Abstract
BACKGROUND AND PURPOSE
Improvement in recovery rates may contribute to increase in healthy life expectancy. It is unclear, however, whether such changes take place because health researchers traditionally deal with changes in incidence and survival from diseases. The purpose of this paper is to test the presence of time trends in the recovery rate from stroke.
METHOD AND DATA
We compared age patterns of recovery rates from stroke evaluated in two subsequent sub-cohorts represented in the NLTCS data linked with the Medicare service use files.
RESULTS
We found statistically significant increase in recovery rate between 1994 and 1999 for females, but not for males.
CONCLUSION
Time trends in recovery rate from stroke exist and can be detected from available data. The roles of influential factors and causes of gender difference in recovery improvement deserve further studies.
Keywords: recovery trends, sensitivity analyses, healthy life span, compression of morbidity, survival after stroke
Despite the noticeable decline in mortality from stroke between 1981 and 2004 the causes of this decline remain controversial1–5. Earlier we found that survival from stroke significantly improved, while incidence rate almost did not change between 1984 and 2001 among the U.S. elderly4, 5. Recent study6 provided evidence that long-term outcome after first ischemic stroke depends on the quality of treatment and subsequent rehabilitation, indicating that under appropriate conditions functional recovery from stroke is possible. Improvement in recovery from disease would contribute to increasing the quality of life for stroke victims, by increasing their healthy life span. At the population level, the progress in recovery would reduce disease burden and contribute to public health improvement. To our knowledge, no studies of temporal changes in the recovery rate from stroke have been performed so far. In this paper we introduce working definition of the recovery from stroke, evaluate time trends in recovery rates, and investigate sensitivity of findings to different factors, including variable definitions of recovery and incidence rates, age- and co-morbidity structures in compared cohorts using the Medicare files linked with the National Long Term Care Survey (NLTCS) data.
Data and Methods
The NLTCS file contains longitudinal and cross-sectional data on a nationally-representative sample of about 49,000 U.S. elderly persons aged 65+ years. All NLTCS records are linked to Medicare data for 1982–2005 to allow for tracking mortality, morbidity, and HMO/MCO enrollment/disenrollment. Individual medical histories of stroke including information on life span were reconstructed from Medicare files linked with the NLTCS data, using records containing respective ICD-9 codes: 431.xx, 433.x1, 434.x1, and 436.xx and ages at onset of stroke and subsequent recovery were identified. Then, two cohorts of patients having the disease onset between 1994–1996 and 1999–2001 have been formed to investigate time trends in the recovery rates. Patients in these two cohorts were followed-up until death or the recovery event. An individual was considered to be in recovery (or sustained remission) if he/she did not have a Medicare record containing the respective ICD code(s) during one year after the last inpatient or outpatient visit related to this disease. Age at death that happened before recovery and ages of the last available data (at the end of 2005) were treated as censoring variables for the recovery time.
Survival and not-yet-recovery (i.e., the analogue of survival function constructed for recovery events) functions were calculated using the Kaplan-Meier approach. The difference between survival functions in the two sub-cohorts was tested using the log-rank test.
Results
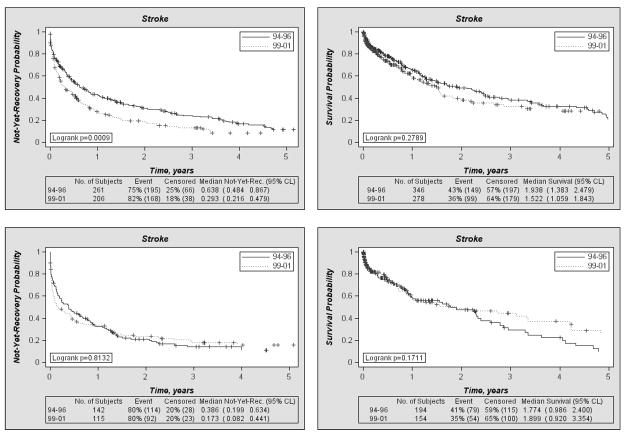
The analyses revealed statistically significant improvement in the recovery rate between 1994 and 1999 for females, but not for males. Fig. 1 (left panel) shows the graphs of the two not-yet-recovery functions constructed using two recovery rates evaluated for patients with stroke in the two (1994–1996 and 1999–2001) sub-cohorts for males and females, respectively. This figure shows that respective recovery rate is higher for the 1999–2001 sub-cohort than that for the 1994–1996 sub-cohort. The total numbers of subjects in each sub-cohort, the percents of recovery and censored events, as well as the median values with respective 95% confidence intervals, are shown in the tables below the graphs.
Figure 1.
Not-yet-recovery probability functions (left panels) and survival probabilities (right panels) for females (top panels) and males (bottom panels) in the two sub-cohorts of the NLTCS participants having age at onset of stroke in 1994–1996 and in 1999–2001.
The survival functions after stroke (where the occurrences of recovery were treated as censoring times) for the same sub-cohorts did not reveal substantial changes during the five year period for each gender (right panel of Fig. 1). Together with the results shown in Fig. 1, left panel, these indicate that the progress in coping with the disease was mainly due to changes in recovery from this disease in females.
Sensitivity analyses
To test sensitivity of our findings to different factors capable of affecting trends in recovery rate, we repeated our calculations using i) several different operational definitions of recovery and incidence rates, ii) explicit representation of observed heterogeneity effects stratifying individuals on age, comorbidity (Charlson index7), or disability (the numbers of self-reported ADL/IADLs), and iii) other approaches to censoring strategies, selection of individuals, and study design effects. The results of the analyses, presented in Table 1 for both genders, indicate that positive trends in the recovery rate from stroke take place in all cases independently from the definition of such rates. One source of bias in findings could be partial coverage by HMO which may reduce the number of stroke diagnoses in Medicare records. In our calculations this source of uncertainty could be important because the fraction of coverage by HMO is different for two considered time periods. So, in 1994 the fraction of person-months additionally covered by HMO was at the level of 3–5% while in 1999 the fraction exceeds 15%.
Table 1.
Results of sensitivity analysis (N94 and N99 shows the size of the cohorts of respective years)
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| N94,N99 | log-rank | Median (years) | N94,N99 | log-rank | Median (years) | |||
| 94–96 | 99–01 | 94–96 | 99–01 | |||||
| Base calculation | 261,206 | 0.0009 | 0.64 | 0.29 | 142,115 | 0.8132 | 0.39 | 0.17 |
| 1. Approaches to identification of recovery events | ||||||||
| Time to recovery (1.5 years) | 237,192 | 0.0049 | 1.05 | 0.36 | 135,109 | 0.9988 | 0.69 | 0.41 |
| Time to recovery (0.5 years) | 286,223 | 0.0043 | 0.34 | 0.21 | 155,129 | 0.9686 | 0.24 | 0.17 |
| Alzheimer’s disease and dementia | 261,206 | 0.0178 | 0.61 | 0.41 | 142,115 | 0.4345 | 0.42 | 0.17 |
| 2. Approaches to identification of incident cases | ||||||||
| All Medicare sources used | 286,221 | 0.0013 | 0.64 | 0.31 | 159,125 | 0.6977 | 0.39 | 0.24 |
| Keeping not only primary diagnoses | 323,269 | 0.0009 | 0.49 | 0.24 | 182,145 | 0.4876 | 0.36 | 0.09 |
| No requirement of second visit | 523,416 | 0.0111 | 0.09 | 0.05 | 276,242 | 0.4220 | 0.05 | 0.01 |
| Acceptable lag of pre-diagn.=0.5year | 261,206 | 0.0009 | 0.64 | 0.29 | 146,117 | 0.8363 | 0.39 | 0.20 |
| Only 436 ICD-9 code included | 228,182 | 0.0017 | 0.61 | 0.25 | 123,90 | 0.8276 | 0.39 | 0.24 |
| Exclude hemorrhagic (ie, 431) stroke | 258,202 | 0.0016 | 0.62 | 0.29 | 141,109 | 0.7406 | 0.39 | 0.25 |
| Only hemorrhagic (i.e., 431) stroke | 13,14 | 0.5680 | 0.016 | 0.063 | 10,12 | 0.1711 | 0.227 | 0.027 |
| Include ICDs: 430–432 | 23,28 | 0.0871 | 0.112 | 0.067 | 20,20 | 0.6478 | 0.153 | 0.114 |
| Exclude not completely covered | 229,188 | 0.0041 | 0.61 | 0.27 | 136,108 | 0.5108 | 0.39 | 0.14 |
| 3. Factors of observed heterogeneity | ||||||||
| 65≤Age≤72 | 56,43 | 0.0140 | 0.61 | 0.21 | 31,31 | 0.4543 | 0.25 | 0.32 |
| 73≤Age≤84 | 139,110 | 0.0096 | 0.72 | 0.30 | 95,66 | 0.8493 | 0.39 | 0.20 |
| 85≤Age | 66,53 | 0.4828 | 0.61 | 0.46 | 16,18 | 0.3374 | 0.15 | 0.09 |
| Charlson Index=0 | 107,77 | 0.0522 | 0.61 | 0.24 | 55,36 | 0.9565 | 0.18 | 0.23 |
| 1≤Charlson Index≤3 | 120,95 | 0.0232 | 0.76 | 0.36 | 66,56 | 0.7897 | 0.46 | 0.14 |
| Charlson Index≥4 | 34,34 | 0.1317 | 0.61 | 0.25 | 21,23 | 0.8836 | 0.79 | 0.25 |
| Disability group=1 | 143,128 | 0.0007 | 0.60 | 0.24 | 97,87 | 0.4733 | 0.39 | 0.13 |
| Disability group=2 | 47,27 | 0.0504 | 1.01 | 0.21 | 22,13 | 0.4767 | 0.13 | 0.28 |
| Disability group=3 | 71,51 | 0.9775 | 0.80 | 0.90 | 23,15 | 0.5246 | 1.32 | 1.49 |
| 4. Censoring strategies | ||||||||
| Cases of death are recovery cases | 261,206 | 0.0059 | 0.50 | 0.25 | 142,115 | 0.4704 | 0.39 | 0.16 |
| Cases of death are never recovered | 346,278 | 0.0478 | 2.61 | 0.93 | 194,154 | 0.5400 | 1.20 | 1.17 |
| 5. Disenrollment from Medicare and coverage by HMO | ||||||||
| All covered by HMO are included | 284,215 | 0.0125 | 0.61 | 0.33 | 149,124 | 0.8593 | 0.37 | 0.17 |
| Fraction of HMO enrollment≤0.3 | 264,209 | 0.0015 | 0.62 | 0.31 | 145,118 | 0.9811 | 0.39 | 0.17 |
| 6. Study Design effect | ||||||||
| Using NLTCS weights | 472524, 427500 | 0.62 | 0.31 | 270628, 255493 | 0.39 | 0.13 | ||
Note that the definition of recovery index used in this paper does not take into account some important factors affecting the recovery rate, such as rehabilitation therapy. These details, however, were not among the goals of this study focused on detecting the presence of positive trends in the recovery from stroke. An important property of our results is that they are robust to changes in definitions of recovery or incidence events, as well as to many other potential confounders. Note that the estimate of an increase in healthy life span would be more sensitive to the definitions of incidence and recovery events.
Conclusion
Time trends in the recovery rate from stroke exist and can be detected from available data. The detected gender difference in such trends may partly be caused by different attitude towards the use of health care services in males and females6, as well as a small time difference between compared sub-cohorts. Note that gender differences are also documented in stroke incidence and case-fatality8. More studies are needed to evaluate the changes in the quality of life in post-stroke individuals.
Acknowledgments
Sources of Funding
This study was supported by the NIA/NIH grants R01AG027019, R01AG028259, and R01AG032319. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA or NIH.
Footnotes
Conflicts of Interest/Disclosures
None
References
- 1.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, Gutnikov SA, Edwards P, Mant D, Sackley CM, Farmer A, Sandercock PAG, Dennis MS, Warlow CP, Bamford JM, Anslow P. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 2.Pessah-Rasmussen H, Engstrom G, Jerntorp I, Janzon L. Increasing stroke incidence and decreasing case fatality, 1989–1998: A study from the Stroke Register in Malmo, Sweden. Stroke. 2003;34:913–918. doi: 10.1161/01.STR.0000063365.10841.43. [DOI] [PubMed] [Google Scholar]
- 3.Sarti C, Stegmayr B, Tolonen H, Mahonen M, Tuomilehto J, Asplund K. Are changes in mortality from stroke caused by changes in stroke event rates or case fatality? Results from the WHO MONICA Project. Stroke. 2003;34:1833–1840. doi: 10.1161/01.STR.0000081224.15480.52. [DOI] [PubMed] [Google Scholar]
- 4.Ukraintseva S, Sloan F, Arbeev K, Yashin A. Increasing rates of dementia at time of declining mortality from stroke. Stroke. 2006;37:1155–1159. doi: 10.1161/01.STR.0000217971.88034.e9. [DOI] [PubMed] [Google Scholar]
- 5.Akushevich I, Yashin AI. Circulatory Diseases and Aging. In: Heggenhougen K, Quah S, editors. International Encyclopedia of Public Health. San Diego: Academic Press; 2008. [Google Scholar]
- 6.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, Elkind MSV. Long-term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke. 2009;40:2805–2811. doi: 10.1161/STROKEAHA.109.549576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]