disease in (NZBxNZW)F1 mice by sirolimus treatment. Lupus. 2007;16:775–781. doi: 10.1177/0961203307081401. [DOI] [PubMed] [Google Scholar]
- 130.Lui SL, et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol. Dial. Transplant. 2008;23:2768–2776. doi: 10.1093/ndt/gfn216. [DOI] [PubMed] [Google Scholar]
- 131.Montano Loza AJ, Czaja AJ. Current therapy for autoimmune hepatitis. Nature Clin. Pract. Gastroenterol. Hepatol. 2007;4:202–214. doi: 10.1038/ncpgasthep0768. [DOI] [PubMed] [Google Scholar]
- 132.Massey DC, Bredin F, Parkes M. Use of sirolimus (rapamycin) to treat refractory Crohn’s disease. Gut. 2008;57:1294–1296. doi: 10.1136/gut.2008.157297. [DOI] [PubMed] [Google Scholar]
- 133.Yong PL, Russo P, Sullivan KE. Use of sirolimus in IPEX and IPEX-like children. J. Clin. Immunol. 2008;28:581–587. doi: 10.1007/s10875-008-9196-1. [DOI] [PubMed] [Google Scholar]
- 134.Dames SA, Mulet JM, Rathgeb-Szabo K, Hall MN, Grzesiek S. The solution structure of the FATC domain of the protein kinase target of rapamycin suggests a role for redox-dependent structural and cellular stability. J. Biol. Chem. 2005;280:20558–20564. doi: 10.1074/jbc.M501116200. [DOI] [PubMed] [Google Scholar]
- 135.Kim DH, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 136.Chen EJ, Kaiser CA. LST8 negatively regulates amino acid biosynthesis as a component of the TOR pathway. J. Cell. Biol. 2003;161:333–347. doi: 10.1083/jcb.200210141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frias MA, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 138.Donahue AC, Fruman DA. Proliferation and survival of activated B cells requires sustained antigen receptor engagement and phosphoinositide 3-kinase activation. J. Immunol. 2003;170:5851–5860. doi: 10.4049/jimmunol.170.12.5851. [DOI] [PubMed] [Google Scholar]
- 139.Li HL, Davis W, Pure E. Suboptimal cross-linking of antigen receptor induces Syk-dependent activation of p70S6 kinase through protein kinase C and phosphoinositol 3-kinase. J. Biol. Chem. 1999;274:9812–9820. doi: 10.1074/jbc.274.14.9812. [DOI] [PubMed] [Google Scholar]
- 140.Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur. J. Immunol. 2007;37:2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- 141.Sakata A, Kuwahara K, Ohmura T, Inui S, Sakaguchi N. Involvement of a rapamycin-sensitive pathway in CD40-mediated activation of murine B cells in vitro. Immunol. Lett. 1999;68:301–309. doi: 10.1016/s0165-2478(99)00053-x. [DOI] [PubMed] [Google Scholar]
- 142.Heidt S, et al. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation. 2008;86:1292–1300. doi: 10.1097/TP.0b013e3181874a36. [DOI] [PubMed] [Google Scholar]
- 143.Aagaard-Tillery KM, Jelinek DF. Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell. Immunol. 1994;156:493–507. doi: 10.1006/cimm.1994.1193. [DOI] [PubMed] [Google Scholar]
- 144.Hornung N, Raskova J, Raska K, Jr., Degiannis D. Responsiveness of preactivated B cells to IL-2 and IL-6. Effect of cyclosporine and rapamycin. Transplantation. 1993;56:985–990. doi: 10.1097/00007890-199310000-00039. [DOI] [PubMed] [Google Scholar]
- 145.Wai LE, Fujiki M, Takeda S, Martinez OM, Krams SM. Rapamycin, but not cyclosporine or FK506, alters natural killer cell function. Transplantation. 2008;85:145–149. doi: 10.1097/01.tp.0000296817.28053.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gourlay WA, Chambers WH, Monaco AP, Maki T. Importance of natural killer cells in the rejection of hamster skin xenografts. Transplantation. 1998;65:727–734. doi: 10.1097/00007890-199803150-00021. [DOI] [PubMed] [Google Scholar]
- 147.Gomez-Cambronero J. Rapamycin inhibits GM-CSF-induced neutrophil migration. FEBS Lett. 2003;550:94–100. doi: 10.1016/s0014-5793(03)00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lehman JA, Calvo V, Gomez-Cambronero J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J. Biol. Chem. 2003;278:28130–28138. doi: 10.1074/jbc.M300376200. [DOI] [PubMed] [Google Scholar]
- 149.Lorne E, et al. Participation of mTOR Complex 1 in TLR2 and TLR4 Induced Neutrophil Activation and Acute Lung Injury. Am. J. Respir. Cell. Mol. Biol. 2009 doi: 10.1165/rcmb.2008-0290OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de Paulis A, et al. Characterization of the anti-inflammatory effect of FK-506 on human mast cells. J. Immunol. 1991;147:4278–4285. [PubMed] [Google Scholar]