Abstract
Background
To evaluate whether increased neuroimaging use is associated with increased brain arteriovenous malformation (BAVM) detection, we examined detection rates in Kaiser Permanente Medical Care Program (KPMCP) of Northern California between 1995 and 2004.
Methods
We reviewed medical records, radiology reports and administrative databases to identify BAVM, intracranial aneurysms (IA: subarachnoid hemorrhage, SAH, and unruptured aneurysms) and other vascular malformations (OVM: dural fistulas, cavernous malformation, Vein of Galen malformation, venous malformation). Poisson regression (with robust standard errors) was used to test for trend. Random effects meta-analysis generated a pooled measure of BAVM detection rate from six studies.
Results
We identified 401 BAVMs (197 ruptured, 204 unruptured), 570 OVMs, and 2,892 IAs (2,079 SAH, 813 unruptured IA). Detection rates per 100,000 person-years were 1.4 (95% CI=1.3-1.6) for BAVM, 2.0 (1.8-2.3) for OVM, and 10.3 (9.9-10.7) for IA. Neuroimaging utilization increased 12% per year during the time period (P<0.001). Overall, rates increased for IAs (P<0.001), remained stable for OVMs (P=0.858), and decreased for BAVMs (P=0.001). Detection rates increased 15% per year for unruptured IA (P<0.001), with no change in SAH (P=0.903). However, rates decreased 7% per year for unruptured BAVM (P=0.016) and 3% per year for ruptured BAVM (P=0.005). Meta-analysis yielded a pooled BAVM detection rate of 1.3 (1.2-1.4) per 100,000, without heterogeneity between studies (P=0.25).
Conclusions
Rates for BAVM, OVM and IA in this large, multiethnic population were similar to other series. During 1995-2004, a period of increasing neuroimaging utilization, we did not observe an increased rate of detection of unruptured BAVM, despite increased detection of unruptured IA.
Keywords: brain AVM, cerebral aneurysm, vascular malformation, incidence, MRI
Introduction
Brain arteriovenous malformations (BAVM) are lesions of the cerebral vasculature in which arterial blood flow is shunted directly into the venous system without passing through a capillary system, resulting in high-flow lesions prone to rupture. The estimated detection rate of BAVMs has been reported to be about 1 per 100,000 person-years, accounting for 1-2% of all strokes.1 In order of decreasing frequency, the clinical presentations of BAVMs include hemorrhage, seizures, headaches, and neurological deficits.2 The primary management aspect of BAVM is prevention of rupture and resulting intracranial hemorrhagic event.3 The annual risk of intracranial hemorrhage after diagnosis of BAVMs is around 2-4% per year; the rate is higher for those who present initially ruptured and lower for those with unruptured presentations.1, 4, 5 BAVMs that do not bleed can cause seizures, headaches, or neurological deficits due to mass effect and involvement of neighboring eloquent brain regions.3, 6
Initial discovery of BAVMs in patients usually follows clinical presentation, most commonly hemorrhage from a ruptured lesion. Advances in neuroimaging techniques, including magnetic resonance imaging (MRI), computed tomography (CT), and cerebral angiography, have provided improved resolution for detecting and evaluating BAVMs.7-9 With the ability to detect smaller lesions with newer neuroimaging technology, the detection rate of BAVMs may potentially increase.10 Furthermore, over the last decade, utilization of both MR and CT imaging has increased dramatically in the U.S.,11 including for brain,12 which may increase incidental brain vascular malformation findings. Whether or not the increasing use of these imaging modalities has increased the detection rate of BAVMs, however, remains unclear.
There have been several studies that have reported detection rates for BAVMs in defined populations located in Minnesota,9 New York,13 Sweden,14 Scotland,15 and the Netherlands Antilles.16 However, none of these studies have evaluated recent changes or trends in detection over a long period of time. Here, we report our findings regarding detection rate of BAVMs from a large multiethnic population in Northern California between 1995 and 2004.
Methods
Study Population
This study included enrollees in the Kaiser Permanente Medical Care Program (KPMCP) of Northern California from 1995 through 2004. KPMCP is an integrated health care organization that includes around 3 million members or approximately 30% of the population of the geographical area covered. Membership characteristics are representative of the northern California region covered with the exception of underrepresentation at both extremes of the socioeconomic spectrum.17
We used multiple modalities to identify BAVM cases in the KPMCP population.5, 18-20 These modalities included computerized search of all inpatient (physician-coded) and outpatient (analyst-coded) databases, including the Admission Discharge and Transfers database, Claims Adjustment Tracking system database, Outpatient Summary Clinical Records database, and the Radiology database. For BAVM cases identified after 1998, complete text reports for all radiological procedures were searched using Current Procedural Terminology (CPT)-4 codes for various neuroradiological procedures and screened for text strings germane to BAVM. Chart review of all BAVM cases were reviewed by a study neurologist (VS) to confirm the diagnosis. Unique identifiers prevented recounting from multiple patient visits.
In addition, using International Classification of Diseases 9th edition codes, we identified KPMCP cases with a diagnosis of vascular malformations (747.81), subarachnoid hemorrhage (430), and unruptured aneurysms (437.3) as an internal comparison group for BAVMs. These cases serve as a positive control since we hypothesized that increasing neuroimaging use would uniformly increase detection rates for all brain vascular malformations over the study period. Intracranial aneurysm (IA) includes patients with unruptured aneurysms and ruptured aneurysms (subarachnoid hemorrhage, SAH). Other vascular malformations (OVM) include patients with diagnoses of dural arteriovenous fistulas, cavernous malformations, Vein of Galen malformations, or venous malformations.
The annual number of neuroimaging studies performed in KPMCP over the study period was determined from CPT-4 codes for CT scans of the brain (70450, 70460, 70470), MRI scan of the brain (70551, 70552, 705534) and cerebral angiography (75660, 75662, 75665, 75671, 75685).
Statistical Analysis
Annual detection rates (incidence) were calculated as the number of cases detected divided by total KPMCP membership in the corresponding year. We use the term detection rate rather than incidence, as suggested by others,15 since the denominator may include undetected prevalent cases in the population. However, given the rarity of the disease and a population prevalence of approximately 10 per 100,000,21 the effect of inclusion of prevalent cases on the detection rate is expected to be negligible. The denominator takes into account person-time that members are actively enrolled in the program. We calculated overall rates (per 100,000 person-years) and exact 95% Poisson confidence intervals (CI). Poisson regression analysis was used to test for trend over the 10-year period, using robust standard errors to allow for overdispersion; rate ratios (RR) and 95% confidence intervals (CI) are reported. For BAVMs, we further adjusted yearly rates by mean age of BAVM patients detected in the corresponding year. Determination of neuroimaging utilization rates (per 100,000 person-years), and BAVM detection rates per 100,000 neuroimages per year were calculated and trends analyzed in a similar fashion.
We conducted a random effects meta-analysis to generate a pooled measure of BAVM detection rate from our study and five other published studies.9, 13-16 Articles reporting detection rates of BAVMs were systematically identified via a PubMed search using a large number of terms relating to BAVMs and incidence, including “arteriovenous malformation,” “AVM,” “population-based study,” “detection rate,” and “incidence.” Inclusion criteria included both retrospective and prospective studies between 1965 to the present that: 1) reported annual detection rates specifically of symptomatic and/or incidental BAVMs in a defined population; 2) included both surgical and non-surgical cases (i.e., radiation therapy, embolization, or conservative management); and 3) determined diagnosis of BAVM either through radiological (i.e., MRI or angiogram, etc.) and/or pathological analysis (i.e., surgical pathology or autopsy). Because some studies did not report 95% CIs and to standardize methods used, we generated rates and exact 95% Poisson CI for each published study using reported data. A moment-based estimate of between-study variance and test for heterogeneity were performed. All statistical analyses were conducted using Intercooled Stata version 10 (StataCorp LP; College Station, TX).
Results
Neuroimaging utilization in KPMCP
Between 1995 and 2004, neuroimaging utilization rates per 100,000 person-years was 41 (95% CI=40-42) for angiography, 870 (95% CI=867-874) for MRI, and 1808 (95% CI=1803-1813) for CT. These rates increased linearly over the 10-year period (Figure 1) for an average increase of 12% per year (RR=1.12, 95% CI=1.08–1.15, P< 0.001). Utilization of all three neuroimaging modalities increased: 13% per year for CT (RR=1.13, 95% CI=1.09–1.16, P< 0.001), 10% per year for MRI (RR=1.10, 95% CI=1.06–1.14, P< 0.001), and 12% per year for angiography (RR=1.12, 95% CI=1.07–1.17, P<0.001).
Figure 1.
Neuroimaging utilization rates of computed tomography (CT), magnetic resonance imaging (MRI), angiography, and total imaging per 100,000 person-years in the KPMCP membership population from 1995 to 2004.
Detection Rate of BAVMs
A total of 401 BAVMs were identified, of which 197 (49.1%) were ruptured and 204 (50.9%) were unruptured cases. The majority of these cases (49-60%) were identified by radiology reports, were female (52.8%), and of white race/ethnicity (55.5%). During the 10-year period, there was a total of 28,175,520 person-years of observation, resulting in an overall BAVM detection rate of 1.42 per 100,000 person-years (95% CI=1.29-1.57; Table 1). The BAVM detection rate decreased over this period (RR=0.95, 95% CI=0.92-0.98, P=0.001); on average, a 5% decrease per year, as shown in Figure 2B.
Table 1.
Crude detection rates per 100,000 person-years for total, ruptured, and unruptured BAVMs in the Kaiser Permanente Medical Care Program (KPMCP) of Northern California population, between 1995 and 2004. 10-year rates (exact 95% Poisson confidence intervals) are reported.
| Year | Total KPMCP population | Total BAVM | Ruptured BAVM | Unruptured BAVM |
|---|---|---|---|---|
| 1995 | 2,398,562 | 1.46 | 0.79 | 0.67 |
| 1996 | 2,474,392 | 1.66 | 0.77 | 0.89 |
| 1997 | 2,615,712 | 1.61 | 0.61 | 0.99 |
| 1998 | 2,750,346 | 1.64 | 0.87 | 0.76 |
| 1999 | 2,848,935 | 1.65 | 0.74 | 0.91 |
| 2000 | 2,875,745 | 1.91 | 0.87 | 1.04 |
| 2001 | 3,051,435 | 1.41 | 0.59 | 0.82 |
| 2002 | 3,052,714 | 0.98 | 0.52 | 0.46 |
| 2003 | 3,064,036 | 1.04 | 0.69 | 0.36 |
| 2004 | 3,043,647 | 1.02 | 0.59 | 0.43 |
| 1995-2004 | 2,817,552 | 1.42 (1.29-1.57) | 0.70 (0.60-0.80) | 0.72 (0.63-0.83) |
Figure 2.
A) Total number of intracranial aneurysm (IA), brain arteriovenous malformation (AVM), and other vascular malformation (OVM) cases detected from 1995 to 2004; B) Detection rate of IA, AVM, and OVM per 100,000 person-years.
The declining trend of BAVM detection could not be explained by neuroimaging volume or age of cases. BAVM detection rates per 100,000 neuroimages per year resulted in a similar decreasing trend (RR=0.82, 95% CI=0.79-0.85, P<0.001), despite a 4-fold increase in neuroimaging volume over the 10-year period (Supplemental Figure). Adjusting for mean age of BAVM cases per year also had little effect on the declining trend (RR=0.96, 95% CI=0.93-0.99, P=0.021), even though increasing mean age was a significant predictor for BAVM detection (RR=1.03, 95% CI=1.01-1.05, P=0.008). Gender was not associated with detection rate (P=0.347).
For ruptured and unruptured BAVMs, the mean detection rate per 100,000 person-years was 0.70 (95% CI=0.60-0.80) and 0.72 (95% CI=0.63-0.83), respectively. A small but significant decreasing trend for detection was observed for both ruptured BAVM (P=0.005) and unruptured BAVM (P=0.016, Figure 3). On average, the rates decreased by 3% per year (RR=0.97, 95% CI=0.95-0.99) for ruptured BAVM and by 7% per year (RR=0.93, 95% CI=0.87-0.99) for unruptured BAVM.
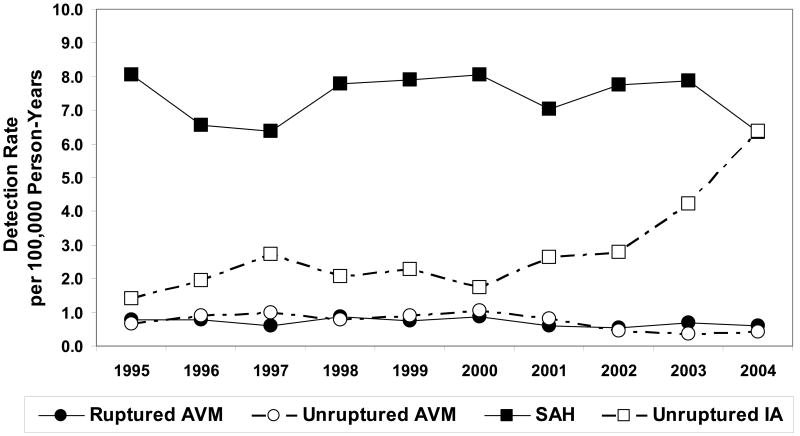
Figure 3.
Detection rate of ruptured and unruptured BAVM and intracranial aneurysms per 100,000 person-years.
Detection Rate of OVMs and IAs
For reference, we compared the BAVM detection rates during this 10-year period with those for OVMs and IAs. There were 570 OVM cases, including 377 (66.1%) cavernous malformations, 98 (17.2%) dural AVMs, 94 (16.5%) venous malformations, and 1 (0.2%) case of anomalous venous drainage. The overall detection rate of OVMs was 2.02 (95% CI=1.86-2.20) per 100,000 person-years, with fairly stable rates over the 10-year period (P=0.858, Figure 2B).
A total of 2,892 IA cases was identified, of which 2,079 (72%) were related to SAH and 813 (28%) were unruptured IAs. The mean annual detection rates per 100,000 person-years were 10.26 (95% CI=9.89-10.65) for total IA, 7.38 (95% CI=7.06-7.70) for SAH, and 2.89 (95% CI=2.69-3.09) for unruptured IAs. The overall IA detection rate increased by 4% per year (RR=1.04, 95% CI=1.02-1.05, P<0.001) during this 10-year period (Figure 2B). When examining rates by rupture status (Figure 3), there was a 15% increase per year for unruptured aneurysms (RR=1.15, 95% CI=1.08-1.23, P<0.001), accompanied by no significant change in SAH detection (P=0.903).
Detection Rate of BAVMs in Comparison to Other Studies
To compare our results to the literature, we reviewed all studies that measured detection rates for BAVMs. Our calculated detection rate of 1.42 was similar to but higher than five other studies,9, 13-16 which reported values ranging from 1.10 to 1.34 per 100,000 person-years. A random effects meta-analysis, including our study estimate along with these five other studies (Figure 4), yielded a combined BAVM detection rate of 1.31 (95% CI=1.21-1.41) per 100,000 person-years. Test for heterogeneity between studies included in the meta-analysis was not significant (P=0.246).
Figure 4.
Crude BAVM detection rate in KPMCP, a large HMO population, and other population-based studies with 95% confidence intervals. The area of each point estimate box is weighted by the inverse variance. Random effects meta-analysis was performed to obtain a combined estimate of BAVM detection rate; no significant heterogeneity between studies was observed (P=0.25).
Discussion
Using multiple modalities for BAVM ascertainment, we report an overall BAVM detection rate of 1.42 (95% CI=1.29-1.57) per 100,000 person-years in this large multiethnic population during 1995 through 2004. Despite an increasing trend for neuroimaging utilization during this 10-year period, the overall detection rates did not uniformly increase for all brain vascular malformations. In particular, this is the first report noting a recent declining trend for detection of both unruptured and ruptured BAVMs.
Our BAVM detection rate was similar to previously published prospective,13-15 as well as retrospective population-based studies.9, 16 Al-Shahi et al reported a detection rate for first-in-a-lifetime diagnosis of BAVM of 1.12 (95% CI=0.90-1.37) per 100,000 person-years in their prospective study following the Scotland population for two years between 1999 and 2000.15 Hillman et al report a detection rate for de novo diagnosed BAVMs of 1.24 per 100,000 person years in Linköping, Sweden, between 1989 and 1999.14 Stapf et al reported a detection rate of 1.34 (95% CI=1.18-1.49) per 100,000 person-years in their prospective study of a defined population in the New York islands during 27 months from 2000 to 2002.13 Furthermore, detection rates were also similar to older retrospective studies based in Olmsted County, Minnesota from 1965 to 1992,9 and the Netherlands Antilles between 1980 and 1990.16 Despite different study populations and methods used, there was no significant heterogeneity between studies allowing us to combine estimates together with our study for a pooled BAVM detection rate of 1.31 (95% CI=1.21-1.41) per 100,000.
Detection rates for unruptured (0.70 per 100,000) and ruptured BAVMs (0.72 per 100,000) in our study were also similar but slightly higher than those reported in the New York Islands AVM Study (0.51; 95% CI=0.41-0.61),13 the Northern Manhattan Stroke Study (0.55; 95% CI=0.11-1.61),22 and the Scottish Intracranial Vascular Malformation Study (0.51; 95% CI=0.37-0.69). However, the 95% CI intervals overlap with estimates from our study.
In the KPMCP study population, we observed an overall decreasing trend for detection of BAVMs, an increasing trend for IAs, and no change in OVMs over the 10 year period. Interestingly, the rate of unruptured BAVM detection was decreasing at the same time the rate of unruptured aneurysms was increasing. This finding was surprising to us as we had expected the rate of all unruptured vascular malformations to increase given the improved neuroimaging resolution to detect smaller lesions (especially for aneurysms) and increasing rate of utilization for CT, MRI, and angiography in the KPMCP population over this time period. Our detection rate and trends for total aneurysms (10.3, 95% CI=9.9-10.7) and SAH (7.4 per 100,000) were consistent with other published studies. In a population-based study conducted in Olmsted County, Minnesota between 1965 to 1995, for example, the detection rate for total IA and SAH were 9.0 (95% CI=7.8-10.2) and 6.9 (95% CI=5.9-8.0), respectively.23
The declining detection rate of BAVM observed in our study population, despite increasing neuroimaging volume or age of cases, is more difficult to explain. The results may be due to a general decrease in the true incidence of BAVM, or some fundamental change in how newer lesions become symptomatic or are diagnosed, e.g., greater specificity with newer imaging technology. Perhaps previous BAVM cases diagnosed in the pre-MRI era would now be ruled out as a true case, resulting in fewer diagnosed BAVM cases. Another interpretation would be related to biases in case ascertainment and referral algorithms, although less likely because BAVM cases would be captured in the KPMCP databases even if diagnosed outside the Kaiser system, as long as the patient remained an active Kaiser member.
Our results should be interpreted in light of several study limitations. Even though KPMCP is a community-based system and we evaluated both outpatient and in-hospital medical records, true detection may be underestimated because of sudden death before medical evaluation was possible (e.g., survival bias). Results may not be generalizable to enrollees that permanently drop out of the KPMCP system. However, 8-year retention rates in KPMCP for members between 35-84 years are high (>70%), and morbidity and mortality from BAVM hemorrhage is significantly less than other forms of brain hemorrhage.24 By definition, all BAVM cases receive some form of diagnostic imaging; however, not all cases would necessarily receive confirmatory angiography in addition to MRI or CT. Thus, some of these cases classified as BAVM may have been misdiagnosed; detailed neuroimaging data on cases (e.g., type) was not available for analysis. Our estimates for IA and OVM cases may also be subject to misclassification because these cases were identified using ICD-9 codes only and not verified by chart review as with BAVMs. However, relative to one another, we nevertheless were able to detect increased detection of unruptured IA vs. decreased detection of unruptured BAVM.
Summary
In conclusion, we observed a trend for declining detection rates for total, ruptured, and unruptured BAVMs in a large multiethnic population based in Northern California from 1995 to 2004. The reported detection rate of this brain vascular malformation was similar to other published studies for a combined rate of 1.3 per 100,000. Despite the similarity of detection rates of BAVMs among different geographic locations, our data suggest a slight decrease in the detection of unruptured BAVM and an increased detection of unruptured IA during this 10-year period.
Supplementary Material
Acknowledgments
The authors would like to thank members of the UCSF-KPMCP Brain AVM Study Project, and all the patients who participated in the study.
Sources of Funding: This study was supported by NIH grants R01 NS034949 (WLY) and K23 NS058357 (HK), and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Al-Shahi R, Warlow C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001;124:1900–1926. doi: 10.1093/brain/124.10.1900. [DOI] [PubMed] [Google Scholar]
- 2.Hofmeister C, Stapf C, Hartmann A, Sciacca RR, Mansmann U, terBrugge K, Lasjaunias P, Mohr JP, Mast H, Meisel J. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke. 2000;31:1307–1310. doi: 10.1161/01.str.31.6.1307. [DOI] [PubMed] [Google Scholar]
- 3.Choi JH, Mohr JP. Brain arteriovenous malformations in adults. Lancet Neurol. 2005;4:299–308. doi: 10.1016/S1474-4422(05)70073-9. [DOI] [PubMed] [Google Scholar]
- 4.Yamada S, Takagi Y, Nozaki K, Kikuta K, Hashimoto N. Risk factors for subsequent hemorrhage in patients with cerebral arteriovenous malformations. J Neurosurg. 2007;107:965–972. doi: 10.3171/JNS-07/11/0965. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Sidney S, McCulloch CE, Poon KY, Singh V, Johnston SC, Ko NU, Achrol AS, Lawton MT, Higashida RT, Young WL. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38:2430–2437. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- 6.Mast H, Mohr JP, Osipov A, Pile-Spellman J, Marshall RS, Lazar RM, Stein BM, Young WL. “Steal” is an unestablished mechanism for the clinical presentation of cerebral arteriovenous malformations. Stroke. 1995;26:1215–1220. doi: 10.1161/01.str.26.7.1215. [DOI] [PubMed] [Google Scholar]
- 7.Bambakidis NC, Sunshine JL, Faulhaber PF, Tarr RW, Selman WR, Ratcheson RA. Functional evaluation of arteriovenous malformations. Neurosurg Focus. 2001;11:e2. doi: 10.3171/foc.2001.11.5.3. [DOI] [PubMed] [Google Scholar]
- 8.Riina HA, Gobin YP. Grading and surgical planning for intracranial arteriovenous malformations. Neurosurg Focus. 2001;11:e3. doi: 10.3171/foc.2001.11.5.4. [DOI] [PubMed] [Google Scholar]
- 9.Brown RD, Jr, Wiebers DO, Torner JC, O'Fallon WM. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology. 1996;46:949–952. doi: 10.1212/wnl.46.4.949. [DOI] [PubMed] [Google Scholar]
- 10.Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237–1243. doi: 10.1056/NEJM199304293281706. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JM. Utilization trends for advanced imaging procedures: evidence from individuals with private insurance coverage in California. Med Care. 2008;46:460–466. doi: 10.1097/MLR.0b013e31815dc5ae. [DOI] [PubMed] [Google Scholar]
- 12.Rao VM, Parker L, Levin DC, Sunshine J, Bushee G. Use trends and geographic variation in neuroimaging: nationwide medicare data for 1993 and 1998. AJNR Am J Neuroradiol. 2001;22:1643–1649. [PMC free article] [PubMed] [Google Scholar]
- 13.Stapf C, Mast H, Sciacca RR, Berenstein A, Nelson PK, Gobin YP, Pile-Spellman J, Mohr JP. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34:e29–33. doi: 10.1161/01.STR.0000068784.36838.19. [DOI] [PubMed] [Google Scholar]
- 14.Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001;95:633–637. doi: 10.3171/jns.2001.95.4.0633. [DOI] [PubMed] [Google Scholar]
- 15.Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC, Sellar RJ, Warlow CP. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS) Stroke. 2003;34:1163–1169. doi: 10.1161/01.STR.0000069018.90456.C9. [DOI] [PubMed] [Google Scholar]
- 16.Jessurun GA, Kamphuis DJ, van der Zande FH, Nossent JC. Cerebral arteriovenous malformations in The Netherlands Antilles. High prevalence of hereditary hemorrhagic telangiectasia-related single and multiple cerebral arteriovenous malformations. Clin Neurol Neurosurg. 1993;95:193–198. doi: 10.1016/0303-8467(93)90123-x. [DOI] [PubMed] [Google Scholar]
- 17.Gordon NP. Oakland, CA: Kaiser Permanente Division of Research; 2006. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? Available from: http://www.dor.kaiser.org/dor/mhsnet/public/kpnc_community.htm. [Google Scholar]
- 18.Kim H, Hysi PG, Pawlikowska L, Choudhry S, Burchard EG, Kwok PY, Sidney S, McCulloch CE, Young WL. Population stratification in a case-control study of brain arteriovenous malformation in Latinos. Neuroepidemiology. 2008;31:224–228. doi: 10.1159/000160215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullerton HJ, Achrol AS, Johnston SC, McCulloch CE, Higashida RT, Lawton MT, Sidney S, Young WL. Long-term hemorrhage risk in children versus adults with brain arteriovenous malformations. Stroke. 2005;36:2099–2104. doi: 10.1161/01.STR.0000181746.77149.2b. [DOI] [PubMed] [Google Scholar]
- 20.Halim AX, Johnston SC, Singh V, McCulloch CE, Bennett JP, Achrol AS, Sidney S, Young WL. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697–1702. doi: 10.1161/01.STR.0000130988.44824.29. [DOI] [PubMed] [Google Scholar]
- 21.Berman MF, Sciacca RR, Pile-Spellman J, Stapf C, Connolly ES, Jr, Mohr JP, Young WL. The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000;47:389–396. doi: 10.1097/00006123-200008000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Hernesniemi J, Keranen T. Microsurgical treatment of arteriovenous malformations of the brain in a defined population. Surg Neurol. 1990;33:384–390. doi: 10.1016/0090-3019(90)90149-j. [DOI] [PubMed] [Google Scholar]
- 23.Stapf C, Labovitz DL, Sciacca RR, Mast H, Mohr JP, Sacco RL. Incidence of adult brain arteriovenous malformation hemorrhage in a prospective population-based stroke survey. Cerebrovasc Dis. 2002;13:43–46. doi: 10.1159/000047745. [DOI] [PubMed] [Google Scholar]
- 24.Menghini VV, Brown RD, Jr, Sicks JD, O'Fallon WM, Wiebers DO. Incidence and prevalence of intracranial aneurysms and hemorrhage in Olmsted County, Minnesota, 1965 to 1995. Neurology. 1998;51:405–411. doi: 10.1212/wnl.51.2.405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.