Abstract
Introduction
We describe a compact, portable dual-gamma camera system (named “MONICA” for MObile Nuclear Imaging CAmeras) for visualizing and analyzing the whole-body biodistribution of putative diagnostic and therapeutic single photon emitting radiotracers in animals the size of mice.
Methods
Two identical, miniature pixelated NaI(Tl) gamma cameras were fabricated and installed “looking up” through the tabletop of a compact portable cart. Mice are placed directly on the tabletop for imaging. Camera imaging performance was evaluated with phantoms and field performance was evaluated in a weeklong In-111 imaging study performed in a mouse tumor xenograft model.
Results
Tc-99m performance measurements, using a photopeak energy window of 140 keV ± 10%, yielded the following results: spatial resolution (FWHM at 1-cm), 2.2-mm; sensitivity, 149 cps/MBq (5.5 cps/μCi); energy resolution (FWHM), 10.8%; count rate linearity (count rate vs. activity), r2 = 0.99 for 0–185 MBq (0–5 mCi) in the field-of-view (FOV); spatial uniformity, < 3% count rate variation across the FOV. Tumor and whole-body distributions of the In-111 agent were well visualized in all animals in 5-minute images acquired throughout the 168-hour study period.
Conclusion
Performance measurements indicate that MONICA is well suited to whole-body single photon mouse imaging. The field study suggests that inter-device communications and user-oriented interfaces included in the MONICA design facilitate use of the system in practice. We believe that MONICA may be particularly useful early in the (cancer) drug development cycle where basic whole-body biodistribution data can direct future development of the agent under study and where logistical factors, e.g. limited imaging space, portability, and, potentially, cost are important.
Keywords: MONICA, small animal imaging, cancer drug development, mouse whole-body imaging, miniature gamma cameras, single photon imaging
INTRODUCTION
Small animal imaging has become commonplace in biomedical research and very high performance PET, SPECT, CT, MRI, US and optical imaging systems (and combinations of these systems) have been, and continue to be, developed. These systems typically offer state-of-the-art performance and are designed to maximize the functional capabilities available to the user within each technology. However, certain experimental questions can potentially be answered with simpler, more limited and less expensive devices. For example, our laboratory commonly performs whole-body single photon projection imaging in mice as a precursor to further development of tumor-directed diagnostic and therapeutic single photon radiotracers in humans. In these kinds of preliminary studies the goal is not to determine the exact quantitative biodistribution of a putative diagnostic or therapeutic agent but rather to determine if the tracer meets minimal biodistribution requirements that support further development of the compound. If so, more advanced tomographic imaging systems can be used to further characterize such an agent.
With this reasoning in mind, we constructed a compact, portable dual gamma camera system for performing whole-body single photon projection imaging of mice. The system is capable of image acquisition with either camera individually or both cameras simultaneously and of processing the resulting image data. The unit can be easily moved from one location to the next while powered by an onboard uninterruptible power supply to avoid warm-up variations in detector performance. In addition, the user controls and monitors the system wirelessly through a Macintosh laptop computer (MacBook Pro, Apple, Inc., Cupertino, CA). When combined with portability and compact size, this arrangement allows considerable flexibility in choice of experimental venue.
In the current report, the technical features of this system are described and its performance characterized by phantom measurements as well as by a mouse imaging study.
MATERIALS AND METHODS
System Overview
The MONICA system is comprised of two major components: (1) a wheeled cart, shown in Figure 1, that contains the two gamma cameras looking up through the tabletop and (2) a Macintosh laptop computer through which the user controls and monitors the system (not shown).
Figure 1.
MONICA cart. Yellow arrows (left panel) point to the two upward-looking gamma cameras. Router (white box, left panel) enables wireless communication between user Macintosh laptop computer and interior system computers. Tabletop is 62-cm wide × 55-cm deep and 97-cm above the floor.
Gamma Cameras
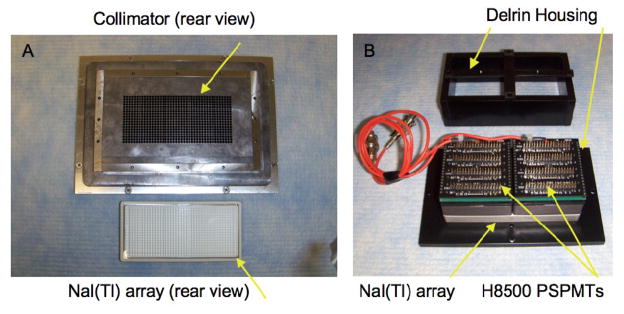
Each gamma camera is comprised of a custom-made 2.54-cm thick lead (Pb) collimator composed of 19 × 42 circular parallel holes, 2.0-mm in diameter with 2.2-mm pitch, drilled in a rectangular array to yield a field-of-view (FOV) of approximately 42-mm × 92-mm (Figure 2A). Maximum septal penetration of this collimator is approximately 4% at 140 keV and 12% at 170 keV. Each collimator is mechanically coupled to a 19 × 42 array (2.2-mm pitch) of individual square thallium-doped sodium iodide (NaI(Tl)) scintillation crystals enclosed in a hermetically sealed box (Figure 2A). Each crystal is 2-mm × 2-mm in cross-section × 10-mm deep, surrounded on five sides by white specular reflector and optically isolated from its neighboring crystals. Importantly, the collimator holes and crystals in each array are aligned such that each collimator hole exactly matches the location of one and only one crystal.
Figure 2.
(A): collimator and matching scintillator array for one of MONICA’s gamma cameras; (B): partially assembled detector module to be mechanically attached to the rear of the collimator such that holes and crystals are exactly aligned; PSPMT = position-sensitive photomultiplier tube.
Each scintillator array with 3-mm thick optical output window is, in turn, coupled with optical grease to two side-by-side position-sensitive photomultiplier tubes (PSPMTs, Figure 2B) (Model H8500, Hamamatsu, Japan). Custom-made resistive divider and amplifier boards with footprints equal to the footprint of the PSPMTs are mounted at the rear of each PSPMT to complete the detector package/collimator assembly.
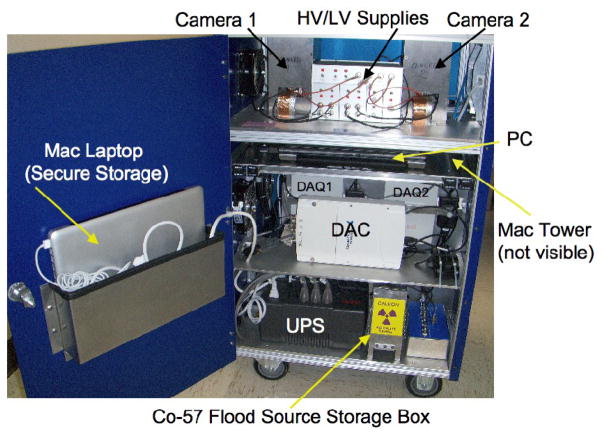
Each of these gamma cameras is inset into the aluminum tabletop of a wheeled cart (Figure 1) such that each collimator surface is flush with the tabletop. The tabletop, in turn, is covered with a 0.8-mm thick sheet of aluminum to protect the collimators from physical damage and from fluid leaks onto the detectors. Below the tabletop each detector module is surrounded on five sides by a 6.4-mm thick tungsten box to prevent radiation from animals and other external sources from reaching the detector modules (Figure 3).
Figure 3.
Interior view of the MONICA cart. Camera 1/Camera 2 arrows point to tungsten boxes surrounding each gamma camera on five sides; HV/LV Supplies: high and low voltage supplies powering PSPMTs and support electronics in each gamma camera; DAQ1/2: data acquisition modules for cameras 1 and 2; DAC: digital-to-analog converter; PC: personal (laptop) computer for camera signal processing; UPS: uninterruptible power supply; Mac Tower: Macintosh tower-type computer (not visible) running NucLear Mac® software for data acquisition and processing.
Gamma Camera Readout and Supporting Electronics
The H8500 PSPMT is comprised of an 8 × 8 anode array potentially yielding 64 output signals for each scintillation event. In order to reduce this number and simplify the readout electronics, we combined these signals (by resistive charge division) into 8 row and 8 column signals that, followed by amplification, yield 16 signals per event per PSPMT or 32 signals per event per detector module.
High voltages (HV) for the four PSPMTs and low voltages (LV) for their amplifier boards are supplied by a computer-controlled, custom-made HV/LV supply (Figure 3).
Data Acquisition
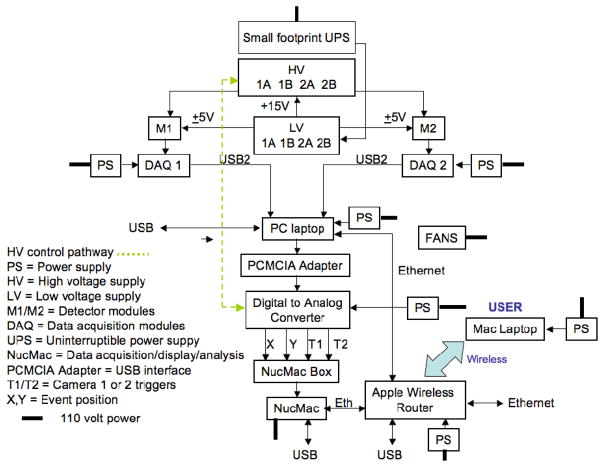
Data flow through the MONICA system is shown in Figure 4. Each detector module is serviced by its own 32-channel data acquisition (DAQ) system [1]. Each DAQ digitizes the 32 incoming analog row and column signals and transmits these digital data by USB2 connection to a PC laptop computer (Dell Precision M2300, Dell, Inc., Round Rock, TX) for processing.
Figure 4.
MONICA system diagram. Components are described in the text.
Data Processing
The PC connected to the two DAQs performs the following tasks for each scintillation event: (1) identifies the camera of origin of the event, (2) computes the raw location of the event using a 16 × 8 signal centroid algorithm, (3) identifies the crystal in that camera’s array in which the event occurred by consulting an event-to-crystal lookup table created during detector calibration, (4) corrects the magnitude of the event for spatial variations in apparent gain for that crystal using a table of scale factors determined during detector calibration, (5) compares the corrected magnitude (energy) of the event with the user-selected energy window and accepts the event if inside, rejects if outside the window and, (6) passes the spatial linearity corrected (digital) X and Y coordinates of the (accepted) event to a digital-to-analog (DAC) converter along with a trigger signal (T1 if event in Camera 1, T2 if in Camera 2; see Figure 4) that indicates a valid event.
Crystal Regions-of-Interest, Pedestal and Gain Calibration Procedures
Crystal regions-of-interest (ROIs) are determined automatically by irradiating each camera with a thin, custom-made Co-57 (half-life = 272 days) field flood source (5.5 cm × 10.5 cm active area, 185 MBq (5 mCi) on delivery, Eckert and Ziegler, Valencia, CA) and identifying the location of each of the individual 19 × 42 crystals (each appearing as a point in the raw, unprocessed images). A small region around each point is automatically identified and all events falling into this region are assigned to the (known) position of the crystal at this location thereby removing any spatial non-linearity in event positioning. These ROIs are contiguous and collectively cover the entire FOV of each detector module. The algorithm for ROI identification requires supervision and possible intervention by a system administrator and is not available to the user.
DAQ pedestal values for each of the 32 signal channels in each detector module are determined automatically from a short (less than one minute) data collection using triggers generated internally to the DAQ. These new pedestal values are stored and used in all subsequent data collection until changed in the next pedestal acquisition.
After pedestal calibration, spatial variations in apparent gain (energy) across the FOV of each camera are removed by first collecting energy spectra for each individual crystal using the Co-57 field flood source centered on the FOV of each camera. The location of the maximum counts in each spectrum is identified and this region is fit with a Gaussian function to estimate the photopeak location. A scale factor is then determined for each crystal that scales the photopeak location of that crystal along the energy axis to the same channel number. These scale factors are stored and used to correct subsequent events to the same apparent gain so that a single energy window can be supplied by the user to reject non-photopeak events.
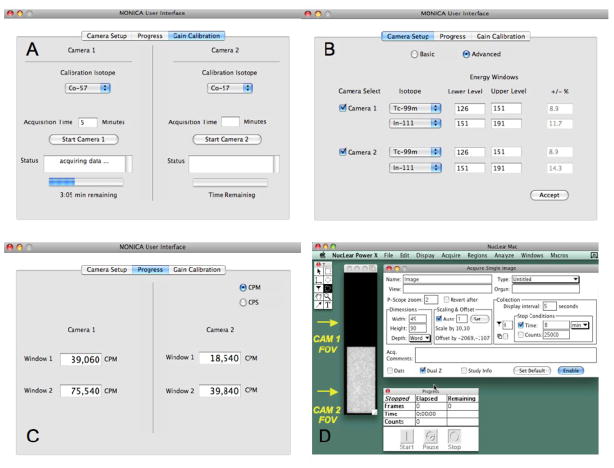
Both the pedestal and gain calibration procedures are initiated by the user through the “Gain Calibration” interface displayed to the user on the Macintosh laptop computer (Figure 5A). With the exception of manual positioning of the flood source in each camera’s FOV, these procedures together run automatically to completion in one camera in less than 10 minutes and can be repeated on a regular basis to correct for drifts in phototube gain and support electronics. To facilitate this process, the Co-57 field flood source is stored inside the MONICA enclosure in a tungsten box (Figure 3) and is transported with the system for use at any time or location.
Figure 5.
(A): User interface for Gain Calibration with the onboard Co-57 field flood source. (B): Camera Setup for selection of cameras to be used in the study and their associated energy windows. (C): Progress window for monitoring count rates in each energy window during a study. (D): NucLear Mac® interface for entering data acquisition parameters, for analyzing acquired data and for viewing persistence images of the fields-of-view (FOV) of both cameras during data collection. All of these interfaces are continuously visible to the user on the Macintosh laptop computer.
Camera Setup and Progress Interfaces
Once the cameras are fully calibrated and the system ready for imaging, the user selects the cameras to be used in the study and the energy windows for those cameras (up to two windows per camera). Once the study is underway, the user can display the instantaneous count rate in each energy window. The Camera Setup and Progress interfaces that perform these functions (Figures 5B and 5C) are always visible to the user on the Macintosh laptop computer.
Acquisition, Display, and Analysis
Analog converted signals from the digital-to-analog converter (DAC) are sent to the X and the Y analog-to-digital converter (ADC) inputs of the NucLear Mac® (Scientific Imaging, Crested Butte, CO), a commercial Macintosh-based hardware/software platform developed for acquiring and processing analog scintillation data from clinical gamma cameras. This system is designed to digitize the continuous stream of spatially corrected and energy accepted X,Y analog signals from the detector heads of commonly used commercial clinical gamma cameras and to organize these X,Y values according to temporal and spatial acquisition formats selected by the user. In the present case, the D-to-A step, though redundant, makes the dual gamma cameras of the MONICA system appear to the NucLear Mac® to be conventional commercial gamma cameras, thereby permitting use of all NucLear Mac® functions without any internal modifications to the NucLear Mac® software. Importantly, this conversion from digital to analog and then back to digital does not detectably degrade image quality.
Software present on the NucLear Mac® allows static, dynamic, and other common forms of single photon data acquisition combined with a comprehensive set of image analysis and visualization tools (http://www.scientificimaging.com/). The NucLear Mac® user interface is shown in Figure 5D. Note that a persistence image of each camera’s FOV is continuously presented to the user during data acquisition.
Image Display
Since the gamma cameras are “pixelated”, raw images of the FOV of each camera will appear to be made up of 19 × 42 discrete points rather than a continuous distribution of scintillation events. Although such discontinuous images do, in fact, contain all the information available in the acquired data, human perception is not well suited to interpreting such images. As a result, each of the 798 image pixels was divided into four quadrants. An event at a particular point is then randomly assigned to one of these four quadrants before display and the X,Y value of this new location output by the PC to the DAC. The final image acquired by the NucLear Mac® thus contains four times more pixels (3192) than the original and appears spatially “smooth(er)” to the eye. Other interpolation schemes can also be used for this purpose but this method is easily implemented in real-time. The images shown in the Results section have been created in this manner.
Evaluation of Imaging Performance
Tc-99m-filled phantoms were used to measure the camera performance parameters listed below.
Spatial Resolution: apparent FWHM width (in mm) of a 1.2 mm diameter line source one cm from the camera face (half mouse thickness) aligned along a short, long and diagonal axis of the collimator.
Energy Resolution: after field flood irradiation and gain correction, the energy spectra for all crystals in the camera were summed and the ratio FWHM (keV)/140 keV (in percent) of the photopeak was determined from this single summed spectrum.
Spatial Uniformity: after field flood irradiation, representative plots of counts across the resulting image in the X and Y directions were created and the variation in these plots expressed as (± standard deviation/mean) in percent.
Sensitivity and Count Rate Linearity: two side-by-side 3 cc syringes, placed directly on the camera face and each initially filled with approximately 1 ml of fluid, were imaged while the fluid volume was stepwise reduced to span an activity range of approximately 0–185 MBq (5 mCi) (more than 10x larger than activities encountered in practice); count rate at each activity level was plotted as a function of combined syringe activity over this range and the resulting plot fit with a straight line; sensitivities, determined from the slope of this line and as the average of individual measurements of sensitivity at each activity level, were also calculated.
Performance measurements for both cameras were essentially identical in all modes of operation so that only a single value for each parameter is reported.
Mouse Imaging Study
The MONICA system was employed in a mouse imaging study designed to evaluate over a 7-day period the time dependence of the whole-body activity distribution and tumor xenograft uptake of 111In-CHX-A-Panitumumab (PT), a human monoclonal antibody that targets tumors expressing the epidermal growth factor receptor [2,3]. Mice (n = 10) were injected subcutaneously with 2 × 106 human colon carcinoma cells (LS-174T) on the right flank. The imaging study was begun 10–14 days after tumor cell injection when the tumor xenografts averaged approximately 200 mg in weight. Animals received between 3 and 3.7 MBq (81–100 μCi) of In-111 PT by tail vein injection on day zero and were imaged in pairs for 5 minutes starting on day zero and on five subsequent days for a total elapsed time of 168 hours post-injection. Imaging examples were selected from two animals at the extremes of tumor weight. These images were corrected for radioactive decay to the time of injection.
In order to assess field performance, MONICA was first disconnected from house power in the imaging physics laboratory and rolled to the Molecular Imaging Program animal holding and imaging area, a distance of some 200 m. During transit, power continued to be supplied to the two detector modules and to certain subsystems, e.g. the two DAQs, by the onboard uninterruptible power supply (UPS) to avoid system shutdown and possible detector warm-up variations on restart. The outer surfaces and wheels of the unit were sprayed with disinfectant and wiped down at the entrance to the secure mouse facility, moved to the imaging room and reconnected to house power. Energy windows in both cameras were set to 171 keV ± 12%, the lower energy gamma emission emitted by In-111, and the study begun.
RESULTS
Gamma Camera Performance
Performance characteristics for Tc-99m are listed in Table 1.
Table 1.
Gamma Camera Performance (Tc-99m)
| Parameter | Value |
|---|---|
| Spatial resolution | 2.2 mm FWHM (all source directions) |
| Energy resolution | 10.8% (at 140 keV) |
| Sensitivity | 149 cps/MBq (± 10% energy window) |
| Count rate linearity | r = 0.996, slope = 149 cps/MBq, straight line fit |
| Spatial uniformity | < ± 3% variation across UFOV |
Mouse Imaging Study
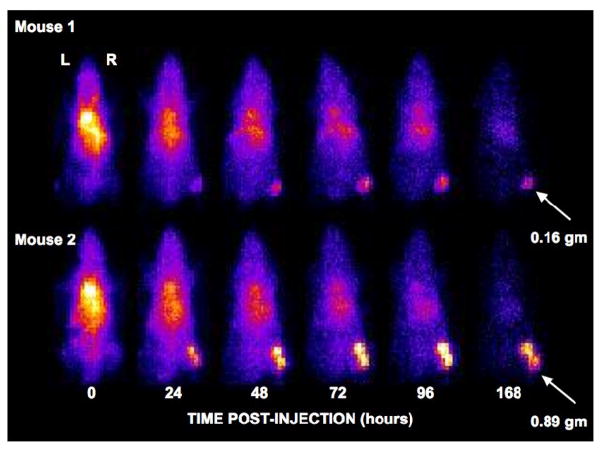
Temporally paired (top and bottom), representative MONICA images of two animals obtained over the 7-day study period are shown in Figure 6. Blood pool and liver activity dominate during the first 24–48 hrs with gradual accumulation of radioactivity in the tumor. Tumors are readily detectable on day 7 and much of the activity outside the tumors has been cleared from the body.
Figure 6.
Anterior projection images of the time variation of tumor (arrows) and body distribution of In-111 Panitumumab in two mice imaged with MONICA. Animals were injected with less than 3.7 MBq (100 μCi) of In-111 at time zero and were imaged in pairs (top and bottom) for 5 minutes at each time point during isoflurane inhalation. Images have been decay corrected to time zero. Mouse 1 had the smallest tumor mass and Mouse 2 the largest tumor mass of all animals studied.
DISCUSSION
Design goals for the MONICA system were set primarily by the specific and immediate needs of the Molecular Imaging Program. In particular, any new imaging system was required to be very compact, portable and capable of imaging at least two animals at once (to increase throughput). The size and portability requirements arose directly from the fact that imaging space in the animal facility was extremely limited (less than a square meter) and that animals held by the Molecular Imaging Program and its collaborators could be at physically different locations (separated by several hundreds of meters).
The performance values measured for the MONICA system, the animal images reflecting field performance and the design characteristics of the device suggest that these practical goals have largely been realized. The performance measurements (Table 1) are consistent with the performance of other small FOV pixilated gamma cameras [4] and with the performance of modern commercial gamma cameras equipped with similar parallel hole collimation [5]. The animal study (Figure 6) illustrates how an imaging system designed for a specific range of applications can answer certain kinds of experimental questions in an environment dominated by logistical constraints. Our laboratory, for example, is engaged in development of new single photon-labeled diagnostic and therapeutic agents intended to target a wide range of human cancers. An important first step in this drug development process is to determine by planar projection imaging whether a new drug meets minimal whole-body and tumor biodistribution requirements in tumor-bearing mice. Inspection of Figure 6 suggests that MONICA imaging of a whole mouse containing a strategically placed tumor xenograft answers this question positively and definitively for 111In PT under circumstances where planar projection imaging might not otherwise be possible due to space limitations.
Although not the purpose of this work, the qualitative changes seen in these images can, in principle, be made (approximately) quantitative by applying ROI and background correction methods sometimes employed in the analysis of clinical projection images. For example, the absolute activities (in MBq) in the tumors shown in Figure 6 could be estimated by first placing an ROI over the tumor and determining the gross count rate in this ROI. This gross count rate, however, will also contain contributions from activity in non-tumor tissue above and below the tumor but within the ROI and from radiation scattered into the ROI from activity elsewhere in the animal. (In the case of 111In there is an additional scatter contribution from the high-energy gamma ray (245 keV) also emitted by 111In). If the sum of these extraneous count rates can be estimated and subtracted from the gross tumor ROI count rate, the resulting net tumor count rate can be multiplied by a small correction factor for radiation attenuation and the (known) system sensitivity at the study photon energy to obtain an estimate of absolute tumor activity in MBq. The accuracy of such time-dependent heuristic correction methods, e.g. peri-tumor ROIs, dual energy windowing for 111In, however, is not currently known for this experimental model. Accordingly, future work will be directed towards validating the accuracy of correction methods tailored to this mouse imaging application.
The MONICA system design provides several procedural options to the user that can enhance other aspects of the imaging process. For example, the wireless Macintosh laptop connection to the MONICA system allows the user to visually monitor study progress in real-time (count rates in each energy window and persistence images of both cameras’ FOVs) from any nearby remote location simply by bringing the laptop along to that location. This capability can, for example, allow the user to carry out other tasks, such as preparing the next animal for study in a nearby room, while continuing to monitor the ongoing study.
The MONICA system was designed for a specific application and to recognize certain logistical constraints and, as a result, possesses limitation outside this application and constraint range. The FOV of the gamma cameras, while adequate for whole-body mouse imaging, permits only portions of larger animals to be visualized, e.g. the rat head or turning a rat sideways to image body sections. In addition, the MONICA collimators cannot be changed to alter the tradeoff among resolution, sensitivity and useful gamma ray energy range, as is the case with continuous slab gamma cameras. Since the scintillation detectors of the MONICA system are pixelated, each collimator hole must match one and only one scintillation pixel or spatial resolution will suffer. The size, pitch and arrangement of the scintillator pixels and the collimator holes, on the other hand, are strongly dependent on manufacturing technology and the sizes chosen for MONICA were largely determined by these manufacturing constraints. Although these constraints are not absolute, cost factors set a practical limit with current technology.
The choice of pixels rather than slabs for the MONICA detectors was made, ultimately, to allow two cameras to be positioned in the cart with reasonable surrounding workspace and separation (to reduce cross-animal scatter), yet be small enough that the overall cart footprint was minimal. Two side-by-side H8500 PSPMTs possess the dimensions and useful fields-of-view (UFOV) to allow whole-body mouse imaging if the entire UFOV of each tube can be used. However, if slabs rather than pixels are used, “edge packing”, an effect that causes erroneous centroid positioning of events in the peripheral FOV of slab detectors, would significantly reduce the UFOV below the required area. This effect can be overcome in practice by simply increasing the size of the slab scintillator and the number of phototubes to achieve the requisite UFOV and ignore peripheral events. This choice, however, would increase the physical size of the scintillator area and would require the addition of more phototubes thereby increasing not only the size of the detectors but also the cost and complexity of the system. Pixelated arrays do not suffer this effect to the same degree and the UFOV of the MONICA detectors much more closely matches the (small) footprint of the paired PSPMTs leading to a compact detector module capable of whole-body mouse imaging.
It should be noted that several single photon planar projection imaging systems have been developed specifically for small animal studies by both the academic [4, 6–9] and commercial communities (Biospace, Paris, France; eV Products, Saxonburg, PA; Carestream Health, New Haven, CT). Portable clinical instruments also exist that can be adapted to small animal imaging [5]. While none of these systems simultaneously meets all of the design conditions set for the MONICA system, each has characteristics that may be of particular value in different experimental settings. Such devices may offer a potentially lower cost alternative to advanced tomographic imaging system when planar projection imaging is able to answer the experimental question at hand.
Acknowledgments
Financial support: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The authors are indebted to, and wish to thank, the following individuals for their critical contributions to the MONICA project: J. Powell, R. Clary, H. Metger, F. Sharpnack (Mechanical Instrumentation Design and Fabrication Branch) and B. Chidakel (Laboratory Equipment and Computer Services Branch), OD/NIH; A. Bumb and G. Kramer-Marek (Radiation Oncology Branch, NCI/NIH); L. Brown and M. Spencer (Medical Arts Division, OD/NIH); C. A. Beegle and K. Cheng (Department of Nuclear Medicine, CC/NIH).
Footnotes
Animal Care: Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, D.C.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Proffitt J, Hammond W, Majewski S, et al. A Flexible High-rate USB2 Data Acquisition System for PET and SPECT Imaging. IEEE Nuclear Science Symposium Conference Record. 2005;5:2971–2975. [Google Scholar]
- 2.Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA Drug Approval Summary: Panitumumab (Vectibix) Oncologist. 2007;12:577–583. doi: 10.1634/theoncologist.12-5-577. [DOI] [PubMed] [Google Scholar]
- 3.Ray GL, Baidoo KE, Wong KJ, et al. Pre-Clinical Evaluation of a Monoclonal Antibody Targeting the Epidermal Growth Factor Receptor for Radioimmunodiagnostic and Radioimmunotherapeutic Applications. British Journal of PharMacintoshology. 2009 doi: 10.1111/j.1476-5381.2009.00327.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loudos G, Majewski S, Wojcik R, et al. Performance Evaluation of a Dedicated Camera Suitable for Dynamic Radiopharmaceutical Evaluation in Small Animals. IEEE Transactions on Nuclear Science. 2007;50:454–460. [Google Scholar]
- 5.MEDX, Inc; Arlington Heights, IL: T-Quest™ gamma camera product information. http://www.medx-inc.com/tquest.html. [Google Scholar]
- 6.Kiyono Y, Kuge Y, Katada Y, Kawashima H, Magata Y, Sajj H. Applicability of a High-resolution Small Semiconductor Gamma Camera to Small Animal Imaging. Nucl Med Commun. 2007;28:736–41. doi: 10.1097/MNM.0b013e32828d8cbc. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S, Hatazawa J. Development of a Small FOV LaBr3(Ce) Gamma Camera for Low Energy Single Photon Imaging. J Nucl Med. 2007;48 (Suppl 2):94P. [Google Scholar]
- 8.Pani R, Pellegrini R, Cinti MN, et al. New Devices for Imaging in Nuclear Medicine. Cancer Biotherapy Radiopharmaceuticals. 2004;19:121–128. doi: 10.1089/108497804773391766. [DOI] [PubMed] [Google Scholar]
- 9.Notaristefani FD, Pani R, Scopinaro F, et al. First Results from a YAP:Ce Gamma Camera for Small Animal Studies. IEEE Transactions on Nuclear Science. 1996;43:3264–3271. [Google Scholar]