There is considerable interest in psychological/behavioral variables that determine the behavioral and physiological impact of stressors, as well as experiential factors that alter the organism's vulnerability to subsequent aversive experiences (Southwick et al., 2005). Interestingly, the opportunity to exert behavioral control over an aversive event not only blunts the behavioral and neurochemical consequences of that event (Maier & Watkins, 1998; Ravindran et al. 2002; Weiss, 1971), but also reduces the behavioral and neurochemical consequences of exposure to subsequent stressors over which the organism does not have behavioral control (Amat et al., 2006; Williams & Maier, 1977). The latter “immunizing” effect of behavioral control has received only recent attention. In the reported experiments rats exposed to a series of tailshocks, each of which terminated whenever the rat turned a small wheel in the front of the chamber (escapable shock, ES), later failed to show the typical behavioral and neurochemical consequences of exposure to inescapable (uncontrollable) shocks (IS) occurring in a different, but similar apparatus. More specifically, the initial ES treatment was shown to completely block a) the interference with shuttlebox escape learning (Williams & Maier, 1977) and reduction in social investigation of a juvenile (Christianson et al., 2008b) that is normally produced by IS, and b) the intense activation of dorsal raphe nucleus (DRN) serotonergic (5-HT) neurons that is typically produced by IS (Amat e t al., 2006). DRN 5-HT activity was explored because prior work had found IS-induced activation of these cells to be critical to the mediation of both the escape interference (Maier et al., 1995) and reduced social investigation (Christianson et al., 2008a) produced by IS. An initial experience with exactly yoked inescapable tailshocks (IS) in the wheel-turn boxes did not blunt these behavioral and neurochemical sequelae of later IS, demonstrating that the stress-resistance produced by the initial experience was caused by the controllability of the tailshocks.
The seeming “resilience” in the face of later uncontrollable stress produced by experiencing a controllable aversive event is striking as the controllable events (ES) are nevertheless quite “stressful”. For example, ES produces ACTH and corticosterone increases that are as large as those produced by yoked IS (Maier et al., 1986), as well as comparable increases in CRH and AVP mRNA in the PVN (Helmreich et al., 1999). However, very little is known concerning this immunization phenomenon. Perhaps the most obvious question is whether the blunting effects of a control episode are restricted to subsequent events that are the same as, or similar to, the events over which the organism is given control, or whether instead exerting control over an aversive stimulus such as tailshock confers a more general resistance to the effects of stressors. In selecting a stressor subsequent to the initial ES it seemed desirable to choose an aversive situation that has as few stimuli in common with ES and the ES environment as possible, and one known to activate DRN 5-HT neurons. Social defeat (SD) meets both requirements. Pilot work indicated that SD produces both shuttlebox escape deficits and reduced juvenile social investigation 24 hr later, as does IS, and Gardner et al. (2005) in rats, and Cooper et al. (2009) in Syrian hamsters, reported that SD induces Fos in DRN 5-HT neurons. Thus here we sought to determine whether prior ES would block the escape deficits, reduced social investigation and DRN 5-HT activation produced by SD.
Experimental Procedures
Subjects
Male Sprague–Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) weighing 275–350 g, were housed 4 per cage on a 12-h light/dark cycle (on at 07:00 h and off at 19:00 h). Long Evans retired breeders weighing 600-800 g housed individually, under a similar lighting schedule, were selected as alpha males for SD encounters (see below) if they attacked a Sprague Dawley rat within the first 5 min of several practice encounters. Experiments were conducted between 09:00 and 1600 h. All procedures conformed to international guidelines on the ethical use of animals and were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder.
Overall organization
This report contains two types of experiments: a) some examined the effects of SD on DRN 5-HT efflux during the SD encounters, and on behavior 24 h later (learned helplessness (LH)), and b) some examined the effects of an initial experience with controllable tail shock (escapable stress (ES)) in modulating the effects of SD occurring 7 days later, both on DRN 5HT efflux during the SD encounter and on changes in behavior 24 h after SD. The behaviors measured in both types of experiments were: Attack/Defeat Index (ADI, see below) during the SD encounters, and shuttlebox escape learning and social exploration 24 h after SD.
Surgery and cannulation
Rats to be used for microdialysis underwent surgery using anesthesia with a mixture of 100 mg kg−1 Ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA) and 6.4 mg kg−1 xylazine and 1.6 mg kg−1 acepromazine (Vedco Inc., St. Joseph, MO, USA). A cannula guide for microdialysis probes (CMA 12), was stereotaxically implanted with the tip terminating just above the caudal DRN: 8.3 mm caudal to bregma and 5 mm ventral from the dura matter at the midline (Amat et al., 2005). A screw cap of a 15-ml conical centrifuge tube, whose central portion was removed, was also affixed to the skull so that its threads were exposed and it encircled the cannulae guide. This was done so that the skull assembly could be protected during microdialysis. Rats were allowed to recover for 1–2 wk before experimentation. All surgical procedures to any given animal were performed the same day.
Wheel-turn escape learning
Each rat was placed in a Plexiglas box (14×11×17 cm) with a wheel mounted in the front and a Plexiglas rod extending from the back. The rat's tail was taped to the Plexiglas rod and affixed with copper electrodes. Rats received shocks in yoked pairs (ES and IS). The treatment consisted of 100 trials with an average intertrial interval of 60 s. Shocks began simultaneously for both rats in a pair and terminated for both whenever the ES rat met a response criterion. Initially, the shock was terminated by a quarter turn of the wheel. The response requirement was increased by one quarter turn when each of three consecutive trials was completed in less than 5 s. Subsequent latencies under 5 s increased the requirement by 50% up to a maximum of four full turns. If the requirement was not reached in less than 30 s, the shock was terminated and the requirement reduced to a single quarter turn. This procedure was used to insure that the ES rats learned an operant response. Shock intensity was 1.0 mA for the first 30 trials, 1.3 mA for the second 30 trials and 1.6 mA for the last 40 trials, to maintain good escape responding. Non-shocked home cage control (HC) rats remained undisturbed in the colony, except during the microdialysis experiments, where they remained undisturbed in the dialysis room.
In vivo microdialysis
The afternoon before the experiment, a CMA 12 microdialysis probe (0.5 mm in diameter, 1 mm membrane with a 20-kD molecular weight cut-off; CMA/Microdialysis Inc., North Chelmsford, MA, USA) was introduced through the cannula guide so that the membranous tip of the probe was within the DRN. A portion of a 15-ml Eppendorf tube was screwed onto the skull-mounted screw cap, through which the dialysis tubing, protected within a metal spring, entered and attached to the probe. Each animal was placed individually in a Plexiglas bowl (Bioanalytical Systems, West Lafayette, IN, USA) and infused with isotonic Ringer's solution (Baxter, Portage, MI, USA) at a rate of 0.2 μl min−1 overnight. At 09:00 h the next day, the flow rate was increased to1.5 l min−1 and a 90-min stabilization period was allowed. The infusion flow remained constant throughout the experiment and samples were collected every 15 min. After taking 4 baseline samples, 45 min social encounters took place, with either an alpha male, or with a Sprague Dawley rat similar in size to the target subject. The same size Sprague-Dawley never fought with the dialyzed target rat. During collection of the last sample, brisk movements of the skull-mounted screw cap were performed to test for possible 5-HT increases due to rat head movement during the dialysis. The data from the rat were discarded if that procedure caused 5-HT increases.
5-HT analysis
5-HT concentration was measured in dialysates by HPLC with electrochemical detection. The system consisted of an ESA 5600A Coularray detector with an ESA 5014B analytical cell and an ESA 5020 guard cell. The column was an ESA MD-150 (C-18, 3 μm, 150×3.2 mm) maintained at 37 °C, and the mobile phase was the ESA buffer MD-TM. The analytical cell potentials were kept at −75 mV and +250 mV and the guard cell at +300 mV. Dialysate (25 μl) was injected with an ESA 542 autosampler that kept the dialysates at 6 °C. External standards (Sigma) were run each day to quantify 5-HT by means of peak height, using ESA software, the experimenter being blind to experimental condition.
Dialysis probe verification
At the end of the experiment an overdose of pentobarbital was administered and brains were removed and frozen. A cryostat was used to take 40-μm sections, which were then stained with Cresyl Violet for cannula placement verification. Only rats with the dialysis probe at least 70% within the intermediate and caudal DRN (−7.8 to −8.8 from bregma) were included (see Fig, 1).
Figure 1.
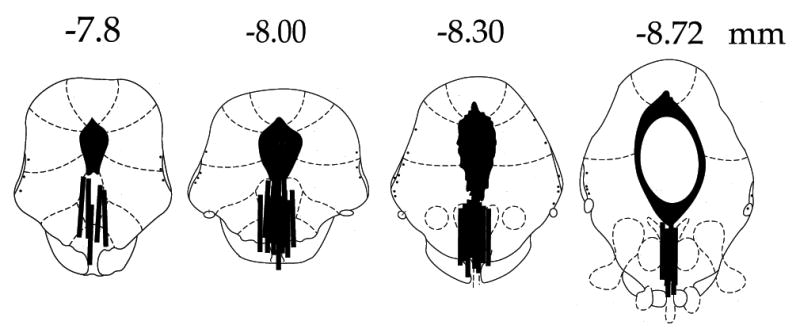
Schematic representation of microdialysis cannula placements within the DRN (black bars). Numerals indicate in mm distance from bregma.
Social defeat
Social defeat encounters were carried out both with freely moving rats as well as during microdialysis. When freely moving, the encounters took place in 45×60×40 cm plastic boxes with rat bedding. The Long Evans alpha males were placed in the boxes 1 h in advance and then the target Sprague Dawley was introduced. The encounters lasted 45 min and were recorded on DVD for later analysis by a researcher unaware of the treatment previously received by the target rat. Two types of behaviors were timed: a) biting or attempted biting and side pushing by the alpha male to the target rat, and b) partial submissive posture (the target rat crouches below the alpha male and turns to expose part of its belly) and full submission (the target rat lies on its back with its belly exposed). All target rats displayed either full or partial submissive posture at least once. As an estimate of the outcome of the SD encounter, an ADI was calculated by dividing the total duration of attack bouts by the total duration of submissive postures (partial and full) displayed during the 45 min. During microdialysis, the 45 min SD encounters took place in the microdialysis bowls after taking the baseline samples. Only partial submissive postures were displayed by the target rats when attacked during microdialysis, due to the movement restriction imposed by the tether. Forty-five min exposure to a same age Sprague Dawley rat during dialysis served as controls for SD.
Shuttlebox escape learning
Escape learning was tested in shuttle boxes measuring 46.0 × 20.7 × 20.0 cm. Shock intensity was 0.6 mA. The subjects were first given 5 min to adapt to the boxes. This was followed by 5 fixed ratio 1 (FR-1) escape trials at 1 min intervals. Here, the rat was required to cross to the other side of the box to terminate shock. The 5 FR-1 trials were followed by 25 FR-2 trials that were analyzed as five blocks of five trials. Here, the rat was required to cross to the other side and then return to terminate shock. It is here that shuttle-box escape deficits occur after IS. Each shock terminated after 30 s if an escape response had not occurred.
Juvenile social exploration test
Experimental adult rats were allocated a transparent plastic cage with shaved wood bedding and a wire lid located in a brightly lit testing room. After 60 min a juvenile stimulus rat was added to the cage. Investigative behaviors, including sniffing, pinning, and allogrooming, initiated by the adult rat were timed by an observer who was blind to group membership. After 3 min, the juvenile was removed and the adult was returned to the homecage. Food and water were not available during the 3 min test. Juvenile stimulus rats were used for multiple tests but were never exposed to the same adult more than once. This social exploration pretest was used to habituate the subjects to the procedure and to screen for rats with unusual baseline social exploration. In our experience, healthy naive rats typically explore for approximately 80 s; thus, rats with pretest social exploration times less than 50 s were excluded.
Experimental Designs
Shuttle escape behavior after SD in rats previously exposed to ES/IS/HC
3 groups received either ES, yoked IS, or HC treatment. 7 days later all received SD followed by shuttlebox escape testing 24 hr later as described above. A fourth group received HC treatment at both times before shuttle testing There were 8 rats in each group. The questions were whether SD would produce a shuttle escape deficit 24 hr later and whether prior ES or IS would alter this effect.
Juvenile social investigation after SD in rats previously exposed to ES/IS/HC
3 groups received either ES (n=12), yoked IS (n=12), or HC (n=8) treatment. 7 days later all received SD followed by juvenile social investigation testing 24 hr later as described above. A fourth group received HC treatment at both times before juvenile investigation testing (n=8). The questions were whether SD would produce a reduction in social investigation 24 hr later and whether prior ES or IS would alter this effect.
DRN 5-HT efflux during SD in rats previously exposed to ES/IS/HC
Two experiments were conducted during which DRN 5-HT levels were measured. In the first, rats received either a 45 min SD treatment (defeated group, n=9), or a 45 min placement with a same size non-aggressive Sprague-Dawley during microdialysis (not-defeated group, n=6), as described above. In the second experiment 3 groups of rats received either ES (n=12), yoked IS (n=8), or HC (n=7) treatment as described above. 7 days later they received a 45 min SD treatment in the microdialysis bowls and extracellular levels of 5-HT within the DRN measured as above. The questions were whether SD would increase DRN 5-HT and whether prior ES or IS would alter this response.
Different animals were used for each of the above experiments
Statistical analysis
Data were analyzed by repeated measures analysis of variance (ANOVA) followed by Fisher's protected least significant difference test (PLSD) post hoc comparison (alpha set at 0.05).
Results
Behavior during Social defeat
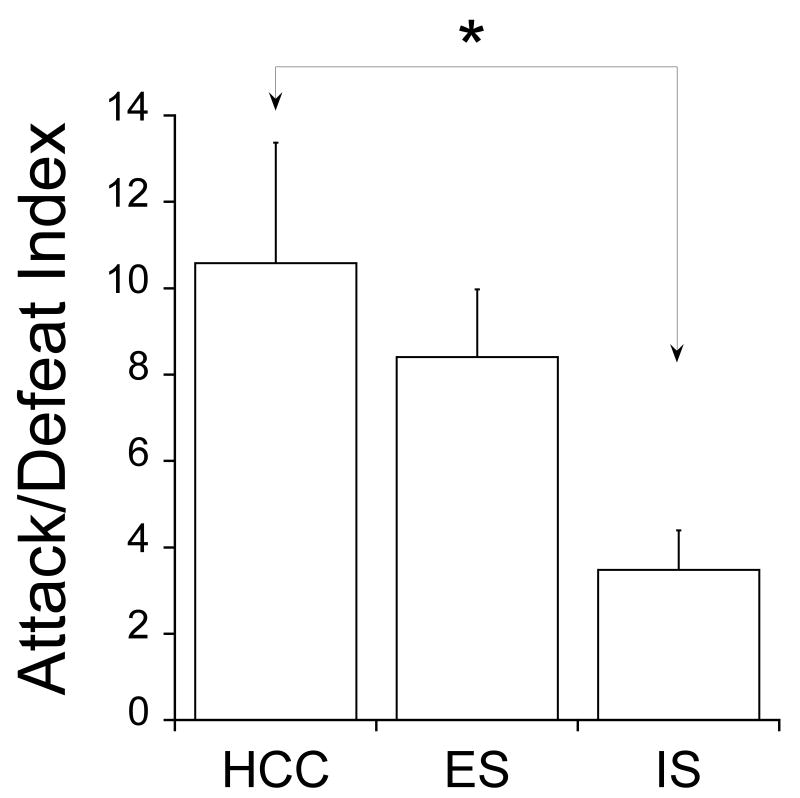
Fig. 2 shows the ADI for subjects given either ES, yoked IS, or HC control treatment 7 days earlier, from the social exploration experiment (see below). Clearly, rats that had received IS displayed more defeat behavior relative to attacks than did either ES or HC controls. ES had no effect on defeat behavior relative to controls. ANOVA showed a significant effect of prior stress condition (F2,18= 3.874, P=0.0399). Fisher's PLSD post-hoc comparison showed that the IS group differed from the HCC group, but the IS group was not different from the ES group (P=0.055).
Figure 2.
Attack/defeat Index (ADI) during social defeat (SD). HCC, ES and IS were treatments administered 7 days earlier. *Indicates that HCC is different from IS (P<0.02).
Escape learning
Here the question was how a prior experience with either ES or IS would impact on the effect of SD on learning to escape 24 h after SD. Fig. 3 shows the mean FR-2 escape latencies across blocks of 5 trials. SD without a prior stress experience (HC/SD, triangles) produced a deficit in learning to escape similar to the usual effect of IS (“learned helplessness”). Because the trials automatically terminated after 30 s, mean latencies near 30 indicate that most subjects in that group failed to escape. The HC/HC group provides baseline escape behavior, and clearly, SD interfered with this behavior. Prior exposure to IS did not alter the interference with escape behavior produced by SD (IS/SD, squares). It can be concluded that the poor escape was produced by the SD rather than the IS because IS does not interfere with escape learning 8 days later (Maier et al., 1979), the interval here. However, when ES preceded SD (ES/SD, circles), the rats learned to escape as well as did the group that was never exposed to stress (HC/HC). It can be concluded that ES blocked the effects of SD because ES by itself has no effect on later escape behavior (Maier et al., 1979). Thus, experiencing ES, but not IS, 7 days earlier protected the rats from the learning impairing effect of SD. This conclusion was confirmed by ANOVA. The effects of groups (F3,28= 10.23, P=0.0001), trial blocks (F4,112= 5.918, P= 0.0002), and the interaction between groups and trial blocks (F12,112= 2.835, P= 0.0002) were significant. Post-hoc Fisher's PLSD tests (P< 0.05) indicated that the ES/SD and the control HC/HC groups did not differ from each other, but did differ from the IS/SD and HC/SD groups. The IS/SD and HCC/SD groups did not differ from each other.
Figure 3.
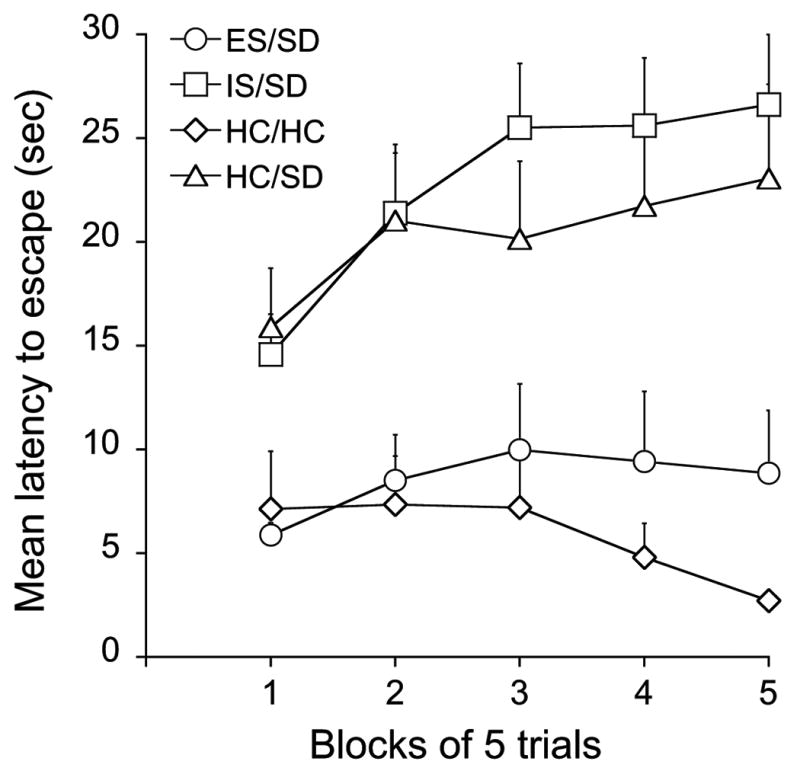
Mean shuttlebox escape latencies across blocks of 5 shuttlebox FR-2 escape trials 24 h after SD or HC, in groups of rats expose 8 days earlier to ES (ES/SD), IS (IS/SD), HC (HC/HC) or HC (HC/SD). ES/SD and HC/HC are different from IS/SD and HC/SD (P<0.05).
Social exploration
The juvenile social exploration results were similar to those from escape behavior. Fig. 4 shows the time spent in social investigation for the various groups. The HC/HC group provides basal levels of interaction. SD without prior stress experience (HC/SD) resulted in a decrease in the amount of social exploration 24 h after SD, and prior IS did not alter this effect. However, prior ES (ES/SD) blocked the decrease of social interaction caused by SD. As with escape learning, ES by itself has no effect on social investigation (Christianson et al, 2009). The amount of social interaction after ES/SD was not different from that in the non-stressed control group. ANOVA showed a significant effect of the stress condition (F3,36= 10.101, P< 0.0001), and post-hoc Fisher's PLSD tests (P<0.05) indicated that the ES/SD and the control HC/HC groups did not differ from each other, but did differ from the IS/SD and HC/SD groups. IS/SD and HC/SD groups did not differ from each other. Pooling together the results of the 3 groups (HC, IS and ES) there was no correlation between social exploration scores and ADI (r2=0.12).
Figure 4.
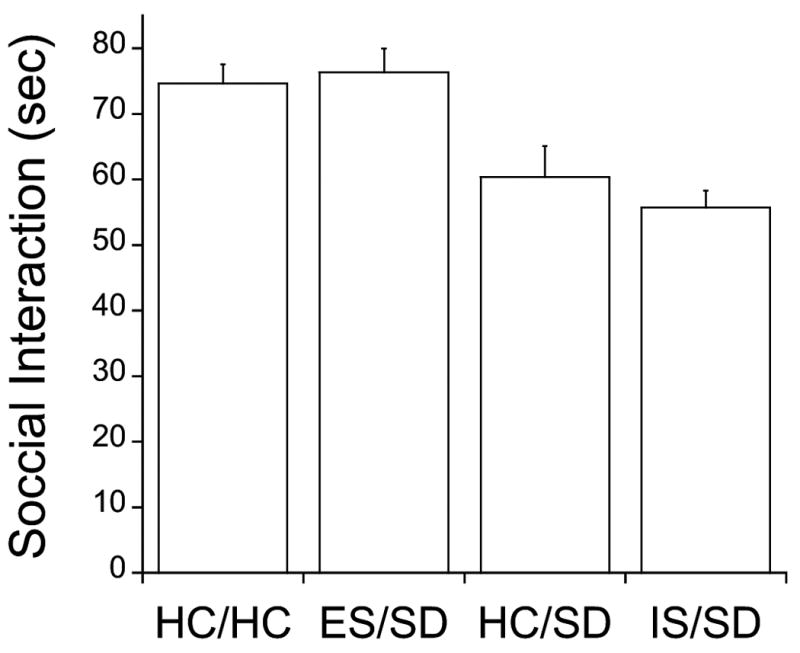
Juvenile social exploration in sec, 24 h after SD or HC, in groups of rats exposed 8 days earlier to ES (ES/SD), IS (IS/SD), HC (HC/HC) or HC (HC/SD). ES/SD and HC/HC are different from IS/SD and HC/SD (P<0.05).
Extracellular 5-HT in the caudal DRN
Social defeat
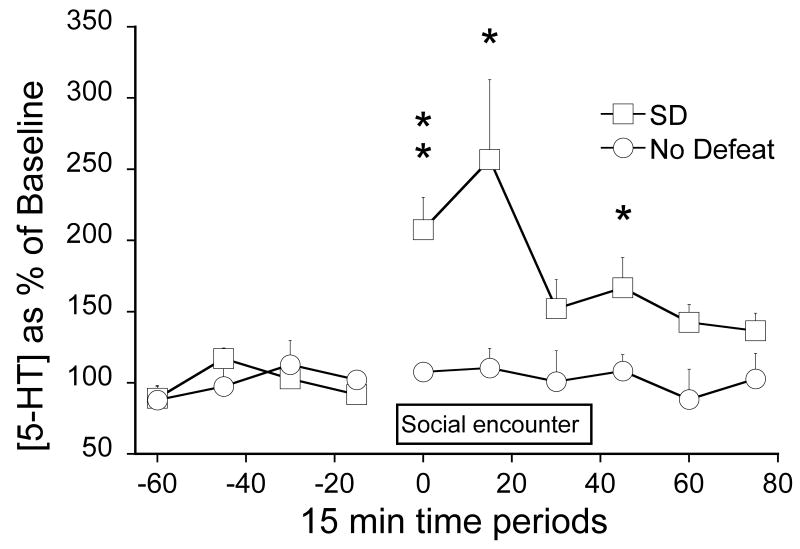
Fig. 5 shows extracellular levels of 5-HT within the DRN as a percentage of baseline for a control group in which the target subjects were paired with non-aggressive same strain and weight animals, and a group given SD (paired with an aggressive larger Long-Evans rat). There were no differences in baseline levels of 5-HT between the 2 experimental groups (means in pg/20 μl ±sem): 0.348±0.071 for the SD and 0.686±0.083 for the No Defeat groups. SD increased 5-HT efflux to a level comparable to that typically produced by IS (Amat et al., 2005). Social interaction not involving did not change 5-HT efflux (circles, Fig. 5). ANOVA showed that there was a significant effect of SD (F1,13= 13.94, P=0.0025).
Figure 5.
5-HT in the DRN as a percentage of baseline before, during and after social encounters either involving social defeat (SD, squares) or without SD (No Defeat, circles). **And * indicate that SD is different from No Defeat with P<0.005 and P<0.05 respectively.
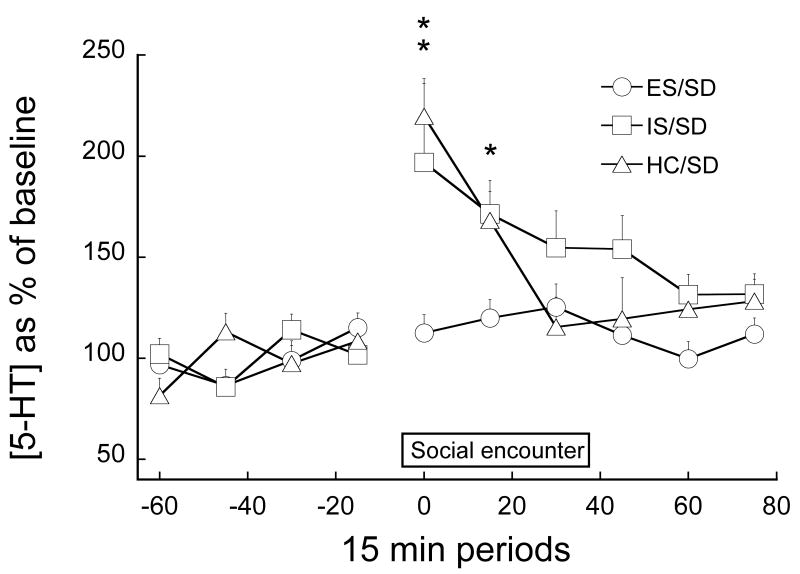
Immunizing effect of ES
There were no differences in 5-HT baseline levels among the 3 experimental groups: HC/SD= 0.606±0.077, ES/SD= 0.719±0.16 and IS/SD= 0.705±0.084. Fig. 6 shows 5-HT levels during SD in animals that had received either ES, yoked IS, or HC treatment 7 days earlier. As before, SD produced a large increase in 5-HT efflux in the DRN. Interestingly, prior IS appeared to prolong the 5-HT response to SD. However, the primary new finding is that prior ES blocked the increase in 5-HT produced by SD. These conclusions are confirmed by ANOVA. The effects of stress condition (F2,24= 7.381, P= 0.0032), time period (F5,120= 8.979, P<0.0001) and the interaction between stress condition and time (F10,120= 3.006, P=0.002) are significant. Post-hoc Fisher's PLSD tests indicated that HC/SD and IS/SD groups are different from the ES/SD group, but do not differ from each other. There was no correlation between 5-HT efflux during SD and the ADI during the encounters when the results of the 3 groups (HC, IS and ES) were pooled (r2=0.00).
Figure 6.
5-HT in the DRN as a percentage of baseline before, during and after social defeat (SD) in groups of rats exposed 7 days earlier to ES (ES/SD), IS (IS/SD) or HC (HC/SD). ** And * indicate that IS/SD and HC/SD are different from ES/SD with P<0.01 and P<0.02 respectively.
Discussion
It is of interest that SD produced the same behavioral consequences as does IS. That is, SD both interfered with shuttlebox escape failure and reduced social investigation 24 h later (Amat et al., 2005). Furthermore, the magnitudes of these behavioral effects were comparable to those typically produced by IS. IS produces dramatic behavioral effects relative to other stressors (e.g., restraint, which does not produce escape deficits or reduced social investigation 24 hr later e.g., Christianson et al., 2008a), and there has been a question as to whether there exists a more “naturalistic” stressor that would also produce these outcomes, and clearly SD does so. It can be argued that SD is uncontrollable as the intruder or target subject is invariably defeated. It would be difficult to manipulate controllability within the SD paradigm, and so this suggestion must remain speculative. Relatedly, IS produces large increases in 5-HT efflux within the DRN and in projection regions, while many other stressors (e.g., restraint) fail to do so (Rueter & Jacobs 1996; 1997). DRN 5-HT levels correlate well with DRN 5-HT neuronal firing as activated DRN 5-HT neurons release 5-HT within the DRN from axon collaterals, as well as in projection regions (Tao et al., 2000). Here we report that SD also produces a large increase in 5-HT efflux within the DRN. Interestingly, Gardner et al. (2005) reported that SD increased Fos expression in 5-HT neurons the middle and caudal part of the DRN, as does IS (Grahn et al., 1999). Thus, SD produces at least some of the same outcomes as does IS.
The major question addressed was whether the immunizing effects of behavioral control over tail shock would extend beyond reducing the behavioral and neural impact of subsequent tailshock or other similar stimuli. SD was selected because it is potent enough to produce persistent behavioral changes that could be measured and seemed maximally dissimilar to IS. In the SD paradigm that was used here there was no shock, no restraint, different environment, different odors, another rat present, etc. The handling routine to bring the subjects to testing was even quite different. For IS the rats were brought from the colony room to a different floor of the building by elevator, and temporarily kept in a “holding room” while waiting for their IS session. SD was conducted on the same floor as the colony, just down the hall. Nevertheless, ES 7 days earlier completely blocked the interference with escape behavior and reduced social investigation produced by SD. Yoked IS did not have this effect, and even slightly, but non-significantly, exaggerated the behavioral effects of SD. Thus, the immunizing effects of ES are attributable to the presence of behavioral control. It might have been possible that the immunizing effects of ES occurred because ES reduces the subject's propensity to be defeated during the encounters, but this proved not to occur.
A variety of data supports the idea that the behavioral sequelae of IS are dependent on IS-induced activation of DRN 5-HT neurons. IS produces a much larger activation of DRN 5-HT neurons than does equated ES as measured by both Fos expression in 5-HT labeled neurons (Grahn et al., 1999) and 5-HT efflux within the DRN (Maswood et al., 1998). Moreover, escape deficits and reduced social investigation do not occur 24 hr after IS if DRN 5-HT activity is pharmacologically inhibited during IS (Maier et al., 1995) or if the DRN is lesioned (Maier et al., 1993). With regard to immunization, prior exposure to ES blocks the activation of DRN 5-HT neurons that IS would normally produce (Amat et al., 2006). It is thus noteworthy that prior ES reduced the DRN 5-HT efflux produced by SD, supporting a role for the DRN in mediating the effects of SD as well as IS. Clearly, further work manipulating the DRN is required.
As noted above, it has been known for some years that an initial experience with ES blocks the typical behavioral and neurochemical effects of later exposure to IS (Seligman & Maier, 1967; Williams & Maier, 1977). The interpretation first suggested was that having learned that the stressor (tailshock) was controllable interfered with later learning that the stressor is uncontrollable, thereby preventing the usual behavioral and neurochemical consequences of the IS (Maier & Seligman, 1976). It might be noted here that such an explanation presupposes that the consequences of IS occur, at least in part, because the organism learns that the IS is uncontrollable. This associative interpretation of the proactive blunting effect of IS made sense because the stimuli controlled in the first phase and not in the second were the same physical events—tailshocks. If an organism has learned that it can exert behavioral control over a stimulus it makes sense to think that this might interfere with learning the opposite about the same stimulus. However, in the present studies prior ES blocked both a critical neurochemical change during SD, as well as the behavioral consequences of SD. The SD situation and the ES situation have few if any identifiable stimuli in common. Thus, this sort of interpretation of the effects of prior ES encounters difficulty.
However, more recent evidence has led to a different view concerning the impact of stressor controllability. Amat et al. (2005) reported that inactivation of the vmPFC with muscimol during exposure to ES and yoked IS altered only the neurochemical and behavioral consequences of ES—now ES produced the same high level of DRN 5-HT activation and later behavioral effects, such as poor escape learning and reduced social investigation (Christianson et al., 2008b) as did IS. Conversely, activation of the vmPFC with picrotoxin during ES and yoked IS altered only the neurochemical and behavioral consequences of IS—now IS produced the same low level of DRN 5-HT activity as did ES, and no longer interfered with later escape or reduced social investigation (Amat et al., 2008, Christianson et al., 2008b). That is, inactivation of the vmPFC during the stressor led both ES and IS to act as if they were IS, and activation of the vmPFC led both ES and IS to act as if they were ES. Since the vmPFC sends glutamatergic afferents to the DRN (Vertes, 2004) and these projections synapse preferentially on GABAergic interneurons that inhibit the 5-HT cells (Jankowski & Sesack, 2004), it was suggested that a) intense stressors per se drive activity of DRN 5-HT neurons, whether the stressor is controllable or uncontrollable (Amat et al., 2001), and b) the presence of behavioral control activates vmPFC output to the DRN, actively inhibiting the 5-HT activity induced by the stressor, thereby preventing stress-induced behavioral changes that depend on intense DRN 5-HT activation. Baratta et al. (2009) directly tested the idea that control activates vmPFC neurons that project to the DRN. The retrograde tracer fluorogold was injected into the DRN, thereby labeling neurons in the vmPFC that project to the DRN. ES led to considerably greater stimulation of these DRN projecting neurons, as assessed by Fos expression, than did IS.
The foregoing also suggests an interpretation of the blockade of IS effects by prior ES. Perhaps the activity within neurons projecting to the DRN (and perhaps to other structures (Baratta et al. 2008)) produced by ES alters these neurons in such a way that they are later now also activated by IS, thereby inhibiting the DRN and preventing the behavioral changes that depend on intense DRN activation. Consistent with this hypothesis, intra-vmPFC microinjection of muscimol before either the initial ES or the later IS completely prevented the blunting effects of the ES (Amat et al., 2006). These data suggest that the immunizing effects of ES are mediated by plasticity within the vmPFC, and vmPFC microinjection of the protein synthesis inhibitor anisomycin after ES prevented the blunting of later reactions to IS (Amat et al., 2006). It was suggested that the conjunction of vmPFC activity and the stressor, as occurs during ES, “ties” vmPFC and the stressor together, so that later occurrences of the stressor (tailshock) will now activate the projecting vmPFC neurons, even if the stressor is uncontrollable and so would not normally activate these cells. By this hypothesis even IS should be immunizing if the vmPFC were to be activated during its presentation, as this would associate the stressor with vmPFC activity. Indeed, Amat et al. (2008) found that the vmPFC administration of picrotoxin during IS led IS to block the DRN 5-HT activation and behavioral consequences of subsequent IS. That is, the tailshock need not be controllable to block the effects of subsequent IS, it is only necessary that the vmPFC be activated during the tailshock.
Within the above framework, perhaps SD activates the vmPFC in subjects that have previously received ES, but not in subjects that had received IS or no prior treatment. If this is so, it might suggest that ES sensitizes the vmPFC-DRN pathway, rather than associatively connecting activity of this pathway to some aspect of ES. Certainly, prior ES reduced the DRN activation produced by SD, and an ES-induced increase in vmPFC inhibitory control over the DRN is one potential mechanism. However, Amat et al., (2008) found that simply activating the vmPFC in the absence of tailshock did not produce immunization against later IS—activation and tailshock had to go together. This finding favors an associative interpretation, rather than sensitization. If ES does lead to associatively mediated plasticity between the vmPFC-DRN pathway and some aspect of tailshock, the present data suggest that it must be an aspect that is common to SD.
In sum, the experience of behavioral control over a stressor, here tailshock, seems to alter how the organism responds to subsequent stressors that are quite different and have no obvious stimuli in common. This suggests a protective or blunting effect of control that is more general than has been previously supposed.
List of abbreviations
- 5-HT
Serotonin
- ADI
Attack/Defeat Index
- DRN
Dorsal raphe nucleus
- ES
Escapable stress
- FR-
Fixed ratio
- HC
Home cage controls
- IS
Inescapable stress
- LH
Learned helplessness
- SD
Social defeat
- vmPFC
Ventral medial prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8(3):365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26(51):13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154(4):1178–86. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917(1):118–26. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Lucero TR, Amat J, Watkins LR, Maier SF. Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learn Mem. 2008;15(2):84–7. doi: 10.1101/lm.800308. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat Stress. 2008 Dec 2;1 doi: 10.1080/10253890802510302. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphé nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193(1):87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter activation of serotonergic neurons and the expression of 5-HT1A mRNA in hamster dorsal raphe nucleus. Neuroscience. 2009;191(3):680–90. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on erotonergic systems. Neuroscience. 2005;126(1):181–91. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, et al. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826(1):35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Watkins LR, Deak T, Maier SF, Akil H, Watson SJ. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 1999;11(2):121–8. doi: 10.1046/j.1365-2826.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468(4):518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res 2. 1998;783(1):115–20. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107(2):377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109(3):404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness:Theory and evidence. Journal of Experimental Psychology:General. 1976;105:3–46. [Google Scholar]
- Maier SF, Coon DJ, McDaniel MA, Jackson RL. The time course of learned helplessness, inactivity, and nociceptive deficits in rats. Learning and Motivation. 1979;10:467–488. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability, anxiety, and serotonin. Cognitive Therapy and Research. 1998;22:595–613. [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behav Neurosci. 1986;100(5):669–74. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Ravindran J, Eapen A, Kar I. Evaluation of repellent action of neem oil against the filarial vector, Culex quinquefasciatus (Diptera: Culicidae) Indian J Malariol. 2002;39(12):13–7. [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res 11. 1996;739(12):57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Fornal CA, Jacobs BL. A critical review of 5-HT brain microdialysis and behavior. Rev Neurosci. 1997;8(2):117–37. doi: 10.1515/revneuro.1997.8.2.117. Review. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;74(1):1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948. Review. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential effect of local infusion of serotonin reuptake inhibitors in the raphe versus forebrain and the role of depolarization-induced release in increased extracellular serotonin. J Pharmacol Exp Ther. 2000;294(2):571–579. [PubMed] [Google Scholar]
- Weiss B. On the regulation of adenyl cyclase activity in the rat pineal gland. Ann N Y Acad Sci. 1971;185:507–19. doi: 10.1111/j.1749-6632.1971.tb45277.x. [DOI] [PubMed] [Google Scholar]
- Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:240–253. [Google Scholar]