Abstract
Bipolar disorder has been conceptualized as an outcome of dysregulation in the behavioral activation system (BAS), a brain system that regulates goal-directed activity. On the basis of the BAS model, the authors hypothesized that life events involving goal attainment would promote manic symptoms in bipolar individuals. The authors followed 43 bipolar I individuals monthly with standardized symptom severity assessments (the Modified Hamilton Rating Scale for Depression and the Bech-Rafaelsen Mania Rating Scale). Life events were assessed using the Goal Attainment and Positivity scales of the Life Events and Difficulties Schedule. As hypothesized, manic symptoms increased in the 2 months following goal-attainment events, but depressed symptoms were not changed following goal-attainment events. These results are congruent with a series of recent polarity-specific findings.
Bipolar disorder exacts a devastating toll from affected individuals. Most strikingly, this disorder leads to suicide in almost one out of every five diagnosed individuals (Isometsa, 1993). With adequate blood serum levels of lithium, one third of bipolar individuals relapse within 3 years (Keller et al., 1992), but in naturalistic studies with varied levels of patient compliance, two thirds of patients relapse within 2 years (Silverstone, McPherson, Hunt, & Romans, 1998). Given these high rates of relapse and the sustained unemployment rates following each episode of mania (Harrow, Goldberg, Grossman, & Meltzer, 1990), it is not surprising that bipolar disorder has been ranked as the sixth leading cause of disability among both physical and psychiatric disorders worldwide (Murray & Lopez, 1996). Fiscal costs for adult Americans with the disorder were estimated in 1991 at $45 billion (Wyatt & Henter, 1995).
Although the modal course is severe, bipolar individuals experience substantial heterogeneity in symptom expression. For instance, as many as 25% of bipolar I individuals will never experience a depressive episode (Goodwin & Jamison, 1990). Further, whereas some individuals experience daily shifts between episode poles, others remain well for a decade or longer (Angst, 1984).
For many years, biological models have dominated attempts to understand this heterogeneity. The importance of genetic influences for this disorder is supported by findings of a concordance rate of .84 for monozygotic twins compared with .35 for dizygotic twins among 110 twin pairs (Bertelsen, Harvald, & Hauge, 1977); similar findings have emerged across 12 twin studies (Vehmanen, Kaprio, & Loennqvist, 1995).
Beyond the important role of genes, the psychosocial environment appears to be a trigger of episodes. For example, expressed emotion (Miklowitz et al., 1988), social support (Johnson, Winett, Meyer, Greenhouse, & Miller, 1999), and life events (Ellicott, 1989; Johnson & Miller, 1997) each have been shown to predict symptom changes in bipolar disorder. Recent models of bipolar course have incorporated biological and psychosocial variables (cf. Goodwin & Jamison, 1990) within a diathesis stress framework (Johnson & Roberts, 1995).
The behavioral activation and inhibition model, one attempt to integrate biological and environmental predictors, has been described independently by Depue and Iacono (1989) and Gray (1975, 1982, 1987, 1990, 1991). This theory concerns two broadband motivational systems, regulating approach and withdrawal behavior in response to environmental cues. Both systems are hypothesized to correspond to specific neural pathways in the brain. The behavioral inhibition system (BIS) is sensitive to environmental cues of punishment and novelty and regulates inhibition or avoidance of these cues as well as correspondent feelings of anxiety and nonspecific arousal. This dimension is an important component of anxiety but is less relevant for bipolar episodes of mania and depression (Meyer, Johnson, & Carver, 1999).
The behavioral activation system (BAS) is sensitive to environmental cues of reward and regulates approach behaviors and the correspondent feelings of hope, elation, and happiness.1 Self-reported BAS levels (Carver & White, 1994) appear highly correlated with sensitivity to cues of reward in a conditioning task but not to cues of punishment (Zinbarg & Mohlman, 1998).
Depue and his colleagues proposed that manic symptoms are the manifestation of dysregulation in the BAS (Depue, Collins, & Luciana, 1996; Depue & Iacono, 1989; Gray, 1994). To facilitate goal-directed behavior, the BAS enhances positive affect (Watson & Tellegen, 1985), incentive–reward motivation, sociability and social potency, desire for excitement, and motor activity and arousal (Depue, Krauss, & Spoont, 1987). Depue has noted the strong correspondence between these BAS-regulated behaviors and the manic symptoms of mood change, inflated self-esteem, decreased need for sleep, increased talkativeness, flight of ideas, increased goal-directed activity, and excessive involvement in pleasurable activities.
In preliminary research, support for behavioral implications of the BAS model was found within bipolar II and cyclothymic populations (Depue, 1987) as well as an undergraduate sample with a history of hypomanic symptoms (Meyer et al., 1999). Among bipolar I individuals, self-reported BAS sensitivity levels predict increased mania over time (Meyer, Johnson, & Winters, 2000). Cognitive–behavioral theorists, drawing on the clinical phenomenology of bipolar disorder, have noted that manic cognition involves elevated belief in the ability to achieve reward (Leahy, 1999). This further links mania to BAS function, as BAS is presumed to be central to reward responsiveness. In short, evidence has begun to emerge that mania is tied to increases in BAS activity (Depue & Iacono, 1989; Depue, Krauss, & Spoont, 1987; Depue & Zald, 1993).
One important tenet of this BAS model, however, remains untested. If BAS activity becomes elevated in response to cues of reward, then one would expect mania to be triggered by the reward that is embodied in life events involving goal attainment. We hypothesized that among individuals with bipolar disorder, goal-attainment life events would predict increases in mania. To test this hypothesis, we interviewed individuals diagnosed with bipolar I disorder by using standardized symptom interviews on a monthly basis. We measured goal-attainment life events by using the Brown and Harris Life Events and Difficulties Schedule. Then, we assessed whether goal-attainment life events predicted increases in manic symptoms. As a measure of discriminant validity, we also examined more general positive events that did not specifically involve goal attainment. Finally, we examined the impact of both goal attainment and positive events on depressive symptoms. Given our previous research suggesting more robust effects for BAS on mania than depression (Meyer et al., 1999, 2000), we anticipated that goal-relevant events would predict only manic increases.
Method
Participants
Previous articles reported on severe negative life events and social support in this sample and one other sample (Johnson & Miller, 1997; Johnson et al., 1999; Johnson, Meyer, Winett, & Small, 2000). The targeted population was individuals admitted for bipolar disorder or related conditions to a private psychiatric hospital in Rhode Island, Selection criteria included bipolar disorder (manic, depressed, or mixed episode) as diagnosed by the Structured Clinical Interview for DSM–III–R (SCID; Spitzer, Williams, Gibbon, & First, 1990), a Modified Hamilton Rating Scale for Depression score (MHRSD) ≥ 17, or Bech–Rafaelsen Mania Rating Scale (BRMS) ≥ 15, and age between 18 and 65. Individuals were excluded if they met criteria for substance abuse or dependence within the past year, had central nervous system diseases other than bipolar disorder, or were unable to complete self-report measures independently because of mental retardation or language barriers.
Within this sample, 47% of participants were male and 12% were minority individuals. Fifty-eight percent were married or cohabiting, 26% were single, and 16% were divorced or separated. The mean educational attainment was 16 years (SD = 13). Comparable with other bipolar samples (Harrow et al., 1990), only 49% were employed.
At study entry, approximately 72% of the participants were diagnosed as manic, 23% were depressed, and 5% were mixed, reflective of overall inpatient admissions. On average, individuals had experienced 9.05 lifetime episodes (SD = 9.92) and 4.16 depressions (SD = 9.41). Congruent with other naturalistic studies (Goodwin & Jamison, 1990; Depue & Monroe, 1978), 33% of the participants reported no previous depressive episodes.
Procedures
Once attending psychiatrists provided permission to approach potential participants, candidates were approached about the study. Interested individuals completed written informed consent procedures. Symptom severity assessments were administered every month by telephone, and the life events interviews were conducted at the 6-, 12-, 18-, and 24-month face-to-face follow-ups. In general, rates of inclusion and follow-up (see Johnson & Miller, 1997) were congruent with naturalistic studies of bipolar disorder (Hunt, Bruce-Jones, & Silverstone, 1992; Ellicott, 1989). On average, participants completed 18 months of follow-up.
Measures
Bedford College Life Events and Difficulties Schedule (LEDS; Brown & Harris, 1978a, 1978b)
The LEDS is a semistructured interview system for rating life events while taking into account the context of each life event. A series of studies have documented the reliability and validity of this measure (Brown & Harris, 1989; Gorman, 1995).
Several procedures are used to avoid potential bias in recall and dating of events. For instance, calendars with “anchors,” such as birthdays, holidays, or other significant dates, are used during the interview, strategies which appear to enhance recall accuracy (Brown & Harris, 1982; Katschnig, 1986; Loftus & Marburger, 1983; McQuaid et al., 1992; Shum, 1998; Sobell, Toneatto, Sobell, Schuller, & Maxwell, 1990).
One author (Sheri L. Johnson) has received LEDS training from Scott Monroe and Ellen Frank, and she trained all raters. All ratings were anchored by using Brown's “dictionaries,” which provide tens of thousands of sample ratings (Brown & Harris, 1978a). Life event raters were unaware of symptom status. Each event was rated on the LEDS scales of illness-related nature, goal attainment, and positivity.
Illness-related nature of events
One of the concerns in life event research is that symptoms of psychopathology may contribute to the generation of stress; for example, manic symptoms could increase goal striving and thereby increase goal-attainment life events. To control for this possibility, raters evaluated each event on the LEDS Illness scale, which considers whether events could be the result of symptomatic behavior. Events that were rated as related or possibly related to either current or previous symptoms were excluded from analyses.
Goal Attainment scale
The 4-point Goal Attainment scale has been used in previous research on recovery from unipolar depression and anxiety (Leenstra, Ormel, & Giel, 1995). Ratings are based on the achievement of a desired goal and the amount of commitment or striving toward that goal. Examples of goal attainments rated a 1 included acceptance into graduate school and passing an exam in which 50% of students were cut from a graduate training program. Examples of ratings of 2 included winning a poetry award and obtaining a new job. Ratings of 3 included activities in which the attainments were more limited, such as starting a training program, and a 4 event would include no elements of goal attainment. Our team achieved an intraclass correlation of .94 (N = 33, 4 raters; F = 17.32, p < .00005). Only 5% of individuals within this sample experienced an event rated 1, and only 14% experienced an event rated below 4 on goal attainment. The mean goal attainment rating was 3.72 (SD = 1.77). This low base rate of goal-attainment events limits power, so only relatively robust effect sizes could be detected.
Positive Events scale
Theoretical models of affect have often emphasized the importance of a two-dimensional approach, in which valence is differentiated from activation (cf. Lang, 1995). Whereas goal-attainment life events include only events that appeared positive and activating, the Positive Events scale was designed to capture positivity without regard to activation. Positive events include the creation of new positive experiences, alleviation of ongoing difficulty or stress, filling a vacuum or emptiness in a person's life, and creating stability in the environment. Although obtaining a new job or achieving other goals would be included as positive events, this category was considerably broader than goal attainment, including events that had little to do with the participant's personal efforts. Examples of positive events rated 1 included winning the lottery, whereas 2-level events included starting a new position, and 3-level events included receiving a tax refund. Many more individuals experienced positive events than goal-attainment life events: 32 individuals experienced a positive event rated less than 4. The mean positive event rating was 2.56 (SD = 1.05). High interrater reliability has been obtained in other studies and by our team. As expected, levels of goal attainment and positivity were modestly correlated (r = .32, p = .04, N = 43).
Diagnosis
The SCID was used to assess diagnoses. We also used the DSM–IV criterion (Diagnostic and Statistical Manual of Mental Disorders 4th edition; American Psychiatric Association, 1994), that manic episodes were not triggered by antidepressant medication. Interrater reliability for this measure has been strong: Williams et al. (1992) achieved a kappa for bipolar disorder of .84, and our team achieved a kappa of 1.0 for mania in seven interviews evaluated by two raters (r = .94 for specific manic symptoms, p < .0001, N = 74). Sheri L. Johnson supervised all diagnostic decisions, and a psychiatrist was consulted for differential diagnosis of organic brain syndrome.
Symptom severity
The MHRSD was used to assess the severity of depressive symptoms (Miller, Bishop, Norman, & Maddever, 1985). This modification of the original Hamilton Rating Scale for Depression highly correlates with the original but was revised to include standardized probes and behavioral anchors for ratings. This widely used scale is sensitive to changes in depression severity (cf. Keitner, Ryan, Miller, & Norman, 1992; Miller, Norman, & Keitner, 1989). Our interrater reliability for the scale was high (intraclass correlation = .93, calculated using methods described by Shrout & Fleiss, 1979). Internal consistency was also high (α = .92, N = 164).
The BRMS was used to assess the severity of manic symptoms (Bech, Bolwig, Kramp, & Rafaelsen, 1979). This 11-item interview measures mood, self-esteem, motor activity, verbal activity, sleep, hostility, and sexual interest. In previous research, the average intercorrelation of 4 raters as assessed by the Kendall coefficient of concordance was high, ω = .95, p = .0001, N = 12, (Bech et al., 1979), and the scale showed strong sensitivity for the severity of manic states, χ2 = 41.95, p = .001. Our team achieved an intraclass correlation of .92. The internal consistency was also high (α = .94, N = 164). The MHRSD and the BRMS assessed the most severe week of symptoms within the month.
For analyses, we identified the most substantial goal attainment for each individual. On average, this occurred during the 6th month of the study (M month = 6.56, SD = 3.99). Baseline symptom severity was defined as the score one month before the goal-attainment life event. Follow-up symptom severity was defined as the average level of symptoms in the 2 months after the goal-attainment life event, on the basis of previous research suggesting that manic episodes typically last for 2 months or less (Rosenberg, Winett, & Johnson, 1998). Separate indices were constructed for depressed and manic symptoms. We defined parallel symptom severity baseline and follow-up indices for the month in which the most positive event occurred (M month = 6.05, SD = 3.73).
The Somatotherapy Index (Bauer, McBride, Shea, & Gavin, 1997) was used to categorize the adequacy of outpatient treatment. This scale was developed to evaluate treatment of bipolar disorder and is based on the Pharmacotherapy Adequacy scale used in the National Institute of Mental Health Collaborative Program on the Psychobiology of Depression Clinical Studies Project (Mueller et al., 1996). Medication adequacy is rated on a 5-point scale, using prescribed dosages, reported compliance, and blood serum levels. For example, to obtain a rating of 4, a patient must maintain a minimum of 4 weeks of lithium levels ≥ 0.8 mEq/L or carbamazapine levels ≥ 8 μg/dl or valproate level ≥ 75 μg/dl or ≥ 300 mg/day of imipramine hydrochloride or its equivalent. This scale has obtained high interrater reliability scores, and complicated treatments were rated by consensus.
Results
We conducted all tests using a two-tailed p value of .05. Before examining the primary hypotheses, we conducted analyses to identify potential confounds. Then, we conducted bivariate correlations of life event and symptom variables. To examine hypotheses, we conducted partial correlations of level of goal-attainment life events with follow-up symptoms, controlling for baseline symptom levels. We conducted separate analyses for depression (MHRSD) and mania (BRMS) and for goal attainment and positivity.
To determine whether goal-attainment life events were confounded with demographic or illness characteristics, we computed Pearson product–moment correlations. No significant correlations emerged between degree of goal attainment and age, gender, years of education, Hollingshead (1957) occupational status, onset age, number of hospitalizations, number of episodes, number of depressions, or adequacy of medication levels.
Means and standard deviations of all dependent variables are presented in Table 1. Relatively few individuals were experiencing clinical levels of symptoms at the time of the baseline symptom assessment; approximately 10% were experiencing a major depressive episode (MHRSD > 17) and 7% were experiencing a full manic episode (BRMS > 16). For the overall sample means, neither depression nor mania changed significantly from baseline to follow-up: for mania, dependent t(42) = .45, ns; for HRSD, t(42) = .07, ns. Baseline symptoms were not correlated with follow-up symptoms for mania (r = .07, ns, N = 43) but were moderately correlated for depression (r = .56, two-tailed p ≤ .0005, N = 43).
Table 1.
Means and Standard Deviations of Mania and Depression at Baseline and Follow-Up
| Scale | M | SD |
|---|---|---|
| Little or no goal attainment (n = 37) | ||
| BRMS baseline | 4.67 | 7.02 |
| BRMS follow-up | 3.30 | 4.03 |
| MHRSD baseline | 6.68 | 6.46 |
| MHRSD follow-up | 7.09 | 6.04 |
| At least some goal attainment (n = 6) | ||
| BRMS baseline | 2.50 | 2.17 |
| BRMS follow up | 6.83 | 12.44 |
| MHRSD baseline | 11.00 | 6.87 |
| MHRSD follow-up | 8.00 | 5.37 |
| Little or no positively (n = 11) | ||
| BRMS baseline | 5.54 | 4.82 |
| BRMS follow-up | 4.86 | 5.60 |
| MHRSD baseline | 5.91 | 6.16 |
| MHRSD follow-up | 4.82 | 4.98 |
| At least some positivity (n = 32) | ||
| BRMS baseline | 5.25 | 7.61 |
| BRMS follow-up | 3.14 | 6.62 |
| MHRSD baseline | 8.44 | 6.73 |
| MHRSD follow-up | 7.86 | 7.25 |
Note. BRMS = Bech-Rafaelsen Mania Rating Scale; MHRSD = Modified Hamilton Rating Scale for Depression.
Next, we examined bivariate correlations between goal attainment and symptom severity scores. Goal attainment was not significantly related to baseline manic (r = .13, p = .40, N = 43) or depressive symptoms (r = −.17, p = .28, N = 43). These results suggested that the severity of symptoms in the month before the life event was not an influence on the magnitude of goals achieved. Goal attainment was not significantly related to follow-up depressive symptoms (r = .02, p = .86, N = 43). As predicted, goal attainment was significantly related to higher levels of follow-up manic symptoms (r = −.36, p ≤ .05, N = 43).
Next, we examined the change in manic and depressive symptoms following goal attainment, using partial correlations to control for baseline symptoms. The partial correlation of goal attainment and manic symptoms, controlling for baseline manic symptoms, was significant and in the expected direction, r(40) = −.37, p = .01. The partial correlation of goal attainment with depressive symptoms, controlling for baseline depressive symptoms, was not significant, r(40) = .15, p = .36. As assessed using z transformations to compare the difference between partial correlations (Meng, Rosenthal, & Rubin, 1992), manic symptoms increased more than depressive symptoms did after goal-attainment life events (z = 2.02, p < .05).
As a point of comparison, we examined whether positive events predicted changes in manic and depressive follow-up symptoms. As above, we first checked whether symptoms changed from baseline to follow-up (positive events involved different symptom assessments). Matched t tests revealed that neither manic nor depressive symptoms changed from before to after the event period. Bivariate correlations revealed no relation between positive events and baseline manic (r = .10, ns, N = 43) or depressive symptoms (r = −.16, ns, N = 43) nor between positive events and follow-up manic (r = −.003, ns, N = 43) or depressive symptoms (r = −.07, ns, N = 43). The partial correlations, controlling for baseline symptoms, also revealed no relation between positive events and manic follow-up symptoms, r(40) = −.02, ns, or depressive follow-up symptoms, r(40) = −.02, ns. Goal-attainment life events were more related to manic symptoms than positive events were (z = −1.95, p < .05).
Discussion
Goal-attainment life events, but not general positive events, are associated with relative elevations in subsequent manic symptoms. Our findings are polarity specific, in that neither goal-attainment nor positive life events were associated with changes in depression.
These results are congruent with recent findings suggesting that the predictors of depression versus mania in bipolar individuals do not overlap. For example, negative life events, low social support, and low self-esteem predict bipolar depression but not manic symptoms (Johnson et al., 1999; Johnson, 2000). Mania has been found to he preceded by schedule-disrupting life events (Malkoff-Schwartz et al., 1998). Current findings indicate that manic symptoms are predicted by goal-attainment life events.
This study and previous studies have shown that positive events, defined broadly, do not appear to be tied to mania (Alloy, Reilly-Harrington, Fresco, Whitehouse, & Zechmeister, 1999; Reilly-Harrington, Alloy, Fresco, & Whitehouse, 1999). The personal striving involved in goal-attainment events compared with positive events appears to be an important consideration.
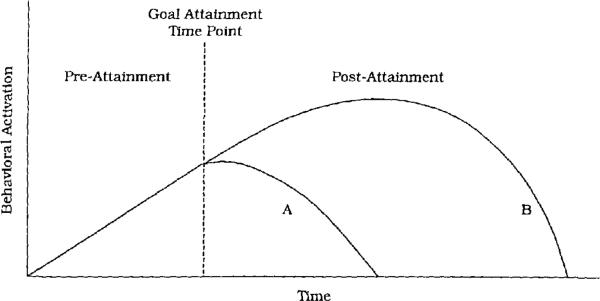
In some ways, the finding that goal-attainment events trigger manic symptoms is not surprising; goal-attainment events increase positive affect and energy in nonbipolar individuals as well (Anderman, 1999; Carver & Scheier, 1998; Emmons & Kaiser, 1996). We theorize, along with Depue and Iacono (1989), that bipolar individuals are not differentiated from nondisturbed individuals on the nature of important triggers but, rather, in their ability to regulate motivation and affect following these triggers (see Figure 1). Whereas most individuals appear to shift temporarily into a “coasting” mode after goal attainment (Carver & Scheier), bipolar individuals seem to spiral excessively into increasing positive affect and continued goal seeking.
Figure 1.
Theoretical time course of behavioral activation before and after goal-attainment life events. Among nondisturbed individuals (A), behavioral activation increases during the pre-attainment phase and then reduces to baseline as individuals switch to “coasting” after goal attainment. Among individuals with bipolar disorder (B), behavioral activation may also increase during striving but then continue to increase even after goal attainment.
Our ability to interpret this spiral is severely limited by the absence of direct measures of mediators. These events may interfere with sleep or other unmeasured mechanisms. However, integrating these findings with previously discussed research on self-reported BAS levels in bipolar disorder (Meyer et al., 1999, 2000), we tentatively hypothesize that this dysregulation following goal-attainment life events is the result of increases in BAS activity (Depue & Zald, 1993).
Interactions of the nucleus accumbens and the dopamine-secreting neurons from the ventral tegmental area (VTA DA) appear to be the neural substrate of the BAS, as evidenced by a broad range of research (Depue & Collins, 1999; Hestenes, 1992; Mirenowicz & Schultz, 1996; Porrino, 1987; Swerdlow & Koob, 1987). Individuals with bipolar disorder are hypothesized to have a deficit in the serotonergic regulation of this pathway (Depue & Zald, 1993; Winters, Scott, & Beevers, 2000), which could explain the more intense reactivity to cues of reward within this population.
Parallel to the biological dysregulation, shifts in information processing may also increase reactivity to goal attainment among bipolar individuals. First, extremely high levels of VTA DA activity produce deficits in selective attention (Weiner, 1990; Weiner & Feldon, 1997), documented during mania (Johnson, 1986, 1988). Second, positive moods enhance recall of positive memories (Blaney, 1986; Weingartner, Miller, & Murphy, 1977). Third, a history of hypomania is associated with higher expectations for ongoing success after an initial success experience (Stern & Berrenberg, 1979). As attention deficits occur, and access to positive memories and expectations for success increase, one might expect increasing distractibility, self-esteem, and confidence. Behaviorally, these information-processing shifts could increase engagement in goal-oriented behavior, thereby enhancing positive affect. In short, at a cognitive and behavioral level, we anticipate that recursive loops maximize the goal-setting behavior and positive affect, spiraling into mania. One fundamental goal for future research, therefore, is the study of information processing and positive affect among bipolar individuals, particularly in the context of goal attainments.
Understanding how goal-attainment life events influence manic symptoms has important clinical implications. Monitoring goal-attainment events, such as promotions and new romances, could allow individuals to counter the impact of these events, through careful pharmacological adjustments, sleep regulation (Wehr et al., 1998), and behavioral strategies. We do not believe that bipolar individuals should avoid goal attainment. Rather, goal attainment forms the first part of a cycle. This recursive cycle is more problematic then the trigger.
It is also important to recognize that goal-attainment life events accounted for a modest proportion of the variance in manic symptoms. Variables other than goal-attainment life events predict mania, including sleep deprivation, medication changes, and expressed emotion; whereas some of these variables may influence the BAS system, other pathways to mania probably exist as well. Some characteristics, such as medication and BAS levels, may interact with goal-attainment life events in determining mania. The current study contains too few individuals to allow for an examination of such multifactorial models. In addition, because so few individuals experienced full manic episodes, current results are more relevant for understanding increases in subsyndromal symptoms than onsets of full episodes. Further research is also needed to understand whether current findings explain specific symptom patterns associated with mania as well as whether or not current findings generalize to other subtypes of mood disorders, such as bipolar II disorder.
In summary, current results indicate that goal-attainment life events increase the risk of manic symptoms among bipolar individuals. Taken with other recent findings, results highlight the potential promise of using the BAS model to understand bipolar disorder.
Acknowledgments
This study was supported by grants from the National Alliance for Research on Schizophrenia and Depression and by National Institute of Mental Health Grams R29 MH55950 and R01 MH48171.
We thank Julie Dykstra for her help in interviewing and Charles Carver and Aaron T. Beck for their helpful feedback on this article.
Footnotes
Depue refers to a behavioral facilitation system (BFS), whereas Gray refers to a behavioral activation system (BAS). Because these appear to be essentially the same system, for simplicity we refer in the text only to the BAS.
References
- Alloy LB, Reilly-Hanington N, Fresco DM, Whitehouse WG, Zechmeister JS. Cognitive styles and life events in subsyndromal unipolar and bipolar disorders: Stability and prospective prediction of depressive and hypomanic mood swings. Journal of Cognitive Psychotherapy. 1999;13:21–40. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Anderman LH. Classroom goal orientation, school belonging and social goals as predictors of students' positive and negative affect following the transition to middle school. Journal of Research and Development in Education. 1999;32:89–103. [Google Scholar]
- Angst J. The course of affective disorders. Mood Disorders: Pharmacologic Prevention of Recurrences; Consensus Development Conference; Bethesda, MD: National Institute of Mental Health; 1984. [Google Scholar]
- Bauer MS, McBride L, Shea N, Gavin C. Impact of an easy-access VA clinic-based program for patients with bipolar disorder. Psychiatric Services. 1997;48:491–496. doi: 10.1176/ps.48.4.491. [DOI] [PubMed] [Google Scholar]
- Bech P, Bolwig TG, Kramp P, Rafaelsen OJ. The Bech–Rafaelsen Mania Scale and the Hamilton Depression Scale: Evaluation of homogeneity and inter-observer reliability. Acta Psychiatrica Scandinavica. 1979;59:420–430. doi: 10.1111/j.1600-0447.1979.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Bertelsen A, Harvald B, Hauge M. A Danish twin study of manic–depressive disorders. British journal of Psychiatry. 1977;130:330–351. doi: 10.1192/bjp.130.4.330. [DOI] [PubMed] [Google Scholar]
- Blaney PH. Affect and memory: A review. Psychological Bulletin. 1986;99:229–246. [PubMed] [Google Scholar]
- Brown GW, Harris TO. The Bedford College Life Events and Difficulty Schedule: Directory of contextual threat of events. Bedford College, University of London; London: 1978a. [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. Free Press; New York: 1978b. [Google Scholar]
- Brown GW, Harris TO. Fall-off in the reporting of life events. Social Psychiatry. 1982;17:23–28. [Google Scholar]
- Brown GW, Harris TO. Life events and illness. Guilford Press; New York: 1989. [Google Scholar]
- Carver CS, Scheier MF. On the self-regulation of behavior. Cambridge University Press; New York: 1998. [Google Scholar]
- Carver CS, White T. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The B1S/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extra-version. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF, Luciana M. A model of neurobiology: Environment interaction in developmental psychopathology. In: Lenzenweger MF, Haugaard JJ, editors. Frontiers of developmental psychopathology. Oxford University Press; New York: 1996. pp. 44–76. [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Review of Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss SP, Spoont MR. A two-dimensional threshold model of seasonal bipolar affective disorder. In: Magnuson D, Ohman A, editors. Psychopathology: An interactional perspective. Academic Press; San Diego, CA: 1987. pp. 95–123. [Google Scholar]
- Depue RA, Monroe SM. The unipolar-bipolar distinction in the depressive disorders. Psychological Bulletin. 1978;85:1001–1029. [PubMed] [Google Scholar]
- Depue RA, Zald DH. Biological and environmental processes in nonpychotic psychopathology: A neurobiological perspective. In: Costello CG, editor. Basic issues in psychopathology. Guilford Press; New York: 1993. pp. 127–237. [Google Scholar]
- Ellicott AG. Doctoral dissertation. University of California; 1989. A prospective study of life stress and bipolar illness. 1989. [Google Scholar]
- Emmons RA, Kaiser HA. Goal orientation and emotional well-being: Linking goals and affect through self. In: Martin LL, Tesser A, editors. Striving and feeling: Interactions among goals, affect, and self-regulation. Erlbaum; Mahwah, NJ: 1996. pp. 79–98. [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness. Oxford University Press; Oxford, England: 1990. [Google Scholar]
- Gorman DM. A review of studies comparing checklist and interview methods of data collection in life event research. Behavioral Medicine. 1995;19:66–73. doi: 10.1080/08964289.1993.9937567. [DOI] [PubMed] [Google Scholar]
- Gray JA. Elements of a two-process theory of learning. Academic Press; London, England: 1975. [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford University Press; Oxford, England: 1982. [Google Scholar]
- Gray JA. The psychology of fear and stress. 2nd cd. Cambridge University Press; Cambridge, England: 1987. [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition and Emotion. 1990;4:269–288. [Google Scholar]
- Gray JA. Neural systems, emotion, and personality. In: Madden J IV, editor. Neurobiology of learning, emotion, and affect. Erlbaum; New York: Hillsdale NJ: 1991. pp. 273–306. [Google Scholar]
- Gray JA. Framework for a taxonomy of psychiatric disorder. In: Van Goozen HM, Van De Poll NE, Sergeant JA, editors. Emotions- Essays on emotion theory. Erlbaum; Hillsdale, NJ: 1994. pp. 29–59. [Google Scholar]
- Harrow M, Goldberg JF, Grossman LS, Meltzer HY. Outcome in manic disorders: A naturalistic follow-up study. Archives of General Psychiatry. 1990;47:665–671. doi: 10.1001/archpsyc.1990.01810190065009. [DOI] [PubMed] [Google Scholar]
- Hestenes D. A neural network theory of manic-depressive illness. In: Levine DS, Leven SJ, Samuel J, editors. Motivation, emotion, and goal direction in neural networks. Erlbaum; Hillsdale, NJ: 1992. pp. 209–257. [Google Scholar]
- Hollingshead AB. Two-Factor Index of Social Position. Yale University; New Haven, CT: 1957. [Google Scholar]
- Hunt N, Bruce-Jones WD, Silverstone T. Life events and relapse in bipolar affective disorder. Journal of Affective Disorders. 1992;25:13–20. doi: 10.1016/0165-0327(92)90088-n. [DOI] [PubMed] [Google Scholar]
- Isometsa ET. Course, outcome, and suicide risk in bipolar disorder: A review. Psychiatric Fennica. 1993;24:113–124. [Google Scholar]
- Johnson FN. Different treatment modalities for recurrent bipolar affective disorders: An integrative approach. Psychotherapy and Psychosomatics. 1986;46:13–22. doi: 10.1159/000287958. [DOI] [PubMed] [Google Scholar]
- Johnson FN. Signal detection analysis of information processing in patjents receiving prophylactic lithium therapy. Human Psychopharmacology: Clinical and Experimental. 1988;3:95–100. [Google Scholar]
- Johnson S, Meyer B, Winett C, Small J. Social support and self-esteem predict changes in bipolar depression but not mania. Journal of Affective Disorders. 2000;58:79–86. doi: 10.1016/s0165-0327(99)00133-0. [DOI] [PubMed] [Google Scholar]
- Johnson S, Miller I. Negative life events and recovery from episodes of bipolar disorder. Journal of Abnormal Psychology. 1997;106:449–457. doi: 10.1037//0021-843x.106.3.449. [DOI] [PubMed] [Google Scholar]
- Johnson S, Roberts JR. Life events and bipolar disorder: Implications from biological theories. Psychological Bulletin. 1995;117:434–449. doi: 10.1037/0033-2909.117.3.434. [DOI] [PubMed] [Google Scholar]
- Johnson S, Winett C, Meyer B, Greenhouse W, Miller I. Social support and the course of bipolar disorder. Journal of Abnormal Psychology. 1999;108:558–566. doi: 10.1037//0021-843x.108.4.558. [DOI] [PubMed] [Google Scholar]
- Katschnig H, editor. Life events and psychiatric disorders Controversial issues. Cambridge University Press; Cambridge, England: 1986. [Google Scholar]
- Keitner GL, Ryan CE, Miller IW, Norman WH. Recovery and major depression: Factors associated with twelve-month outcome. American Journal of Psychiatry. 1992;149:93–99. doi: 10.1176/ajp.149.1.93. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Kane JM, Gelenberg AJ, Rosenbaum JF, Walzer EA, Baker LA. Subsyndromal symptoms in bipolar disorder. Archives of General Psychiatry. 1992;49:371–376. doi: 10.1001/archpsyc.1992.01820050035005. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Leahy RL. Decision-making and mania. Journal of Cognitive Psychotherapy. 1999;13:83–105. [Google Scholar]
- Leenstra AS, Ormel J, Giel R. Positive life change and recovery from depression and anxiety: A three-stage longitudinal study of primary care attenders. British Journal of Psychiatry. 1995;166:333–343. doi: 10.1192/bjp.166.3.333. [DOI] [PubMed] [Google Scholar]
- Loftus EF, Marburger W. Since the eruption of Mt. St. Helens, has anyone beat you up? Improving the accuracy of retrospective reports with landmark events. Memory & Cognition. 1983;11:114–120. doi: 10.3758/bf03213465. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson B, Sherrill JT, Siegel L, Patterson D, Kupfer DJ. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes. Archives of General Psychiatry. 1998;55:702–707. doi: 10.1001/archpsyc.55.8.702. [DOI] [PubMed] [Google Scholar]
- McQuaid JR, Monroe SM, Roberts JR, Johnson SL, Garamoni G, Kupfer DJ, Frank E. Toward the standardization of life stress assessments: Definitional discrepancies and inconsistencies in methods. Stress Medicine. 1992;8:47–56. [Google Scholar]
- Meng X, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111:172–175. [Google Scholar]
- Meyer B, Johnson S, Carver C. Exploring behavioral activation and inhibition sensitivities among college students at-risk for mood disorders. Journal of Psychopathology and Behavioral Assessment. 1999;21:275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Johnson S, Winters R. Responsiveness to threat and incentive in bipolar disorder. Relations of the BIS/BAS scales with symptoms. 2000. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Goldstein MJ, Nuechterlein KH, Snyder KS, Mintz J. Family factors and the course of bipolar affective disorder. Archives of General Psychiatry. 1988;45:225–231. doi: 10.1001/archpsyc.1988.01800270033004. [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop S, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: Reliability and validity. Psychiatry Research. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Miller IW, Norman WH, Keitner GI. Cognitive-behavioral treatment of depressed inpatients: Six- and twelve-month follow-up. American Journal of Psychiatry. 1989;146:1274–1279. doi: 10.1176/ajp.146.10.1274. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Mueller TI, Keller MB, Leon AC, Solomon DA, Shea MT, Coryell W, Endicott J. Recovery after 5 years of unremitting major depressive disorder. Archives of General Psychiatry. 1996;53:794–799. doi: 10.1001/archpsyc.1996.01830090040006. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Harvard University Press; Boston: 1996. [Google Scholar]
- Porrino L. Cerebral metabolic changes associated with activation of reward systems. In: Engel J, Oreland L, editors. Brain reward systems and abuse. Raven Press; New York: 1987. [Google Scholar]
- Reilly-Harrington NA, Alloy LB, Fresco DM, Whitehouse WG. Cognitive styles and life events as predictors of bipolar and unipolar symptomatology. Journal of Abnormal Psychology. 1999;108:567–578. doi: 10.1037//0021-843x.108.4.567. [DOI] [PubMed] [Google Scholar]
- Rosenberg DG, Winett CA, Johnson SL. Validating definitions of recovery in bipolar disorder. 13th Annual Meeting of the Society for Research in Psychopathology; Cambridge, MA. Nov, 1998. [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Shum MS. The role of temporal landmarks in autobiographical memory processes. Psychological Bulletin. 1998;124:423–442. doi: 10.1037/0033-2909.124.3.423. [DOI] [PubMed] [Google Scholar]
- Silverstone T, McPherson H, Hunt N, Romans S. How effective is lithium in the prevention of relapse in bipolar disorder? A prospective naturalistic follow-up study. Australian and New Zealand Journal of Psychiatry. 1998;32:61–66. doi: 10.3109/00048679809062707. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Toneatto T, Sobell MB, Schuller R, Maxwell M. A procedure for reducing errors in reports of life events. Journal of Psychosomatic Research. 1990;34:163–170. doi: 10.1016/0022-3999(90)90050-e. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. User's guide for the Structured Clinical Interview for DSM–III–R: SCID. American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- Stern GS, Berrenberg JL. Skill-set, success outcome, and mania as determinants of the illusion of control. Journal of Research in Personality. 1979;13:206–220. [Google Scholar]
- Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania, and depression: Toward a unified hypothesis of cortico-striato-pallidothalamic function. Behavioral & Brain Sciences. 1987;10:197–245. [Google Scholar]
- Vehmanen L, Kaprio J, Loennqvist J. Twin studies of bipolar disorder. Psychiatria Fennica. 1995;26:107–116. [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98:219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Turner EH, Shimada JM, Lowe CH, Barker C, Leibenluft E. Treatment of a rapidly cycling bipolar patient by using extended bed rest and darkness to stabilize the timing and duration of sleep. Biological Psychiatry. 1998;43:822–828. doi: 10.1016/s0006-3223(97)00542-8. [DOI] [PubMed] [Google Scholar]
- Weiner I. Neural substrates of latent inhibition: The switching model. Psychological Bulletin. 1990;108:442–461. doi: 10.1037/0033-2909.108.3.442. [DOI] [PubMed] [Google Scholar]
- Weiner I, Feldon J. The switching model of latent inhibition: An update of neural substrates. Behavioral Brain Research. 1997;88:11–25. doi: 10.1016/s0166-4328(97)02314-0. [DOI] [PubMed] [Google Scholar]
- Weingartner H, Miller H, Murphy DL. Mood-state dependent retrieval of verbal associations. Journal of Abnormal Psychology. 1977;86:276–284. doi: 10.1037//0021-843x.86.3.276. [DOI] [PubMed] [Google Scholar]
- Williams JBW, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Rounsaville B, Wittchen H. The Structured Clinical Interview for DSM–III–R (SCID): II. Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Winters R, Scott W, Beevers C. Affective distress as a central and organizing symptom in depression: Neurobiological mechanisms. In: Johnson SL, Hayes AM, Field T, Schneiderman N, McCabe P, editors. Stress, coping and depression: Proceedings of the 15th Annual Stress and Coping Conference; Mahwah, NJ: Erlbaum; 2000. pp. 177–222. [Google Scholar]
- Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness: 1991. Social Psychiatry Epidemiology. 1995;30:213–219. doi: 10.1007/BF00789056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinbarg RE, Mohlman J. Individual differences in the acquisition of affectively valenced associations. Journal of Personality and Social Psychology. 1998;74:1024–1040. doi: 10.1037//0022-3514.74.4.1024. [DOI] [PubMed] [Google Scholar]