Abstract
A number of mammalian antimicrobial proteins produced by neutrophils and cells of epithelial origin have chemotactic and activating effects on host cells, including cells of the immune system. Eosinophil granules contain an antimicrobial protein known as eosinophil-derived neurotoxin (EDN), which belongs to the RNase A superfamily. EDN has antiviral and chemotactic activities in vitro. In this study, we show that EDN, and to a lesser extent human pancreatic RNase (hPR), another RNase A superfamily member, activates human dendritic cells (DCs), leading to the production of a variety of inflammatory cytokines, chemokines, growth factors, and soluble receptors. Human angiogenin, a RNase evolutionarily more distant to EDN and hPR, did not display such activating effects. Additionally, EDN and hPR also induced phenotypic and functional maturation DCs. These RNases were as efficacious as TNF-α, but induced a different set of cytokine mediators. Furthermore, EDN production by human macrophages could be induced by proinflammatory stimuli. The results reveal the DC-activating activity of EDN and hPR and suggest that they are likely participants of inflammatory and immune responses. A number of endogenous mediators in addition to EDN have been reported to have both chemotactic and activating effects on APCs, and can thus amplify innate and Ag-specific immune responses to danger signals. We therefore propose these mediators be considered as endogenous multifunctional immune alarmins.
Mammalian antimicrobial proteins are important for innate host defense (1). Many of these proteins also have stimulating effects on cells of the immune system (2, 3). Human antimicrobial proteins stored in neutrophil granules, such as α-defensins and cathelicidins, are chemotactic for distinct subsets of leukocytes (4–7). The β-defensins, predominantly produced by epithelial cells and keratinocytes, act as chemoattractants for immature dendritic cells (iDCs),3 memory T cells, mast cells, and macrophages (8–12). Additionally, defensins and cathelicidins activate diverse types of leukocytes and epithelial cells, resulting in degranulation, and release of various mediators including cytokines and chemokines (13–16). In murine experimental models, neutrophil granule-derived α-defensins and epithelial cell-derived β-defensins have been demonstrated to enhance Ag-specific immune responses when administered together with Ags or as Ag-defensin fusion conjugates (9, 16, 17).
Human eosinophil granules contain four major cationic proteins, two of which, namely eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP), are members of the RNase A superfamily. Based on their evolutionary relationship, the RNase A superfamily has been classified into five subfamilies: the eosinophil-associated RNases (EARs), including human EDN and human ECP, the pancreatic-type RNases (PRs), the RNase 4s, the angiogenins (ANGs), and the RNases from the bullfrog Rana, including onconase (18). They are characterized by a specific disulfide-bond structure that is crucial for the relative positioning of amino acid residues contributing to the catalytic site, and they all function to varying degrees in endo- and/or exonucleolytic cleavage of polymeric RNA (18). In addition to their common RNase activity, members of the RNase A superfamily have been reported to have diverse biologic actions, including antitumor, antimicrobial, and angiogenic properties (18–20).
Of the two human EARs, EDN is less cationic (pI ≈ 8) than ECP and has no cellular toxicity, except for that observed against rabbit Purkinje cells, which accounts for its unusual name (18, 21). EDN was cloned in 1989 (22), and its x-ray crystallographic structure was elucidated in 1996 (23). EDN was shown to have in vitro antiviral activity against respiratory syncytial virus in 1998 (24), thus marking EDN as a host gene-encoded antimicrobial protein. In addition, EDN has also been shown to be responsible in part for the anti-HIV-1 activity found in the supernatant of MLCs (25).
Very recently, we have shown that EDN functions as a selective chemoattractant for dendritic cells (DCs) (26). Because certain mammalian antimicrobial proteins that are chemotactic for leukocytes can induce the activation of leukocytes (2, 3, 9, 16), the initial aim of this study was to examine whether a variety of DC chemotactic antimicrobial proteins, including EDN, could serve as activators for DCs. Using a very sensitive Ab-based microarray capable of quantifying numerous soluble mediators (including cytokines, chemokines, growth factors, and soluble receptors) (27), we found that, among several chemotactic antimicrobial proteins tested, EDN was as capable as TNF-α in inducing soluble mediator production by DCs. To our surprise, human PR (hPR) (intended as a negative control for EDN) was also active in this respect. EDN and hPR not only stimulated the production of many soluble mediators by DCs, but were also capable of inducing the maturation of DCs. EDN is not only produced by eosinophils, its expression by macrophages was induced by a combination of TNF-α and LPS. These results demonstrate that EDN and hPR, two closely related RNase A superfamily members, can act as endogenous activators of Ag-presenting DCs.
Materials and Methods
Reagents
Recombinant human TNF-α, GM-CSF, M-CSF, IL-4, Flt3-L, stem cell factor, thrombopoietin, RANTES, and CCL21/secondary lymphoid tissue chemokine (SLC) were purchased from PeproTech (Rocky Hill, NJ). FITC-conjugated goat anti-mouse IgG Ab, BSA, and LPS (Escherichia coli, serotype O55:B5) were from Sigma-Aldrich (St. Louis, MO). [3H]TdR (specific radioactivity = 2 Ci/mmol) was purchased from NEN (Boston, MA). Human ANG (hANG) was purchased from R&D Systems (Minneapolis, MN). IMDM was purchased from Invitrogen Life Technologies (Rockville, MD). RPMI 1640, glutamine, penicillin, streptomycin, and HEPES were from BioWhittaker (Walkersville, MD). FBS was purchased from HyClone (Logan, UT). mAbs against human CD11c, CD14, CD40, CD83, CD86, and HLA-DR and isotype-matched control Abs were purchased from BD Pharmingen (San Diego, CA).
The genes coding for human EDN or hPR were cloned into the vector, pET-11d (Novagen, Madison, WI) (28), and expressed in BL21 (DE3) E. coli cells (Novagen), as described (29). Recombinant proteins were isolated from inclusion bodies, denatured, renatured, and dialyzed against 20 mM Tris-HCl, pH 7.5, containing100 mM urea before application to a heparin-Sepharose column (Amersham Biosciences, Piscataway, NJ) for human rEDN or a chemotaxis medium (CM)-Sephadex C-50 column (Amersham Biosciences) for rhPR using 2 ml of the respective resin per liter of dialyzed protein. Following elution from the respective columns with 20 mM Tris-HCl, pH 7.5, containing 10% glycerol and 0.5 M NaCl, the proteins were dialyzed vs 20 mM Tris-HCl, pH 7.5, 5% glycerol, lyophilized, resuspended in H2O, and applied to a Sephadex G100 column (Amersham Biosciences) equilibrated and eluted with 20 mM Tris-HCl, pH 7.5, containing 10% glycerol. Fractions containing the proteins of interest were pooled, dialyzed, lyophilized, and stored as a dry powder at −20°C. Natural human EDN was purified from commercial preparations of human urinary gonadotrophin, as described (30). Briefly, resuspended urinary gonadotrophin preparations were dialyzed extensively at 4°C against 50 mM Tris (pH 8.0) + 1 mM NaCl, and were then fractionated by heparin-Sepharose column chromatography (Amersham Biosciences) using a salt-gradient (1 mM to 1 M NaCl in 50 mM Tris, pH 8.0) for the elution. The fractions containing EDN were pooled, concentrated (Centricon 10; Millipore, Beverly, MA), and subjected to purification by Superdex 200 (Amersham Biosciences) chromatography. The concentration of purified EDN and recombinant proteins was determined by bicinchoninic acid assay (Pierce, Rockford, IL).
Limulus amebocyte lysate assay
The endotoxin level in the various RNase samples was measured by the use of Limulus Amebocyte Lysate Pyrogent test kit (BioWhittaker) following the manufacturer’s protocol. The sensitivity of the test kit for endotoxin was 0.06 IU/ml.
Isolation, purification, and preparation of cells
Human PBMC were isolated by Ficoll density gradient centrifugation from leukopacks supplied by the Department of Transfusion Medicine (Clinical Center, National Institutes of Health, Bethesda, MD). Monocytes were purified (>95%) from human PBMC with MACS CD14 monocyte isolation kit (Miltenyi Biotec, Auburn, CA). Human cord blood CD34+ progenitors (>90%) were purchased from Cambrex (Walkersville, MD). The DC precursors were amplified from CD34+ hemopoietic progenitor cells by culturing the cells at 5 × 104 cells/ml in IMDM supplemented with 20% FBS, 10−5 M DTT, 25 ng/ml Flt3-L, 10 ng/ml TPO, and 20 ng/ml stem cell factor for 4 wk (31). The amplified DC precursors were cryopreserved in IMDM containing 20% FBS and 10% DMSO for later usage.
Macrophages were generated by incubating purified human peripheral blood monocytes (1 × 106/ml) in the presence of M-CSF, as described previously (32). Macrophages were treated in the absence or presence of TNF-α (100 ng/ml) and LPS (100 ng/ml) for a period of time, as specified.
DC culture
DCs were generated, as described previously (31, 32). In brief, monocyte-derived iDCs were generated by incubating purified monocytes at 1 × 106/ml in G4 medium (RPMI 1640 containing 10% FBS, 2 mM glutamine, 25 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 ng/ml GM-CSF, and 50 ng/ml IL-4) at 37°C in a CO2 (5%) incubator for 7 days. To generate CD34+ hemopoietic progenitor cell-derived iDCs (CD34-iDCs), the amplified DC precursors were cultured in G4 medium for 48 h at 37°C in a CO2 (5%) incubator. All of the cultures were fed with the same cytokine-containing medium every 2–3 days.
To examine the effect of various RNase samples on DCs, iDCs were suspended at 1 × 106/ml in G4 medium alone or in the presence of various samples at concentrations as specified, and incubated at 37°C in a CO2 (5%) incubator for indicated periods of time. Subsequently, the culture supernatants and/or the cells were collected and analyzed.
ELISA
IL-6, IL-12p70, TNF-α, IFN-γ-inducible protein-10 (IP-10), RANTES, MCP-2, and MIP-1α in the culture supernatants were measured by Pierce using MultiPlex ELISA kits. The concentrations of IL-6, TNF-α, and MIP-1α in some culture supernatants were measured using ELISA kits purchased from R&D Systems strictly following the protocols provided by the manufacturer.
Microarray manufacture and rolling-circle amplification (RCA) immunoassay
Ab microarrays were printed using a bicinchoninic acid-II piezoelectric microarray dispenser (Packard Biosciences, Downers Grove, IL) on cyanosilane-coated glass slides divided by Teflon boundaries into sixteen 0.5-cm-diameter circular subarrays. mAbs for 78 analytes were dispensed in quadruplicate at a concentration of 0.5 mg/ml. Printed slides were blocked, as described previously (27), and stored at 4°C until use. Batches of slides were subjected to a quality control consisting of incubation with a fluorescent-labeled anti-mouse Ab, followed by washing, scanning, and quantitation. Typically, the coefficient of variability of Ab deposition in printing was <5%.
The RCA immunoassay was performed by a liquid-handling Biomek 2000 robot (Beckman Coulter, Fullerton, CA), which was enclosed in 80% humidified, high efficiency particulate air-filtered, plexiglass chamber. For each sample, duplicates were tested either undiluted or diluted at 1/10. A total of 20 μl of samples was applied to each subarray, and RCA immunoassay was performed, as previously described (27). Slides were scanned by the use of GenePix (Axon Instruments, Foster City, CA) at 10 μm resolution with a laser setting of 100 and photomultiplier tube setting of 550. Mean pixel fluorescence was quantified using the fixed circle method in GenePix Pro 3.0 (Axon Instruments). The fluorescence intensity of eight microarray features (duplicate subarrays and quadruplicate spots in each subarray) was averaged for each feature and sample, and the resulting cytokine values as Cy5 intensity were determined. For each slide, a set of blanks was run as a negative control. Analyses were performed using the levels of all 78 analytes (cytokines, chemokines, growth factors, and soluble receptors) measured in relative fluorescent intensity units or by fold increase for the purpose of comparison. Sensitivity of RCA immunoassay was femtomolar (27, 33): 46 (60%) of the 78 analytes had a sensitivity of ≤10 pg/ml, 24 (30.8%) had a sensitivity of ≤100 pg/ml, 5 (6.4%) had a sensitivity of ≤1 ng/ml, and 3 (3.8%) had a sensitivity of ≤10 ng/ml. The dynamic range (~3 orders of magnitude) and precision of RCA immunoassay were similar to unamplified immunoassays (27, 33).
RNA extraction and RT-PCR
Total RNA was isolated from macrophages by the use of TRIzol reagent (Invitrogen Life Technologies), according to the manufacturer’s recommendation. The RNAs were treated with RNase-free DNase I (Stratagene, La Jolla, CA) at 37°C for 30 min to eliminate possible genomic DNA contamination and purified by phenol:chloroform extraction and ethanol precipitation. The concentration and purity of RNA samples were examined by measuring their absorbance at 260 and 280 nm, and the integrity of RNA samples was confirmed by agarose gel electrophoresis. All of the RNA samples used were pure (A260/280 > 1.8) and without degradation. The EDN mRNA expression by macrophages was measured by RT-PCR by the use of GeneAmp kit (Invitrogen Life Technologies). Briefly, total RNA was reverse transcribed in a volume of 20 μl. Subsequently, the reverse-transcription product was divided in half and used for the amplification of EDN or GAPDH by 35 cycles of PCR (denature at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min) with the last extension being performed at 72°C for 10 min. The sense and antisense primers for EDN were 5′-TCTCACAGGAGCTACAGCGCG-3′ (nt 326~346, exon 1) and 5′-AACATGTTTGCTGGTGTCTGC-3′ (nt 967~987, exon 2), which enabled the amplification of a 420-bp EDN-specific cDNA fragment. The primers for human GAPDH were 5′-GATGACATCAAGAAG GTGGTGAA-3′ and 5′-GTCTTACTCCTTGGAGGCCATGT-3′, which resulted in the amplification of a fragment of 246 bp. PCR products were identified on 1.8% agarose gel, stained with ethidium bromide, and photodocumented after washing off excessive dye with water.
Flow cytometry
DCs (106/sample) were first washed three times with FACS buffer (PBS, 1% FBS, 0.02% NaN3, pH 7.4), and then stained with mouse mAbs against human CD14, CD40, CD83, CD86, HLA-DR, or isotype-matched control Ab (final concentration of 5 μg/ml) at room temperature for 30 min. After washing three times with FACS buffer, the cells were suspended in FACS buffer containing FITC-conjugated goat anti-mouse IgG for 30 min at room temperature. Finally, the stained cells were washed twice with FACS buffer, twice with PBS, fixed with 1% paraformaldehyde in PBS, and analyzed the next day with a flow cytometer (Coulter Epics, Miami, FL).
Chemotaxis assay
Cell migration was assessed using a 48-well microchemotaxis chamber (32). The cells were washed three times and resuspended in CM (RPMI 1640 containing 1% BSA). RANTES and SLC diluted with CM were placed in wells of the lower compartment of the chamber (NeuroProbe, Cabin John, MA), and DCs suspended in CM at 1 × 106 cells/ml were added into wells of the upper compartment. The lower and upper compartments were separated by a 5-μm polycarbonate filter (Osmonics, Livermore, CA). After incubation at 37°C for 1.5 h in humidified air with 5% CO2, the filters were removed, scraped, and stained. The cells migrated across the filter were counted with the use of a Bioquant semiautomatic counting system (Nashville, TN) and presented as the number of cells per high-power field.
Mixed leukocyte reaction
Allogeneic MLR was performed, as described (34). Briefly, purified allogeneic T cells (105/well) were cultured with different numbers of DCs in a 96-well flat-bottom plate for 7 days at 37°C in humidified air with 5% CO2. The proliferative response of T cells was examined by pulsing the culture with [3H]TdR (0.5 μCi/well) for the last 8 h before harvesting. [3H]TdR incorporation was measured with a microbeta counter (PerkinElmer Wallac, Gaithersburg, MD).
SDS-PAGE and Western blot
Each 20 μg of proteins from the culture supernatants of macrophages treated with TNF-α and LPS for indicated period of time was mixed with 4× SDS-PAGE sample buffer to achieve a final concentration of 62.5 mM Tris-HCl (pH 6.8 at 25°C), 2% w/v SDS, 10% glycerol, 50 mM dithiothretol, and 0.01% bromphenol blue in a final volume of 20 ml. The samples were boiled for 5 min, cooled on ice, loaded (20 μl/lane), and separated on a 4–12% NuPAGE bis-Tris gel (Invitrogen Life Technologies). SeeBlue Plus2 (Invitrogen Life Technologies) was used as molecular size marker. After the electrophoresis, proteins in the gel were electrotransferred (30 V constant for 1 h) onto a piece of Immobilon membrane (Millipore) with a Xcell II Blot Module (NOVEX, San Diego, CA) using 1× NuPAGE transfer buffer (12 mM Tris, 96 mM glycine, pH 8.3, 0.1% v/v antioxidant, 10% v/v methanol). The Western blot was conducted at room temperature, unless specified otherwise. The polyvinylidene difluoride membrane was sequentially washed with 25 ml of 1× TBS for 5 min, incubated for 1 h in 25 ml of blocking buffer (1× TBS, 0.1% Tween 20, 5% w/v nonfat milk, 5 μg/ml human IgG, and 10% FCS), washed three times for 5 min each with TBST (1× TBS, 0.1% Tween 20), and incubated at 4°C overnight in blocking buffer containing 1/1000 dilution of rabbit anti-EDN polyclonal Ab (Assay Research, College Park, MD). On the next day, the membrane was washed three times for 5 min each with 15 ml of TBST and incubated with 10 ml of 1/2000 dilution of HPR-conjugated anti-rabbit IgG (Cell Signaling Technology, Beverly, MA; catalogue 7074) for 1 h. After washing five times for 5 min each with 15 ml of TBST, the membrane was incubated with 10 ml of working solution of ECL Plus Detection Reagents (Amersham, Piscataway, NJ) for 5 min, immediately wrapped with Saran wrap, and exposed to a piece of BioMax x-ray film (Kodak, Rochester, NY) for 5 s. The x-ray film was developed using an automatic processor (Kodak X0OMAT 200A).
Statistical analysis
All experiments were performed 2~3 times, and the data of one representative experiment are shown. The statistical significance of difference between groups was analyzed using paired Student’s t test.
Results
EDN and hPR induce CD34+ progenitor-derived iDCs to produce inflammatory mediators
Previously, we have shown that EDN is selectively chemotactic for both human and mouse DCs (26). To evaluate whether EDN could activate DCs, a number of DC chemotactic factors, including several antimicrobial proteins, were screened for their capacity to stimulate the production of a dozen prominent inflammatory cytokines and chemokines by human CD34+ progenitor-derived iDCs. Of the DC chemoattractants, EDN treatment of CD34+ progenitor-derived DCs for 16 h resulted in the induction of all of the cytokines and chemokines measured by 2- to 36-fold (Fig. 1). In these experiments, hPR was intended to be a negative control because both the chemotactic and antiviral activities of EDN were not shared by hPR (24–26). To our surprise, hPR also induced the production of a similar set of inflammatory cytokines and chemokines (Fig. 1). Other DC chemoattractants, including murine EAR2 (mEAR2), showed negligible effects on the production of 12 inflammatory cytokines and chemokines by CD34+ progenitor-derived DCs (Fig. 1 and data not shown).
FIGURE 1.
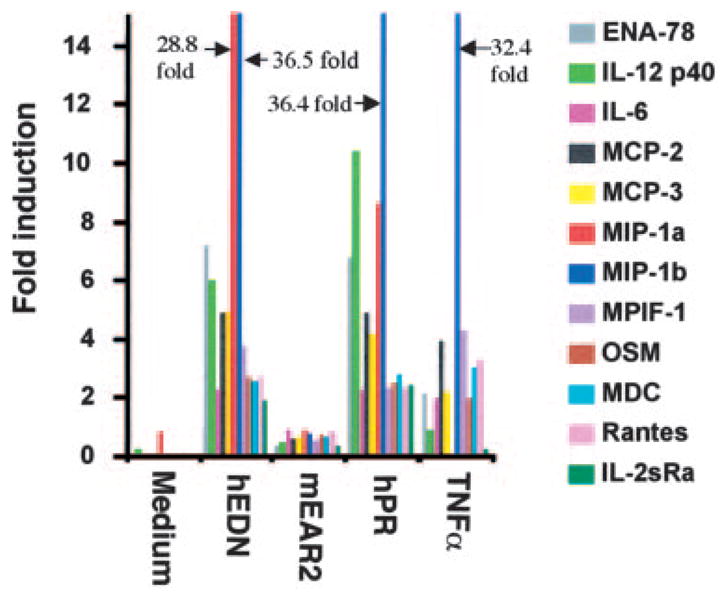
EDN and hPR induction of soluble mediator production by DCs. CD34+ progenitor-derived iDCs (106/ml) were cultured in G4 medium alone or in the presence of EDN (1 μg/ml), mEAR2 (1 μg/ml), hPR (1 μg/ml), or TNF-α (100 ng/ml) for 16 h at 37°C in humidified air containing 5% CO2. A dozen of soluble mediators in the culture supernatants was measured by RCA immunoassay, as described in Materials and Methods. The results are shown as fold induction, which, for a given soluble mediator, was calculated by the formula: amount produced by DCs in the presence of a stimulant/amount produced by DCs in G4 medium alone. IL-2sRa: soluble IL-2R α-chain.
EDN and hPR stimulated the production of various mediators by both CD34+ progenitor-derived and monocyte-derived DCs
To gain a thorough view of DC-produced soluble mediators induced by EDN and hPR, we investigated the soluble mediators that EDN and hPR stimulated in human DCs using Ab-based microarrays capable of simultaneously measuring 78 different soluble mediators. Table I summarizes the induction of soluble mediators by EDN or hPR from both CD34+ progenitor-derived and monocyte-derived iDCs when treated for 48 h. EDN and hPR treatment of CD34+ progenitor-derived iDCs induced ≥3-fold increased secretion of 17, and 12 of the 78 soluble mediators, respectively. Monocyte-derived iDCs produced 15 soluble mediators in response to EDN, while 7 of 78 were induced by hPR (with ≥3-fold increase). Several characteristics stand out. 1) EDN and hPR preferentially induced mostly proinflammatory mediators. 2) The soluble mediators induced by hPR largely overlapped with those induced by EDN; however, hPR appears to be less efficacious than EDN in stimulating soluble mediator production given the fact that molecular mass of EDN (≈16 kDa) is slightly larger than that of hPR (≈14 kDa). 3) The profile of soluble mediator induction of CD34+ progenitor-derived iDCs was similar to that of monocyte-derived iDCs in response to EDN or hPR, with the exception of three chemokines. CD34+ progenitor-derived iDCs up-regulated epithelial-derived neutrophil-activating protein (ENA) 78, and MCP-3, but not MIP-1β, whereas monocyte-derived iDCs showed the reverse. This difference in the production of ENA-78, MCP-3, and MIP-1β by CD34+ progenitor-derived vs monocyte-derived DCs most likely reflects one of a few distinctive features between these two populations of DCs (31, 35, 36).
Table I.
Comparison of mediator production by CD34+ cell-derived and monocyte-derived DCs in response to EDN or hPRa
| CD34+ Cell-Derived DCs |
Monocyte-Derived DCs |
|||
|---|---|---|---|---|
| Mediatorsb | EDN | hPR | EDN | hPR |
| ENA-78 | 91 | 26 | 2 | 1 |
| Eot2 | 1 | 1 | 3 | 3 |
| I-309 | 4 | 3 | 4 | 1 |
| IFN-α | 1 | 1 | 5 | 0 |
| IL-10 | 1 | 2 | 6 | 0 |
| IL-12p40 | 32 | 4 | 3 | 1 |
| IL-12p70 | 7 | 2 | 2 | 1 |
| IL-13 | 1 | 2 | 3 | 1 |
| IL-16 | 1 | 1 | 0 | 3 |
| IL-6 | 182 | 74 | 181 | 15 |
| IP-10 | 74 | 10 | 67 | 6 |
| MCP-1 | 4 | 4 | 2 | 1 |
| MCP-2 | 26 | 8 | 18 | 1 |
| MCP-3 | 97 | 18 | 1 | 1 |
| M-CSF | 5 | 2 | 1 | 1 |
| MIG | 4 | 2 | 6 | 1 |
| MIP-1α | 14 | 7 | 10 | 2 |
| MIP-1β | 2 | 2 | 61 | 19 |
| MPIF-1 | 3 | 2 | 6 | 6 |
| NAP-2 | 4 | 2 | 2 | 1 |
| RANTES | 22 | 12 | 44 | 7 |
| sCD23 | 2 | 3 | 1 | 1 |
| TNF-α | 25 | 5 | 10 | 1 |
| sTNF-RI | 3 | 2 | 1 | 2 |
CD34+ cell-derived and monocyte-derived iDCs were cultured at 37°C in humidified air containing 5% CO2 in G4 medium alone or G4 medium plus EDN or hPR (both at 1 μg/ml) for 48 h. The culture supernatants were collected and stored at −70°C. The level of cytokines, chemokines, and soluble receptors (a total of 78) in each supernatant was measured by RCA immunoassay and presented as fold increase, which was calculated by the following formula: fold increase = Cy5 fluorescence in the presence of EDN or human pancreatic RNase/Cy5 fluorescence in the absence of EDN or hPR. Only those with fold increase ≥3 by EDN or hPR are shown.
MPIF, Myeloid progenitor inhibitory factor; NAP, neutrophil-activating protein; s, soluble.
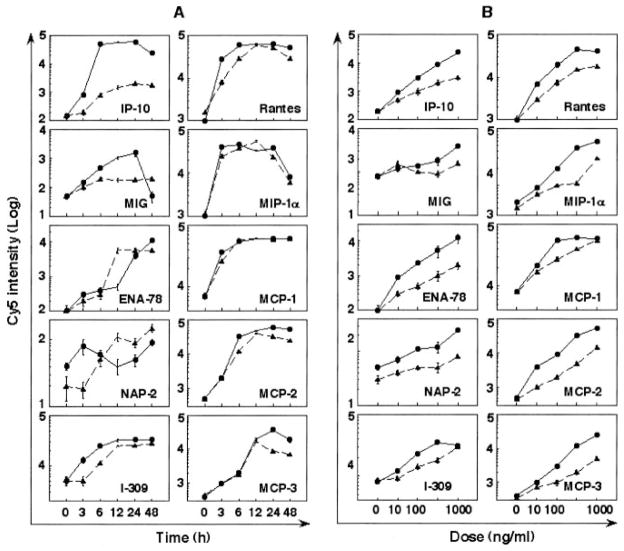
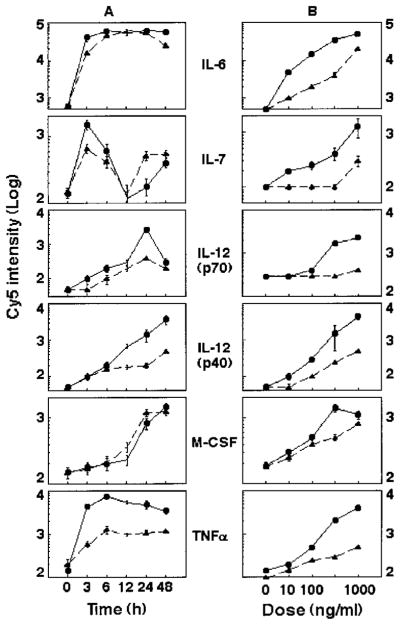
To further characterize the capacity of EDN and hPR to induce soluble mediator production by DCs, we investigated their dose-and time-dependent effects. The time course and dose response of EDN- and hPR-induced production of 10 chemokines and 5 cytokines by CD34+ progenitor-derived DCs are shown in Figs. 2 and 3, respectively. The mediator induction was dose dependent and peaked at different times. For example, EDN induced MIP-1α, RANTES, IL-6, IP-10, and MCP-1, and TNF-α maximally at 3~6 h; monokine induced by IFN-γ (MIG), MCP-2, MCP-3, and IL-12 p70 at 24 h; and neutrophil-activating peptide-2, IL-12 p40, and M-CSF at 48 h (Figs. 2 and 3A). Although the induction of most cytokines and chemokines by EDN and hPR was similar, only EDN induced considerable production of MIG and IL-12p70 (Figs. 2 and 3), suggesting that EDN may favor Th1 polarization. Again, EDN was more efficacious than hPR in the induction of cytokine and chemokine production (Figs. 2 and 3). EDN- and hPR-induced mediator production by monocyte-derived DCs showed a similar dose dependence and kinetics (data not shown), indicating considerable similarity between the panel of mediators produced by the two DC populations generated in vitro in response to EDN and hPR.
FIGURE 2.
Chemokine production induced by EDN (●) and hPR (▲) from CD34+ progenitor-derived DCs. A, Time course; iDCs (106/ml) were cultured in G4 medium containing 1 μ g/ml EDN or hPR for various time points, as specified. B, Dose response; iDCs (106/ml) were cultured for 48 h in G4 medium in the absence or presence of various concentrations of EDN or hPR, as specified. The culture supernatants were collected and measured by RCA immunoassay, as described in Materials and Methods.
FIGURE 3.
Cytokine induction by EDN (●) and hPR (▲) from CD34+ progenitor-derived DCs. iDCs (106/ml) were cultured in G4 medium containing 1 μg/ml recombinant EDN or hPR for various times (A, time course) or for 48 h in G4 medium containing various concentrations of EDN or hPR (B, dose response). The culture supernatants were collected and measured by RCA immunoassay. Five cytokines induced by EDN or hPR are shown.
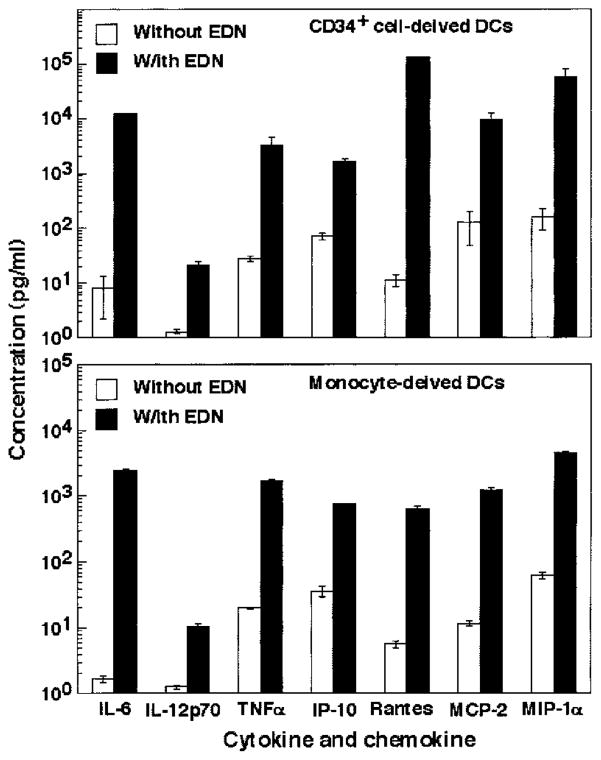
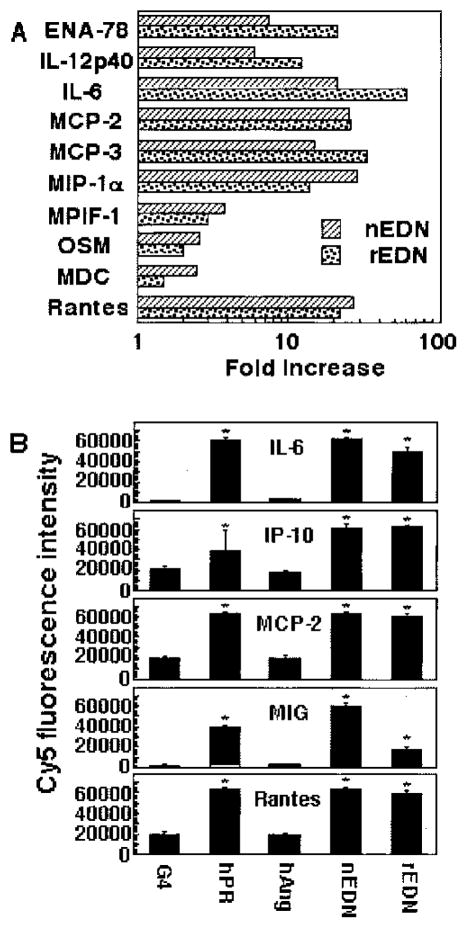
To both confirm and validate EDN induction of inflammatory cytokines and chemokines from DCs, DCs were generated from either CD34+ progenitors or monocytes of various healthy individuals, treated with or without EDN for 24 h, and the production of seven inflammatory mediators (which were shown to be induced by EDN using the Ab-based microarray immunoassay) in the culture supernatants was measured by ELISA (Fig. 4). Similar to the results obtained using the microarray (Figs. 2 and 3), EDN induced the production of IL-6, IL-12p70, TNF-α, IP-10, RANTES, MCP-2, and MIP-1α from both CD34+ progenitor-derived and monocyte-derived DCs, as measured by ELISA (Fig. 4), validating both the reliability of Ab-based microarray assay as well as authenticating stimulation of DC production of cytokines and chemokines by EDN.
FIGURE 4.
ELISA confirmation of EDN-stimulated DC production of cytokines and chemokines. iDCs generated from either bone marrow CD34+ progenitors (upper panel) or monocytes (lower panel) were cultured in triplicate in the absence (□) or presence (■) of EDN at 1 μg/ml for 48 h. The supernatants were harvested for performance of ELISA measurement on seven mediators, as indicated. Shown is the average (mean ± SD) of mediator production by CD34+ cell-derived DCs from two (upper panel) and monocyte-derived DC from four (lower panel) individuals.
Mediator induction by EDN and hPR was not due to endotoxin contamination
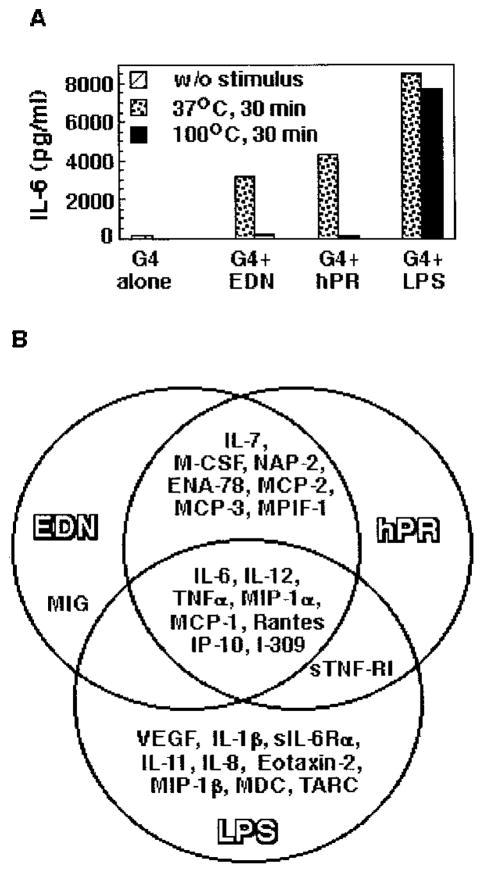
Because E. coli-derived EDN and hPR samples were used in most of the experiments, it was critical to determine whether the observed effect was due to possible endotoxin contamination. The endotoxin level in EDN and hPR samples used in the present study was below the detection limit of the Limulus amebocyte lysate assay, indicating <0.6 ng of endotoxin per mg of protein. The highest concentrations of EDN or hPR used in our experiments were 1 μg/ml, which could only contain <0.6 pg/ml endotoxin. In addition, heating samples for 30 min at 100°C destroyed the capacity of EDN or hPR to induce IL-6 production by DCs, while having no significant effect on the IL-6-inducing capacity of LPS (Fig. 5A). We further compared the spectra of mediators induced by EDN, hPR, and LPS from CD34+ progenitor-derived iDCs. Although EDN and hPR induced a similar battery of mediators by CD34+ progenitor-derived iDCs, LPS stimulates a different set of mediators, half of which were not induced by EDN and hPR (Fig. 5B). Therefore, the effect of EDN or hPR on iDCs was not due to endotoxin contamination.
FIGURE 5.
A, The effect of heating on the IL-6-inducing capacity of recombinant EDN or hPR. EDN, hPR, and LPS samples were placed at 37°C or heated at 100°C for 30 min, followed by rapid cooling down on ice before addition to CD34+ progenitor-derived iDCs (106/ml). The final concentration for EDN, hPR, and LPS was 1 μg/ml, 1 μg/ml, and 100 ng/ml, respectively. After incubation at 37°C for 24 h in humidified air containing 5% CO2, the supernatants were collected and measured by ELISA for IL-6 production. B, Comparison of production of mediators by CD34+ progenitor-derived iDCs cultured for 48 h in the presence of recombinant EDN (1 μg/ml), hPR (1 μg/ml), and LPS (100 ng/ml). Only those mediators increased by ≥3-fold over control levels were included.
Both natural and recombinant EDNs exhibit similar cytokine-inducing effects on DCs
Because rEDN generated in E. coli is not glycosylated as is natural EDN, we examined whether EDN purified from a natural source was also able to induce the production of cytokines and chemokines. When human CD34+ progenitor-derived iDCs were used, both natural and recombinant EDNs induced the production of similar amounts of 10 different cytokines (Fig. 6A). We also compared the effects of hANG, hPR, and natural and recombinant EDNs on the production of five cytokines by monocyte-derived iDCs (Fig. 6B). hPR and EDN (both natural and recombinant), albeit slightly varied in terms of extent, all induced statistically significant production of IL-6, IP-10, MCP-2, MIG, and RANTES. In contrast, hANG, although also belonging to the RNase A superfamily, did not induce cytokine and chemokine production, indicating that not all members of the RNase A superfamily share the capacity to induce cytokine production by DCs.
FIGURE 6.
Comparison of the production of selected cytokines and chemokines by DCs in response to various RNase samples. A, CD34+ progenitor-derived iDCs were incubated in G4 medium alone or in the presence of natural (nEDN) or rEDN at 1 μg/ml at 37°C in humidified air containing 5% CO2. After 48 h of culture, the supernatants were assayed for 10 selected cytokines and chemokines by RCA immunoassay. The induction of cytokines and chemokines is presented as fold increase, which was calculated by the following formula: fold increase = Cy5 fluorescence in the presence of EDN or human pancreatic RNase/Cy5 fluorescence in the absence of EDN or hPR. B, Monocyte-derived iDCs were incubated in triplicates in G4 medium alone or in the presence of hPR, hANG, and natural and recombinant EDNs (at 1 μg/ml) at 37°C for 48 h in humidified air containing 5% CO2. The production of IL-6 and IL-4 chemokines in the culture supernatants was measured by RCA immunoassay and shown as the average (mean ± SEM) Cy5 fluorescence intensity. *, p < 0.05 when compared with G4-treated group.
EDN and hPR induced phenotypic and functional maturation of DCs
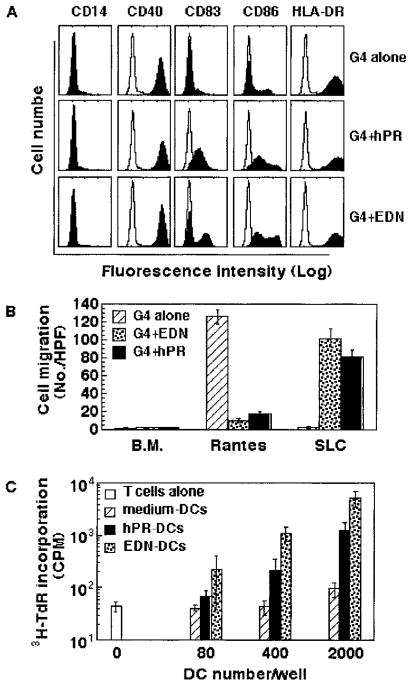
Activation of iDCs is usually accompanied by DC maturation. We therefore investigated whether treatment of iDCs with EDN or hPR could induce maturation of DCs. Monocyte-derived iDCs were incubated in G4 medium alone or in the presence of either EDN or hPR for 48 h and then investigated for their maturational status (Fig. 7). iDCs cultured in G4 medium alone for 48 h maintained an immature phenotype (CD14−, CD40+, CD83−, CD86low, HLA-DRhigh) (Fig. 7A, upper panel). In contrast, iDCs cultured in G4 plus hPR or EDN for 48 h, although still CD14−, up-regulated the surface expression of CD83, CD86, and HLA-DR, indicating that hPR and EDN treatment induced phenotypic maturation of DCs (Fig. 7A). In contrast to hPR, EDN-treated DCs appeared to generate two subsets of DCs differing in levels of surface CD83 and CD86 expression. This phenomenon was not due to donor variability because cells from three independent donors demonstrated identical results.
FIGURE 7.
Induction of DC maturation by hPR and EDN. Monocyte-derived iDCs were incubated in G4 medium alone or in the presence of recombinant hPR or EDNs (at 1 μg/ml) at 37°C for 48 in humidified air containing 5% CO2. The cells were collected and analyzed by flow cytometry, chemotaxis assay, or allogeneic MLR, as described in Materials and Methods. A, Surface expression of CD14, CD40, CD83, CD86, and HLA-DR by DCs cultured under conditions, as specified. B, Comparison of the chemotatic responses of DCs cultured in the absence or presence of 1 μg/ml EDN or hPR at 37°C for 48 h to optimal concentration (100 ng/ml) of RANTES or SLC. C, Stimulation of the proliferation of allogeneic human peripheral blood T lymphocytes by EDN- or hPR-treated DCs. T cells (105/well) were cultured in triplicate alone or with treated DCs at concentrations specified in 96-well plates for 7 days with the addition of [3H]TdR (0.5 μCi/well) in the last 8 h of incubation. The cells were harvested and measured for the incorporation of [3H]TdR (mean ± SD).
The capacity of hPR or EDN to induce DC maturation was also evidenced by a change in chemokine responsiveness of treated DCs (Fig. 7B). DCs cultured in G4 medium alone migrated in response to RANTES, but not to SLC, indicating expression of functional CCR1 and/or CCR5, but not functional CCR7. However, DCs cultured in G4 in the presence of EDN or hPR lost responsiveness to RANTES and acquired SLC-responsive CCR7. Because the down-regulation of a number of chemokine receptors (including CCR1 and CCR5) and up-regulation of functional CCR7 are another hallmark of DC maturation (36, 37), these results provide additional evidence that treatment with EDN or hPR induced the maturation of DCs.
To determine whether EDN- and hPR-induced maturation of DCs is also reflected at the functional level, we determined whether EDN- or hPR-treated DCs could stimulate the proliferation of allogeneic human peripheral blood T cells in an MLR assay. In contrast to medium-treated DCs that did not stimulate allogeneic T cells, both EDN- and hPR-treated DCs stimulated the proliferation of allogeneic T lymphocytes in a concentration-dependent manner, as judged by the increase of tritiated thymidine incorporation (Fig. 7C). Therefore, treatment of iDCs with either EDN or hPR induces both phenotypic and functional maturation of DCs.
EDN expression by macrophages is inducible
The activating effects of EDN on DCs suggest that EDN has cytokine-like activities. A common feature for all cytokines is inducibility, so we studied whether EDN expression can be up-regulated. Besides being stored in eosinophil granules, EDN is also expressed by neutrophils and macrophages (38, 39). To investigate whether the expression of EDN by macrophages is inducible, we treated human monocyte-derived macrophages with a combination of TNF-α and LPS (both at 100 ng/ml) and measured EDN expression at both mRNA and protein levels (Fig. 8). As shown by Fig. 8A, RT-PCR revealed that unstimulated macrophages displayed a faint band (second lane, upper panel), confirming the previous report (39). Treatment of macrophages with TNF-α plus LPS up-regulated the expression of EDN mRNA in a time-dependent manner. EDN mRNA level began to increase at 30 min, peaked at 2 h, and subsided gradually thereafter (Fig. 8A, upper panel). Amplification of GAPDH using the other half of reverse-transcription products as template under identical PCR conditions yielded GAPDH bands of nearly identical intensity, confirming that equal amounts of reverse-transcription products were used in the PCR for EDN amplification (lower panel). Furthermore, Western blot analysis (Fig. 8B) demonstrated that upon treatment by a combination of TNF-α and LPS for 2 h, macrophage began to secrete EDN into the culture supernatant, which peaked at 8 h. The EDN protein appeared as two bands of ~31 and 37 kDa, which were larger than the size of rEDN (17.5 kDa, without glycosylation). The appearance of two bands larger than the rEDN was due presumably to differential glycosylation of EDN because, using the same polyclonal antiserum, Rugeles et al. (25) also found that EDN generated by cultured human PBMC appears on a SDS-PAGE gel as bands of 31–40 kDa. Therefore, EDN expression by macrophages can be rapidly induced.
FIGURE 8.
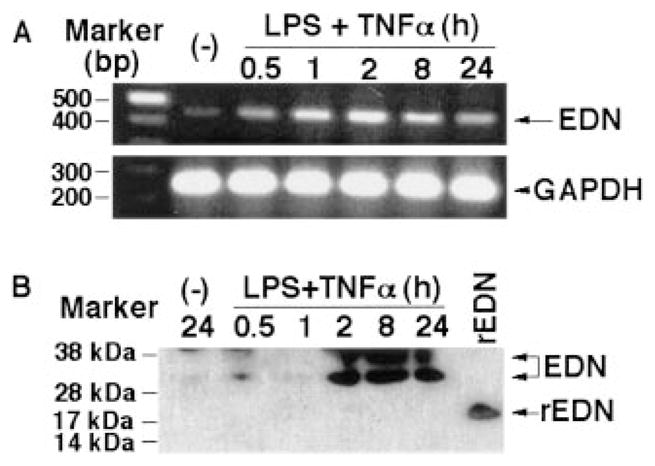
Up-regulation of EDN expression by macrophages in response to TNF-α and LPS. A, Human monocyte-derived macrophages were incubated in RPMI 1640 medium alone or in the presence of a combination of LPS and human rTNF-α (each at 100 ng/ml) at 37°C in humidified air containing 5% CO2 for a period of time, as indicated. Subsequently, total RNA was extracted and used as the template for the amplification of EDN or GAPDH by RT-PCR, as described in Materials and Methods. Upper and lower panels, EDN and GAPDH, respectively. B, Macrophage culture supernatant was harvested under conditions as specified, and the amount equivalent to 20 μg of protein was loaded onto each lane of a SDS-PAGE gel. rEDN (100 ng) generated in E. coli was used as a control (last lane). After transfer, the polyvinylidene difluoride membrane was blotted with rabbit anti-EDN polyclonal Ab, as detailed in Materials and Methods.
Discussion
In the present study, we have shown that EDN (both natural and recombinant forms) and hPR stimulated human CD34+ progenitor-derived and monocyte-derived DCs to produce a variety of soluble mediators, including cytokines, chemokines, growth factors, and soluble receptors. The soluble mediators induced by EDN and, to a lesser extent, hPR, consisted mostly of proinflammatory cytokines and chemokines. In addition, treatment of human iDCs with EDN or hPR also resulted in maturation of DCs, as evidenced by up-regulation of CD83, CD86, and HLA-DR; switch of chemokine receptor expression from CCR5+ to CCR7+; and acquisition of the capacity to stimulate allogeneic MLR. These activities of EDN and hPR were not due to endotoxin contamination. Thus, both EDN and hPR can act as activators for DCs. This is the first report that demonstrates EDN and hPR act as activator for DCs.
How EDN or hPR signals activation of DCs is currently under investigation. The chemotactic effect of EDN on DCs is mediated by a pertussis toxin-sensitive Giα protein-coupled receptor (GPCR) (26). We could not determine whether the DC-activating effect of EDN was also mediated by a pertussis toxin-sensitive mechanism because pertussis toxin treatment of DCs by itself induces the activation of DCs in vitro, leading to DC maturation and cytokine production (40, 41). It is unlikely that the capacity of EDN and hPR to activate DCs relies on a pertussis toxin-sensitive GPCR-dependent mechanism because hPR is not chemotactic for DCs (26). Conversely, although mEAR2, like EDN, is chemotactic for human DCs (26), mEAR2 could not activate DCs to produce cytokines (Fig. 1). Another issue is whether EDN and hPR use different mechanisms for the activation of DCs. Unlike EDN, hPR does not have either antiviral or chemotactic activities (18, 24–26). Human ANG, another member of RNase A superfamily that is evolutionarily more distant from EDN and hPR (18), also did not activate DCs to produce any cytokines and chemokines (Fig. 6). Thus, these RNase A superfamily members have essential functional differences, implying that EDN and hPR may use different mechanisms to activate DCs.
hPR, produced in the pancreas and excreted into the gut, is believed to degrade the large amounts of RNA present in food (19). The observation that hPR is also an activator of DCs was unexpected. However, hPR is known to be expressed by tissues other than the pancreas (19), including activated monocytes (39), α-galactosylceramide-stimulated monocyte-derived DCs (42), and human endothelial cells from arteries, veins, and capillaries (43). Thus, hPR may have nondigestive biological roles that have not been previously appreciated. Our results suggest that hPR may contribute to inflammation. In vivo, hPR secretion is likely to be tightly regulated. Consequently, the effect of hPR may not be evident unless it is quickly produced and exposed to target cells in high amounts. Such is likely to be the case during acute pancreatitis, a disorder that results from exposure of pancreatic parenchyma to digestive enzymes, including hPR. It remains to be determined whether or not hPR is generated under various inflammatory conditions.
EDN is a multifunctional mediator because it acts as an activator (present study) and a chemoattractant of DCs (26), as well as an antiviral effector and an RNase (24, 25). EDN, in addition to being expressed by liver, spleen, neutrophils, and monocytes/macrophages (22, 38, 39), is released in large amounts upon eosinophil degranulation (21, 22). Furthermore, the expression of EDN by macrophages is rapidly induced in response to TNF-α and LPS (Fig. 8). Substantial infiltration and activation of eosinophils and monocytes/macrophages are most often seen in tissues with helminth or viral infections and allergic and atopic reactions (18, 44). It is thus conceivable that EDN is produced at considerable levels at sites of inflammation associated with such disorders. Based on its multifunctional activities, EDN may contribute to both innate and adaptive antimicrobial immunity of the host. EDN may contribute to innate immunity by killing/inactivating helminthic/viral invaders and inducing the production of proinflammatory mediators to amplify local inflammatory responses. EDN may participate in adaptive immunity in at least two ways. EDN may act synergistically with chemokines to promote the recruitment of DCs to the sites of inflammation, hereby enhancing Ag uptake and processing. Additionally, because it has the capacity to induce phenotypic and functional maturation (Fig. 7), EDN may also contribute to promoting both the trafficking of DCs from inflammatory sites to regional lymph nodes and the Ag presentation to naive T cells. This suggests that the primary role of EDN is to eliminate the microbial invaders and restore host homeostasis. Conversely, persistent generation of EDN in considerable amount may contribute to the pathophysiology associated with eosinophil infiltration in asthma and other eosinophil-associated diseases due to its capacity to cause DC recruitment and production of inflammatory cytokines and chemokines.
Taking advantage of their RNase activities, both EDN and hPR have been used as fusion partners of immunotoxins for experimental tumor therapy (45–47). In these studies, targeted delivery of EDN or hPR into tumor cells causes the death of tumor cells both in vitro and in vivo, the underlying mechanism of which is thought to be due to the degradation of RNAs of tumor cells by EDN or hPR. The possibility that the DC-activating effects of EDN or hPR are maintained by the fused immunotoxins has not been evaluated. It is therefore possible that immunoadjuvant effects of EDN and hPR may contribute to the immunotherapeutic antitumor effects of EDN- or hPR-based immunotoxins in immunocompetent mice.
In addition to EDN, a number of endogenous mediators, including defensins (4, 5, 8, 9), high mobility group box 1 (48), cathelicidins (3, 6), aminoacyl-tRNA synthetases (49, 50), urokinase (51, 52), and ribosomal protein S19 (53), have also recently been shown to be chemotactic based on interactions with GPCRs expressed by mononuclear leukocytes, including DCs, monocytes/macrophages, and/or lymphocytes. These mediators are structurally diverse and distinct from conventional chemokines because they are present in the intracellular compartment or on the surface of keratinocytes and epithelial cell lining, where they function as enzymes, DNA-binding protein, or antimicrobial effectors under normal or steady state. These mediators are rapidly released or induced to produce in response to danger signals generated by tissue injury or various (viral, bacterial, fungal, or parasitic) forms of infection. All of these mediators are capable of stimulating the production of proinflammatory cytokines by monocytes/macrophages and/or DCs, and some, such as murine β-defensin 2 (16), high mobility group box 1 (48), and EDN (present study), have been shown to stimulate the maturation of DCs. Because the recruitment and subsequent activation of monocytes/macrophages and DCs are pivotal for the initiation of both innate and adaptive immune responses (37, 54), these mediators may function to mobilize and galvanize the immune system in response to danger signals. Consequently, to aid in the recognition of these structurally diverse, but functionally similar mediators, we propose to call them “endogenous multifunctional immune alarmins.”
Footnotes
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract NO1-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. The publisher or recipient acknowledges right of the U.S. Government to retain a nonexclusive, royalty-free license in and to any copyright covering the article.
Abbreviations used in this paper: iDC, immature dendritic cell; ANG, angiogenin; CM, chemotaxis media; DC, dendritic cell; EAR, eosinophil-associated RNase; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; GPCR, Giα protein-coupled receptor; hANG, human ANG; hPR, human pancreatic-type RNase; IP-10, IFN-γ-inducible protein-10; mEAR, murine EAR; MIG, monokine induced by IFN-γ; PR, pancreatic-type RNase; RCA, rolling-circle amplification; SLC, CCL21/secondary lymphoid tissue chemokine.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci. 2001;58:978. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott MG, Hancock REW. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit Rev Immunol. 2002;20:407. [PubMed] [Google Scholar]
- 4.Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 5.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukocyte Biol. 2000;68:9. [PubMed] [Google Scholar]
- 6.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OMZ, Oppenheim JJ. β-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 9.Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E, Oppenheim JJ, Kwak LW. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 10.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human β-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol. 2002;14:421. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JR, Krause A, Schulz S, Rodriguez-Jimenez FJ, Kluver E, Adermann K, Forssmann U, Frimpong-Boateng A, Bals R, Forssmann WG. Human β-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819. [PubMed] [Google Scholar]
- 12.Garcia JR, Jaumann F, Schulz S, Krause A, Rodriguez-Jimenez J, Forssmann U, Adermann K, Kluver E, Vogelmeier C, Becker D, et al. Identification of a novel, multifunctional β-defensin (human β-defensin 3) with specific antimicrobial activity: its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001;306:257. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- 13.Van Wetering S, Mannesse-Lazeroms SPG, van Sterkenburg MAJA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:L888. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 14.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human β-defensin-1/2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur J Immunol. 2001;31:1066. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock REW. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune response. J Immunol. 2002;169:3883. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 16.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 17.Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg HF, Domachowke JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukocyte Biol. 2001;70:691. [PubMed] [Google Scholar]
- 19.Sorrentino S. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell Mol Life Sci. 1998;54:785. doi: 10.1007/s000180050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton DL, Rybak SM. Unique recombinant human ribonuclease and inhibition of Kaposi’s sarcoma cell growth. J Natl Cancer Inst. 1998;90:1787. doi: 10.1093/jnci/90.23.1787. [DOI] [PubMed] [Google Scholar]
- 21.Durack DT, Ackerman SJ, Loegering DA, Gleich GJ. Purification of human eosinophil-derived neurotoxin. Proc Natl Acad Sci USA. 1981;78:5165. doi: 10.1073/pnas.78.8.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg HF, Tenen DG, Ackerman SJ. Molecular cloning of human eosinophil-derived neurotoxin: a member of the ribonuclease gene family. Proc Natl Acad Sci USA. 1989;86:4460. doi: 10.1073/pnas.86.12.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosimann SC, Newton DL, Youle RJ, James MN. X-ray crystallographic structure of recombinant eosinophil-derived neurotoxin at 1.83 A resolution. J Mol Biol. 1996;26:540. doi: 10.1006/jmbi.1996.0420. [DOI] [PubMed] [Google Scholar]
- 24.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 25.Rugeles MT, Trubey CM, Bedoya VI, Pinto LA, Oppenheim JJ, Rybak SM, Shearer GM. Ribonuclease is partly responsible for the HIV-1 inhibitory effect activated by HLA alloantigen recognition. AIDS. 2003;17:481. doi: 10.1097/00002030-200303070-00002. [DOI] [PubMed] [Google Scholar]
- 26.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 27.Schweitzer B, Roberts S, Grimwade B, Shao W, Wang M, Fu Q, Shu Q, Laroche I, Zhou Z, Tchernev VT, et al. Multiplexed protein profiling on microarrays by rolling-circle amplification. Nat Biotechnol. 2002;20:359. doi: 10.1038/nbt0402-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zewe M, Rybak SM, Dubel S, Coy JF, Welschof M, Newton DL, Little M. Cloning and cytotoxicity of human pancreatic RNase immunofusion. Immunotechnology. 1997;3:127. doi: 10.1016/s1380-2933(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 29.Newton DL, Rybak SM. Antibody-enzyme fusions. In: Dubel S, Kortermann R, editors. Antibody Engineering Lab Manual. Springer; Heidelberg, Germany: 2001. p. 667. [Google Scholar]
- 30.Rosenberg HF, Domachowske JB. Eosinophil-derived neurotoxin. In: Nicholson AW, editor. Methods in Enzymology. Vol. 341. Academic Press; San Diego: 2001. p. 273. [DOI] [PubMed] [Google Scholar]
- 31.Arrighi JF, Hauser C, Chapuis B, Zubler RH, Kindler V. Long-term culture of human CD34+ progenitors with Flt3-ligand, thrombopoietin, and stem cell factor induces extensive amplification of a CD34−CD14− and a CD34−CD14+ dendritic cell precursor. Blood. 1999;93:2244. [PubMed] [Google Scholar]
- 32.Yang D, Chen Q, Le Y, Wang JM, Oppenheim JJ. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages. J Immunol. 2001;166:4092. doi: 10.4049/jimmunol.166.6.4092. [DOI] [PubMed] [Google Scholar]
- 33.Kingsmore SF, Patel DD. Multiplexed protein profiling on antibody-based microarrays by rolling circle amplification. Curr Opin Biotechnol. 2003;14:74. doi: 10.1016/s0958-1669(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 34.Yang D, Chen Q, Stoll S, Chen X, Howard OMZ, Oppenheim JJ. Differential regulation of responsiveness to fMLP and C5a upon dendritic cell maturation: correlation with receptor expression. J Immunol. 2000;165:2694. doi: 10.4049/jimmunol.165.5.2694. [DOI] [PubMed] [Google Scholar]
- 35.Dilioglou S, Cruse JM, Lewis RE. Costimulatory function of umbilical cord blood CD14+ and CD34+ derived dendritic cells. Exp Mol Pathol. 2003;75:18. doi: 10.1016/s0014-4800(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 36.Sozzani S, Allavena P, Vecchi A, Mantovani A. The role of chemokines in the regulation of dendritic cell trafficking. J Leukocyte Biol. 1999;66:1. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 38.Sur S, Glitz DG, Kita H, Kujawa SM, Peterson EA, Weiler DA, Kephart GM, Wagner JM, George TJ, Gleich GJ, Leiferman KM. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukocyte Biol. 1998;63:715. doi: 10.1002/jlb.63.6.715. [DOI] [PubMed] [Google Scholar]
- 39.Egesten A, Dyer KD, Batten D, Domachowske JB, Rosenberg HF. Ribonucleases and host defense: identification, localization and gene expression in adherent monocytes in vitro. Biochim Biophys Acta. 1997;1358:255. doi: 10.1016/s0167-4889(97)00081-5. [DOI] [PubMed] [Google Scholar]
- 40.Ausiello CM, Fedele G, Urbani F, Lande R, Di-Carlo B, Cassone A. Native and genetically inactivated pertussis toxins induce human dendritic cell maturation and synergize with lipopolysaccharide in promoting T helper type 1 responses. J Infect Dis. 2002;186:351. doi: 10.1086/341510. [DOI] [PubMed] [Google Scholar]
- 41.Tonon S, Goriely S, Aksoy E, Pradier O, Del Giudice G, Trannoy E, Willems F, Goldman M, De Wit D. Bordetella pertussis toxin induces the release of inflammatory cytokines and dendritic cell activation in whole blood: impaired responses in human newborns. Eur J Immunol. 2002;32:3118. doi: 10.1002/1521-4141(200211)32:11<3118::AID-IMMU3118>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 42.Lapteva N, Nieda M, Ando Y, Nicol A, Ide K, Yamaura A, Hatta-Ohashi Y, Egawa K, Juji T, Tokunaga K. Gene expression analysis in human monocytes, monocyte-derived dendritic cells, and α-galactosylceramide-pulsed monocyte-derived dendritic cells. Biochem Biophys Res Commun. 2001;289:531. doi: 10.1006/bbrc.2001.6003. [DOI] [PubMed] [Google Scholar]
- 43.Landre JB, Hewett PW, Olivot JM, Friedl P, Ko Y, Sachinidis A, Moenner M. Human endothelial cells selectively express large amounts of pancreatic-type ribonuclease (RNase 1) J Cell Biochem. 2002;86:540. doi: 10.1002/jcb.10234. [DOI] [PubMed] [Google Scholar]
- 44.Walsh GM. Eosinophil granule proteins and their role in disease. Curr Opin Hematol. 2001;8:28. doi: 10.1097/00062752-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Newton DL, Nicholls PJ, Rybak SM, Roule RJ. Expression and characterization of recombinant human eosinophil-derived neurotoxin and eosinophil-derived neurotoxin-anti-transferrin receptor sFv. J Biol Chem. 1994;269:26739. [PubMed] [Google Scholar]
- 46.Newton DL, Ilercil O, Laske DW, Oldfield E, Rybak SM, Youle RJ. Cytotoxic ribonuclease chimeras: targeted tumoricidal activity in vitro and in vivo. J Biol Chem. 1992;267:19572. [PubMed] [Google Scholar]
- 47.Rybak SM, Newton DL. Natural and engineered cytotoxic ribonucleases: therapeutic potential. Exp Cell Res. 1999;253:325. doi: 10.1006/excr.1999.4718. [DOI] [PubMed] [Google Scholar]
- 48.Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukocyte Biol. 2002;72:1084. [PubMed] [Google Scholar]
- 49.Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science. 1999;284:147. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- 50.Howard OMZ, Dong HF, Yang D, Raben N, Nagaraju K, Rosen A, Casciola-Rosen L, Hartlein M, Kron M, Yang D, et al. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med. 2002;196:781. doi: 10.1084/jem.20020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blasi F. The urokinase receptor: a cell surface, regulated chemokine. AP-MIS. 1999;107:96. doi: 10.1111/j.1699-0463.1999.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 52.Resnati M, Pallavicini I, Wang J, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 2002;99:1359. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shibuya Y, Shiokawa M, Nishiura H, Nishimura T, Nishino N, Okabe H, Takagi K, Yamamoto T. Identification of receptor-binding sites of monocyte chemotactic S19 ribosomal protein dimer. Am J Pathol. 2001;159:2293. doi: 10.1016/S0002-9440(10)63079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]