Abstract
Benzodiazepines and alcohol are widely used psychoactive substances that have performance-impairing effects. Research suggests that the impairment profiles for benzodiazepines and alcohol differ, though few cognitive psychopharmacological studies have directly compared these drugs. This double-blind, double-dummy, placebo-controlled, repeated measures study directly compared the acute dose effects of triazolam (0.125 mg, 0.25 mg/70 kg) and alcohol (0.40 g, 0.80 g/kg) in 20 social drinkers. At doses that produced comparable psychomotor impairment, triazolam was more likely to impair several objective measures of cognitive performance (e.g., episodic memory, divided attention) and to slow performance across several measures. However, only alcohol impaired accuracy on the digit symbol substitution and semantic memory tasks. In addition to objective measures, both drugs impaired awareness of performance impairments (i.e., metacognition) such that participants over-estimated impairment, and the magnitude of this effect was generally larger for alcohol. Only triazolam impaired other measures of metacognition (e.g., error detection on a choice reaction time task). Future research might examine the clinical implications of the performance impairments reported here given the widespread use of benzodiazepines and alcohol.
Keywords: Performance, Cognition, Benzodiazepines, Triazolam, Alcohol
Introduction
Benzodiazepines (e.g., diazepam: Valium, triazolam: Halcion) are prescription medications commonly used among the general population for medical (e.g., anxiety, insomnia), as well as recreational purposes (Kaufman, Kelly, Rosenberg, Anderson, & Mitchell, 2002; McCabe, 2005; Yang, Simoni-Wastila, Zuckerman, & Stuart, 2008). In fact, recent estimates suggest that nonmedical use of prescription benozodiazepines is on the rise among college students (Mohler-Kuo, Lee, Wechsler, 2003). Alcohol, another drug commonly used by college students, is one of the most widely used drugs in the world with total adult per capita alcohol consumption estimated to be about five liters of pure alcohol (World Health Organization, 2004). In addition to their widespread use, benzodiazepines and alcohol are similar in that both drugs impair cognitive performance (for reviews see Buffett-Jerrott & Stewart, 2002; Curran, 1991; Drummer, 2002; Koelega, 1989; 1995; Ogden & Moskowitz, 2004). The cognitive impairing effects of these drugs are thought to exist, at least in part, because both drugs influence activity at the gamma-aminobutyric acid (GABAA) receptor (Berry, Chandra, Diaz-Granados, Homanics, & Matthhews, 2009; Kotlinska &Langwinski, 1995). However, unlike the GABA-specific activity of benzodiazepines, alcohol has more varied effects across several other receptor systems including acetylcholine and glutamate (Julien, 2001; Malcolm, 2003).
Based on these neurochemical differences, benzodiazepines and alcohol may differ in the specific pattern of cognitive impairment they produce. A large body of research has explored the cognitive effects of benzodiazepines and alcohol; however, few studies have directly compared these effects by incorporating both drugs into a single study. As reviewed below, the few studies that have directly compared benzodiazepines and alcohol suggest that the impairment profiles for these drugs differ (Lombardi, Sirocco, Andreason, & George, 1997; Mattila, Vanakoski, Kalska, & Seppala, 1998; Mintzer & Griffiths, 2002; Roache, Cherek, Schenkler, Cowan, & Bennett, 1993; Schuckit, Greenblatt, Gold, & Irwin, 1991; Tiplady et al., 1998).
Episodic memory (conscious long-term memory for a personally experienced event; Tulving, 1972, 1983) is consistently impaired by both benzodiazepines and alcohol (Mintzer & Griffiths, 2002; Soderlund, Parker, Schwartz, & Tulving, 2005; Tiplady, Hiroz, Holmes, & Drummond, 2003). However, in some studies, the magnitude of episodic memory impairment is greater for benzodiazepines relative to alcohol (Mattila et al., 1998; Mintzer & Griffiths, 2002; Roache et al., 1993; Schuckit et al., 1991; but see Tiplady et al., 1999). For example, a repeated-measures, placebo-controlled study of triazolam (0.25mg/70kg) and alcohol (0.80g/kg) in our laboratory revealed that both drugs decreased sensitivity in discriminating between old and new words on a recognition memory task (d′) relative to placebo; however, d′ was significantly lower for triazolam relative to alcohol at doses that produced comparable levels of psychomotor impairment (Mintzer & Griffiths, 2002).
Working memory, a system that assists in the temporary holding and manipulation of information (Baddeley, 1999), is another type of memory that has been examined in studies of benzodiazepines and alcohol. Roache and colleagues (1993) directly compared the effects of triazolam (0, 2, 4, and 8 μg/kg) and alcohol (0. 0.25, 0.50, and 1 g/kg) on a number recall task in which participants were required to recall an 8-digit number after a 10 sec delay. Only triazolam (4 and 8 μg/kg) impaired performance while comparable doses of alcohol (0.50 and 1 g/kg) did not alter performance. However, it should be noted that using an identical number recall task, Simpson and Rush (2002) found that neither triazolam (0.125 and 0.25 mg) nor alcohol (0.50 g/kg) impaired performance.
In contrast to the sizeable amount of research on episodic and working memory, awareness and knowledge of one’s performance (i.e., metacognition; Flavell, 1971; Metcalfe & Shimamura, 1994) is less often examined. Measuring drug-induced changes in metacognition is particularly important given that benzodiazepines and alcohol impair gross motor activity; awareness of impairment, or lack thereof, might have serious consequences (e.g., risking injury or death by choosing to drive a car). The previously described study by Roache and colleagues (1993) included one metacognitive outcome on which participants estimated their performance on a circular lights task (a measure of psychomotor performance) immediately after task completion.. Though both drugs impaired performance on the circular lights task, awareness of this performance impairment was only present in the alcohol condition (1.0 g/kg) and not the comparable high dose triazolam (8.0 μg/kg) condition. In other words, metacognitive processes were disrupted by triazolam (performance impairment was underestimated) and not altered by alcohol. These findings are consistent with other studies showing that benzodiazepines tend to impair metacognition (Bacon et al., 1998; Massin-Krauss, Bacon, & Danion, 2002; Mintzer & Griffiths, 2003a; Roache & Girffiths, 1985, 1987; Roache et al., 1993), whereas for the most part alcohol does not (Acons, Chan, Drummond, & Tiplady, 2006; Harrison & Filmore, 2005; Mintzer, Guarino, Kirk, Roache, & Griffiths, 1997b; Nelson, McSpadden, Fromme, & Marlatt, 1986; Roache et al., 1993). Although in one study alcohol did impair performance ratings (performance was overestimated) on a task that measured knowledge of general information (i.e., semantic memory; Tiplady, Franklin, & Scholey, 2004), alcohol did not impair objective performance (i.e., semantic memory) in that study, making it difficult to compare the performance rating results to those of studies in which drug-induced impairment was observed on objective measures of performance. Additional research on this subject would help to confirm the hypothesis that benzodiazepines are more likely to disrupt metacognition relative to alcohol.
Lastly, another cognitive function, divided attention, was examined in a study comparing triazolam (0.25 mg) and alcohol (0.6 g/kg), though that study was primarily designed to examine the influence of nap taking on performance (Roehrs, Claiborue, Knox, & Roth, 1993). However, even with this limitation, some inferences can be drawn. Triazolam impaired performance relative to placebo on both components (central visual tracking and peripheral visual monitoring) of the task independent of nap taking whereas alcohol’s impairing effects were reduced by nap taking and were smaller in magnitude relative to triazolam (comparisons of the active drugs were not reported). This study suggests that triazolam might impair divided attention to a greater degree than alcohol – a surprising conclusion given that divided attention is considered to be the most sensitive measure for detecting alcohol-related impairment (Moskowitz & Robinson, 1987). However, additional research is needed before firm conclusions regarding the relative effects of these drugs on measures of divided attention can be drawn.
In summary, the existing literature comparing the effects of benzodiazepines and alcohol suggests that these drugs have different cognitive impairment profiles. Specifically, the above review suggests that benzodiazepines may produce more impairment in episodic memory, working memory, metacognition, and divided attention relative to alcohol. However, additional studies are needed to fully elucidate the effects of benzodiazepines and alcohol on these cognitive processes. In addition, some aspects of cognition, such as semantic memory (conscious long term memory that is not associated with a specific spatial or temporal context; i.e., knowledge of the world; Baddeley, 1999) have yet to be examined in a study directly comparing benzodiazepines and alcohol. A review of research that has examined the effect of either benzodiazepines or alcohol on semantic memory provides little clarity on their relative effects: some studies suggest that both drugs impair performance (Bacon et al., 1998, Bacon, Schwartz, Paire-Ficout, & Izaute, 2007; Brown, Brown, & Bowes, 1983, Massin-Kraus et al., 2002; Nelson et al., 1986; Wendt & Risberg, 2001) and others suggest null effects (Curran, 1991; 1999; Tiplady et al., 2004; Weingartner et al., 1993).
The purpose of the present study was to systematically compare the cognitive effects of triazolam and alcohol using tasks specifically designed to assess the cognitive processes reviewed above. Alcohol and triazolam were compared across multiple doses in a double-blind, double-dummy, placebo-controlled, repeated measures design. Doses of triazolam (0.125 mg and 0.25 mg/70 kg) and alcohol (0.40 g and 0.80 g/kg) were selected to produce comparablesedative effects (as reflected by similar levels of psychomotor performance impairment based on previous studies in our laboratory; Mintzer & Griffiths, 2000; 2007; Mintzer et al., 2001). Based on the existing literature, we hypothesized that the cognitive performance impairing effects of triazolam would be greater in magnitude relative to alcohol across multiple measures (i.e., episodic memory, working memory, metacognition, divided attention).
Method
General Study Design
Participants completed an initial screening interview/practice session in which they were familiarized with the experimental procedures and tasks. Participants were informed that during their participation in the study, they would receive various drugs and that these could include placebo, various sedatives (e.g., alcohol, diazepam, triazolam, zolpidem, etc), antihistamines, stimulants, and weight loss medications. Participants were otherwise blind to the type of drug administered. After the screening interview, participants completed five outpatient sessions in which placebo (PL), triazolam (TRZ; 0.125 mg and 0.25 mg/70 kg) and alcohol (ALC; 0.40 g and 0.80 g/kg) were administered in a double-blind, double-dummy, cross-over design; successive sessions were separated by a minimum of 48 h.
Participants
Twenty adult participants (12 women) completed the experiment. The participants ranged in age from 21 to 50 years (M = 24.5, SD = 6.6) and in weight from 50.9 to 87.2 kg (M = 68.4, SD = 9.5), and reported having completed 13 to 19 years of education (M = 16.1, SD = 1.7). All participants were in good health (as assessed by medical history and personal interview) with no contraindications to sedative drugs. Individuals with psychiatric disorders were excluded. For the female participants, urine pregnancy tests during screening were negative. All participants reported drinking alcohol socially (mean number of drinks per week = 8.6, SD = 5.7), but none reported histories of drug or alcohol abuse. Three participants reported daily cigarette use (mean cigarettes per day = 4.5, SD = 0.5) and 18 participants reported daily caffeine use (mean mg per day = 168.2, SD = 133.2).
Results of urine drug tests (opiates, methadone, cocaine, and benzodiazepines) using an EMIT system (Syva Co., Palo Alto, CA) and alcohol breathalyzer tests conducted at screening and prior to drug administration during each session were negative for all participants. The study was approved by the Institutional Review Board of the Johns Hopkins Bayview Medical Center, all participants gave their written informed consent before beginning the study, and all were paid for their participation.
Procedures
Sessions began in the morning and lasted approximately 6 hours. Measures (see below) were administered before and repeatedly following drug administration (except for the episodic and semantic memory tasks, which were administered at single time points estimated to coincide with peak drug effects). Non-computerized psychomotor performance tasks (i.e., circular lights, balance) were administered and breath alcohol level was tested approximately 50, 75, 155, 200, and 265 min after capsule administration. The Digit Symbol Substitution Test (DSST) was administered approximately 60, 115, 165, 225, and 260 min after capsule administration and the error detection task was administered approximately 55, 80, 160, and 215 min after capsule administration. The working memory and divided attention tasks were administered consecutively, starting at approximately 120 and 230 min after capsule administration. Lastly, participant ratings of subjective effects were collected before each administration of the DSST. Data from the participant ratings will be presented in a separate manuscript due to the complexity of the cognitive performance and participant rating findings. However, data from participant ratings of “Drug Strength” will be described in the present manuscript to compare the effects of triazolam and alcohol on a global rating of drug effect. We expected to replicate the pattern of results observed previously that participant ratings of overall strength of drug effect are higher for alcohol relative to triazolam at doses that produce comparable levels of psychomotor impairment (Mintzer & Griffiths, 2002). All computerized measures were administered on an Apple Macintosh microcomputer (Apple Computer, Cupertino, CA).
Drug Administration
To achieve balance in the presentation order and in the order of drug conditions relative to one another, the drug order across the five sessions was determined by two Latin squares using the Williams (1949) method. Participants received a set of capsules (containing either TRZ or PL) and a beverage (containing either alcohol or placebo). In order to synchronize the time of peak effects of TRZ and ALC based on pharmacokinetic and behavioral data, the capsules were administered 30 min earlier than the start of beverage consumption. TRZ doses were prepared from commercially available 0.25 mg tablets (Halcion; Pharmacia and UpJohn Company, Bridgewater, NJ, USA). Tablets were crushed and doses were adjusted by participant body weight. All doses were dispensed in a size 0 capsule. Lactose was used to fill the remainder of all the capsules; PL capsules contained only lactose. Capsules were taken orally with approximately 150 ml of water. ALC doses were administered as 95% ethanol (USP, Florida Distillers Co., Lake Alfred, FL, USA) mixed with fruit juice; PL beverage consisted of fruit juice. In all conditions, beverage was administered in a covered opaque beverage cup and was consumed through a straw that was wrapped with an ALC-soaked band in order to mask the olfactory and gustatory cues of the beverage. In addition, 2 ml of 95% ALC was added into the straw to further mask taste differences between PL and active beverage. Initially, participants (n = 13) were given a total volume of 400 ml in a single cup to be consumed within 5 minutes. However, because several participants took over 5 minutes to complete consumption of the beverage, the procedure was changed such that the remaining participants (n = 7) were given a total volume of 600 ml split equally into two cups across a total of 10 min (5 min per cup).
Experimental Measures
Participant Rating of Drug Strength
Participants rated how strong a drug effect they felt on a 5-point scale (coded numerically from 0 to 4) using a computer mouse.
Psychomotor Performance Tasks
Psychomotor tasks were circular lights (a measure of psychomotor speed and hand-eye coordination; Griffiths, Bigelow, & Liebson, 1983) and a standing balance task. The dependent measures were the number of correct button presses during a 60-s trial for circular lights and the total seconds balanced across both feet.
Attention
Digit symbol substitution task (DSST)
A computerized version of the DSST (a measure of focused attention and processing speed) was utilized (McLeod, Griffiths, Bigelow, & Yingling, 1982; based on Wechsler, 1981). In response to randomly selected digits (1–9) appearing on the screen, participants used a numeric keypad to reproduce the geometric symbol pattern associated with that digit by using the digit-symbol code displayed continuously at the top of the screen. The dependent measures were number attempted (speed) and proportion correct (accuracy) during a 90-s trial.
Choice reaction time task
During the choice reaction time task participants responded to 160 trials during which three randomly selected digits (1–9) were presented in the center of the computer screen for 1 sec. Simultaneously, a “1”, “2”, or “3” was shown above the three digits to indicate which digit in the series was the target stimulus (i.e., position 1, 2, or 3). Participants indicated whether the target stimulus was odd or even by using the computer mouse to click on an “Odd” or “Even” button on the screen. The response intervals were either 2 s or 6 s and were randomly distributed across the 160 trials (80 trials per response interval condition). If participants did not respond during the response interval, then the response was counted as a “timeout”. If the participant did respond within the response interval, then they indicated whether they thought they made an error on that trial (“oops” button) or had not made an error (“go” button). Participants were told that they were to avoid making errors when possible but that speed was more important than accuracy and it was important to respond as quickly as possible to each item even if this meant that they were not able to avoid an error. Dependent measures were median response time for correct responses (time between stimulus display offset and the participant’s “odd” or “even” response) and proportion of correct, incorrect, and timeout responses. In addition, proportion correct on the error detection component of the task (i.e., proportion of “oops” responses for errors and “go” responses for correct responses) was calculated to provide a measure of error detection accuracy (i.e., metacognition).
Divided attention
The divided attention task included simultaneous completion of visual tracking and monitoring components. Tracking took place in the center of the computer screen while monitoring required the participant to attend to the corners of the computer screen (as described below). For the tracking component, the participant used the computer mouse to track a diamond stimulus that moved horizontally in the center of the computer screen. For the monitoring component, the participant attended to a green digit (1–9; target digit) that was presented for 60 s at the bottom, center of the screen. The target digit was chosen randomly and changed 4 times per trial. Participants clicked a mouse each time the digit matched the target digit (6 target presentations per corner, resulting in 24 opportunities to correctly click the mouse). Participants were told that the tracking and the monitoring components were equally important. Dependent measures associated with tracking were: tracking moves (number of times the cross hair was moved on the screen), tracking deviation (distance in pixels between the diamond stimulus and cross hair), and tracking overlap (number of times the cross hair and diamond overlapped). Dependent measures associated with monitoring were: total responses (total number of times the mouse was clicked), mean RT (time between the appearance of a digit in the corner of the computer screen that matched the target digit and the participant’s mouse click), and proportion correct (number of times a mouse press was made when the target digit was presented in the corner of the screen out of a total possible of 24). Outcomes for this task can be categorized as assessing either speed (tracking moves, total responses, mean RT) or accuracy (tracking deviation, tracking overlap, and proportion correct).
Working Memory
During completion of the working memory task (Mintzer & Griffiths, 2007; Postle, Berger, & D’Esposito, 1999; based on Sternberg, 1969)) participants were presented with a memory set consisting of seven randomly selected and randomly ordered consonant letters (excluding L and Y; e.g., PCZMSTF) that was followed by a probe consisting of a letter-digit pair (e.g., c-2). Participants then decided whether the probed letter had appeared in the memory set in the ordinal position represented by the digit (e.g., 2 represented the second position in memory set). To prevent simple perceptual matching to the memory set stimuli (which were presented in upper case), the probe was always presented in lower case. Effects on working memory rehearsal processes were tested by varying the delay between memory-set presentation and testing (i.e., probe presentation; 0 s and 12 s). There were 12 trials in each of the two delay conditions and 12 trials in a control condition in which the memory set remained on the screen during probe presentation (i.e., 36 trials total); trials in all conditions were randomly intermixed. The control condition was designed to control for drug effects on non-memory processes (e.g., motor coordination, perception, etc.). Within each condition, the probed digit represented the correct position of the probed letter on half of the trials. The memory set appeared on the screen for 4 s followed by the predetermined delay and presentation of the probe for a period of 7 s during which the participant responded by using the computer mouse to click on a button labeled ‘yes’ or ‘no’. RT from onset of the probe was recorded. After each response, participants were required to return the mouse cursor to a screen position equidistant between the two response buttons during a 2 s intertrial interval. Dependent measures were proportion of correct responses and median RT on correct trials as a function of delay condition.
Episodic Memory
Following an initial study phase, episodic memory was tested via free recall and recognition tests. Stimuli for each session consisted of a unique set (i.e., no stimulus was repeated across sessions) of 140 common concrete nouns selected from the Thorndike and Lorge (1944) word corpus (i.e., total of 700 words across five sessions); the sets were equated across the five sessions for mean word frequency in the language (Thorndike & Lorge, 1944) and word length. The 140-word stimulus set assigned to each session was randomly divided into two 70-word subsets equated for mean word frequency in the language and word length. One subset was assigned to the old condition and one was assigned to the new condition (see below); the subsets assigned to the two conditions were counterbalanced such that, across participants, each subset appeared equally often in the old and new conditions.
During the study phase, which was conducted 80 min after capsule administration (i.e., 45 min after beverage administration), participants were presented with a set of 70 words (those assigned to the old condition) that appeared on the computer screen one at a time. Each word appeared on the screen for two seconds. In order to ensure that participants would attend to and encode the words presented during the study phase, participants were asked to perform a conceptual categorization task on each word (to categorize each word as representing an “artificial” or “natural” object; e.g., Mintzer et al., 2001) and to make their responses by using the computer mouse to click on the appropriately labeled button on the screen.
Two hours after completing the study phase, participants were administered a free recall test followed by a recognition memory test. For free recall, participants were given five minutes to write down all the words they remembered seeing during the initial study phase. In the recognition memory test, participants were presented with a set of 140 words that appeared on the screen one at a time in random order (70 old words that had been presented during the study phase randomly mixed with 70 new words that had not been presented during the study phase). Participants made judgments about the words using a 6-point confidence scale (definitely old, probably old, maybe old, maybe new, probably new, definitely new), and were encouraged to distribute their responses across the entire confidence range. Each test word remained on the screen until the participant responded. In addition to measuring episodic memory, this version of the recognition memory test also provides a measure of participants’ metamemory (awareness and knowledge of one’s own memory; Flavell, 1971; Metcalfe & Shimamura, 1994) by comparing confidence ratings given to correct versus incorrect recognition responses; greater awareness of the state of one’s memory should result in greater confidence in correct responses and lower confidence in incorrect responses. Performance on the conceptual categorization task (study phase) was assessed by analyzing the proportion of correct categorization responses. Two sets of analyses were conducted on the data from the recognition memory test: one in which data were collapsed across confidence ratings to provide measures of episodic memory, and one in which the confidence ratings themselves were analyzed to provide a measure of metamemory. Dependent measures for the free recall test were number of correct responses (i.e., number of old words; out of a total of 70 possible). Dependent measures for recognition memory included proportion of old responses made to old words (hit rate), proportion of old responses to new words (false alarm rate), and signal detection measures of sensitivity in distinguishing between old and new words (d′) and response bias (C; Snodgrass & Corwin 1988). Metamemory was measured by calculating the Goodman–Kruskal gamma correlation (between confidence ratings and recognition memory accuracy computed for each participant; Goodman & Kruskal 1954). Values for gamma can range from 1 (complete concordance between confidence ratings and recognition memory accuracy as reflected in high confidence ratings given to correct responses/low confidence ratings given to incorrect responses) to −1 (complete discordance between confidence ratings and recognition memory accuracy). To ensure the availability of the data from a sufficient number of items per participant to enable the performance of these analyses, data were collapsed across old and new words.
Semantic Memory
The semantic memory procedure used in the present study was based on Koriat and Goldsmith (1996) and consisted of two phases that centered on 45 general knowledge questions (unique across five sessions). The questions were selected based on initial piloting in our laboratory from a pool of 300 general knowledge questions developed by Nelson and Narens (1980). The questions were formulated such that the correct answer was always a single word or a proper name (e.g., Q: “What is the name of a large hairy spider that lives near bananas?”; A: tarantula). The five lists of 45 questions were balanced for probability of recall (based on normative data collected by Nelson & Narens, 1980), and the order of questions was randomized for each participant in each phase of the task.
Phase 1 (forced report)
The 45 questions appeared on the computer screen one at a time, and participants were required to provide an answer to every question by typing a one-word response, even if they had to guess (i.e., forced-report). Immediately after responding to each question participants assessed the likelihood that their answer was correct using a 0 (definitely not correct) to 100% (definitely correct) visual analogue scale. There were no time constraints.
Phase 2 (free report)
In Phase 2, participants could choose whether to provide an answer to a given question (by typing in a one-word response) or to not provide an answer (by typing in the word “pass”). Participants were told that they would not be penalized (but neither would they receive any bonus) for omitted items. Accurate responding was reinforced by paying the participant 25 cents for each correct answer (for a total possible of $11.25 per session) and penalizing the same amount for each incorrect answer. The amount of money that participants earned in this portion of the task was added to their total pay at the end of the study; participants were assured that they would not have to pay any losses. To ensure that participants understood the task, they were required to repeat the instructions in detail to the research assistant during each session
Dependent measures
Dependent measures were as described in Koriat and Goldsmith (1996). Retrieval of correct answers to the general knowledge questions (i.e., semantic memory) was measured using quantity and accuracy measures. Quantity is calculated as the proportion of correct responses out of the total number of items presented (i.e., 45), whereas accuracy is calculated as the proportion of correct responses out of the total number of non-“pass” responses provided by the participant (i.e., 45 in the forced report phase; variable in the free report phase). This task also permits the measurement of several aspects of metamemory (described in detail in Koriat & Goldsmith, 1996), which will not be described here because no significant drug effects were observed on any of these measures.
Performance Estimation
In addition to the above objective measures of performance, participants were asked to rate how well they thought they would perform/did perform on the DSST, choice reaction time, and working memory tasks in terms of speed and accuracy compared to normal, before (pre-performance estimates) and after (post-performance estimates) each task. Participants made these separate speed and accuracy ratings by clicking along a line on the screen labeled “much worse” at the left extreme, “normal” in the middle, and “much better” at the right extreme; scores range from 0 (much worse) to 100 (much better). These ratings are measures of the participants’ awareness of their performance (i.e., metacognition). The participant estimates of performance were compared to the actual task scores by calculating difference scores for each post-drug time point as the percentage of pre-drug estimate minus the percentage of pre-drug actual performance. In addition to speed and accuracy, participants were asked to estimate the number of items correct out of total items possible before and after completing the choice reaction time task (160 items possible) and the working memory task (36 items possible). Difference score calculations were carried out between these ratings and actual number correct. For all performance estimates, a positive score represents an under-estimation of performance impairment, while a negative score represents an over-estimation.
It should be noted that after calculating difference scores for speed performance estimates as described above we realized that estimates of speed and actual measures of speed for the choice reaction time task and the working memory task (i.e., RT) are on different scales: performance estimates range from 0 to 100, whereas actual performance (i.e., RT) has an unlimited upward bound. This scale difference sometimes resulted in distorted difference scores. Consequently, we decided to remove the speed estimates for choice reaction time and working memory from the performance estimate analyses.
Data Analysis
All outcomes included complete data sets (N=20) with the exception of semantic memory, episodic memory gamma, and performance estimation for which data were missing for one volunteer each (N = 19; one participant was removed from semantic memory and performance estimate data due to noncompliance with task instructions; one participant was excluded from the gamma analysis due to insufficiently distributed data). All data were analyzed using PROC MIXED in SAS (SAS Institute Inc., Cary, NC). The analyzed data were either raw values (participant rating of drug strength, episodic memory, semantic memory), percentage of predrug values (psychomotor tasks, DSST, divided attention, working memory task, performance estimation: accuracy and speed), or difference from predrug values (choice reaction time task, performance estimation: number of items correct; difference scores were calculated for these measures because predrug values of 0 for some participants made calculation of percentage of predrug values impossible).
Two sets of analyses were conducted. First, data from all measures that were completed before and repeatedly following drug administration were analyzed by repeated measures analysis of variance (ANOVA) with drug condition (PL, TRZ 0.125, 0.25, ALC 0.4, 0.8) and time (see Method section for specific times) as factors. Second, data from tasks that were administered at single time points following drug administration (episodic and semantic memory) and peak effects data were analyzed by repeated measures ANOVA with drug condition as the factor. Peak effects were calculated for each participant as the minimum value observed after drug administration for the following outcomes: circular lights, balance, DSST, divided attention (total responses, proportion correct, tracking overlap, tracking moves), working memory task (proportion correct), and choice reaction time task (proportion correct, error detection accuracy), and as the maximum value observed after drug administration for the following outcomes: participant rating of drug strength, divided attention (tracking deviation, RT), working memory task (RT), and choice reaction time task (proportion incorrect, proportion timeouts, RT). Additional factors were included in the ANOVA for the following: 1) Choice reaction time task (response interval of 2 s and 6 s), 2) working memory task (delay condition of 0 s and 12 s; note that the nonmemory control condition was entered as a covariate for this task), and 3) Semantic memory (phase 1 and 2). Performance estimate peak effects were calculated as the largest absolute change from pre-drug (either maximum or minimum in each case) rather than the minimum or maximum post-drug value. Unlike objective measures of performance for which the direction of impairment can be predicted (e.g., for response time, higher values indicate greater impairment; for accuracy, lower values indicate greater impairment), the direction of impairment for performance estimates could be a maximum or minimum change relative to pre-drug (i.e., either an over-estimation or an under-estimation of performance impairment is theoretically plausible). Therefore, peak effects for performance estimate outcomes are best captured using absolute change from pre-drug values.
Significant main effects and interactions were followed up with simple effects tests with modified Bonferroni corrections (Keppel, 1991). The simple effects tests were limited to comparisons between active drug conditions and placebo, and comparisons between low dose (TRZ 0.125 vs. ALC 0.40) and high dose conditions (TRZ 0.25 vs. ALC 0.80) of the active drugs. For all statistical tests, p ≤ 0.05 was considered significant.
Results
Breath Alcohol Level
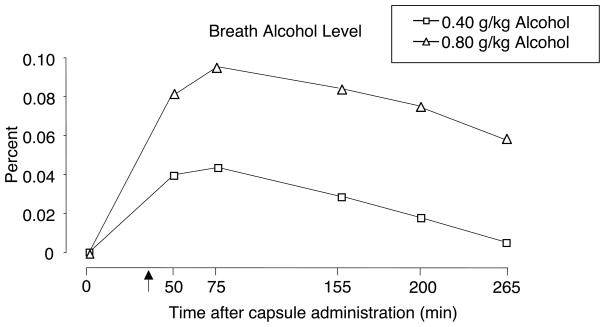
As shown in Figure 1, ALC produced orderly dose- and time-related increases in breath alcohol level (BAL). BAL peaked approximately 40 min after beverage administration (75 min after capsule) and showed modest monotonic decreases through the end of the session.
Figure 1.
Alcohol time-course functions for breath alcohol level. X-axis: time in minutes after capsule administration; arrow indicates start of beverage administration.
Time Course Analyses
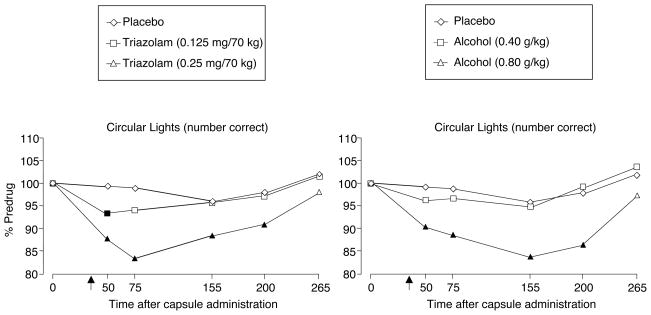
Time course analyses revealed that on all tasks on which triazolam and alcohol produced comparable effects relative to placebo (see dose effect analyses below), the effects of triazolam and alcohol did not differ as a function of time, and the only measure for which a significant drug condition by time interaction was observed was circular lights [F (4, 76) = 1.75; see Figure 2]. Simple effects follow-up tests for circular lights revealed that high doses of both TRZ and ALC significantly decreased the number of correct responses relative to PL across all time points except the final time point, and no significant differences were observed between corresponding doses of TRZ and ALC at any time point (see Figure 2). Given that the effects of triazolam and alcohol did not differ as a function of time, the remainder of the results section will focus on dose effect analyses.
Figure 2.
Triazolam (left panel) and alcohol (right panel) time-course functions for performance on the circular lights task. X-axis: time in minutes after capsule administration; arrow indicates start of beverage administration. Filled symbols indicate active drug values that are significantly different from placebo at that time point (p ≤0.05)
Dose Effect Analyses
Participant Rating of Drug Strength
A significant main effect of drug condition was observed for ratings of drug strength [F (4, 76) = 24.48; see Table 1]. Peak values were significantly higher for high dose TRZ and both ALC doses relative to PL. In addition, peak values were significantly higher in high and low dose ALC conditions relative to comparable TRZ doses.
Table 1.
Descriptive statistics for participant rating, psychomotor, and attention outcomes as a function of dose.
| Placebo | TRZ 0.125 mg/70kg | TRZ 0.25 mg/70kg | ALC 0.40 g/kg | ALC 0.80 g/kg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | |
| Participant rating | ||||||||||
| Drug Strength | 1.25 | (0.24) | 1.40 | (0.31) | 2.45* | (0.18) | 2.55*† | (0.01) | 3.45*† | (0.11) |
| Psychomotor | ||||||||||
| Circular Lights (number correct) | 92.45 | (1.59) | 87.83 | (2.66) | 80.46* | (2.11) | 90.94 | (1.47) | 80.99* | (2.49) |
| Balance (seconds) | 70.77 | (6.74) | 72.09 | (15.87) | 30.25* | (4.20) | 65.43 | (6.71) | 36.06* | (6.53) |
| Attention | ||||||||||
| DSST | ||||||||||
| Attempted | 95.46 | (2.57) | 88.32*† | (2.31) | 79.89* | (2.46) | 97.92 | (1.41) | 82.71* | (2.24) |
| Proportion correct | 93.89 | (1.38) | 94.42 | (1.40) | 92.97 | (3.09) | 94.30 | (1.07) | 87.82* | (1.71) |
| Choice reaction time task | ||||||||||
| Median RT | 83.54 | (22.10) | 124.79 | (19.05) | 241.25* | (23.68) | 56.25 | (15.83) | 261.67* | (42.60) |
| Proportion correct | −0.04 | (0.02) | −0.07 | (0.01) | −0.13* | (0.02) | −0.07 | (0.01) | −0.10* | (0.02) |
| Proportion incorrect | 0.03 | (0.01) | 0.05 | (0.01) | 0.08* | (0.01) | 0.06 | (0.01) | 0.08* | (0.01) |
| Proportion timeouts (2 sec) | 0.05 | (0.03) | 0.05 | (0.02) | 0.13* | (0.03) | 0.03 | (0.01) | 0.09 | (0.02) |
| Proportion timeouts (6 sec) | 0.00 | (0.00) | 0.00 | (0.00) | 0.01 | (0.01) | 0.00 | (0.00) | 0.00 | (0.00) |
| Error detection accuracy (2 sec) | −0.06 | (0.03) | −0.06 | (0.02) | −0.19* | (0.03) | −0.06 | (0.02) | −0.12 | (0.02) |
| Error detection accuracy (6 sec) | −0.02 | (0.02) | −0.05 | (0.02) | −0.09* | (0.02) | −0.08 | (0.02) | −0.08 | (0.02) |
| Divided attention | ||||||||||
| Tracking moves | 98.59 | (1.83) | 95.87 | (2.37) | 87.18*† | (2.38) | 95.77 | (1.97) | 96.46 | (1.45) |
| Tracking deviation | 115.46 | (6.93) | 137.79 | (14.04) | 147.23*† | (10.03) | 111.71 | (5.25) | 123.88 | (5.55) |
| Tracking overlap | 94.98 | (2.72) | 87.81 | (3.46) | 78.06*† | (3.47) | 95.55 | (2.31) | 89.45 | (2.71) |
| Total responses (monitoring) | 93.60 | (1.86) | 92.66 | (2.39) | 86.06 | (4.96) | 93.91 | (2.47) | 92.62 | (2.51) |
| Mean RT (monitoring) | 119.38 | (5.10) | 133.98† | (6.60) | 127.53 | (7.39) | 109.31 | (6.83) | 132.05 | (4.27) |
| Proportion correct (monitoring) | 91.34 | (2.11) | 87.74 | (2.84) | 82.20*† | (4.49) | 95.04 | (2.47) | 91.31 | (2.34) |
Data are either peak raw values (participant rating), peak percentage of predrug values (psychomotor, DSST, and divided attention) or difference from predrug values (choice reaction time).
indicates a significant difference between active drug and placebo (p ≤0.05).
indicates a significant difference between alcohol and triazolam at corresponding dose level (p ≤0.05).
Psychomotor Performance Tasks
Circular lights and balance
A significant main effect of drug condition was observed for circular lights [F (4, 76) = 13.99] and balance [F (4, 76) = 7.25]. For both outcomes, peak values were significantly lower in the high dose TRZ and ALC conditions relative to PL (see Table 1); there were no significant differences between TRZ and ALC at corresponding dose levels, confirming that the drugs produced similar levels of dose-related impairment of psychomotor function.
Attention
DSST
Significant main effects of drug condition were observed for number attempted [F (4, 76)= 26.02] and proportion correct [F (4, 76) = 4.41] for the DSST. Simple effects tests revealed that the number of trials attempted was significantly lower for both doses of TRZ and the high dose of ALC relative to PL (see Table 1). In addition, a difference between TRZ and ALC was observed such that the number of trials attempted was significantly lower in low dose TRZ relative to low dose ALC. Proportion correct was significantly lower in the high dose ALC condition relative to PL; no other significant differences among means were observed.
Choice reaction time task
Main effects of drug condition were observed for median RT, proportion correct, proportion incorrect, and timeouts [Fs (4, 76) ≥ 3.32] on the choice reaction time task. For all outcomes, with the exception of timeouts for which a significant drug condition by response interval interaction is described in greater detail below, performance was impaired in the high dose TRZ and ALC conditions relative to PL (see Table 1). That is, collapsed across response interval, RT and proportion incorrect were higher for the high dose conditions relative to placebo, and proportion correct was lower for the high dose conditions relative to placebo; no other significant differences among means were observed. In addition, a significant drug condition by response interval (2 s and 6 s) interaction was observed for timeouts [F (4, 76) = 3.54] such that timeouts in the 2 sec condition were significantly higher for high dose TRZ relative to PL; these differences were not observed in the 6 sec condition and no other significant differences were observed (see Table 1).
In addition, a significant drug condition by response interval interaction was observed for the error detection portion of the choice reaction time task (i.e., metacognition) [F (4, 75) = 2.55]. Simple effects tests revealed that error detection accuracy in the high dose TRZ condition was significantly impaired relative to PL in both the 2 sec and 6 sec conditions (see Table 1). In addition, in the 2 sec condition, error detection accuracy in the high dose TRZ condition was significantly impaired relative to high dose ALC.
Divided attention
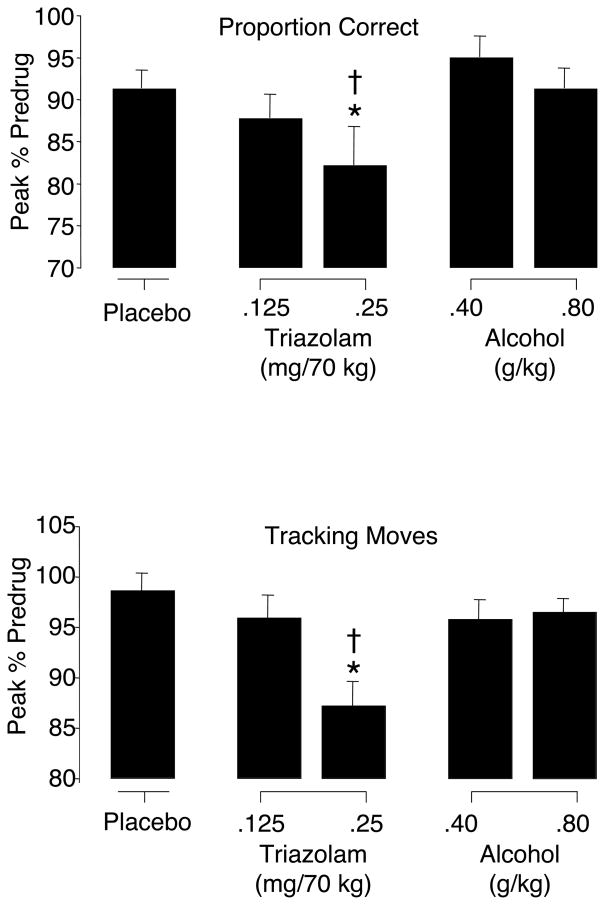
Main effects of drug condition were observed for all central tracking component measures (tracking moves, tracking deviation, and tracking overlap) and one measures for the peripheral monitoring component (proportion correct) [Fs (4, 76) ≥ 3.32]. For these measures, the high dose of TRZ impaired performance relative to PL and high dose ALC (see Table 1 and Figure 3); no other significant differences among means were observed.
Figure 3.
Mean peak percent predrug values for proportion correct and tracking moves on the divided attention task as a function of drug condition. Brackets show 1 S.E.M. An asterisk (*) indicates an active drug value that is significantly different from placebo and a dagger (†) indicates a significant difference between triazolam and alcohol at corresponding dose levels (p ≤ 0.05).
Working Memory
Working memory task
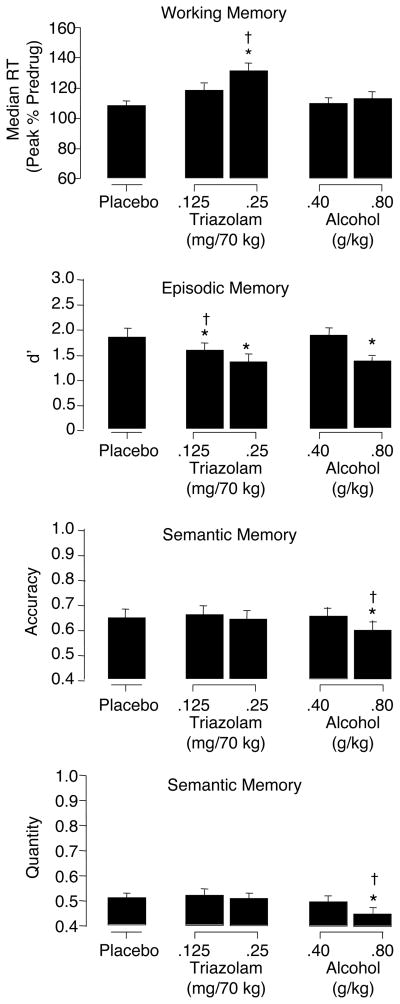
A significant main effect of drug condition was observed for median RT [F (4, 76) = 6.49], but not proportion correct [F (4, 76) = 1.40], with the non-memory control condition as a covariate. Collapsed across delay condition, median RT was significantly higher (i.e., slower) for high dose TRZ relative to PL and high dose ALC (see Table 2 and Figure 4).
Table 2.
Descriptive statistics for memory outcomes as a function of dose.
| Placebo | TRZ 0.125 mg/70kg | TRZ 0.25 mg/70kg | ALC 0.40 g/kg | ALC 0.80 g/kg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | |
| Working Memory | ||||||||||
| Sternberg task | ||||||||||
| Proportion correct | 92.43 | (2.87) | 96.68 | (2.57) | 90.66 | (2.47) | 97.53 | (2.31) | 94.19 | (2.03) |
| Median RT | 108.07 | (2.73) | 118.03 | (4.26) | 131.43*† | (4.28) | 109.28 | (2.82) | 112.74 | (3.76) |
| Episodic Memory | ||||||||||
| Conceptual categorization | ||||||||||
| Proportion correct | 0.97 | (0.00) | 0.97 | (0.01) | 0.95* | (0.01) | 0.97 | (0.01) | 0.97 | (0.01) |
| Recognition task | ||||||||||
| Hits | 0.81 | (0.23) | 0.74*† | (0.03) | 0.70* | (0.03) | 0.80 | (0.03) | 0.71* | (0.02) |
| d′ | 1.85 | (0.15) | 1.59*† | (0.13) | 1.35* | (0.14) | 1.88 | (0.13) | 1.35* | (0.12) |
| False alarm rate | 0.20 | (0.03) | 0.23 | (0.03) | 0.24 | (0.04) | 0.20 | (0.03) | 0.24 | (0.03) |
| Response bias | 0.00 | (0.06) | 0.10 | (0.10) | 0.13 | (0.09) | 0.04 | (0.09) | 0.09 | (0.07) |
| Gamma | 0.61 | (0.05) | 0.54 | (0.06) | 0.40 | (0.08) | 0.60 | (0.06) | 0.50 | (0.06) |
| Free Recall | ||||||||||
| Number recalled | 10.15 | (1.22) | 7.35* | (1.09) | 3.90* | (0.78) | 8.60 | (1.18) | 4.55* | (0.87) |
| Semantic Memory | ||||||||||
| General information task | ||||||||||
| Accuracy | 0.65 | (0.03) | 0.66 | (0.04) | 0.64 | (0.03) | 0.65 | (0.04) | 0.60*† | (0.04) |
| Quantity | 0.51 | (0.02) | 0.52 | (0.02) | 0.51 | (0.02) | 0.50 | (0.02) | 0.45*† | (0.02) |
Data are either peak percentage of predrug values (working memory) or raw values (episodic memory and semantic memory).
indicates a significant difference between active drug and placebo (p ≤0.05).
indicates a significant difference between alcohol and triazolam at corresponding dose level (p ≤0.05).
Figure 4.
Mean data for working memory (Sternberg: median RT), episodic memory (recognition: d′), and semantic memory (general information: accuracy and quantity) measures as a function of drug condition. Brackets show 1 S.E.M. An asterisk (*) indicates an active drug value that is significantly different from placebo and a dagger (†) indicates a significant difference between triazolam and alcohol at corresponding dose levels (p ≤ 0.05).
Episodic Memory
Conceptual categorization task (study phase)
During the study phase of the episodic memory task, the proportion of correct categorization responses was significantly lower in the high dose TRZ condition relative to PL [F (4, 76) = 2.73; see Table 2]; no other significant differences among means were observed.
Recognition task
A main effect of drug condition was observed for hits [F = (4, 76) = 9.78] and d′ [F = (4, 76) = 11.72] such that these values were significantly lower for both doses of TRZ and the high dose of ALC relative to PL (see Table 2 and Figure 4). In addition, for both hits and d′, low dose TRZ was significantly lower relative to low dose ALC. No significant effects were observed for false alarm rates or response bias [Fs (4, 76) < 2.06; see Table 2]. Likewise, no significant differences among means were observed for the Goodman-Kruskal gamma correlation (i.e., metamemory) [F (4, 76) = 2.43; see Table 2].
Free recall task
Similar to the pattern observed for the recognition task, significantly fewer words were recalled for both doses of TRZ and the high dose of ALC relative to PL [F (4, 76) = 12.67; see Table 2]. No other significant differences among means were observed.
Semantic Memory
Accuracy and quantity
A main effect of drug condition was observed for both accuracy [F (4, 75) = 3.11] and quantity [F (4, 75) = 5.82] such that, collapsed across phase, values in the high dose ALC condition were significantly lower relative to high dose TRZ and PL (see Table 2 and Figure 4). In addition, a main effect of phase was observed for each outcome [Fs (1, 19) > 46.12]: Collapsed across drug condition, performance increased from Phase 1 to Phase 2 for both accuracy (phase 1 M = 0.46, SE = 0.15; phase 2 M = 0.81, SE = 0.12) and quantity (phase 1 M = 0.46, SE = 0.15; phase 2 M = 0.53, SE = 0.13), and the magnitude of this increase was larger for accuracy.
Metamemory
No significant effects were observed for any metamemory outcome measure [Fs (4, 75) ≤ 1.15; data not shown].
Performance Estimation
For all performance estimates, negative values indicate that ratings of performance were lower than actual performance and thus suggest an over-estimation of performance impairment. Likewise, larger positive values indicate that ratings of performance were higher than actual performance and thus suggest under-estimation of performance impairment.
Main effects of drug condition were observed for all performance estimation outcomes [Fs (4, 72) ≥ 4.23; see Table 3]. On most measures, participants overestimated their performance impairment in the high dose ALC condition relative to placebo and high dose TRZ. TRZ and ALC produced a similar degree of over-estimation of performance impairment on ratings of the number of items correct on the Sternberg task. In addition, a significant interaction of drug condition and time (pre-performance/post-performance) was observed for ratings of accuracy on the Sternberg task (see Table 3). Simple effects tests revealed that prior to completing the task (pre-performance estimates), volunteers significantly over-estimated performance impairment in the high dose ALC condition relative to placebo and high dose TRZ. In contrast, after completing the task (post-performance estimates), volunteers significantly over-estimated performance impairment in the high dose TRZ condition relative to placebo and high dose ALC.
Table 3.
Descriptive statistics for performance estimate outcomes as a function of dose.
| Placebo | TRZ 0.125 mg/70kg | TRZ 0.25 mg/70kg | ALC 0.40 g/kg | ALC 0.80 g/kg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | |
| DSST | ||||||||||
| Speed | −1.23 | (4.18) | 4.66 | (2.88) | 2.33 | (5.10) | −9.56† | (3.36) | −16.73*† | (5.35) |
| Accuracy | −6.17 | (4.72) | −6.73 | (3.25) | −10.41 | (4.69) | −10.29 | (3.87) | −33.63*† | (3.12) |
| Choice reaction time task | ||||||||||
| Items correct | 8.68 | (3.72) | 5.05 | (3.96) | −4.29* | (4.93) | 5.71 | (3.95) | −19.61*† | (5.30) |
| Accuracy | −2.66 | (5.37) | −1.27 | (3.11) | −14.12 | (5.99) | −13.30 | (4.44) | −35.61*† | (5.36) |
| Sternberg task | ||||||||||
| Items correct | −0.50 | (0.84) | −2.21 | (0.93) | −4.16* | (0.96) | −1.63 | (0.89) | −3.42* | (1.11) |
| Accuracy (pre-performance estimates) | −5.41 | (5.53) | −6.34 | (3.26) | −8.08 | (4.46) | −14.68 | (4.67) | −28.88*† | (5.89) |
| Accuracy (post-performance estimate) | −8.93 | (7.00) | −16.47 | (5.71) | −32.30*† | (5.46) | −6.16 | (5.34) | −11.05 | (7.28) |
Data are either peak percentage of predrug values (speed and accuracy) or difference from predrug values (correct items).
Difference scores were calculated as: Speed = speed rating minus trials completed; Accuracy = accuracy rating minus proportion correct; Items correct = items correct rating minus actual items correct).
indicates a significant difference between active drug and placebo (p ≤0.05).
indicates a significant difference between alcohol and triazolam at corresponding dose level (p ≤0.05).
Discussion
The present study compared the acute dose effects of triazolam and alcohol in healthy volunteers across a variety of cognitive performance outcomes using a repeated measures, placebo-controlled, double-dummy, double-blind design. As discussed below, at doses that produced similar levels of impairment on psychomotor and choice reaction time (see Figure 2 and Table 1), triazolam and alcohol had differing effects on other outcomes.
The finding that triazolam and not alcohol impaired divided attention in the present study (see Figure 3 and Table 1) is consistent with previous research showing that triazolam had more robust impairing effects on a similar type of divided attention task relative to alcohol (Roehrs et al., 1993). Nevertheless, the absence of alcohol-related impairment on divided attention is striking given that several previous reports suggest that alcohol impairs divided attention performance (Hamilton & Copeman, 1970; Leigh, Tong, & Campbell, 1977; Maylor, Rabbitt, James, & Kerr, 1990; Schulte, Muller-Oehring, Strasburger, Warzel, & Savel, 2001, Wilkinson, 1995). However, all of the above studies included tasks that incorporated two sensory modalities into the design (e.g., auditory and visual) or tasks that included auditory stimuli only. In contrast, the present study (and Roehrs et al, 1993) utilized visual stimuli only. Thus, the absence of an alcohol effect on divided attention in the present study may be related to sensory modality, although a previous review of the effects of alcohol on divided attention suggests that sensory modality does not mediate alcohol’s impairing effects (Moskowitz, 1984).
Triazolam and alcohol also had differing effects across measures of memory in the present study (see Figure 4 and Table 2). First, the high dose of alcohol and not triazolam significantly impaired semantic memory performance. This finding is consistent with previous research showing alcohol-related impairment on a similar measure of semantic memory (i.e., general information task; Nelson et al., 1986). The finding that triazolam did not impair semantic memory replicates results of another study in our laboratory that examined triazolam using the same task (Mintzer, Kleykamp, & Griffiths, 2008). However, it should be noted that Bacon and colleagues have found impairment with the benzodiazepine lorazepam in several studies with a similar general information task (Bacon et al., 1998, 2007; Massin-Krauss et al., 2002).
In contrast to the pattern of effects on semantic memory, only high dose triazolam but not alcohol increased working memory response time after controlling for a non-memory control condition. More robust effects of triazolam on working memory were reported previously in a study directly comparing these drugs (i.e., number recall task; Roache et al., 1993). In addition, some studies examining alcohol alone have reported minimal to no effects on working memory (Finn, Justus, Mazas, & Steinmetz, 1999; Paulus, Tapert, Pulido, & Schuckit, 2006; Schweizer et al., 2006; but see also Grattan-Miscio & Vogel-Sprott, 2005). Lastly, triazolam produced relatively greater effects on episodic memory measures. That is, both doses of triazolam but only the high dose of alcohol impaired hits and d′ on the recognition memory task and number correct on the free recall task. This finding is consistent with previous reports suggesting more robust effects of benzodiazepines relative to alcohol on episodic memory outcomes (Matilla et al., 1998; Mintzer & Griffiths, 2002; Roache et al., 1993; Schuckit et al., 1991). The finding of differences between triazolam and alcohol in dose effects on episodic memory is interesting in conjunction with results of a study in our laboratory examining the dose effects of the NMDA antagonist ketamine (Lofwall, Griffiths, & Mintzer, 2006), which suggest that ketamine may also produce less robust effects on episodic memory than benzodiazepines. Robust subjective ratings of drug effect and impaired psychomotor performance were observed at a ketamine dose (0.2 mg/kg) that did not produce significant episodic memory or working memory impairment. Although the effects of a benzodiazepine were not examined in that study, this pattern of effects appears to differ from that observed with benzodiazepines, which produce memory impairment even at relatively low doses.
More robust effects of triazolam relative to alcohol were also observed for the number of attempted trials on the DSST. Similar to the pattern observed with episodic memory, both doses of triazolam but only high dose alcohol decreased the number of attempted trials on the DSST suggesting that this speed of processing measure is more sensitive to triazolam. However, only high dose alcohol and not triazolam impaired accuracy (proportion correct) on the DSST. Taken together, these DSST findings are in agreement with a previously proposed hypothesis that benzodiazepines are more likely to slow performance relative to alcohol, whereas alcohol is more likely to impair accuracy relative to benzodiazepines (a pattern of effects that might reflect a speed/accuracy tradeoff; Tiplady et al., 1998; 2003). The findings that triazolam and not alcohol increased the number of timeouts in the 2-sec response interval condition of the choice reaction time task and increased response time on the working memory task lend additional support to the idea that benzodiazepines are more slowing than alcohol (although the pattern of effects on these measures does not indicate a speed/accuracy tradeoff)/Interestingly, research suggests that benzodiazepines are also more slowing relative to the anticholinergic drug scopolamine (Curran, Schifano, & Lader, 1991; Mintzer & Griffiths, 2003a; 2007).
In addition to objective measures of performance, the present study revealed that triazolam and alcohol significantly impaired subjective ratings of performance, or metacognition. Across most performance estimates, both drugs were associated with an over-estimation of performance impairment, regardless of whether volunteers were asked to rate performance before or after the task, and the magnitude of this over-estimation effect was generally larger for alcohol. For example, for DSST accuracy, the performance estimate difference score value was 4.24 for high dose triazolam and 27.46 for high dose alcohol (see Table 3). The tendency for participants to estimate performance as less impaired in the triazolam condition relative to the alcohol condition is consistent with results of a previous study that showed triazolam was associated with under-estimation of performance impairment, while alcohol had no effect on performance estimates (Roache et al., 1993). Interestingly, participants tend to estimate performance as less impaired under benzodiazepine conditions when compared to other performance-impairing drugs as well (e.g., pentobarbital, scopolamine; Mintzer & Grifiths, 2003a; Roache & Griffiths, 1985).
While the tendency to estimate performance as less impaired for triazolam relative to alcohol is consistent with previous work, the finding that both drugs produced over-estimation of impairment relative to placebo is in contrast to reports that triazolam produces under-estimation of impairment (Bacon et al., 1998; Massin-Krauss, et al., 2002; Mintzer & Griffiths, 2003b; Roache et al., 1985, 1987, 1993) and alcohol generally produces no effects on performance estimates (Acons et al., 2006; Harrison & Filmore, 2005; Mintzer, Frey, Yingling, & Griffiths, 1997a; Nelson et al., 1986; Roache et al., 1993). One explanation for the present findings is that participants might have been sensitized to their level of performance because they were asked to rate their performance before and after completing multiple tasks and these tasks were administered at multiple time points in each study session. Thus, over-estimation of performance impairments might have been driven by hyper vigilance to performance. Indeed, previous research suggests an “under-confidence-with-practice (UWP)” effect for learning new information such that the more volunteers completed a task, such as word recollection, the less confident they were with regards to their performance (Koriat, 1997; Koriat, Ma’ayan, Sheffer, & Bjork, 2006, Koriat, Sheffer, Ma’ayan, 2002). One explanation for this UWP effect suggests that memory for past task performance increases under-confidence (Finn & Metcalfe, 2008). Therefore, volunteers in the present study might have remembered their performance on a task earlier in the session and this memory could have increased their under-confidence or over-estimation of performance impairment. Further, this UWP effect might have been enhanced in active drug conditions because volunteers experienced disorienting drug effects (e.g., dizziness) and thus were even more conservative when rating their performance.
Interestingly, a significant time by drug interaction for the working memory task suggests that practice with the task, at least for this measure, has differing effects on estimates of performance depending on drug type. That is, ratings of accuracy on the working memory task revealed that, prior to completing the task, volunteers significantly over-estimated impairment in the high dose alcohol condition relative to high dose triazolam and placebo conditions. In contrast, after completing the task, volunteers significantly over-estimated impairment in the high dose triazolam condition relative to high dose alcohol and placebo. This finding suggests that in the high dose alcohol condition volunteers were able to use their experience with the task to adjust their ratings such that they more accurately estimated actual performance. In contrast, in the triazolam condition experience with the task contributed to an impairment of metacognition such that individuals were no longer accurate in their ratings and were more likely to over-estimate impairment. More research on task practice and drug effects would be helpful in clarifying whether the UWP effect varies as a function of performance measure and drug type.
A further point to consider is the role that drug experience played in the effects of triazolam and alcohol on metacognition in the present study. Notably, volunteers were not benzodiazepine users, but were regular users of alcohol. Previous research suggests that experience with a drug influences the subjective effects of that drug (Brumback, Cao, & King, 2007; Marczinski, Harrison, & Fillmore, 2008) and perhaps our metacognitive findings might have differed had the participants also been regular users of benzodiazepines.
In contrast to performance estimate outcomes, triazolam and not alcohol impaired other measures of metacognition relative to placebo. First, error detection was significantly impaired by triazolam and not alcohol suggesting that volunteers were less aware of their errors in the high dose triazolam condition (see Table 1). Similarly, a previous study that utilized a similar choice reaction time found that alcohol (0.8 g/kg) did not impair participants’ detection of errors relative to placebo (Acons et al., 2006). In addition, benzodiazepine-induced impairment of error detection has been reported elsewhere using behavioral measures and/or electrophysiological correlates of error detection (deBruijn, Hulstijn, Verkes, Ruigt, & Sabbe, 2004; Johannes, Wieringa, Nager, Dengler, & Munte, 2001; Riba, Rodriguez-Fornells, Munte, & Barbanoj, 2005). Second, the effect of drug condition on metamemory on the episodic memory task, as measured by the correspondence between confidence ratings and accuracy (i.e., gamma correlations), was marginally significant (p < 0.06) in the present study (see Table 2). A closer inspection of the means suggests that high dose triazolam impaired the volunteers’ ability to distinguish between correct and incorrect responses relative to placebo. A similar triazolam-induced impairment of metamemory accuracy has been reported elsewhere suggesting that this effect is reliable (Mintzer & Griffiths, 2003b).
Lastly, of note is the absence of any effects of either drug on the metamemory outcomes associated with the semantic memory (general information) task in the present study (data not shown). The lack of triazolam-related effects on these outcomes is particularly surprising given that previous reports suggest that the benzodiazepine lorazepam impairs some metamemory outcomes on a similar semantic memory task (Bacon et al., 1998; Massin-Krauss et al., 2002). However, results from a separate study in our laboratory confirmed that triazolam had no effects on the same metamemory outcomes included in the present study suggesting that the present findings are reliable (Mintzer et al., 2008). One important distinction to be made among the above studies as noted above is that those from our laboratory did not observe benzodiazepine-related impairment of actual performance (i.e., semantic memory accuracy) on the general information task, whereas the other studies that reported drug-related impairment of metamemory outcomes also found drug-related impairment of actual performance. Therefore, one possibility is that the presence of actual performance impairment may be related to the detection of metamemory impairments. Further research is needed to explore the inconsistencies among the above studies, as well as to further examine alcohol’s effects on metamemory outcomes considering little research has been dedicated to this topic.
In summary, the present study provides insight into the effects of triazolam and alcohol on specific cognitive processes. Overall, the results suggest that triazolam is a more potent disruptor of objective measures of cognitive performance especially with regards to measures of speed. However, only alcohol impaired accuracy on the DSST and semantic memory task. In addition to objective measures, both drugs impaired subjective awareness of performance decrements, though the magnitude of these effects was generally larger for alcohol (i.e., greater over-estimation of impairment), whereas triazolam had greater effects on other measures of metacognition (i.e., error detection and episodic memory gamma). Importantly, the differential effects of triazolam and alcohol across performance outcomes cannot be accounted for by differences in the time course of drug effects because time course analyses did not reveal differences between active drug conditions. In addition, breath alcohol levels (BALs; see Figure 1) confirm that the doses of alcohol chosen for this study produced dose-dependent increases in BALs across the duration of the study sessions. Furthermore, as predicted participant ratings of overall strength of drug effect were higher for alcohol relative to triazolam at corresponding doses. Therefore, an absence of alcohol-related effects on particular measures cannot be accounted for by insufficient alcohol dosing. It is also unlikely that differences between drugs in effects on performance were related to differential effects of alcohol on ascending versus descending limbs of the breath alcohol curve given that most tasks were administered at multiple time points throughout each session. Thus, the absence of an effect of alcohol, for instance on the divided attention outcomes (see Figure 3 and Table 1), cannot be tied to isolated testing on one limb.
Overall, despite the fact that the effects of triazolam and alcohol are both at least partially mediated through the GABAA receptor site, the findings reported here suggest that these drugs have distinct cognitive impairment profiles. In conjunction with an accumulating body of cognitive psychopharmacological research examining these and other drugs (e.g., ketamine, scopolamine, pentobarbital), the observed differences between triazolam and alcohol contribute to a more complete understanding of the neurochemical mechanisms underlying different cognitive processes. In addition, performance impairments observed in the present study could have important clinical implications given the widespread use of benzodiazepines and alcohol. In particular, the triazolam-induced slowing of performance and impairment of divided attention might have detrimental effects on driving performance, and the episodic memory impairments associated with both drugs could impact work-related performance, such as remembering standard operating procedures.
Acknowledgments
This project was supported by National Institute on Drug Abuse Research Grant DA-11936. The authors thank Crystal Barnhouser and Kristina Burns for protocol management and technical assistance, John Yingling for computer programming assistance and technical support, and Paul Nuzzo for assistance with the data analysis. Portions of these data were presented at the 70th annual meeting of the College on Problems of Drug Dependence.
Contributor Information
Bethea A. Kleykamp, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Roland R. Griffiths, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Miriam Z. Mintzer, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
References
- Acons K, Chan L, Drummond G, Tiplady B. Effects of ethanol and promethazine on awareness of errors and judgements of performance. Journal of Psychopharmacology. 2006;20(5):661–669. doi: 10.1177/0269881106060690. [DOI] [PubMed] [Google Scholar]
- Bacon E, Danion JM, Kauffmann-Muller F, Schelstraete MA, Bruant A, Sellal F, Grange D. Confidence level and feeling of knowing for episodic and semantic memory: an investigation of lorazepam effects on metamemory. Psychopharmacology. 1998;138:318–25. doi: 10.1007/s002130050677. [DOI] [PubMed] [Google Scholar]
- Bacon E, Schwartz BL, Paire-Ficout L, Izaute M. Dissociation between the cognitive process and the phenomenological experience of TOT: Effect of the anxiolytic drug lorazepam on TOT states. Consciousness and Cognition. 2007;16:360–373. doi: 10.1016/j.concog.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Essentials of human memory. UK: Psychology Press, Ltd; 1999. [Google Scholar]
- Berry RB, Chandra D, Diaz-Granados JL, Homanics GE, Matthews DB. Investigation of ethanol-induced impairment of spatial memory in gamma2 heterozygous knockout mice. Neuroscience Letters. 2009;455(2):84–87. doi: 10.1016/j.neulet.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Brown MW, Bowes JB. Effects of lorazepam on rate of forgetting, on retrieval from semantic memory and on manual dexterity. Neuropsychologia. 1983;21(5):501–12. doi: 10.1016/0028-3932(83)90006-4. [DOI] [PubMed] [Google Scholar]
- Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug and Alcohol Dependence. 2007;91(1):10–17. doi: 10.1016/j.drugalcdep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Current Pharmaceutical Design. 2002;8:45–58. doi: 10.2174/1381612023396654. [DOI] [PubMed] [Google Scholar]
- Curran HV. Effects of anxiolytics on memory. Human Psychopharmacology: Clinical and Experimental. 1999;14:S72–S79. [Google Scholar]
- Curran HV. Benzodiazepines, memory and mood: A review. Psychopharmacology. 1991;105:1–8. doi: 10.1007/BF02316856. [DOI] [PubMed] [Google Scholar]
- Curran HV, Schifano F, Lader M. Models of memory dysfunction? A comparison of the effects of scopolamine and lorazepam on memory, psychomotor performance and mood. Psychopharmacology. 1991;103(1):83–90. doi: 10.1007/BF02244079. [DOI] [PubMed] [Google Scholar]
- Curran HV. Benzodiazepines, memory and mood: a review. Psychopharmacology. 1991;105:1–8. doi: 10.1007/BF02316856. [DOI] [PubMed] [Google Scholar]
- de Bruijn ERA, Hulstijn W, Verkes RJ, Ruigt GSF, Sabbe BGC. Drug-induced stimulation and suppression of action monitoring in health volunteers. Psychopharmacology. 2004;177:151–160. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- Drummer OH. Benzodiazepines — Effects on human performance and behavior. Forensic Science Review. 2002;14(1):1–14. [PubMed] [Google Scholar]
- Finn B, Metcalfe J. Judgments of learning are influenced by memory for past test. Journal of Memory & Language. 2008;58:19–34. doi: 10.1016/j.jml.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146(4):465–72. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Flavell JH. What is memory development the development of? Human Development. 1971;14:272–278. [Google Scholar]
- Goodman LA, Kruskal WH. Measures of association for cross classifications. J Am Stat Assoc. 1954;49:732–764. [Google Scholar]
- Grattan-Miscio KE, Vogel-Sprott M. Effects of alcohol and performance incentives on immediate working memory. Psychopharmacology. 2005;181(1):188–96. doi: 10.1007/s00213-005-2226-2. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Differential effects of diazepam and pentobarbital on mood and behavior. Arch Gen Psychiatry. 1983;40:865–873. doi: 10.1001/archpsyc.1983.01790070055007. [DOI] [PubMed] [Google Scholar]
- Hamilton P, Copeman A. The effect of alcohol and noise on components of a tracking and monitoring task. British Journal of Psychology. 1970;61:149–156. doi: 10.1111/j.2044-8295.1970.tb01232.x. [DOI] [PubMed] [Google Scholar]
- Harrison ER, Fillmore MT. Social drinkers underestimate the additive impairing effects of alcohol and visual degradation on behavioral functioning. Psychopharmacology. 2005;177:459–464. doi: 10.1007/s00213-004-1964-x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Dengler R, Munte TF. Oxazepam alter action monitoring. Psychopharmacology. 2001;155:100–106. doi: 10.1007/s002130100680. [DOI] [PubMed] [Google Scholar]
- Julien RM. A primer of drug action: A concise nontechnical guide to the actions, uses, and side effects of psychoactive drugs. 9. New York: W.H. Freeman and Company; 2001. [Google Scholar]
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Journal of the American Medical Association. 2002;287(3):337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A research’s handbook. 3. Prentice Hall; Englewood Cliffs: 1991. [Google Scholar]
- Koelega HS. Benzodiazepines and vigilance performance: A review. Psychopharmacology. 1989;98:145–156. doi: 10.1007/BF00444684. [DOI] [PubMed] [Google Scholar]
- Koelega HS. Alcohol and vigilance performance: A review. Psychopharmacology. 1995;118:233–249. doi: 10.1007/BF02245951. [DOI] [PubMed] [Google Scholar]
- Koriat A, Ma’ayan H, Sheffer L, Bjork RA. Exploring a Mnemonic Debiasing Account of the Underconfidence-with-Practice Effect. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2006;32:595–608. doi: 10.1037/0278-7393.32.3.595. [DOI] [PubMed] [Google Scholar]
- Koriat A. Monitoring one’s own knowledge during study: A cue-utilization approach to judgments of learning. Journal of Experimental Psychology: General. 1997;126:349–370. [Google Scholar]
- Koriat A, Sheffer L, Ma’ayan H. Comparing objective and subjective learning curves: Judgments of learning exhibit increased underconfidence with practice. Journal of Experimental Psychology: General. 2002;131:147–162. [PubMed] [Google Scholar]
- Koriat A, Goldsmith M. Monitoring and control processes in the strategic regulation on memory accuracy. Psychological Review. 1996;103:490–517. doi: 10.1037/0033-295x.103.3.490. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Langwinski R. Influence of CGS 8216 on some acute effects of ethanol. Alcohol and Alcoholism. 1995;30(5):601–5. [PubMed] [Google Scholar]
- Leigh G, Tong JE, Campbell JA. Effects of ethanol and tobacco on divided attention. Journal of Studies on Alcohol. 1977;38(7):1233–1239. doi: 10.15288/jsa.1977.38.1233. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Griffiths RR, Mintzer MZ. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Experimental and clinical Psychopharmacology. 2006;14(4):439–49. doi: 10.1037/1064-1297.14.4.439. [DOI] [PubMed] [Google Scholar]
- Lombardi WJ, Sirocco KY, Andreason PJ, George DT. Effects of triazolam and ethanol on proactive interference: evidence for an impairment in retrieval inhibition. Journal of Clinical and Experimental Neuropsychology. 1997;19(5):698–712. doi: 10.1080/01688639708403755. [DOI] [PubMed] [Google Scholar]
- Malcolm RJ. GABA systems, benzodiazepines, and substance dependence. Journal of Clinical Psychiatry. 2003;64(S3):36–40. [PubMed] [Google Scholar]
- Marczinski CA, Harrison ELR, Fillmore MT. Effects of Alcohol on Simulated Driving and Perceived Driving Impairment in Binge Drinkers. Alcoholism: Clinical and Experimental Research. 2008;32(7):1329–1337. doi: 10.1111/j.1530-0277.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- Massin-Krauss M, Bacon E, Danion JM. Effects of the benzodiazepine lorazepam on monitoring and control processes in semantic memory. Consciousness and Cognition. 2002;11(1):123–37. doi: 10.1006/ccog.2001.0538. [DOI] [PubMed] [Google Scholar]
- Mattila MJ, Vanakoski J, Kalska H, Seppala T. Effects of alcohol, zolpidem, and some other sedatives and hypnotics on human performance and memory. Pharmacology, Biochemistry, and Behavior. 1998;59(4):917–923. doi: 10.1016/s0091-3057(97)00506-6. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Rabbitt PMA, James GH, Kerr SA. Effects of alcohol and extended practice on divided-attention performance. Perception & Psychophysics. 1990;48:445–452. doi: 10.3758/bf03211588. [DOI] [PubMed] [Google Scholar]
- McCabe SE. Correlates of nonmedical use of prescription benzodiazepine anxiolytics: results from a national survey of U.S. college students. Drug and Alcohol Dependence. 2005;79(1):53–62. doi: 10.1016/j.drugalcdep.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavioral Research Methods and Instruments. 1982;14:463–466. [Google Scholar]
- Metcalfe J, Shimamura AP. Metacognition: Knowing about knowing. Cambridge: MIT Press; 1994. [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: A comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behavioural Pharmacology. 1997a;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Acute effects of triazolam on false recognition. Memory and Cognition. 2000;28:1357–1365. doi: 10.3758/bf03211836. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Alcohol and triazolam: Differential effects on memory, psychomotor performance and subjective ratings of effects. Behavioural Pharmacology. 2002;13:653–658. doi: 10.1097/00008877-200212000-00007. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: A single-dose comparison of effects on human memory and attentional processes. Experimental and Clinical Psychopharmacology. 2003a;11:56–72. doi: 10.1037//1064-1297.11.1.56. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Triazolam-amphetamine interaction: dissociation of effects on memory versus arousal. Journal of Psychopharmacolgy. 2003b;17:17–29. doi: 10.1177/0269881103017001689. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. A triazolam/amphetamine dose-effect interaction study: dissociation of effects on memory versus arousal. Psychopharmacology. 2007;192:425–440. doi: 10.1007/s00213-007-0726-y. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR, Contoreggi C, Kimes AS, London ED, Ernst M. Effects of triazolam on brain activity during episodic memory encoding: A PET study. Neuropsychopharmacology. 2001;25:744–756. doi: 10.1016/S0893-133X(01)00280-9. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Guarino J, Kirk T, Roache JD, Griffiths RR. Ethanol and pentobarbital: comparison of behavioral and subjective effects in sedative drug abusers. Experimental and Clinical Psychopharmacology. 1997b;5(3):203–15. doi: 10.1037//1064-1297.5.3.203. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Kleykamp BA, Griffiths RR. Dose effects of triazolam and scopolamine on metamemory. Poster presented at the 15th Annual Meeting of Cognitive Neuroscience Society.2008. [Google Scholar]
- Mohler-Kuo M, Lee JE, Wechsler H. Trends in marijuana and other illicit drug use among college students: results from 4 Harvard School of Public Health College Alcohol Study surveys: 1993–2001. Journal of American College Health. 2003;52(1):17–24. doi: 10.1080/07448480309595719. [DOI] [PubMed] [Google Scholar]
- Moskowitz H. Attention tasks as skills performance measures of drug effects. British Journal of Clinical Pharmacology. 1984;18:51S–61S. doi: 10.1111/j.1365-2125.1984.tb02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz H, Robinson C. Driving-related skills impairment at low blood alcohol levels. Alcohol, drugs and traffic safety. 1987;T86:79–86. [Google Scholar]
- Nelson TO, Narens L. Norms of 300 General-information questions: Accuracy of recall, latency of recall, and feeling-of-knowing ratings. Journal of Verbal Learning and Verbal Behavior. 1980;19:338–368. [Google Scholar]
- Nelson TO, McSpadden M, Fromme K, Marlatt GA. Effects of alcohol intoxication on metamemory and on retrieval from long-term memory. Journal of Experimental Psychology: General. 1986;115:247–254. doi: 10.1037//0096-3445.115.3.247. [DOI] [PubMed] [Google Scholar]
- Ogden EJ, Moskowitz H. Effects of alcohol and other drugs on driver performance. Traffic Injury Prevention. 2004;5:185–198. doi: 10.1080/15389580490465201. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcoholism, Clinical and Experimental Research. 2006;30(8):1363–71. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Rodriquez-Fornells A, Munte TF, Barbanoj MJ. A neurophysiological study of the detrimental effects of aprazolam on human action monitoring. Cognitive Brain Research. 2005;25:554–565. doi: 10.1016/j.cogbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Roache JD, Cherek DR, Bennett RH, Schenkler JC, Cowan KA. Differential effects of triazolam and ethanol on awareness, memory, and psychomotor performance. Journal of Clinical Psychopharmacology. 1993;13:3–15. [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Comparison of triazolam and pentobarbital: performance impairment, subjective effects and abuse liability. Journal of Pharmacology and Experimental Therapeutics. 1985;234(1):120–33. [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Lorazepam and meprobamate dose effects in humans: Behavioral effects and abuse liability. The Journal of Pharmacology and Experimental Therapeutics. 1987;243:978–988. [PubMed] [Google Scholar]
- Roehrs T, Claiborne D, Knox M, Roth T. Effects of ethanol, diphenhydramine, and triazolam after a nap. Neuropsychopharmacology. 1993;9(3):239–245. doi: 10.1038/npp.1993.60. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Greenblatt D, Gold E, Irwin M. Reactions to ethanol and diazepam in healthy young men. Journal of Studies on Alcohol. 1991;52(2):180–187. doi: 10.15288/jsa.1991.52.180. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Strasburger H, Warzel H, Savel BA. Acute effects of alcohol on divided and covert attention in men. Psychopharmacology. 2001;154:61–69. doi: 10.1007/s002130000603. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31(6):1301–9. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- Simpson CA, Rush CR. Acute performance-impairing and subject-rates effects of triazolam and temazepam, alone, and in combination with ethanol, in humans. Journal of Psychopharmacology. 2002;16(1):23–34. doi: 10.1177/026988110201600102. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Soderlund H, Parker ES, Schwartz BL, Tulving E. Memory encoding and retrieval on the ascending and descending limbs of the blood alcohol concentration curve. Psychopharmacology. 2005;182:305–317. doi: 10.1007/s00213-005-0096-2. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: Extensions of Donder’s method. ACTA Psychologica. 1969;30:276–315. [Google Scholar]
- Thorndike EL, Lorge I. The Teacher’s Word Book of 30,000 Words. New York: Teacher’s College, Columbia University; 1944. [Google Scholar]
- Tiplady B, Franklin N, Scholey A. Effect of ethanol on judgments of performance. British Journal of Psychology. 2004;95:105–118. doi: 10.1348/000712604322779497. [DOI] [PubMed] [Google Scholar]
- Tiplady B, Harding C, McLean D, Ortner C, Porter K, Wright P. Effects of ethanol and temazepam on episodic and semantic memory: a dose-response comparison. Human Psychopharmacology. 1999;14:263–269. [Google Scholar]
- Tiplady B, Hiroz J, Holmes L, Drummond G. Errors in performance testing: a comparison of ethanol and temazepam. Journal of Psychopharmacology. 2003;17(1):41–49. doi: 10.1177/0269881103017001691. [DOI] [PubMed] [Google Scholar]
- Tiplady B, Faineteau H, Loganathan A, Spiegelberg M, Taylor Z, Wright P. Effects of ethanol and temazepam on performance in memory and psychomotor tasks: a dose-response comparison. Human Psychopharmacology. 1998;13:285–291. [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic; 1972. [Google Scholar]
- Tulving E. Elements of Episodic Memory. New York: Oxford University; 1983. [Google Scholar]
- Wechsler D. WAIS-R manual. New York: Psychological Corporation; 1981. [Google Scholar]
- Weingartner HJ, Joyce EM, Sirocco KY, Adams CM, Eckhardt MJ, George T, Lister RG. Specific memory and sedative effects of the benzodiazepine triazolam. Journal of Psychopharmacology. 1993;7:303–315. doi: 10.1177/026988119300700401. [DOI] [PubMed] [Google Scholar]
- Wendt PE, Risberg J. Ethanol reduces rCFB activation of left dorsolateral prefrontal cortex during a verbal fluency task. Brain and Language. 2001;77:197–215. doi: 10.1006/brln.2000.2434. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Global Status Report on Alcohol. 2004 Retrieved from http://www.who.int/substance_abuse/publications/alcohol/en/
- Wilkinson CJ. The acute effects of zolpidem, administered alone and with alcohol, on cognitive and psychomotor function. Journal of Clinical Psychiatry. 1995;56(7):309–18. [PubMed] [Google Scholar]
- Williams EJ. Experimental designs balanced for the estimation of residual effects of treatments. Australian Journal of Scientific Research. 1949;2:149–168. [Google Scholar]
- Yang HK, Simoni-Wastila L, Zuckerman IH, Stuart B. Benzodiazepine Use and Expenditures for Medicare Beneficiaries and the Implications of Medicare Part D Exclusions. Psychiatric Services. 2008;59(4):384–391. doi: 10.1176/appi.ps.59.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]