Abstract
Social neuroscience is rapidly exploring the complex territory between perception and action where recognition, value, and meaning are instantiated. This review follows the trail of research on oxytocin and vasopressin as an exemplar of one path for exploring the “dark matter” of social neuroscience. Studies across vertebrate species suggest that these neuropeptides are important for social cognition, with gender and steroid-dependent effects. Comparative research in voles yields a model based on inter-species and intra-species variation of the geography of oxytocin receptors and vasopressin V1a receptors in the forebrain. Highly affiliative species have receptors in brain circuits related to reward or reinforcement. The neuroanatomical distribution of these receptors may be guided by variations in the regulatory regions of their respective genes. This review describes the promises and problems of extrapolating these findings to human social cognition, with specific reference to the social deficits of autism.
Social neuroscience has come a long way in a short time. Two decades ago a gap existed between behavioral neuroscience, systems neuroscience, behavioral ecology, and social psychology. Today the field of social neuroscience fills this gap with abundance: social neuroscience now has its own journals, textbooks, societies and, according to PubMed, nearly 3000 research papers.(PubMed, February 22,2010) Much of this stunning growth has been driven by human neuroimaging studies seeking the neural correlates of psychological processes, from face perception to social preferences. Social neuroscience has a different foundation in animal studies, built on molecular and cellular approaches as well as the tools of systems neuroscience. In fact, the history of animal studies of social perception and social behavior with classical lesion and neurophysiological techniques extends back several decades in the venerable literatures of neuroethology and behavioral neuroendocrinology. This review will follow a single thread of social neuroscience spun from this older animal research, recently woven into human studies, and now suggesting potential treatments of human disorders of social behavior, such as autism.
Social Neuroscience in 2010
Most of social neuroscience can be separated into studies of either receptive or expressive processes. Receptive studies, which emerged from neuroethology, focus on sensory processing. From the elegant work on pheromone receptors in mice (Dulac and Torello, 2003) to the careful mapping of face cells in human and non-human primates (Kanwisher, 2006), this arm of social neuroscience has described the neural geography and, in some cases, the cellular landscape by which sensory information is initially encoded as social. A fundamental insight from receptive studies is that in most vertebrates, the brain employs specific receptors or cortical regions for processing social information, whether that information is from pheromonal/olfactory, audio-vocal, somatosensory, or visual cues. That is, social information is not simply complex multi-sensory perception, it is perceived and encoded in unique ways in the brain.
Expressive studies, long the domain of behavioral neuroscience and behavioral neuroendocrinology, focus on social interactions: communication, reproductive behavior (especially parental care and sex), agonistic actions (aggression and predation), and affiliative behaviors (including social play). In vertebrates (and many invertebrates), nearly all of these behaviors are influenced by gonadal steroids (estrogens and androgens), acting via their nuclear receptors. The mapping of gonadal steroid receptor expression in the brain helped identify key regions for social behavior.(McEwen et al., 1979; Pfaff and Keiner, 1973; Pfaff and McEwen, 1983) Additionally, steroid receptors are transcription factors, and by identifying the genes regulated by steroid receptors, a molecular basis for social behavior could be proposed. (Warnmark et al., 2003)
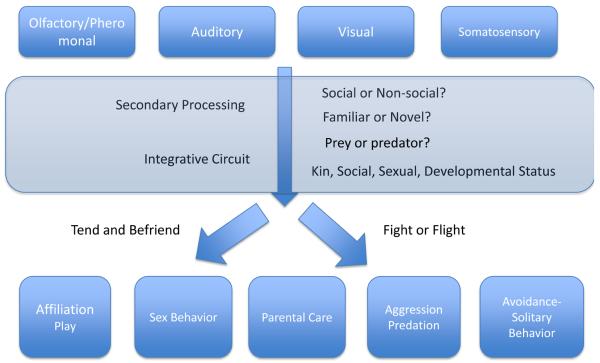
Between the receptive and expressive arms sits the great dark matter of social neuroscience. (Figure 1) What happens between the stage when a percept is encoded as “social” to the stage when a “go” signal is given for initiating social behavior? How does the brain distinguish prey from predator, juvenile from adult, novel from familiar, kin from unrelated conspecific? What are the neural mechanisms that facilitate or inhibit social interaction? These are the questions which have been more difficult to answer.
Figure 1. The Dark Matter of Social Neuroscience.
Social neuroscience has benefitted from the legacy of two venerable traditions: studies of sensory processing from neuroethology and studies of social behaviors from neuroendocrinology. The vast and often mysterious territory in between the sensory input and motor outputs – the “dark matter” – involves integrative circuits that remain to be fully described. At the simplest level, the outputs can be described as approach and affiliation, which Taylor and colleagues have called “tend and befriend” versus agonistic behavior or avoidance, classically called “fight or flight”.(Taylor et al., 2000)
Recently, researchers have begun to address these questions with human neuroimaging studies seeking to map various aspects of higher order processing of social information and even develop computational principles for the neural basis of social cognition.(Behrens et al., 2009) Human neuroimaging studies can describe the cortical patterns but mechanistic studies are still mostly the domain of animal research. In fact, some of the cellular mechanisms for the dark matter of social neuroscience are being explored with greatest success in Caenorhabditis elegans (C. elegans), an invertebrate with a central nervous system (CNS) composed of only 302 neurons but a surprisingly complex behavioral repertoire that includes social feeding and social avoidance. Dr. Cornelia Bargmann's laboratory has recently reported on the first cellular model of social behavior in C. elegans, describing a hub and spoke arrangement of cells for organizing social information processing, regulating the expression of affiliative behavior.(Macosko et al., 2009) The molecular basis of social feeding in C. elegans largely depends on a neuropeptide receptor, encoded by the npr gene.(de Bono and Bargmann, 1998) Gene transfer experiments between strains of worms that differ in their propensity for feeding in social groups demonstrate that this single genetic variant can shift a social strain to become solitary or a solitary strain to become social.(de Bono and Bargmann, 1998) Social feeding is driven by neurons that detect noxious chemicals in the environment.(de Bono et al., 2002; Gray et al., 2004) While we have much to learn about the dark matter of social neuroscience, these seminal studies in C. elegans demonstrate that (a) complex social behaviors may rely on surprisingly simple molecular mechanisms, (b) neuropeptides and their receptors appear to be important mediators of social behaviors, and (c) comparative studies may be a powerful approach for social neuroscience. The remainder of this review applies these three principles to understand the neurobiology of social affiliation involving two neuropeptides: oxytocin and vasopressin.
Neuropeptides as neuromodulators
Of the roughly 100 neuropeptides described in the mammalian brain, most are synthesized and released from the hypothalamus, often with peripheral effects as endocrine hormones. Neuropeptides usually interact with G-protein coupled receptors through which they act as slow neurotransmitters or neuromodulators. Nonapeptides are one of the oldest families of neuropeptides: each with nine amino acids and a genetic structure that includes a large precursor protein known as neurophysin. The nonapeptide lineage has been traced through invertebrates and includes members in virtually every vertebrate taxa. There are two members of this class in vertebrates: arginine vasotocin (arginine vasopressin in mammals) and the oxytocin-like peptides (isotocin in fish, mesotocin in lungfish and non-eutherian tetrapods, and oxytocin in eutherian mammals). Across these diverse species, three aspects appear to be conserved: (a) nonapeptides are usually expressed selectively in brain and gonads; (b) nonapeptides and their receptors are influenced by gonadal steroids, seasonality, and gender; and (c) nonapeptides are important for social behavior, often in a highly species-typical fashion.
The remarkable evolution of nonapeptides and social behavior has been reviewed elsewhere (Donaldson and Young, 2008; Goodson, 2005) A few examples help to illustrate the extraordinary evolutionary conservation of the behavioral effects of this family. In the mollusc Lymnaea stagnalis, a single ancestral nonapeptide (lys-conopressin) is expressed selectively in neuronal and gonadal cells where it binds to a G-protein coupled receptor to influence male copulatory behavior.(Van Kesteren et al., 1995; van Kesteren et al., 1992; van Kesteren et al., 1996) In birds, the representative nonapeptides are vasotocin and mesotocin. In different species of finches, mesotocin receptor distribution in the lateral septum correlates with flock size and administration of mesotocin increases while a mesotocin antagonist reduces social behavior, such as flock formation.(Goodson et al., 2009) In bony fish, arginine vasotocin and isotocin have been studied extensively. The plainfin midshipman is a vocal teleost fish in which grunts are an important aspect of reproductive behavior. Arginine vasotocin, but not isotocin, regulates grunting in males whereas isotocin but not arginine vasotocin influences grunting in females. (Goodson and Bass, 2000)
Oxytocin and Vasopressin in Mammals
The same principles of nonapeptide function observed in invertebrates and non-mammalian vertebrates are evident in rodents. Oxytocin (OT) and arginine vasopressin (AVP) are synthesized in the brain's hypothalamic paraventricular and supraoptic nuclei, with AVP also synthesized in the suprachiasmatic nucleus. Both neuropeptides are transported via large neurosecretory axons to the posterior hypothalamus, hence their common designation as neurohypophyseal peptides. OT is released from the posterior pituitary in response to sexual stimulation, uterine dilatation, nursing, and, in some situations, stress. OT receptors in the uterine and mammary myoepithelium result in labor and lactation. Importantly, expression of these peripheral OT receptors increases markedly in response to the gonadal steroids of late pregnancy. AVP is released in response to sexual stimulation, uterine dilatation, stress, and dehydration. AVP V2 receptors in the kidney are anti-diuretic, whereas V1a as well as V1b receptors in the vascular tree, adrenal gland, uterus, and other tissues mediate the diverse peripheral effects of this peptide.
More relevant to social neuroscience, OT and AVP expressing neurons in the hypothalamus also project centrally and OT, V1a, and V1b receptors are found in the brain. Early studies described central effects of OT that were consistent with the peptide's peripheral effects on labor and lactation.(Insel, 1997; Kendrick, 2004) Similarly, AVP was reported to have central effects on memory and aggression, among many other behaviors.(Keverne and Curley, 2004) As in non-mammals, many of the effects are species-specific, some are gender-specific, and nearly all are dependent on gonadal steroids.(see, for instance, (Choleris et al., 2003) While this review will focus on neuropeptide effects mediated via integrative networks, the reader should note at the outset that OT and AVP can also influence early processing of social perception, as shown in the rat olfactory bulb.(Tobin et al.) Below I summarize studies of OT and maternal behavior as well as AVP and affiliation.
OT and Maternal Behavior
In rats, maternal behavior is initiated only after parturition.(Numan, 1988) Adult virgin females avoid or attack pups. Adult virgin females primed with estrogen and injected centrally with OT were reported to exhibit full maternal behavior, including nest building and crouching over pups in a nursing posture.(Fahrbach et al., 1984; Pedersen et al., 1982); although also see (Rubin et al., 1983). Importantly, an OT receptor antagonist could block the natural postpartum onset of maternal behavior (Fahrbach et al., 1985) As estrogen increased OT receptor expression specifically in the bed nucleus of the stria terminalis and ventral tegmental area, analogous to effects in uterine and mammary tissue, the localized increase in receptors along with the pulsatile central release of the peptide during parturition was thought to initiate maternal behavior.(Insel, 1990) This model has now been supported by neuroimaging studies in lactating rats.(Febo et al., 2005) Similar evidence emerged from physiological studies in sheep, also a species with the onset of maternal care generally only following parturition or with the stimulated release of OT following vagino-cervical stimulation. (Kendrick et al., 1997; Keverne and Kendrick, 1992) While most of the focus has been on OT as a mediator of maternal behavior, a recent study has demonstrated an important role for AVP as well.(Bosch and Neumann, 2008) Note that AVP can bind to all four receptors, including the OTR, so effects of the peptide may not be specific for a single receptor sub-type.
Given the apparent necessity and the parsimony of OT's central effects on maternal behavior (in addition to peripheral effects on lactation and parturition), one might suppose that an OT null mutant (OT-KO) mouse would fail to be maternal. In fact, OT-KO mice exhibit relatively normal maternal behavior.(Nishimori et al., 1996; Young et al., 1998), although subtle deficits have been reported.(Pedersen et al., 2006) OT-KO mice have profound social amnesia (without apparent deficits in non-social memory) and they show altered aggression, but maternal behavior is largely preserved.(Ferguson et al., 2000) Mice with a knock out of the OT receptor show deficits in maternal behavior, suggesting that AVP or some other endogenous ligand may be binding to the OT receptor in OTKO mice.(Takayanagi et al., 2005) It is also important to recognize that mice, unlike rats or sheep, are “promiscuously” maternal, meaning that they do not require parturition or steroid induction to exhibit maternal care. The species difference is instructive: OT may be critical for the initiation of maternal care, permitting female rats and sheep to overcome their avoidance of neonates.(Russell and Leng, 1998) But there is a deeper lesson here as well. There is a profound difference in the forebrain distribution of OT receptors in mice and rats even in the absence of any difference in the distribution or quantity of OT cells.(Insel et al., 2001) The receptor maps may be a useful guide to understanding the function of the peptide in different species. For instance, the gonadal steroid induction of OT receptors specifically in the bed nucleus of the stria terminalis of the rat brain may be important for inhibiting pup avoidance and permitting maternal behavior to emerge.(Insel et al., 2001)
AVP and Affiliation
Voles are Microtine rodents found in diverse habitats in North America. For social neuroscience, voles offer good models for comparative studies because closely related species exhibit marked contrasts in social organization and social behavior.(Aragona and Wang, 2004; Carter et al., 1995; Lim et al., 2005; McGraw and Young, 2009) Prairie voles (Microtus ochrogaster) and pine voles (Microtus pinetorum) are monogamous species living in burrows with extended families; montane voles (Microtus montanus) and meadow voles (Microtus pennsylvanicus) are promiscuous species often living in solitary burrows. Curiously these species differences in sociality are evident in the first days of life: prairie vole pups respond to social isolation with ultrasonic calls and increased corticosterone, whereas montane vole pups do not respond to social isolation as a stressor, although they produce ultrasonic and corticosterone responses to non-social stressors.(Shapiro and Insel, 1990)
Male prairie voles show a striking change in behavior following mating, including an enduring selective preference for their mate (increased affiliation), increased aggression towards other males (mate guarding), and increased paternal care.(Carter et al., 1995; Wang et al., 1994) These changes are not seen in montane or meadow voles following mating, suggesting that these mating induced changes reflect pair bonding and are fundamental to monogamous social organization in prairie voles. As AVP and OT are released with mating (Ross et al., 2009a), does either peptide have a role in the prairie vole's pair bond formation? AVP, given centrally to prairie vole males who have not mated, induces each of these pair bonding behaviors.(Wang et al., 1994; Winslow et al., 1993) A V1a receptor antagonist given centrally blocks each of these behaviors in males allowed to mate, without reducing mating behavior per se.(Wang et al., 1994; Winslow et al., 1993) OT does not induce pair bonding in male prairie voles although OT increases and an OT receptor antagonist decreases partner preference formation and parental behavior in female prairie voles.(Insel and Hulihan, 1995) (Cushing et al., 2001; Ross et al., 2009b) AVP or a V1a receptor antagonist administered to montane voles has no impact on social behavior, although the peptide increases self-grooming.(Insel et al., 1993; Young et al., 1999)
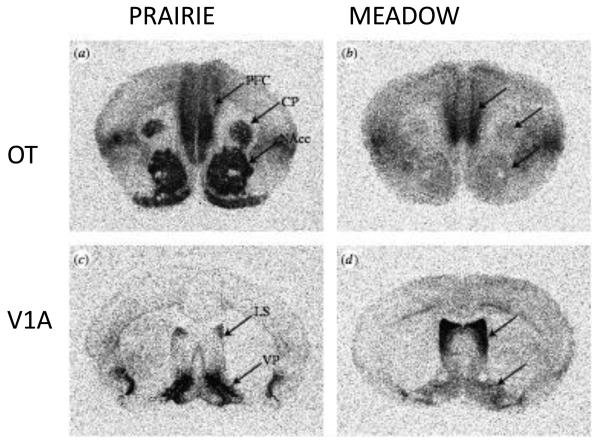
Why does the same peptide have such different effects in two closely related species? The neuroanatomical expression maps for the V1a receptor and the OT receptor are markedly different across vole species.(Insel and Shapiro, 1992; Young et al., 1997b) In prairie voles, which pair bond following mating, V1a receptors are highly concentrated in the ventral pallidum and OT receptors are expressed most heavily in the nucleus accumbens, both regions associated with reward and reinforcement. In montane voles or meadow voles, V1a and OT receptors are more heavily expressed in the lateral septum and amygdala. One model (Figure 2) would suggest that the neuropeptides are released in both species with mating but that the neurobehavioral consequences of mating are different because the neuropeptides are activating different pathways: in pair bonding species mating is reinforcing and leads to attachment. In non-pair bonding species, mating has no enduring effects.(Young et al., 2005)
Figure 2. Contrasting distribution of oxytocin and vasopressin V1a receptors to prairie (monogamous) and meadow (promiscuous) voles.
Receptors are labeled with iodinated ligands by in vitro receptor autoradiography. Levels matched across species with arrows pointing to homologous structures. Prairie voles show higher binding in nucleus accumbens for oxytocin and ventral pallidum for vasopressin. Meadow voles show higher binding for vasopressin in lateral septum. Not shown are differences in other regions including posterior cingulate-retrosplenial cortex (high for vasopressin V1a receptor in prairie vole) and ventral thalamus and amygdala (high for oxytocin receptor in meadow vole). Abbreviations: PFC prefrontal cortex, CP caudate putamen, NAcc nucleus accumbens, LS lateral septum, VP ventral pallidum. Figure adapted from (Hammock and Young, 2006)
Several observations in male prairie voles support this model. Overexpression of V1a receptors with viral vector mediated gene transfer into the ventral forebrain, including the ventral pallidum, facilitates pair bonding even in the absence of mating.(Pitkow et al., 2001) Cells in the ventral pallidum express Fos with mating and selective overexpression of the V1a receptor in the ventral pallidum increases Fos expression with mating.(Lim and Young, 2004) But perhaps the most direct evidence is that local injection of a V1a receptor antagonist into the ventral pallidum (but not into two other limbic regions with V1a receptors) inhibits pair bond formation.(Lim and Young, 2004) In fact, expressing the V1a receptor in the meadow vole ventral pallidum is sufficient to induce pair bond-like behavior after mating in this non-monogamous vole.(Lim et al., 2004) In an analogous fashion, overexpression of the OT receptor in the nucleus accumbens of female prairie voles facilitates partner preference formation.(Ross et al., 2009b) Note that this model that focuses on oxytocin and vasopressin as neuromodulators binding social signals to reward pathways does not take into account effects of these neuropeptides on anxiety and stress pathways (as suggested above for maternal behavior in rats and sheep) nor does it account for neuropeptide effects on social memory (as noted above for the OT-KO mouse).
This model also leaves several questions unanswered. What other brain regions are involved in sociality? Phelps and his colleagues have pointed out that there are several other areas that manifest both intra-species and inter-species variation in V1a receptor distribution in voles studied in the field. (Phelps and Young, 2003) Receptor density in the posterior cingulate-retrosplenial cortex was better than ventral pallidum as a predictor for mating success in male prairie voles in the field, although the correlation was especially found with males that mated but did not form pair bonds.(Ophir et al., 2008) Gobrogge and colleagues have demonstrated recently the role of V1a receptors in the anterior hypothalamus for mediating the aggression or mate guarding associated with pair bonding, with receptors increased in pair bonded males. (Gobrogge et al., 2009)
Why the gender difference in response? There are no striking gender differences in receptor expression, yet the path to pair bonding appears preferentially to use AVP in males and OT in females, just as found for effects on many other socio-sexual behaviors in other species.(Goodson and Bass, 2000)
What are the downstream effects of AVP binding to its receptor in the ventral pallidum or OT binding to the nucleus accumbens? Wang and his colleagues have demonstrated the importance of an interaction between OT and dopamine, specifically the dopamine D2 receptor, in the nucleus accumbens for pair bond formation in female prairie voles.(Aragona et al., 2003; Curtis et al., 2006; Liu and Wang, 2003) In the nucleus accumbens shell, the cellular output appears to involve cAMP (cyclic adenosine monophosphate) signaling: decreased cAMP signaling facilitates pair bond formation and increased cAMP signaling or activation of PKA (protein kinase A) inhibits pair bond formation.(Aragona and Wang, 2007) The cellular nature of the enduring pair bond – whether epigenetic or some form of cellular plasticity - is not yet known, although an intriguing body of work from Wang's lab points to a role for dopamine D1 receptors in the maintenance of pair bonding. (Aragona and Wang, 2009)
Finally, perhaps the most fundamental question is how such closely related species could have evolved such different neuroanatomical receptor expression maps? In fact, the expression of V1a receptors and OT receptors are strikingly different across mammalian species, in contrast to most neuropeptide receptors which have conserved patterns of expression. It may be more than coincidence that other monogamous species, such as marmosets and California mice (Peromyscus californicus), resemble prairie voles in that they also have OT or V1a receptors in nucleus accumbens or ventral pallidum, areas associated with reinforcement and reward.(Bester-Meredith et al., 1999; Insel et al., 1991; Schorscher-Petcu et al., 2009; Wang et al., 1997) Indeed, oxytocin has been reported to influence pair bonding in marmosets.(Smith et al.) But how does one explain the difference between congeners like prairie and montane voles? These species show few differences in the distribution of opiate receptors(Insel and Shapiro, 1992), although species differences for CRF receptors are striking.(Lim et al., 2006)Young and colleagues have done a series of elegant molecular studies to answer this question with the V1a receptor.(reviewed in (Young and Hammock, 2007) Although the coding regions of the V1a receptor genes are virtually identical across vole species, in monogamous species (prairie and pine voles) Young found a variable repeat microsatellite sequence in the V1a promoter region just upstream from the coding region.(Young et al., 1999) Such a large variable region in the promoter is suggestive of a functional role since this region of the gene regulates where and when expression occurs. However, the experimental evidence is still not conclusive. Inserting the prairie vole gene with the promoter into the mouse genome yields patterns of receptor expression that resemble the prairie vole pattern but are not identical.(Young et al., 1999) Prairie voles bred for different length repeat sequences had different anatomical patterns of receptors and different socio-sexual behaviors (including partner preference formation) suggesting that this microsatellite may be important for both individual differences within a species as well as the marked differences observed between species.(Hammock and Young, 2005) Recent studies comparing V1a receptor gene sequences from wild-caught voles suggest it may not be the length of the promoter microsatellite but aspects of the sequence or potentially interactions with distant sequences that drive tissue specific expression.(Ophir et al., 2008) Genetic polymorphisms have also been reported in the vole OT receptor gene, but their function is not clear.(Young et al., 1997a)
In summary, here we have a story that begins to shine some light into the dark matter of social neuroscience by bridging from gene variation to cellular expression to neural network to affiliative behavior. The results are parallel in many ways to the C. elegans research on social feeding, which also involved a neuropeptide receptor and a genetic variant. (de Bono and Bargmann, 1998) There are three critical principles from the vole story that may be relevant generally to a molecular basis for social neuroscience. First, comparative studies have proven important: species differences reveal candidates for individual differences within a species. Second, differences in neuropeptide receptor genes, thus far, appear more informative than differences in the genes for neuropeptides. Importantly, there are no evident differences in the levels or distribution of OT or AVP cells in different vole species.(Wang et al., 1996) Third, genetic sequence differences, especially in promoter regions, can alter patterns of receptor expression and the geography of receptors in the brain appears to be, thus far, the best correlate of social organization. It follows from each of these principles that (a) one must be cautious about generalizing from one species to another, (b) measuring neuropeptide levels may not be as informative as mapping receptor expression patterns, and (c) the effects of administering neuropeptide agonists or antagonists will depend on receptor expression. Can the principles gleaned from these studies in nematodes and voles inform human social neuroscience?
OT and AVP and Human Social Cognition
Humans like other eutherian mammals have both OT and AVP as well as their four receptors: OT, V1a, V1b, and V2 receptors. Most research in humans has been focused either on the variations in the genes of these receptors or behavioral and cognitive effects of administering OT or AVP.
Genetic variants in the OT and V1a receptor genes in primates are found in some of the same regions detected in voles and other mammals.(Ebstein et al., 2009) However, comparative studies of these variants in primates defy a simple relationship to social behavior.(Donaldson et al., 2008; Rosso et al., 2008) One of the most intriguing observations is the increase in V1a receptor expression and spine density of pyramidal cells in the prefrontal cortex of male marmosets as they become fathers. (Kozorovitskiy et al., 2006) Remarkably, there is little information about receptor expression of either OT or V1a receptors in human or non-human primates.(Loup et al., 1991; Schorscher-Petcu et al., 2009) As of 2010, major questions about individual variation, gender differences, and developmental changes have not yet been addressed in human brain.
OT and V1a receptor genetic variation in the population
Although the peptide and precursor peptide genes have been reported to be polymorphic in humans (Xu et al., 2008), the variants that have been studied most extensively are related to the receptor genes. (summarized in Figure 3).(Ebstein et al., 2009; Israel et al., 2008; Knafo et al., 2008; Rodrigues et al., 2009; Walum et al., 2008) The human AVP V1a receptor gene is relatively simple, 2 exons and 1 intron, located at 12q14-15 with 3 polymorphisms located in the 5′ flanking region and one in the intron.(Thibonnier, 2004; Thibonnier et al., 1994) The 5′ flanking region microsatellites, RS1 and RS3, have received the most attention, with links to a diverse set of interpersonal skills from sibling relationship to musical ability to economic decision-making. (Israel et al., 2008) One intriguing study of economic decision-making in an online game found subjects with longer RS3 genotypes were more altruistic and, in a separate cohort longer RS3 polymorphisms were associated with increased V1a mRNA in human post-mortem hippocampus.(Knafo et al., 2008) Studies in 552 healthy Swedish twin pairs reveal significant associations between the 334 allele in the RS3 polymorphism of the V1a gene and several aspects of pair-bonding in men, including marital status, perceived marital problems, and reported marital quality as reported by spouses. The effects were relatively modest (0.27 effect size for carrying one or two 334 alleles vs none), but the prevalence of this allele (40%) suggests that at a population level, this variant could be relevant to social behavior.(Walum et al., 2008)
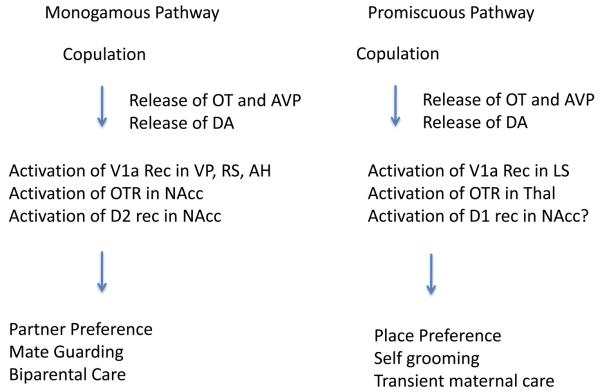
Figure 3. A model for mating-induced pair bonding in voles.
In monogamous prairie voles, pair bond formation usually occurs as a consequence of mating. The model is based on the release of oxytocin and vasopressin with mating. In prairie voles, these neuropeptides activate receptors and interact with dopamine in brain regions associated with reward and reinforcement. The model presumes that the neuropeptides transduce the sensory information about the identity of the mate to a highly salient reinforcer. The pair bond is operationally a conditioned response to the mate. In non-monogamous voles, the same peptides are released with mating but the absence of receptors in reward pathways precludes pair bonding. Experimental evidence supporting this model includes blockade of pair bonding by local administration of antagonists for oxytocin and vasopressin V1a receptors in mating prairie voles and induction of partner preference formation in non-monogamous voles by local viral vector induction of receptors. Abbreviations: OT – oxytocin, AVP – vasopressin, DA – dopamine, VP – ventral pallidum, RS – retrosplenial cortex, AH – anterior hypothalamus, NAcc – nucleus accumbens, D2 –dopamine-2 receptor, LS-lateral septum, Thal – thalamus.
The human OTR gene, located at 3p25.3, spans roughly 17kb, with 4 exons and 3 introns.(Kimura et al., 1992) At least 30 single nucleotide polymorphisms (SNPs) have been reported in the human OTR, mostly in intronic regions. Among the early descriptions of the OTR gene structure, a genomic element in the third intron was implicated in transcriptional repression.(Mizumoto et al., 1997) In a study of this polymorphic region of the third intron of the OT receptor, Rodrigues and colleagues reported a SNP (RS53576) associated with empathy and stress reactivity in both male and female college students (n = 192).(Rodrigues et al., 2009) Again, effects were small, but individuals with GG alleles at RS53576 performed significantly better than those with AA or AG alleles on a test of the ability to read the emotional state of others as well as on self-reported measures of empathy but not on self-reported measures of attachment.(Rodrigues et al., 2009) This same allele has been reported in a study of parental sensitivity, with AA and AG alleles associated with lower parental sensitivity to their toddlers. (Bakermans-Kranenburg and van Ijzendoorn, 2008)
In this era of high throughput genomics, the trend is to scan the entire genome for common variants in large populations. These approaches have yielded many genes of small effect. (Manolio et al., 2009) The candidate gene approach, used with these V1a and OT receptor studies, suffers from the criticism of “looking under the lamppost”. In defense of the candidate gene approach, these studies provide more intense coverage of a small region, identifying both common and rare variants. It is important to remember that relatively subtle changes in the regulatory region of the V1a receptor gene in voles appear to have profound effects on social behavior. (Hammock and Young, 2005) With the functional studies of these variants in the vole, OTR and V1a receptor genes are reasonable candidates to study in humans, recognizing that species differences are the hallmark of nonapeptide evolution.
OT and AVP effects on human social behavior
What are the effects of OT and AVP on human social behavior? The absence of non-peptide agonists that readily cross the blood brain barrier has required investigators to administer the peptides intranasally to explore effects on behavior and cognition. Recognizing that negative studies may be less likely to be reported, the available published literature converges around the notion that OT increases trust, empathy, eye contact, face memory, and generosity. (Domes et al., 2007b; Guastella et al., 2008; Kosfeld et al., 2005; Savaskan et al., 2008; Zak et al., 2005; Zak et al., 2007) In line with animal research showing that OT reduces anxiety (Neumann et al., 2000), there is also evidence that intranasal OT facilitates response to exposure therapy in people with social anxiety disorder.(Guastella et al., 2009b) In an important extension of this work to neuroimaging, Kirsh and colleagues reported that OT reduces the amygdala activation following threatening stimuli.(Kirsch et al., 2005) While not conclusive, the results suggest that the OT effect on amygdala activation could be more evident in the response to social threats (faces) versus non-social threats (scenes)(Kirsch et al., 2005). In fact, OT appears to reduce the amygdala response to emotional expression irrespective of valence.(Domes et al., 2007a)
What about AVP and social behavior? The syndrome of central diabetes insipidus, which involves either a deficiency of AVP (central diabetes insipidus) or abnormal V2 receptors (nephrogenic diabetes insipidus), serves as a natural experiment to begin to answer this question. There is little data to suggest that the absence of AVP, as in central diabetes insipidus, is associated with social deficits although subtle memory problems can be detected (Bruins et al., 2006). Moreover, the admininstration of the mixed V2-V1a receptor agonist desmopressin (dDAVP) as a treatment for central diabetes insipidus, to my knowledge, has not been reported to influence social behavior or social cognition.
The limitations for pharmacological studies are notable. The absence of non-peptide agonists has been a formidable barrier for studying CNS effects. Peripherally administered peptides have a very brief half-life and, following intravenous administration, less than 1% of the dose crosses into the CNS.(Kendrick et al., 1986) Animal studies have benefitted from central administration of a range of highly selective peptide agonists and antagonists. But human studies depending on intra-nasal administration of peptide face variable delivery to the brain of a peptide which has an unknown half-life on central receptors. Non-peptide antagonists and agonists, currently in development, could transform this field.(Pettibone et al., 1993; Ring et al.); as would the advent of a PET tracer for studying receptor expression in human brain.
OT, AVP, and Autism
The vole research on affiliation, the mouse studies of social cognition, and the human research suggesting pro-social effects of OT and AVP all beg the question: are these peptides involved in autism? Autism is a developmental disorder with onset by age three of social deficits, absent or abnormal communication, and a tendency to repetitive or stereotyped behaviors. There have been three lines of evidence exploring the link between OT, AVP, and autism: genetics, plasma levels, and peptide treatment studies.
Based on twin studies, autism is among the most heritable of neuropsychiatric disorders.(Abrahams and Geschwind, 2008) Yet, the genetic basis of autism appears quite complex. There is, thus far, no evidence linking monogenic causes, such as Fragile X and Rett syndrome, to either oxytocin or vasopressin or their receptors. Curiously, one of the most heavily studied autism candidate genes, reelin, has been associated with changes in the expression of brain OTR.(Liu et al., 2005) Large scale case control studies looking for common variants or linkage, have not found associations between any of the known alleles in the genes for OT, AVP, or their receptors, although two of the many genome-wide searches have reported linkage on chromosomal region, 3p25.3, which contains the OTR.(Lauritsen et al., 2006; McCauley et al., 2005) In fact, genome wide association studies (GWAS) in autism have been surprisingly uninformative, beyond findings on chromosome 5 in an area between two cadherin genes.(Glessner et al., 2009; Wang et al., 2009) Rare variants, such as copy number duplications or deletions, have been reported to be about 10-fold more prevalent in autism genomes relative to controls, with many different regions of the genome affected.(Sebat et al., 2007) An interesting case of pervasive developmental disorder and delayed speech associated with duplication of 3p25.3 suggested a role for the OTR as well as several other genes in the phenotype. (Bittel et al., 2006) A few autism cases have been identified with deletions in chromosome 3 that essentially knock-out the human OTR. (Gregory et al., 2009; Sebat et al., 2007) In the best characterized case, Gregory et al reported on a deletion of the OTR in an autism boy and his mother, who had OCD. An affected brother did not have the OTR deletion but exhibited epigenetic silencing of the OTR due to hypermethylation of the OTR promoter. In an independent sample, Gregory et al not only found additional autism cases with hypermethylation of the OTR gene but reported reductions in OTR mRNA in temporal cortex associated with hypermethylation, demonstrating likely epigenetic silencing of the OTR even in the absence of a genetic mutation. The reason for this epigenetic modification is unclear, but this finding reminds us that epigenetic mechanisms may be important regulators of protein expression. Considerable data support the hypothesis that early environmental experience, especially social experience, can have enduring effects on the OTR system.(Carter et al., 2009)
A number of candidate gene studies investigating the OT and V1a receptor genes and autism have been published. Figure 4 summarizes what has been reported for variants in the V1a receptor (Kim et al., 2002; Wassink et al., 2004; Yirmiya et al., 2006) and OTR genes (Jacob et al., 2007; Lerer et al., 2008; Liu et al.; Wu et al., 2005; Yrigollen et al., 2008) While there are many reports for associations between variations in both genes and risk for autism, the data are not entirely consistent, as some find the association with different risk alleles, perhaps reflecting the effects of varying ethnic backgrounds. More importantly, there is still little evidence that these variants are functional. For the OTR gene, a third intron variant has been implicated in transcriptional repression (see above), but this has yet to be shown for any of the intronic variants reported for autism. As noted above, there is one report that a longer RS3 version of the V1a receptor gene was associated with increased levels of hippocampal V1a receptor mRNA.(Knafo et al., 2008) Another report, used 121 healthy volunteers to test for a functional role of the RS1 and RS3 variants reported in autism. Looking at amygdala activation via fMRI BOLD (blood oxygen level dependent functional magnetic resonance imaging) in a face matching task, longer RS1 alleles were associated with higher activation whereas longer RS3 alleles were associated with lower activation.(Meyer-Lindenberg et al., 2009) While this finding is broadly consistent with the vole data as well as evidence for vasopressin receptors in the rat amygdala (Huber et al., 2005), the evidence in humans points to a role for OT rather than AVP on amygdala activation. (Kirsch et al., 2005) The presence or role of V1a receptors in the human amygdala remains to be defined.
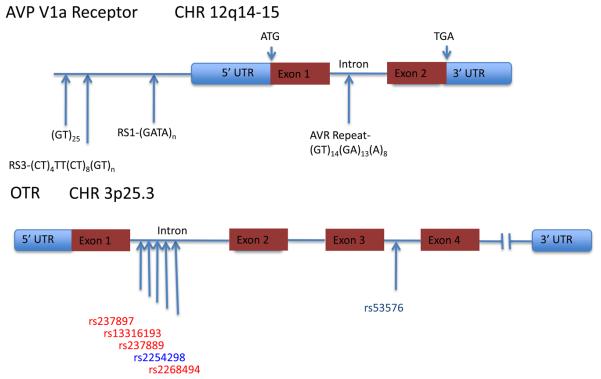
Figure 4. Variations in the human vasopressin V1a receptor and oxytocin receptor genes.
Schematics of genomic structure of V1a and OT receptors show regions of interest for social cognition. Two microsatellites in the 5′ flanking region of the V1a receptor, denoted RS1 and RS3, have been associated with autism (Kim et al., 2002; Wassink et al., 2004; Yirmiya et al., 2006). In particular, the length of RS3 has been associated with a range of interpersonal skills (reviewed by (Israel et al., 2008) as well as several measures of fidelity in men(Walum et al., 2008). The OTR includes many intronic SNPs, with the cluster shown in the first intron linked as a haplotype to autism in Chinese Han (Wu et al., 2005), Japanese (Liu et al.), and Israeli (Lerer et al., 2008) cohorts. In a Caucasian cohort (Jacob et al., 2007), neither this haplotype nor the third intron SNP was associated with autism and in the single positive allele at rs2254298, the G allele was overtransmitted to probands, opposite to the overtransmission of the A allele reported in other populations. The rs53576 SNP in the third intron, which showed the largest effect in a family based association test in the Han Chinese study (Wu et al., 2005) has also been associated with measures of parental sensitivity, altruism, and a test of the ability to read the emotional state of others.(Bakermans-Kranenburg and van Ijzendoorn, 2008; Rodrigues et al., 2009)
Is there any evidence for a decrease in OT or AVP neuropeptide levels in autism? Modahl and colleagues reported a marked reduction in OT in children with autism relative to age matched controls.(Modahl et al., 1998) This decrease in circulating OT could be explained by a deficit in processing the peptide from its precursor prohormone.(Green et al., 2001) There have been few attempts to replicate these findings, although Andari et al noted profoundly reduced OT plasma levels in their study of high functioning autism patients.(Andari et al.) As noted above, children lacking AVP have diabetes insipidus but not autism. Cerebrospinal fluid (CSF) measures of OT or AVP could be informative but are not currently available from individuals with autism. Curiously, CSF OT has been reported to be selectively reduced in both women subjected to childhood abuse and monkeys raised with social deprivation.(Heim et al., 2009; Winslow, 2005)
Currently there are no medical treatments for the social and communications deficits that form the core symptoms of autism. If OT and AVP are “pro-social,” could these peptides improve social behavior in children or adults with autism? While AVP has been more likely to affect male social behavior in other species and autism is 3-fold more common in males(Abrahams and Geschwind, 2008), research thus far has focused on OT. Three studies have examined the effects of OT administered intranasally to high functioning autistic patients. (Andari et al.; Guastella et al., 2009a; Hollander et al., 2007; Hollander et al., 2003) While not a cure, the results are promising. These initial trials report that, relative to placebo, OT improves eye contact, social memory, and use of social information. These reports should be considered a proof of principle. With the advent of non-peptide agonists (Ring et al.) and expanded clinical trial infrastructure allowing research with a broader range of children and adults with autism, there may soon be an opportunity to develop new pharmacological agents tailored to social deficits. Recall however, one of the principles from the vole research. The difference between social and solitary voles is not the amount of peptide but the location of receptors. If autism involves altered receptor distribution, the administration of additional peptide will not reverse the social deficit, just as AVP or OT does not increase social behavior in the montane voles.
Summary
This review began with a brief history of social neuroscience, describing the dark matter as the molecules, cells, and circuits linking sensory information to the motor outputs of social behavior. From research on the npr gene in C. elegans and studies of OT and AVP receptors in voles, a set of principles can be distilled. First, neuropeptides and their receptors appear important mediators of social behavior. Second, comparative studies point to important sites of intra-species as well as inter-species variation. And finally, from the work in voles, the neural geography of receptor distribution appears critical for determining function. A working model posits a role for OT and AVP in social attachment by linking socio-sexual information to pathways for reward and reinforcement, although effects on other aspects of social cognition or anxiety may also contribute to pair bond formation.
The role of OT or AVP in human social cognition remains unclear, but studies of intranasal OT suggest pro-social effects as measured operationally or by self-report. Whether OT, AVP, or their receptors are involved in autism is still not proven from genetic studies. The possibility that OT could improve social cognition in autism is especially intriguing given the absence of effective medical treatments for the social deficits of this syndrome.
Social neuroscience is still a frontier area of neurobiology. In 2010, one of the most exciting areas of this frontier is the opportunity to bridge the insights emerging from studies of social cognition and social behavior in animals to human research. While there is a temptation to think of “animal models” of human disorders or to assume that findings in animals will map directly on to human neurobiology, the translational bridge will need to be built with careful consideration of species differences, based on evolutionary adaptations. While some of the principles may be conserved (i.e. importance of receptor maps and role of gonadal steroids), the details for social organization need to be explored for each species, recognizing the importance of diversity in the neural mechanisms for social cognition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. ILAR J. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Opposing regulation of pair bond formation by cAMP signaling within the nucleus accumbens shell. J Neurosci. 2007;27:13352–13356. doi: 10.1523/JNEUROSCI.3216-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Hunt LT, Rushworth MF. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Dasouki M, Knoll JH, Butler MG. A 9-year-old male with a duplication of chromosome 3p25.3p26.2: clinical report and gene expression analysis. Am J Med Genet A. 2006;140:573–579. doi: 10.1002/ajmg.a.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins J, Kovacs GL, Abbes AP, Burbach JP, van den Akker EL, Engel H, Franken AA, de Wied D. Minor disturbances in central nervous system function in familial neurohypophysial diabetes insipidus. Psychoneuroendocrinology. 2006;31:80–91. doi: 10.1016/j.psyneuen.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci. 2009;31:332–341. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Res. 2006;1126:76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Martin JO, Young LJ, Carter CS. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Horm Behav. 2001;39:48–58. doi: 10.1006/hbeh.2000.1633. [DOI] [PubMed] [Google Scholar]
- de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007a;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007b;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Kondrashov FA, Putnam A, Bai Y, Stoinski TL, Hammock EA, Young LJ. Evolution of a behavior-linked microsatellite-containing element in the 5′ flanking region of the primate AVPR1A gene. BMC Evol Biol. 2008;8:180. doi: 10.1186/1471-2148-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I, Riebold M, Salomon S, Yirmiya N. Arginine vasopressin and oxytocin modulate human social behavior. Ann N Y Acad Sci. 2009;1167:87–102. doi: 10.1111/j.1749-6632.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Oxytocin induction of short-latency maternal behavior in nulliparous, estrogen-primed female rats. Horm Behav. 1984;18:267–286. doi: 10.1016/0018-506x(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol Psychiatry. 2001;50:609–613. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal Oxytocin Improves Emotion Recognition for Youth with Autism Spectrum Disorders. Biol Psychiatry. 2009a doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009b;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR. Regional changes in brain oxytocin receptors post-partum: time-course and relationship to maternal behaviour. J Neuroendocrinol. 1990;2:539–545. doi: 10.1111/j.1365-2826.1990.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gingrich BS, Young LJ. Oxytocin: who needs it? Prog Brain Res. 2001;133:59–66. doi: 10.1016/s0079-6123(01)33005-4. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Williams JR, Hastings N, Shapiro LE, Carter CS. The role of neurohypophyseal peptides in the central mediation of complex social processes--evidence from comparative studies. Regul Pept. 1993;45:127–131. doi: 10.1016/0167-0115(93)90194-d. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, Uzefovsky F, Reibold M, Bachner-Melman R, Granot R, Bornstein G, Knafo A, Yirmiya N, et al. Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: from autism to altruism with some notes in between. Prog Brain Res. 2008;170:435–449. doi: 10.1016/S0079-6123(08)00434-2. [DOI] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N. Neuroscience. What's in a face? Science. 2006;311:617–618. doi: 10.1126/science.1123983. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. The neurobiology of social bonds. J Neuroendocrinol. 2004;16:1007–1008. doi: 10.1111/j.1365-2826.2004.01262.x. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Da Costa AP, Broad KD, Ohkura S, Guevara R, Levy F, Keverne EB. Neural control of maternal behaviour and olfactory recognition of offspring. Brain Res Bull. 1997;44:383–395. doi: 10.1016/s0361-9230(97)00218-9. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA, Sharman DF. Cerebrospinal fluid levels of acetylcholinesterase, monoamines and oxytocin during labour, parturition, vaginocervical stimulation, lamb separation and suckling in sheep. Neuroendocrinology. 1986;44:149–156. doi: 10.1159/000124638. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Kendrick KM. Oxytocin facilitation of maternal behavior in sheep. Ann N Y Acad Sci. 1992;652:83–101. doi: 10.1111/j.1749-6632.1992.tb34348.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Jr., Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356:526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo A, Israel S, Darvasi A, Bachner-Melman R, Uzefovsky F, Cohen L, Feldman E, Lerer E, Laiba E, Raz Y, et al. Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor RS3 promoter region and correlation between RS3 length and hippocampal mRNA. Genes Brain Behav. 2008;7:266–275. doi: 10.1111/j.1601-183X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Lauritsen MB, Als TD, Dahl HA, Flint TJ, Wang AG, Vang M, Kruse TA, Ewald H, Mors O. A genome-wide search for alleles and haplotypes associated with autism and related pervasive developmental disorders on the Faroe Islands. Mol Psychiatry. 2006;11:37–46. doi: 10.1038/sj.mp.4001754. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: potential rodent models of autism. Int J Dev Neurosci. 2005;23:235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lim MM, Tsivkovskaia NO, Bai Y, Young LJ, Ryabinin AE. Distribution of corticotropin-releasing factor and urocortin 1 in the vole brain. Brain Behav Evol. 2006;68:229–240. doi: 10.1159/000094360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu W, Pappas GD, Carter CS. Oxytocin receptors in brain cortical regions are reduced in haploinsufficient (+/−) reeler mice. Neurol Res. 2005;27:339–345. doi: 10.1179/016164105X35602. [DOI] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, Nishida H, Hashimoto O, Nakagami R, Tochigi M, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JL, Li C, Jiang L, Olson LM, Crockett G, Gainer K, Folstein SE, Haines JL, Sutcliffe JS. Genome-wide and Ordered-Subset linkage analyses provide support for autism loci on 17q and 19p with evidence of phenotypic and interlocus genetic correlates. BMC Med Genet. 2005;6:1. doi: 10.1186/1471-2350-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Annu Rev Neurosci. 1979;2:65–112. doi: 10.1146/annurev.ne.02.030179.000433. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2009 doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry. 2009;14:968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto Y, Kimura T, Ivell R. A genomic element within the third intron of the human oxytocin receptor gene may be involved in transcriptional suppression. Mol Cell Endocrinol. 1997;135:129–138. doi: 10.1016/s0303-7207(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Campbell P, Hanna K, Phelps SM. Field tests of cis-regulatory variation at the prairie vole avpr1a locus: association with V1aR abundance but not sexual or social fidelity. Horm Behav. 2008;54:694–702. doi: 10.1016/j.yhbeh.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Clineschmidt BV, Kishel MT, Lis EV, Reiss DR, Woyden CJ, Evans BE, Freidinger RM, Veber DF, Cook MJ, et al. Identification of an orally active, nonpeptidyl oxytocin antagonist. J Pharmacol Exp Ther. 1993;264:308–314. [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, McEwen BS. Actions of estrogens and progestins on nerve cells. Science. 1983;219:808–814. doi: 10.1126/science.6297008. [DOI] [PubMed] [Google Scholar]
- Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J Comp Neurol. 2003;466:564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, Grauer S, Pulicicchio C, Resnick L, Rahman Z, et al. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 58:69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci U S A. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009a;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009b;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso L, Keller L, Kaessmann H, Hammond RL. Mating system and avpr1a promoter variation in primates. Biol Lett. 2008;4:375–378. doi: 10.1098/rsbl.2008.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Menniti FS, Bridges RS. Intracerebroventricular administration of oxytocin and maternal behavior in rats after prolonged and acute steroid pretreatment. Horm Behav. 1983;17:45–53. doi: 10.1016/0018-506x(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Russell JA, Leng G. Sex, parturition and motherhood without oxytocin? J Endocrinol. 1998;157:343–359. doi: 10.1677/joe.0.1570343. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupre A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, Insel TR. Infant's response to social separation reflects adult differences in affiliative behavior: a comparative developmental study in prairie and montane voles. Dev Psychobiol. 1990;23:375–393. doi: 10.1002/dev.420230502. [DOI] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm Behav. 57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Thibonnier M. Genetics of vasopressin receptors. Curr Hypertens Rep. 2004;6:21–26. doi: 10.1007/s11906-004-0006-8. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Auzan C, Madhun Z, Wilkins P, Berti-Mattera L, Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J Biol Chem. 1994;269:3304–3310. [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kesteren RE, Smit AB, De Lange RP, Kits KS, Van Golen FA, Van Der Schors RC, De With ND, Burke JF, Geraerts WP. Structural and functional evolution of the vasopressin/oxytocin superfamily: vasopressin-related conopressin is the only member present in Lymnaea, and is involved in the control of sexual behavior. J Neurosci. 1995;15:5989–5998. doi: 10.1523/JNEUROSCI.15-09-05989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren RE, Smit AB, Dirks RW, de With ND, Geraerts WP, Joosse J. Evolution of the vasopressin/oxytocin superfamily: characterization of a cDNA encoding a vasopressin-related precursor, preproconopressin, from the mollusc Lymnaea stagnalis. Proc Natl Acad Sci U S A. 1992;89:4593–4597. doi: 10.1073/pnas.89.10.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren RE, Tensen CP, Smit AB, van Minnen J, Kolakowski LF, Meyerhof W, Richter D, van Heerikhuizen H, Vreugdenhil E, Geraerts WP. Co-evolution of ligand-receptor pairs in the vasopressin/oxytocin superfamily of bioactive peptides. J Biol Chem. 1996;271:3619–3626. doi: 10.1074/jbc.271.7.3619. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman PM, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Toloczko D, Young LJ, Moody K, Newman JD, Insel TR. Vasopressin in the forebrain of common marmosets (Callithrix jacchus): studies with in situ hybridization, immunocytochemistry and receptor autoradiography. Brain Res. 1997;768:147–156. doi: 10.1016/s0006-8993(97)00636-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Warnmark A, Treuter E, Wright AP, Gustafsson JA. Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation. Mol Endocrinol. 2003;17:1901–1909. doi: 10.1210/me.2002-0384. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC. Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- Winslow JT. Neuropeptides and non-human primate social deficits associated with pathogenic rearing experience. Int J Dev Neurosci. 2005;23:245–251. doi: 10.1016/j.ijdevneu.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xue Y, Asan, Daly A, Wu L, Tyler-Smith C. Variation of the oxytocin/neurophysin I (OXT) gene in four human populations. J Hum Genet. 2008;53:637–643. doi: 10.1007/s10038-008-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- Young LJ, Hammock EA. On switches and knobs, microsatellites and monogamy. Trends Genet. 2007;23:209–212. doi: 10.1016/j.tig.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- Young LJ, Waymire KG, Nilsen R, Macgregor GR, Wang Z, Insel TR. The 5′ flanking region of the monogamous prairie vole oxytocin receptor gene directs tissue-specific expression in transgenic mice. Ann N Y Acad Sci. 1997a;807:514–517. doi: 10.1111/j.1749-6632.1997.tb51955.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci. 1997b;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Shepard E, DeVries AC, Zimmer A, LaMarca ME, Ginns EI, Amico J, Nelson RJ, Hennighausen L, Wagner KU. Targeted reduction of oxytocin expression provides insights into its physiological roles. Adv Exp Med Biol. 1998;449:231–240. doi: 10.1007/978-1-4615-4871-3_30. [DOI] [PubMed] [Google Scholar]
- Yrigollen CM, Han SS, Kochetkova A, Babitz T, Chang JT, Volkmar FR, Leckman JF, Grigorenko EL. Genes controlling affiliative behavior as candidate genes for autism. Biol Psychiatry. 2008;63:911–916. doi: 10.1016/j.biopsych.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]