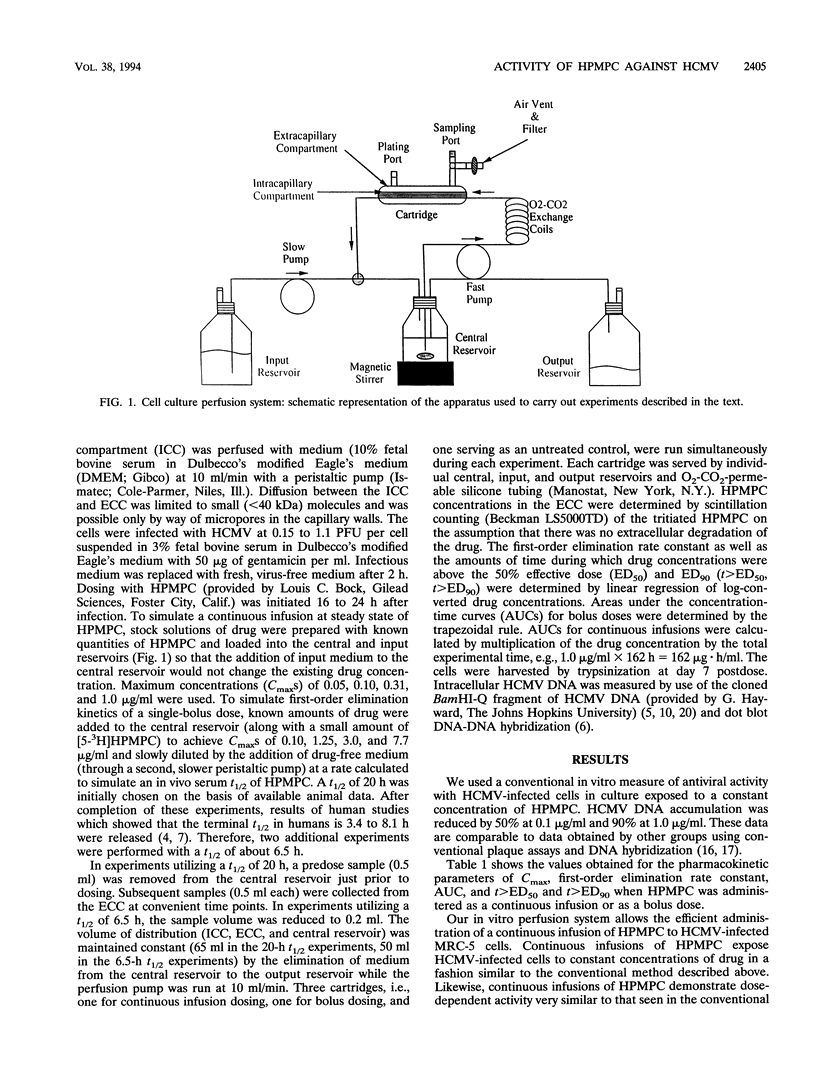
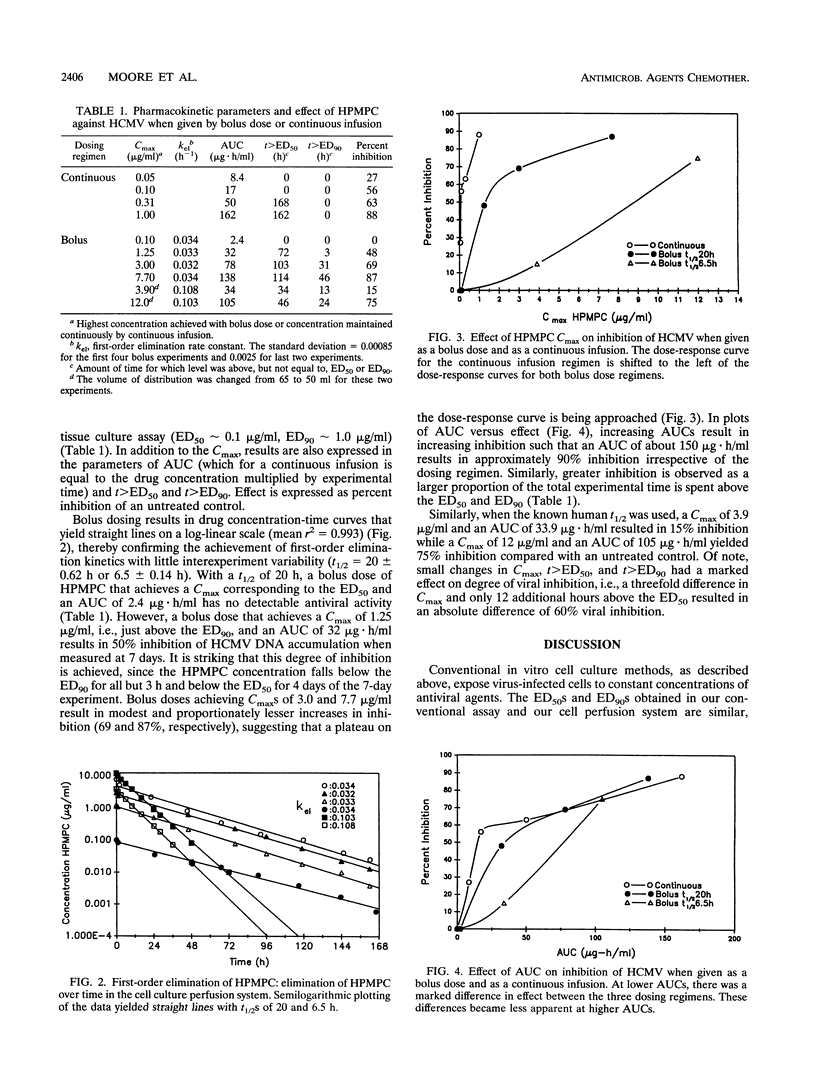
Abstract
HPMPC [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine] is a potent inhibitor of human cytomegalovirus (HCMV) replication as determined by conventional tissue culture methods in which the drug concentration remains constant over time. Previous studies have shown HPMPC to have a long intracellular half-life. Despite its relatively short extracellular half-life, HPMPC might provide significant anti-HCMV activity long after the elimination of the drug by first-order kinetics. We addressed this hypothesis by measuring the activity of HPMPC in a novel cell culture perfusion system. This system allows us to compare the activity of HPMPC when given as a continuous infusion with its activity when given as a single-bolus dose followed by elimination that simulates the drug's in vivo pharmacokinetics. We show that continuous infusions maintaining maximum concentrations (Cmaxs) of 0.05, 0.10, 0.31, and 1.0 micrograms/ml and achieving areas under the drug concentration-time curves (AUCs) of 8.4, 17, 50, and 162 micrograms.h/ml, respectively, result in 27, 56, 63, and 88% inhibition of viral DNA accumulation, respectively, compared with an untreated control. Single-bolus doses achieving Cmaxs of 0.10, 1.25, 3.0, and 7.7 micrograms/ml with an elimination half-life of 20 h achieved AUCs of 2.4, 32, 78, and 138 micrograms.h/ml and resulted in 0, 48, 69, and 87% inhibition of HCMV DNA accumulation. Single-bolus doses achieving Cmaxs of 3.9 and 12 micrograms/ml with an elimination half-life of 6.5 h achieved AUCs of 34 and 105 micrograms.h/ml, respectively, resulting in 15 and 76% inhibition of viral DNA accumulation. Comparison of Cmax-versus-effect curves for these three regimens suggests that maximum concentration is not the only important pharmacokinetic determinant of HPMPC's antiviral activity. Similar comparisons of AUC-versus-effect curves for continuous and bolus dosing suggest that the AUC is an important determinant of antiviral activity for AUCs greater than 100 micrograms . h/ml. We conclude that single-bolus doses of HPMPC potently inhibit HCMV DNA accumulation but that this activity is more heavily influenced by the AUC than the Cmax at the upper end of the AUC range tested. At lower AUCs, some other parameter may be the primary determinant of antiviral activity. Our cell culture perfusion system provides a novel, efficient, and convenient method for addressing questions relating the effects of constantly changing drug concentrations to antiviral effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser J., Stone B. B., Groner M. C., Zinner S. H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987 Jul;31(7):1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser J., Stone B. B., Zinner S. H. Two compartment kinetic model with multiple artificial capillary units. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):131–137. doi: 10.1093/jac/15.suppl_a.131. [DOI] [PubMed] [Google Scholar]
- Bronson J. J., Ghazzouli I., Hitchcock M. J., Webb R. R., 2nd, Martin J. C. Synthesis and antiviral activity of the nucleotide analogue (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine. J Med Chem. 1989 Jul;32(7):1457–1463. doi: 10.1021/jm00127a010. [DOI] [PubMed] [Google Scholar]
- D'Aquila R. T., Hayward G. S., Summers W. C. Physical mapping of the human cytomegalovirus (HCMV) (Towne) DNA polymerase gene: DNA-mediated transfer of a genetic marker for an HCMV gene. Virology. 1989 Jul;171(1):312–316. doi: 10.1016/0042-6822(89)90546-1. [DOI] [PubMed] [Google Scholar]
- Hamzeh F. M., Lietman P. S., Gibson W., Hayward G. S. Identification of the lytic origin of DNA replication in human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990 Dec;64(12):6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H. T., Woods K. L., Bronson J. J., De Boeck H., Martin J. C., Hitchcock M. J. Intracellular metabolism of the antiherpes agent (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine. Mol Pharmacol. 1992 Jan;41(1):197–202. [PubMed] [Google Scholar]
- Martin J. C., Hitchcock M. J. Phosphonomethylether compounds as antiviral agents. Transplant Proc. 1991 Jun;23(3 Suppl 3):156–158. [PubMed] [Google Scholar]
- Murakawa T., Sakamoto H., Hirose T., Nishida M. New in vitro kinetic model for evaluating bactericidal efficacy of antibiotics. Antimicrob Agents Chemother. 1980 Sep;18(3):377–381. doi: 10.1128/aac.18.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyts J., Snoeck R., Balzarini J., De Clercq E. Particular characteristics of the anti-human cytomegalovirus activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in vitro. Antiviral Res. 1991 Jul;16(1):41–52. doi: 10.1016/0166-3542(91)90057-x. [DOI] [PubMed] [Google Scholar]
- Neyts J., Snoeck R., Schols D., Balzarini J., De Clercq E. Selective inhibition of human cytomegalovirus DNA synthesis by (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine [(S)-HPMPC] and 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG). Virology. 1990 Nov;179(1):41–50. doi: 10.1016/0042-6822(90)90271-r. [DOI] [PubMed] [Google Scholar]
- Snoeck R., Sakuma T., De Clercq E., Rosenberg I., Holy A. (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother. 1988 Dec;32(12):1839–1844. doi: 10.1128/aac.32.12.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck R., Schols D., Andrei G., Neyts J., De Clercq E. Antiviral activity of anti-cytomegalovirus agents (HPMPC, HPMPA) assessed by a flow cytometric method and DNA hybridization technique. Antiviral Res. 1991 Jul;16(1):1–9. doi: 10.1016/0166-3542(91)90053-t. [DOI] [PubMed] [Google Scholar]
- Stals F. S., de Clercq E., Bruggeman C. A. Comparative activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine against rat cytomegalovirus infection in vitro and in vivo. Antimicrob Agents Chemother. 1991 Nov;35(11):2262–2266. doi: 10.1128/aac.35.11.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]
- White C. A., Toothaker R. D., Smith A. L., Slattery J. T. In vitro evaluation of the determinants of bactericidal activity of ampicillin dosing regimens against Escherichia coli. Antimicrob Agents Chemother. 1989 Jul;33(7):1046–1051. doi: 10.1128/aac.33.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]



