Summary
Immunity results from a complex interplay between the antigen-nonspecific innate immune system and the antigen-specific adaptive immune system. The cells and molecules of the innate system employ non-clonal recognition receptors including lectins, Toll-like receptors, NOD-like receptors and helicases. B and T lymphocytes of the adaptive immune system employ clonal receptors recognizing antigens or their derived peptides in a highly specific manner. An essential link between innate and adaptive immunity is provided by dendritic cells (DCs). DCs can induce such contrasting states as immunity and tolerance. The recent years have brought a wealth of information on the biology of DCs revealing the complexity of this cell system. Indeed, DC plasticity and subsets are prominent determinants of the type and quality of elicited immune responses. Here we summarize our recent studies aimed at a better understanding of the DC system to unravel the pathophysiology of human diseases and design novel human vaccines.
I. Introduction
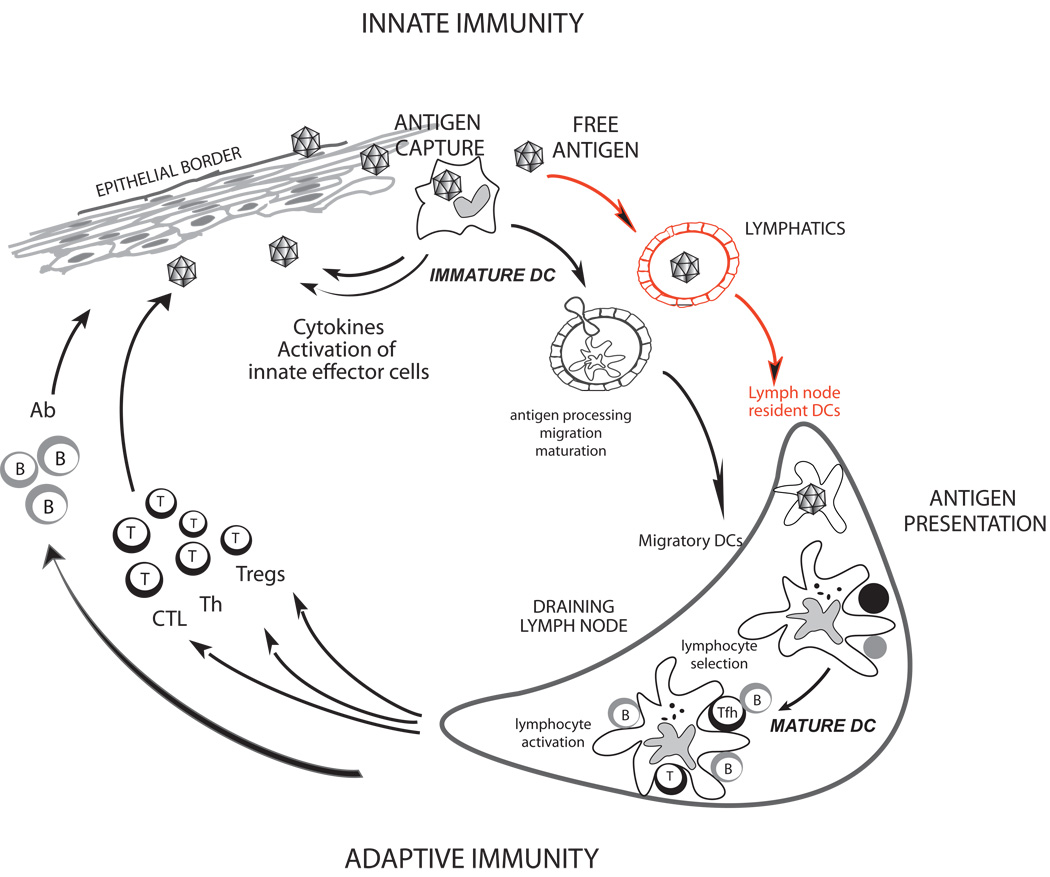
The immune system is endowed with the ability to recognize a universe of diverse molecules called antigens, and to generate responses specific to the recognized antigens. Generating the right type of immune response can be a matter of life and death. In leprosy, for instance, the tuberculoid form of the disease is characterized by a Type 1 response which keeps the disease in check, while the lepromatous form induces an often fatal Type 2 response (1). An immune response involves both the antigen (Ag)-nonspecific innate immunity and Ag-specific adaptive immunity. The innate and the adaptive immune systems act in concert to eradicate pathogens through cells, such as macrophages, granulocytes, dendritic cells (DCs) and lymphocytes, and through effector proteins such as cytokines, antimicrobial peptides, complement, and antibodies (2). Lymphocytes (T, B, NK, and NKT cells) and their products are under the control of DCs (3–6). DCs reside in peripheral tissues where they are poised to capture antigens. In the classical view, antigen-loaded DCs migrate from tissues through the afferent lymphatics into the draining lymph nodes. There, they present processed protein and lipid Ags to T cells via both classical (MHC class I and class II) and non-classical (CD1 family) antigen presenting molecules (5) (Figure 1). The soluble antigens also reach the draining lymph nodes through lymphatics and conduits where they are captured, processed, and presented by lymph-node resident DCs (7). In the steady state, non-activated (immature) DCs present self-antigens to T cells (8–10), which leads to tolerance. Once activated (mature), antigen-loaded DCs are geared towards the launching of antigen-specific immunity (11) leading to the T cell proliferation and differentiation into helper and effector cells with unique functions and cytokine profiles. DCs are also important in launching humoral immunity partly due to their capacity to directly interact with B cells (12, 13). DCs can route antigens into nondegradative recycling compartments, which allow presentation of unprocessed antigens to B cells (14, 15). DCs appear to be essential for both central tolerance in the thymus and peripheral tolerance (16). DCs induce immune tolerance partly through T cell deletion and partly through activation of regulatory T cells (Tregs). Thus, it is not surprising that alterations in DC biology are involved in autoimmune diseases, such as systemic lupus erythematosus (SLE) (17) and in malignant diseases, such as myeloma and breast cancer (18–20).
Figure 1.
DCs are composed of multiple subsets with distinct functions at the interface of the innate and adaptive immunity. The two major subsets are the myeloid DCs (mDCs) and the plasmacytoid DCs (pDCs). In this review, we will focus on our recent progresses in the determination of phenotypical and functional differences among human DC subsets (5, 6, 21). We surmise that the understanding of the biology of each DC subset is essential to unravel the pathophysiology of human diseases and design novel vaccines.
II. Human DC subsets
II-1. Myeloid DCs
In vivo mDCs exist in at least three compartments: 1) peripheral tissue DCs, 2) secondary lymphoid organ-resident DCs, and 3) circulating blood mDCs.
The best studied human DC subsets are those from skin, where three subsets can be identified. The epidermis hosts only Langerhans cells (LCs), while the dermis displays two DC subsets, CD1a+ DCs and CD14+ DCs, as well as macrophages (22). We have concentrated our studies on epidermal LCs and dermal CD14+ DCs. Dermal CD14+ DCs express a large number of surface C-type lectins including DC-SIGN, DEC-205, LOX-1, CLEC-6, Dectin-1 and DCIR, while LCs express the lectins Langerin and DCIR. Dermal CD14+ DCs express multiple Toll-like receptors (TLRs) recognizing bacterial pathogen-associated molecular patterns (PAMPs), such as TLR2, 4, 5, 6, 8, and 10 (23, 24). While LCs have been reported to express TLR1, 2, 3, 6, and 10 (23, 25), our own studies using microarray of highly purified LCs failed to show much TLR expression (24). Thus, additional studies will be necessary.
LCs and CD14+ DCs also differ in their cytokine profiles. While CD14+ DCs produce a large set of soluble factors including IL-1β, IL-6, IL-8, IL-10, IL-12, GM-CSF, MCP and TGF-β in response to stimulation via CD40, LCs produce only a few cytokines, including IL-15 (26). As we discuss hereunder, this different cytokine secretion pattern might contribute to the unique biological functions of these DC subsets.
II-1-1. Dermal CD14+ DCs: potent inducers of antibody response
In the mid 90’s, we observed that CD14+ DCs derived from CD34+ hematopoietic progenitor cells (HPCs) induce CD40-activated naïve B cells to differentiate into IgM-producing plasma cells through the secretion of IL-6 and IL-12 (27). A decade later, we found that CD14+ DCs induce naïve CD4+ T cells to differentiate into effectors sharing properties with T follicular helper cells (Tfh) (26), a CD4+ T cell subset specialized in B cell help (28, 29). There, CD4+ T cells primed by CD14+ DCs (both generated in vitro and isolated from the skin) help naïve B cells to produce large amounts of IgM, and switch isotypes towards IgG and IgA. This ability to regulate B cell differentiation appears unique to CD14+ DCs, as LCs are unable to do so.
Acquisition of Tfh phenotype and function by human CD4+ T cells depends on IL-12p70 secreted by DCs as neutralizing antibodies inhibit their development (30). IL-12 endows activated CD4+ T cells with the capacity to help the differentiation of antibody secreting cells (ASCs) via IL-21 (30), a pleotropic cytokine that promotes B cell growth, differentiation, and class-switch recombination (31). IL-12 induces human naïve CD4+ T cells to differentiate into two different types of IL-21-producing T cells: i) IL-21+ IFN-γ+ Th1 cells expressing T-bet, and ii) IL-21+ IFN-γ− non-Th1 cells. The development of both T cell types is dependent on STAT4 (Figure 2A). Whether both types of IL-21-producing CD4+ T cells display an equivalent capacity to help B cells is currently under investigation.
Figure 2.
Thus, IL-12 appears to contribute to humoral immunity in humans through two different paths: a direct path in DC-B interaction, and an indirect path through DC-T cell interaction and induction of Tfh cells (Figure 2B). These two paths might act simultaneously in vivo, through the “ménage à trois” formation of antigen-presenting DCs with antigen-specific T cells and B cells at extrafollicular sites, as recently illustrated through in vivo imaging in mice (13, 32).
Mouse studies have demonstrated that IL-12, when administered as vaccine adjuvant, enhances the development of tumor-specific CTL and Th1 responses in vivo (33, 34). In humans, systemically administered IL-12 has thus far shown only very modest clinical efficacy (35, 36). In contrast, the injection of IL-12 into tumor sites of head and neck cancer patients resulted in the activation of B cells in the draining lymph nodes, which was associated with their infiltration into tumor sites and tumor regression (37). Thus, adjuvants that promote the secretion of IL-12 might improve vaccines aimed at induction of neutralizing antibodies in humans. On the contrary, blocking of IL-12 might be beneficial to prevent the development of autoreactive B cells in human autoimmune diseases.
II-1-2. Epidermal Langerhans cells: potent activators of CD8+ T cells
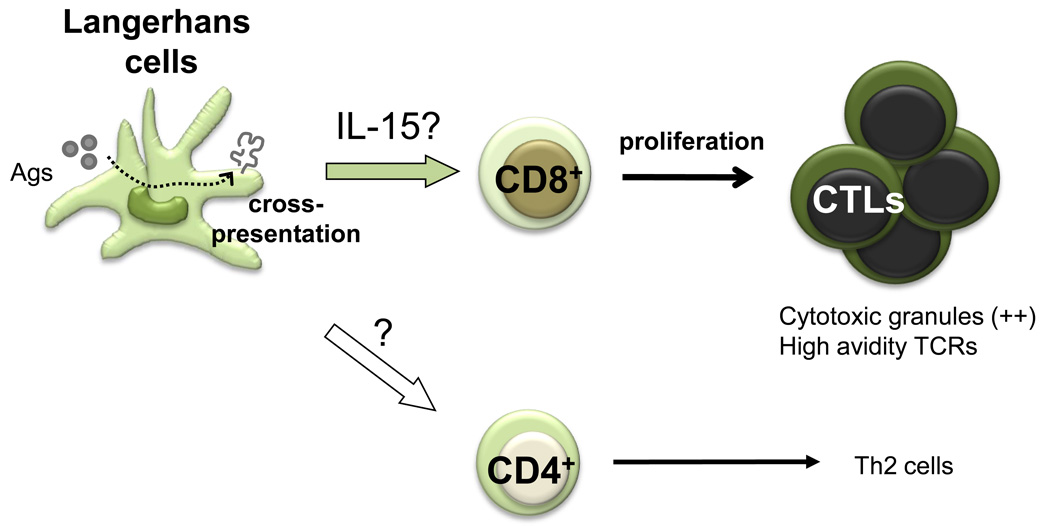
Several observations have led us to conclude that LCs are remarkably efficient at inducing CTL responses (Figure 3). Thus, LCs, either generated in vitro from CD34+ HPCs or isolated from human skin (epidermis), induce a robust proliferation of naïve allogeneic CD8+ T cells when compared to CD14+ DCs (26). When pulsed with MHC class I peptides derived from tumor or viral antigens, LCs are far more efficient than CD14+ DCs in the priming of antigen-specific CD8+ T cells. LCs are also efficient in cross-presenting peptides from protein antigens to CD8+ T cells. When compared to those induced by CD14+ dermal DCs, the CD8+ T cells primed by LCs show high avidity in tetramer binding assays and express higher levels of cytotoxic molecules, such as granzymes and perforin. Accordingly, they are remarkably more efficient in killing target cells; in particular tumor cell lines which express low levels of peptide/HLA complexes (26).
Figure 3.
Our attempts to identify the molecular mechanisms by which LCs induce potent CTL responses are only partially conclusive. LCs, but not CD14+ DCs, express IL-15, a cytokine known to enhance CD8+ T cell responses (38). Addition of IL-15 to co-cultures of CD14+ DCs and naive CD8+ T cells resulted in potent T cell priming. Thus, IL-15 might explain the preferential effects of LCs on the development of CTL responses.
While CD14+ DCs educate naïve CD4+ T cells to become IL-21-producing Tfh-like cells, LCs polarize naïve CD4+ T cells into cells secreting Type 2 cytokines such as IL-4, IL-5 and IL-13. This is consistent with mouse studies showing the preferential induction of Th2 responses upon delivery of an antigen to the LC-rich epidermis (39). Interestingly, IFN-γ-secreting CD4+ T cells are induced at a similar level by human LCs and CD14+DCs. Further studies are necessary to determine whether these IFN-γ-secreting CD4+ T cells share similar biological functions.
The human dermis contains another DC subset, dermal CD1a+ DCs, which in our hands share functional properties of both LCs and dermal CD14+ DCs. The biological role of this DC population remains to be addressed. In mice, Langerin+ DCs found in dermis (40–42) appear to share many properties of lymphoid-resident CD8α+ DCs, a subset efficient at the induction of CTL responses (43).
The heterogeneity of skin mDCs is mirrored by the existence of at least three blood mDC subsets. In human blood, CD1c+ (BDCA-1+) DCs represent a major population of LinnegHLA-DR+CD11c+ mDCs. BDCA3+ LinnegHLA-DR+CD11c+ mDCs (44) represent a minute population that uniquely expresses CLEC9A, a C-type lectin with ITAM-like motif (45). The third mDC subset expresses 6-Sulfo LacNAc, a modified PSGL-1, and CD16 (46). The physiological role of these distinct blood mDC subsets remains to be determined.
II-1-3. DC subsets and the control of humoral and cellular immunity
The results summarized above prompted us to hypothesize that the two different arms of adaptive immunity, i.e., humoral and cellular arms, are differentially regulated by the two skin mDC subsets. Thus, our in vitro studies suggest that humoral immunity is preferentially initiated by CD14+ dermal DCs, while cellular immunity is preferentially regulated by LCs (Figure 4). While the formal demonstration of this hypothesis will require in vivo studies in humans or non-human primates, this proposed dichotomy is supported by certain mouse studies. In particular, one set of studies indicated that dermal DCs migrate into the lymphoid organ outer paracortex just beneath the B cell follicles, whereas LCs migrate into the T cell rich inner paracortex (47, 48). Another set of murine studies demonstrate that LCs cross-present skin-derived exogenous antigen to CD8+ T cells and induce effector functions, such as cytokine production and cytotoxicity (48). Finally, targeting distinct DC subsets in vivo with specific antibodies leads to generation of distinct immune responses (49). While targeting CD8α+ DCs with the conjugates of αDEC205 and an antigen (OVA) preferentially induced CD8+ T cell response, targeting CD8α− DCs with the conjugates of αDCIR2 and an antigen preferentially induced CD4+ T cell response (49). This study clearly demonstrated the functional specialization of mDC subsets in vivo.
Figure 4.
II-1-4. Monocytes exposed to different cytokines generate DCs with distinct properties
Upon microbial invasion, monocytes are induced to migrate into inflammatory sites and differentiate into DCs (50). In vitro studies indicate that different cytokines skew the differentiation of monocytes into DCs with different phenotypes and functions. When activated monocytes, for example by GM-CSF, encounter IL-4 (secreted for example by mast cells), they differentiate into IL4-DCs (51). By contrast, after encounter with IFN-α/β or IL-15 (secreted by pDCs and keratinocytes, respectively) activated monocytes will differentiate into IFN-DCs or IL15-DCs, respectively (52). This spectrum of DCs represents immunestimulatory DCs which generate different types of immune responses. For example, peptide-pulsed IL15-DCs are more efficient than IL4-DCs in the induction of antigen-specific CTL differentiation in vitro (53). This is consistent with our earlier findings on LCs inasmuch as these DCs might be induced when monocytes encounter GM-CSF and IL15 secreted by keratinocytes (54). In vitro, DCs that exhibit immune-regulatory functions can be generated for example by culturing monocytes in the presence of GM-CSF and IL-10. These DCs can render T cells anergic and allow the expansion of suppressor T cells (55, 56). The challenge will be to link these distinct DC phenotypes in vitro with a specific type of immune response and immune pathology in vivo, as exemplified by TNF and IFN-α in autoimmunity.
II-2. Plasmacytoid DCs
pDCs circulate in the blood and enter lymphoid organs through high endothelial venules (HEV) rather than afferent lymphatics (57). These linnegHLA-DR+ cells express high levels of IL-3Rα chain (CD123), as well as some specific markers such as BDCA-2 (58) and ILT7 (59). Compared to mDCs, they express a different set of TLRs (60). pDC recognize viral components through TLR7 and TLR9, leading to the secretion of large amounts of Type I IFN (57).
pDCs can be activated by i) viruses (57, 61); ii) IL-3 and CD40 ligand (IL-3/CD40-L) (62, 63), possibly originating from mast cell triggered for example by parasites; and iii) by microbial components in the form of CpG DNA (64, 65). Similar to mDCs, pDCs appear to display a remarkable functional plasticity. There is evidence in mice that resting plasmacytoid DCs are involved in the induction of tolerance (66–68). However, pDCs exposed to viruses, such as live influenza virus, are able to launch memory responses by inducing the expansion and differentiation of antigen-specific memory B and T lymphocytes into plasma cells (69), and CTLs (70, 71), respectively. On the contrary, pDCs activated with CpG or IL-3/CD40L induce in vitro IL-10-secreting regulatory CD4+ T cells (72) as well as suppressor CD8+ T cells through the expression of ICOS ligand (73).
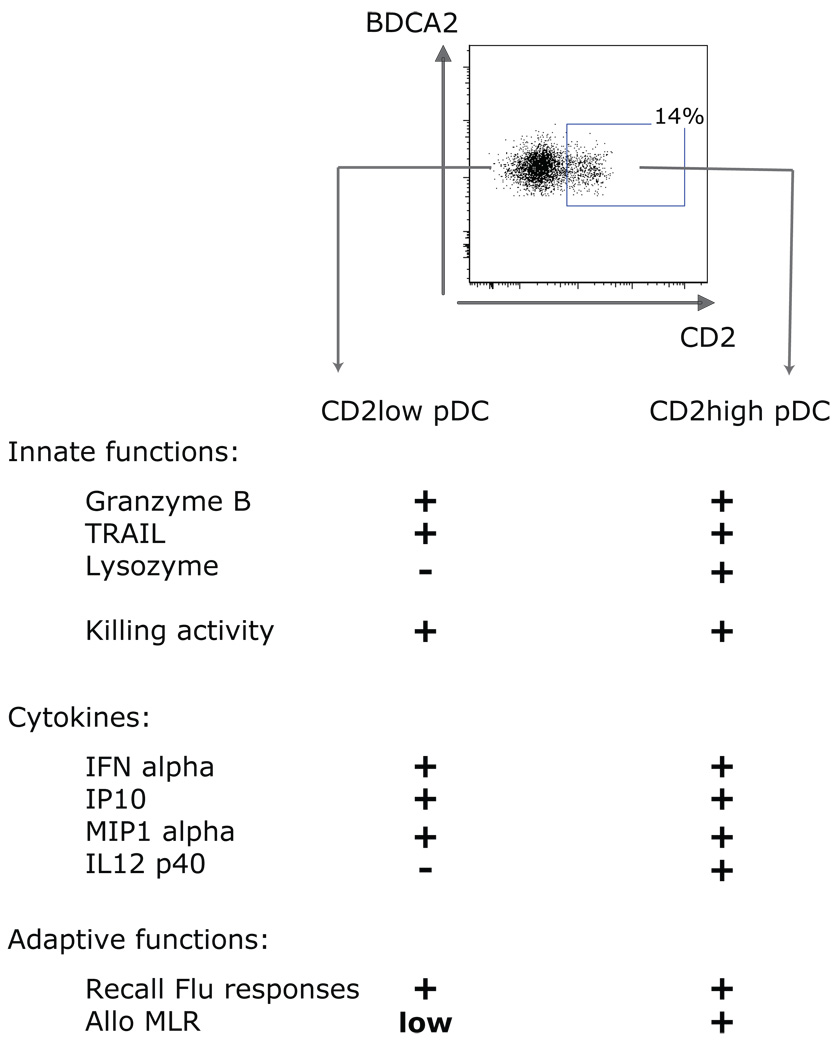
Human pDCs, in fact, are composed of two subsets, distinguished by the expression of CD2 (74)(Figure 5). Both subsets secrete IFN-α and express the cytotoxic molecules Granzyme B and TRAIL. However, the CD2high pDCs are more potent than the CD2low pDCs to induce allogeneic T cell proliferation. These different functional properties of CD2high pDCs and CD2low pDCs are associated to distinct transcription profiles, differential secretion of IL12 p40 and with differential expression of co-stimulatory molecule CD80 on activation. The differential expression of lymphoid-related genes (RAG1 and Ig rearrangement products) (75), CD4 (76), and Ly49Q (77) also indicate the existence of murine pDC subsets. Similar to our results in the human, murine pDC subsets differ in their capacity to trigger allogeneic T cell proliferation and to secrete IL12p70 and IFN-γ (75). Additional studies will be necessary to understand the biological role of these two pDC subsets.
Figure 5.
In humans, pDCs can be mobilized in vivo with cytokines such as Flt3 ligand and G-CSF (78–80), while mDCs are mobilized by Flt3L only. The differential mobilization of distinct DC subsets or DC precursors by these cytokines offers a novel strategy to manipulate immune responses in humans (21, 78).
III. Harnessing dendritic cell subsets: from bench to bedside and back to bench
Translating the new knowledge on the functional specializations of human DC subsets represents an opportunity for the design of novel therapies and novel vaccines. We will first briefly review our key studies on the involvement of DC subsets in different pathologic stimulations. Then, we will discuss how DC subsets can be harnessed for improving human health.
III-1. Alterations of DC function in diseases
III-1-1. DCs in autoimmune diseases
We have shown in the past that blood monocytes from SLE patients behave like mDCs and that exposure of normal monocytes to SLE serum results in the generation of DCs (17). Thus, SLE blood, through its IFN alpha content, represents a DC-inducing environment. Unabated DC maturation could lead to the activation and expansion of autoreactive T cells, thus explaining many of the features of the disease (81).
DCs generated in the presence of SLE sera also drive the differentiation of CD8+ T cells towards fully active cytotoxic effector T lymphocytes able to generate 1) nucleosomes (82), 2) granzyme B-dependent autoantigens (82, 83). These autoantigens could be captured and presented by mDCs, therefore establishing an amplifying loop. Indeed, administration of DCs loaded with apoptotic cells triggers autoimmune responses in mice, although clinical autoimmunity only develops in genetically susceptible recipients (84).
pDC numbers are reduced in SLE blood (17), but these cells massively infiltrate inflamed lupus skin (85). The decrease in SLE blood pDCs might thus result from their accelerated migration to inflammation sites, as demonstrated in allergen challenged nasal mucosa (86).
Together with IL-6, type I IFN promotes the differentiation of mature B cells into plasma cells (12). Thus, the effects of type I IFN on DCs, B and T cells could explain the breakdown of tolerance to nuclear antigens, autoantibody secretion and IC formation characteristic of SLE. Chromatin-containing IC activate i) B cells through the co-engagement of BCR and TLRs and ii) pDCs to secrete more type I IFN through the co-engagement of FcγR and TLRs. How human nucleic acids within IC trigger type I IFN production by pDCs is starting to be understood. HMGB1, a nuclear DNA-binding protein released from necrotic cells, is an essential component of DNA-containing immune complexes that activate pDCs and B cells in response to DNA and contribute to autoimmune pathogenesis. Furthermore, binding of HMGB1 to class A CpG oligodeoxynucleotides also increases cytokine production through the activation of TLR9 and RAGE. This results in an amplification of this pathogenic loop (87). A similar mechanism has been described for the antimicrobial peptide LL37 (also known as CAMP). This peptide, a product of keratinocytes and neutrophils, is the key factor that mediates pDC activation in psoriasis. LL37 converts inert self-DNA into a potent trigger of interferon production by binding the DNA to form aggregated and condensed structures that are delivered to and retained within early endocytic compartments in pDCs to trigger Toll-like receptor 9 (88).
DCs produce cytokines and are susceptible to cytokine-mediated activation. By controlling DC activation, cytokines regulate immune homeostasis and the balance between tolerance and immunity (81). The increased production and/or bioavailability of cytokines and associated alterations in DCs homeostasis have been implicated in various human inflammatory and autoimmune diseases as illustrated above (89). Targeting these cytokines with biological agents as already is the case with TNF (90) and IL-1 (91) represents a success of immunology and the coming years will expand the range of cytokines as therapeutic targets in autoinflammatory and autoimmune pathology.
III-1-2. DCs in tumor environment
Numerous studies in humans have concluded that DC often infiltrate tumors. We found that in >90% breast cancer cases immature mDC infiltrate the tumor beds. In addition, mature DC are found in the peri-tumoral areas in ~60% of cases (92). A number of studies have suggested that DCs can be highjacked by tumors thereby contributing to tumorigenesis. Our studies in breast cancer indicate that tumor cells polarize mDCs into a state which drives the differentiation of naïve CD4+ T cells into IL-13-secreting T cells (93). These type 2 T cells in turn facilitate breast tumor development which can be partly inhibited by IL-13 antagonists (Figure 7). The role of Th2 cells was further corroborated in a spontaneous mouse breast cancer model where Th2 cells facilitate the development of lung metastasis via macrophage activation (94). In several other mouse tumor models, IL-13 produced by NKT cells induces myeloid cells to make TGF-beta that inhibits CTL functions (95). Thus, type 2 cytokines appear involved in tumorigenesis through various mechanisms. mDCs can also have direct interactions with tumor cells as shown in multiple myeloma where they directly promote the survival and clonogenicity of tumor cells (96–99).
Figure 7.
~10% of breast carcinomas are infiltrated with pDCs, and this appears associated with poor prognosis (100). The infiltrating pDCs are functionally altered, as they produce little type I IFN upon TLR ligation (101). This inhibition appears to depend on the ligation of ILT7 on pDCs binding by BST2 expressed on tumor cells (19). Likewise, in ovarian carcinoma, tumor-infiltrating pDCs do not induce effector T cell responses (102), but rather promote the differentiation of IL10+ CCR7+ CD8+ Tregs (103).
Tumors also prevent/skew the development of T cell immunity by altering the antigen capture and presenting pathways in DCs. Tumor glycoproteins such as carcinoembryonic antigen and MUC-1 interact with C-type lectins on the DC surface leading to altered DC functions. For example, MUC-1 glycoproteins, secreted by breast cancer cells, are endocytosed by DC where they are mostly retained in early endosomes leading to inefficient processing and presentation to T cells (104).
DCs have also been associated to the promotion of angiogenesis in human tumors. pDCs may promote angiogenesis for example by the secretion of proangiogenic cytokines (105, 106).
Clearly, understanding the functions of DCs in the tumor bed represents an important area of future investigations. An interesting strategy would be to rewire their molecular pathways from “pro-tumor” DCs into “anti-tumor” DCs. Indeed, DC can fight back to tumors at least through two pathways: an indirect one with the induction of potent CTL responses, and a direct one through DC-dependent tumor cytotoxicity. Different DC subsets express cytotoxic molecules including: granzyme B by pDC, TRAIL by type I interferon-stimulated mDC or pDC, both enabling tumor cell killing. Indeed, pDCs appear to directly contribute to the anti-tumor activity of in vivo-administered Imiquimod (TLR7 ligand), which is used for the treatment of basal cell carcinoma (107, 108).
III-2. Harnessing DCs to generate novel vaccines
Microbiologists, spearheaded by Louis Pasteur, have devised ways to generate vaccines by inactivating pathogens. Most of these vaccines act through the induction of humoral responses. Unfortunately, there are still many pathogens, for which no efficient vaccines are available, including HIV, Hepatitis C virus, Mycobacteria, Chlamydia, and Plasmodium, a parasite causing Malaria. Most of these agents cause chronic diseases where strong cellular immunity, in particular CTL response, is critical for the clearance of pathogens. All what we learned on DC biology in the last three decades encourage us to harness them to design vaccines. DC-based vaccines include two main approaches: ex-vivo generated DC vaccines and DC targeting.
III-2-1. Ex-vivo generated-DC vaccines
Proof-of-concept studies with first generation DC vaccines
Ex vivo-generated DCs have been used as therapeutic vaccines mostly in patients with cancer, and in some cases in HIV-infected patients (109–111). The early studies have been discussed in details elsewhere (112). Hereunder, we briefly summarize what we have learned from phase I/II clinical trials in patients with metastatic melanoma with respect to DC vaccine immunogenicity and clinical efficacy. In our early trials, vaccines were made of DCs generated by culturing CD34+ hematopoietic progenitor cells (CD34+ HPCs) with GM-CSF, TNF and FLT3 ligand (113). These CD34-DCs contain LCs. CD34-DCs were loaded with i) control antigens KLH and Influenza matrix (FluM1) peptide; and ii) multiple HLA-A*0201 binding 9–10AA peptides derived from MART-1/MelanA, gp100, tyrosinase and MAGE3. Initially, patients received four vaccinations over 6 weeks. In the second phase of the trial, patients received four additional boost vaccinations spread over four months or longer. The second type of vaccine was made of DCs generated by culturing enriched monocytes with GM-CSF and IL-4. DCs were loaded with whole tumor cells (killed Colo829 melanoma cells) to exploit their capacity for cross-priming (114). They were also loaded with KLH and activated with TNF and CD40 ligand. Patients received eight vaccinations on a monthly schedule.
The studies of the patient immune responses to vaccines permitted us to classify them into three categories:
i) Patients who fail to mount immune responses to the melanoma antigens presented by the DC vaccines. These patients are usually early clinical progressors. Their responses to control antigens such as KLH or viral peptides (Flu-M1 or CMV) are preserved in most cases, suggesting that their immune system might be specifically tolerant to the tumor antigens.
ii) Patients who mount melanoma-antigen specific immunity but do not experience durable objective tumor regression. This represents the most common outcome of the current DC vaccination protocols. Three scenarios are to be considered: a) the immune responses elicited by the DC vaccines might not be of the quality needed to allow the rejection of the tumors, for example low avidity T cells which are susceptible to suppressive factors and cells within tumor environment; b) the induced T cells might not migrate into the tumor lesions, for example the expression of CXCR3 in blood CD8+ T cells might be necessary as it appears to correlate with survival in patients with melanoma (115); and c) the tumor micro-environment might inhibit effector T cell functions, for example by action of myeloid derived suppressor cells and Tregs (116).
The recent progresses in the immunomonitoring of specific immune responses in the blood and at the tumor site should help us address these questions (114, 117). Modern approaches including polychromatic flow cytometry rather than the analysis of a single cytokine (e.g., IFN-γ ELISPOT) and/or frequency of tetramer positive cells will contribute to better assess the quality of the immune responses elicited in the patients. Indeed, several studies mostly performed in the context of HIV vaccines have lead the conclusion that assessing the frequency of IFN-γ secreting CD8+ T cells is insufficient.
iii) Patients who mount immune responses and experience clinical benefit. In our cross-priming trial where twenty patients were vaccinated with monocyte-derived DCs loaded with killed allogeneic melanoma cells (114), two patients who mounted potent melanoma antigen-specific immune responses showed meaningful objective tumor regressions, i.e., a durable CR (18 months) and a near CR (55+ months). Both patients had failed other therapies while in stage IV (overall 10% objective response rate) (114). Thus, vaccination with DCs can elicit therapeutic immunity and our challenge is to identify approaches that will increase the fraction of patients that will experience durable tumor regression.
The analysis of long-term outcomes in our first trial performed with CD34+ HPC-derived DCs (118) revealed an association between the breadth of melanoma specific immunity and survival, i.e., patients who survived longer are those who showed the expansion of broad repertoire of antigen-specific CD8+ T cells (> 2 melanoma antigens presented on the DC vaccine) (119). Three of these patients who were vaccinated between March 1999 and March 2000 are alive as of November 2009 and show no evidence of disease. Altogether, our retrospective analysis of overall survival in a cohort of 66 patients accrued between 1999 and 2003 shows 20% long-term survival. These data need now to be confirmed in prospective randomized trials testing survival as a pre-defined clinical endpoint.
DC subsets and DC activation as a basis for second generation DC vaccines
The newer generation vaccines are built on the increased knowledge of the DC system. The two concepts which have emerged are the existence of distinct DC subsets and their functional plasticity, properties ultimately leading to the generation of distinct types of immunity (21)(Figure 6).
Figure 6.
Several groups, including our own have used IL4-DCs as DC-based vaccines following pioneering clinical studies in patients with metastatic melanoma by the groups of Nestle (120) and Schuler (121). However, as discussed above, the cytokine combinations used to differentiate monocytes into DCs are critical for the quality of the elicited T cell responses. Thus, DCs generated under alternative culture conditions such as GM-CSF and IL-15 (54, 122) might yield improved clinical responses. Another critical parameter is the DC activation pathway. For example, IL-4 DCs activated with a cocktail of IFN-α, polyI:C, IL-1β, TNF, and IFN-γ induce up to 40 times more melanoma-specific CTLs in vitro than DCs matured with the “standard” cocktail of IL-1β/TNF/IL-6/prostaglandin E2 (PGE2)(123). Furthermore, the addition of PGE2 initially thought beneficial for the induction of CCR7 expression, might in fact be detrimental for the generation of type 1 helper T cells to the benefit of Th2 cells (124, 125). Thus, the conventional “gold standard” DC vaccines established through careful in vitro studies might not perform optimally in vivo. Additional studies will be necessary. These studies are critical to the understanding of the human immune system because they permit us to assess in vivo the functional specialization of human DC subsets.
III-2-2. DC targeting
Another novel approach to vaccination is delivering antigens directly to DCs in vivo using chimeric proteins composed of an anti-DC receptor antibody and an antigen (DC targeting). Recent studies in mice demonstrate that the specific targeting of antigen to DCs in vivo results in dramatic potentiation of antigen-specific CD4+ and CD8+ T cell immunity (126, 127). These pioneering studies have been already extended to demonstrate the targeting of tumor antigens to DCs (128) and LCs in animal models (129, 130) and the generation of anti-tumor immunity (131).
Our studies with human skin DC subsets (26) suggest that targeting LCs for antigen delivery will be optimal for the induction of potent antigen-specific CTL response. There, LC-specific molecule, such as Langerin, can be used as a target DC receptor (132). Dermal CD14+ DCs might represent the appropriate target for the induction of potent humoral response. There, lectin-like oxidized-LDL receptor (LOX-1), and DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN) expressed by this subset will serve as the target DC receptors. In a DC-targeting approach, selection of a right adjuvant is also a critical parameter for the induction of the immunity of the desired type. For example, although TLR-ligands are widely considered to promote protective immunity against infectious agents selecting the appropriate ligand will be critical as illustrated by TLR2 ligation which promotes the induction of Tregs rather than Th1 or Th17 cells (133). DCs activated by different adjuvants might also induce T cells with different migration property, which represents another critical parameter for the design of vaccines.
Furthermore, certain lectins (134–139), including Dectin-1, LOX-1 and DC-SIGN, deliver intracellular signaling to activate DCs. These features of DC-lectins may place them as gatekeepers for controlling the early stage of immune responses. The next challenge is then to determine the quality of the immune responses elicited by DCs activated via lectins and to establish which DC target to choose for which vaccine.
IV. Concluding remarks
The considerable progresses made in the knowledge of DC biology as well as effector/regulatory T cell biology clearly open the avenues for development of considerably improved clinical protocols (Figure 7). Importantly, rather than the quantity of IFN-γ secreting CD8+ T cells, we should aim at generating high quality high avidity poly-functional effector CD8+ T cells able to reject tumors and long-lived memory CD8+ T cells able to prevent relapse. This new knowledge represents a fertile ground to work on to design better strategies for intervening in numerous clinical situations. The capacity of LCs and CD14+ DCs to preferentially prime cellular immunity and humoral immunity respectively has significant implications, most particularly in the context of novel human vaccines. Targeting LCs will be important for the design of vaccines that aim at eliciting strong cellular immunity. Such vaccines might be particularly useful at preventing, and perhaps even treating, chronic diseases including viral (HIV, Hepatitis C Virus), bacterial (mycobacteria) and parasitic (malaria) diseases, as well as cancer (140). The most efficient vaccines might actually be those that will target both CD14+ DCs and LCs, thereby allowing the maximal stimulation of both humoral and cellular immune responses.
Figure 8.
Figure 9.
Acknowledgments
This manuscript is dedicated to all the patients and volunteers who participated in our studies and clinical trials. We thank former and current members of the Institute for their contributions to our progresses. These studies have been supported by the NIH (P01 CA084514, U19 AIO57234, R01 CA089440 and CA078846), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. JB holds the Caruth Chair for Transplant Immunology Research.
References
- 1.Yamamura M, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–2779. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 6.Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 7.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 8.Albert ML, Bhardwaj N. Resurrecting the dead: DCs cross-present antigen derived from apoptotic cells on MHC I. The Immunologist. 1998;6:194–198. [Google Scholar]
- 9.Albert ML, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 12.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–139. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 13.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 14.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 15.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annual review of immunology. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 17.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 18.Aspord C, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. The Journal of experimental medicine. 2007 doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao W, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. The Journal of experimental medicine. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobert M, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer research. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 21.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 22.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 23.van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 24.Klechevsky E, et al. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum Immunol. 2009;70:281–288. doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flacher V, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 26.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caux C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocytemacrophage colony-stimulating factor plus tumor necrosis factor alpha: II.Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 28.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt N, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annual review of immunology. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 32.Germain RN, et al. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunological reviews. 2008;221:163–181. doi: 10.1111/j.1600-065X.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi Y, Richards EC, Chen YT, Old LJ. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tahara H, et al. Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector. J Immunol. 1995;154:6466–6474. [PubMed] [Google Scholar]
- 35.Motzer RJ, et al. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha 2a for patients with advanced renal cell carcinoma. J Interferon Cytokine Res. 2001;21:257–263. doi: 10.1089/107999001750169934. [DOI] [PubMed] [Google Scholar]
- 36.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 37.van Herpen CM, et al. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. International journal of cancer. 2008;123:2354–2361. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- 38.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez D, et al. Cutaneous antigen priming via gene gun leads to skin-selective Th2 immune-inflammatory responses. J Immunol. 2005;174:1664–1674. doi: 10.4049/jimmunol.174.3.1664. [DOI] [PubMed] [Google Scholar]
- 40.Bursch LS, et al. Identification of a novel population of Langerin+ dendritic cells. The Journal of experimental medicine. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginhoux F, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. The Journal of experimental medicine. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. The Journal of experimental medicine. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature immunology. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 44.Dzionek A, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 45.Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. The Journal of biological chemistry. 2008;283:16693–16701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schakel K, et al. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi: 10.1016/s1074-7613(02)00393-x. [DOI] [PubMed] [Google Scholar]
- 47.Kissenpfennig A, et al. Dynamics and Function of Langerhans Cells In Vivo: Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity. 2005;22:643. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Stoitzner P, et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 50.Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Current opinion in immunology. 2008;20:52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romani N, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 53.Dubsky P, et al. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 54.Mohamadzadeh M, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 56.Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–379. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 57.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 58.Dzionek A, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. The Journal of experimental medicine. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao W, et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc{varepsilon}RI{gamma} inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–870. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cella M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nature medicine. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 62.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rissoan MC, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 64.Bauer M, et al. Bacterial CpG-DNA triggers activation and maturation of human CD11c-, CD123+ dendritic cells. J Immunol. 2001;166:5000–5007. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- 65.Krug A, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 66.Goubier A, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Heer HJ, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 69.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 70.Fonteneau JF, et al. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 71.Di Pucchio T, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito T, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilliet M, Liu Y-J. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsui T, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pelayo R, et al. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang GX, et al. CD4- plasmacytoid dendritic cells (pDCs) migrate in lymph nodes by CpG inoculation and represent a potent functional subset of pDCs. J Immunol. 2005;174:3197–3203. doi: 10.4049/jimmunol.174.6.3197. [DOI] [PubMed] [Google Scholar]
- 77.Kamogawa-Schifter Y, Ohkawa J, Namiki S, Arai N, Arai K, Liu Y. Ly49Q defines 2 pDC subsets in mice. Blood. 2005;105:2787–2792. doi: 10.1182/blood-2004-09-3388. [DOI] [PubMed] [Google Scholar]
- 78.Pulendran B, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 79.Maraskovsky E, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. [PubMed] [Google Scholar]
- 81.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 82.Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2005;52:201–211. doi: 10.1002/art.20745. [DOI] [PubMed] [Google Scholar]
- 83.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. The Journal of experimental medicine. 1999;190:815–826. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bondanza A, et al. Cutting edge: dissociation between autoimmune response and clinical disease after vaccination with dendritic cells. J Immunol. 2003;170:24–27. doi: 10.4049/jimmunol.170.1.24. [DOI] [PubMed] [Google Scholar]
- 85.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid Dendritic Cells (Natural Interferon- alpha/beta-Producing Cells) Accumulate in Cutaneous Lupus Erythematosus Lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J Immunol. 2000;165:4062–4068. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- 87.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 88.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 89.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 91.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bell D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. The Journal of experimental medicine. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aspord C, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berzofsky JA, Terabe M. A novel immunoregulatory axis of NKT cell subsets regulating tumor immunity. Cancer Immunol Immunother. 2008;57:1679–1683. doi: 10.1007/s00262-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kukreja A, Radfar S, Sun BH, Insogna K, Dhodapkar MV. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: implications for bone disease. Blood. 2009;114:3413–3421. doi: 10.1182/blood-2009-03-211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kukreja A, et al. Bortezomib disrupts tumour-dendritic cell interactions in myeloma and lymphoma: therapeutic implications. Br J Haematol. 2007;136:106–110. doi: 10.1111/j.1365-2141.2006.06369.x. [DOI] [PubMed] [Google Scholar]
- 98.Kukreja A, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bahlis NJ, et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109:5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Treilleux I, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 101.Hartmann E, et al. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 102.Perrot I, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 103.Wei S, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 104.Hiltbold EM, Vlad AM, Ciborowski P, Watkins SC, Finn OJ. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells [In Process Citation] J Immunol. 2000;165:3730–3741. doi: 10.4049/jimmunol.165.7.3730. [DOI] [PubMed] [Google Scholar]
- 105.Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–1187. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curiel TJ, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–5538. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- 107.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Urosevic M, et al. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst. 2005;97:1143–1153. doi: 10.1093/jnci/dji207. [DOI] [PubMed] [Google Scholar]
- 109.Connolly NC, Whiteside TL, Wilson C, Kondragunta V, Rinaldo CR, Riddler SA. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin Vaccine Immunol. 2008;15:284–292. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia F, et al. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. The Journal of infectious diseases. 2005;191:1680–1685. doi: 10.1086/429340. [DOI] [PubMed] [Google Scholar]
- 111.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nature medicine. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 112.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 113.Banchereau J, et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 114.Palucka AK, et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 115.Mullins IM, et al. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 116.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vence L, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Banchereau J, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer research. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 119.Fay JW, et al. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34(+) progenitor-derived dendritic cells. Cancer Immunol Immunother. 2005:1–10. doi: 10.1007/s00262-005-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nestle FO, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 121.Thurner B, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dubsky P, et al. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. European journal of immunology. 2007 doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 123.Mailliard RB, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 124.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:128–135. [PubMed] [Google Scholar]
- 125.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL- 12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 126.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. The Journal of experimental medicine. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bonifaz LC, et al. In Vivo Targeting of Antigens to Maturing Dendritic Cells via the DEC-205 Receptor Improves T Cell Vaccination. The Journal of experimental medicine. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 129.Flacher V, et al. Epidermal Langerhans Cells Rapidly Capture and Present Antigens from C-Type Lectin-Targeting Antibodies Deposited in the Dermis. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei H, et al. Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009;15:4612–4621. doi: 10.1158/1078-0432.CCR-08-3321. [DOI] [PubMed] [Google Scholar]
- 132.Idoyaga J, et al. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 133.Manicassamy S, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature medicine. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 135.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 136.Jeannin P, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 137.Delneste Y, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 138.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 139.Caparros E, et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 140.Letvin NL. Correlates of immune protection and the development of a human immunodeficiency virus vaccine. Immunity. 2007;27:366–369. doi: 10.1016/j.immuni.2007.09.001. [DOI] [PubMed] [Google Scholar]