Abstract
Background
The incidence of hepatocellular carcinoma (HCC) is rising and radiofrequency ablation (RFA) appears to be increasingly used. The nationwide use and impact of RFA have not been well characterized.
Study Design
Historical cohort study of US patients ≥18 years with a diagnosis of HCC (n=22,103) using the national Surveillance, Epidemiology, and End Results (SEER) limited-use database (1998-2005). Main outcome measures were receipt of different therapeutic interventions (ablation, RFA, resection, or transplantation) and adjusted one- and two- year survival.
Results
4,924 (22%) patients underwent any intervention with a 93% increase over the eight year study period (trend test, p<0.001). RFA accounted for 43% of this increase. Despite increased use of therapeutic interventions, one- and two-year survival did not improve over time for patients in the study cohort (48% and 34%, 52% and 37%, 50% and 36%; in 1998, 2002, and 2004, respectively; p=0.31). Among patients with solitary lesions, adjusted one- and two-year survival remained stable over time after transplantation (97% and 94%, 95% and 89%, 94% and 86%; p=0.99) and RFA (86% and 64%, 76% and 54%; p=0.97) but improved after resection (83% and 71%, 91% and 84%, 97% and 94%; p=0.03).
Conclusions
Utilization of interventions for the treatment of HCC, and specifically RFA, have markedly increased over time. Since increased use of RFA among potentially resectable patients is likely to occur and because of a lack of high-level evidence supporting expanded indications, continued evaluation of the indications for RFA as well as subsequent outcomes among US patients is warranted.
Introduction
Worldwide, hepatocellular carcinoma (HCC) is the third most common cause of cancer death with over half a million new cases diagnosed each year.1 Over the last three decades, the incidence of HCC in the United States has tripled.2, 3 The increase has been attributed to the hepatitis C (HCV) epidemic of the 1970's and 1980's and is expected to peak around 2015.4-6 However, the increase could continue due to the high current rate of obesity, resultant nonalcoholic steatohepatitis, and the concomitant risk for progression to cirrhosis and subsequently HCC.7-11
Liver resection and transplantation are considered curative therapies and are largely performed at tertiary care referral centers. In contrast, ablative modalities, such as radiofrequency ablation (RFA), have conventionally been used to treat patients with non-resectable and non-transplantable disease, to bridge patients to transplant, or with palliative intent. The appeal of a minimally invasive, less morbid approach to the treatment of HCC, such as RFA, is apparent. However, only one study in the English literature has evaluated RFA in a randomized fashion with notable limitations (including small study groups and lack of a non-inferiority design).12 Other studies have recently recommended RFA as a potentially equivalent therapy to resection or transplant, but suffer the limitations inherent to observational data.13-15
There have been no prior population-based evaluations to document the rise in use or impact of RFA in the US. We hypothesize there has been a temporal increase in the use of RFA and in this study we aimed to evaluate changes in the utilization of RFA and the other available therapeutic interventions, to describe changes in the patient population receiving these interventions, and to describe temporal changes in survival. Prior population-based evaluations using the US cohort have either not considered RFA16 or have not performed a temporal evaluation of the growth and utility of RFA relative to other available interventions.17
Methods
A historical cohort study conducted with approval from the University of Washington Institutional Review Board. Data were obtained through the Surveillance, Epidemiology, and End Results (SEER) program (www.seer.cancer.gov), a nationally representative tumor registry sponsored by the National Cancer Institute. We identified 22,959 patients with a histologic diagnosis of HCC (ICD-O-3: 8170, 8171, 8172, 8173, 8174, and 8175) between 1998 and 2005. Sequential exclusions were made for diagnosis at autopsy or from a death certificate (n=473), younger than 18 years (n=62), unknown demographic (age or race) data (n=74), and in whom receipt of therapy was unknown (n=247).
Site-specific surgery codes were used to stratify therapeutic interventions into five categories: resection; transplant; ablation by heat (considered RFA); other ablation; resection and ablation combined. Each SEER patient record contains only one therapeutic procedure corresponding to the most invasive or definitive procedure during the first course of treatment. Age was categorized by quartiles (<55, 55-64, 65-74, and ≥75). We categorized race as white, black, or other because this stratification has been shown to have the highest validity.18
Non-parametric test of trend, multivariate logistic regression, and Kaplan-Meier survival analysis were used. Data from all patients were used in calculating overall rates of procedural utilization (Table 1) as well as rates of utilization associated with specific tumor characteristics (Table 2). In an exploratory regression analyses, only data from treated patients were considered. Receipt of each intervention, relative to the receipt of all other available modalities, was considered separately as the outcome of interest. Odds ratios were adjusted for age, sex, race, marital status, year of diagnosis, tumor size, multifocal disease, prior malignancy, and the presence of metastases. Robust standard errors were used and estimates were adjusted for clustering at the registry level. Patients who underwent resection with concurrent ablation were not considered because of the small number of observations (91 procedures over the eight study years). With regard to the survival analyses, 2004 was considered the most recent year to ensure all patients had at least one full year of follow-up. One- and two-year survival were selected as primary endpoints because follow-up in SEER was only through the end of 2005, meaning patients undergoing RFA in the more recent study years (the majority of cases) had relatively little follow-up.
Table 1.
Temporal Changes in the Rate of Utilization of Surgical Therapy (per 100 HCC Patients)
| 1998 (n=1,373) |
1999 (n=1,465) |
2000 (n=2,682) |
2001 (n=2,967) |
2002 (n=3,042) |
2003 (n=3,326) |
2004 (n=3,527) |
2005 (n=3,721) |
p Value* | |
|---|---|---|---|---|---|---|---|---|---|
| Any intervention | 14.9 | 16.2 | 17.7 | 18.6 | 22.1 | 24.2 | 26.1 | 28.4 | <0.001 |
| Any ablation | 3.2 | 3.6 | 4.9 | 6.0 | 7.7 | 9.5 | 10.5 | 12.1 | <0.001 |
| RFA | 0.4 | 0.3 | 1.0 | 1.8 | 3.2 | 5.6 | 5.8 | 6.2 | <0.001 |
| Other ablative | 2.8 | 3.3 | 3.8 | 4.3 | 4.5 | 3.9 | 4.8 | 6.0 | <0.001 |
| Resection | 8.5 | 9.2 | 9.2 | 8.3 | 7.8 | 7.6 | 7.7 | 9.1 | 0.37 |
| Transplant | 3.1 | 3.3 | 3.6 | 4.3 | 6.5 | 6.4 | 6.9 | 6.3 | <0.001 |
| Resection/ablation | 0 | 0 | 0 | 0 | 0 | 0.7 | 0.9 | 0.9 | <0.001 |
Numbers in the column headings represent total patients diagnosed with HCC in a given year.
Trend test p-value
Table 2.
Rate (per 100 HCC Patients) of Therapeutic Intervention Utilization by Tumor Characteristics
| No Therapy | Other ablation | RFA | Resection | Transplant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1998 | 2002 | 2005 | 1998 | 2002 | 2005 | 1998 | 2002 | 2005 | 1998 | 2002 | 2005 | 1998 | 2002 | 2005 | |
| Tumor size, cm | |||||||||||||||
| ≤2 | 54.7 | 45.5 | 46.8 | 9.4 | 6.2 | 6.4 | 0 | 6.2 | 7.5 | 9.4 | 9.1 | 12.2 | 26.6 | 33.0 | 26.1 |
| 2.1-5 | 66.8 | 60.9 | 53.5 | 7.5 | 8.8 | 8.1 | 1.6 | 7.4 | 14.5 | 17.4 | 10.6 | 10.7 | 6.7 | 12.0 | 12.0 |
| >5 | 82.8 | 80.7 | 75.6 | 1.6 | 3.6 | 6.1 | 0 | 1.7 | 2.4 | 13.7 | 11.9 | 12.6 | 1.9 | 2.1 | 1.9 |
| Missing | 97.3 | 92.0 | 91.4 | 1.1 | 2.3 | 3.6 | 0.2 | 1.3 | 2.6 | 1.3 | 2.3 | 1.6 | 0.2 | 2.1 | 0.8 |
| No. of lesions | |||||||||||||||
| 1 | 62.6 | 56.2 | 55.1 | 7.0 | 7.9 | 7.6 | 1.0 | 5.9 | 9.9 | 22.4 | 19.0 | 16.1 | 7.0 | 10.9 | 9.9 |
| >1 | 90.2 | 82.8 | 76.3 | 1.7 | 3.9 | 5.8 | 0.1 | 2.4 | 4.8 | 5.3 | 5.0 | 6.7 | 2.6 | 5.9 | 5.5 |
| Missing | 96.9 | 93.0 | 94.2 | 1.2 | 1.7 | 2.4 | 0.4 | 2.2 | 2.2 | 1.6 | 1.2 | 1.1 | 0 | 1.9 | 0.2 |
| Solitary lesions * | |||||||||||||||
| 1 lesion & ≤2 cm | 44.4 | 30.5 | 40.1 | 11.1 | 8.6 | 7.8 | 0 | 7.6 | 9.6 | 11.1 | 14.3 | 15.0 | 33.3 | 39.0 | 26.3 |
| 1 lesion & 2.1-5 cm | 50.0 | 51.2 | 42.3 | 11.1 | 10.7 | 8.9 | 2.8 | 10.0 | 17.3 | 28.7 | 17.1 | 16.4 | 7.4 | 10.7 | 14.0 |
| 1 lesion & >5 cm | 66.7 | 63.1 | 66.3 | 3.3 | 5.8 | 6.7 | 0 | 1.2 | 3.9 | 28.3 | 28.2 | 19.5 | 1.7 | 1.7 | 1.7 |
| Metastases | |||||||||||||||
| No | 78.3 | 71.1 | 62.7 | 4.0 | 5.8 | 7.4 | 0.4 | 3.9 | 8.1 | 12.6 | 10.3 | 12.0 | 4.7 | 8.8 | 8.6 |
| Yes | 99.1 | 96.4 | 95.0 | 0 | 1.1 | 1.9 | 0 | 0.6 | 0.6 | 0.4 | 1.9 | 2.4 | 0.4 | 0 | 0 |
| Missing | 96.9 | 93.0 | 93.2 | 1.2 | 1.7 | 2.9 | 0.4 | 2.2 | 2.1 | 1.6 | 1.2 | 1.0 | 0 | 1.9 | 0.8 |
Rates in each year do not equal 100 because patients receiving combined resection/ablation (n=0 in 1998; n=1 in 2002; n=35 in 2005) are not shown.
Records wherein one or both variables were missing are not shown.
Overall survival was ascertained from all patients. All other analyses were restricted to patients with solitary lesions. SEER data only allows categorization of the number of lesions as 1 or >1. Therefore, it is not possible to ascertain how many lesions constitute “>1 lesion” (important information that bears on therapeutic options). Additionally, multifocal disease is a poor prognostic factor with regard to survival after HCC treatment.19, 20 Therefore, restricting the analysis to the subgroup with solitary lesions allowed evaluation of a slightly more homogenous cohort of patients who could be expected to garner the most potential benefit from treatment. Analyses were adjusted for age, race, sex, tumor size, prior malignancy, and the presence of metastases.
There was a high proportion (40.8%) of missing data with regard to tumor characteristics (tumor size, 36.4%; number of lesions, 14.9%; metastases, 15.5%). The degree of missing data was substantially less (12.4%) among treated patients (tumor size, 10.6%; number of lesions, 3.3%; metastases, 3.8%) and even less (5.8%) among treated patients with solitary lesions (tumor size, 5.3%; metastases, 0.6%). Nonetheless, loss of statistical power remained a concern. The missing data indicator method (for categorical variables a separate dummy variable was created for missing values to prevent loss of study subjects from the survival analyses) rather than a case-complete analysis was utilized to deal with missing values.21 Statistical analyses were performed using STATA version 10.1 (STAT Corp, College Station, Texas).
Results
Utilization
Over the study period, there was a significant increase in the utilization of any therapeutic intervention (Table 1). Among the 22,103 patients studied, 4,924 (22.3%) patients underwent a therapeutic intervention (14.9% in 1998 vs 28.4% in 2005; trend test, p<0.001). 43% of this increase was attributable to rising RFA use (15.5-fold increase; trend test, p<0.001). There were also increases in other ablative modalities (2.1-fold; trend test, p<0.001) and transplantation (2-fold; trend test, p<0.001). The frequency of hepatic resection remained stable.
Associated Factors
Table 2 describes temporal changes in the rate of utilization of the different interventions by tumor characteristics. Since RFA was a relatively new modality in the early years of the study, its use increased across nearly all demographic (data not shown) and tumor characteristics. Between 2002 and 2005 there was an increase in RFA use, with a concurrent decrease in resection, among patients with solitary lesions. In particular, RFA use increased by 26% and 73% in patients with solitary tumors measuring ≤2cm and 2.1-5cm, respectively and more than doubled among patients with solitary lesions >5cm.
We used multivariate logistic regression (Table 3) to evaluate the association between patient and tumor characteristics, advancing study year, and receipt of a therapeutic intervention (in particular RFA). After adjusting for relevant demographic and tumor characteristics, incremental increases in the year of diagnosis were associated with significantly higher odds of receiving RFA (OR 1.36; 95% CI [1.24-1.49]) as compared to other therapies. By comparison, increasing year of diagnosis was associated with lower odds of undergoing resection (OR 0.83 [0.78-0.88]). As compared to patients with lesions measuring ≤2cm, those with lesions 2.1-5cm were more likely to undergo RFA (OR 2.03 [1.50-2.74]) while patients with lesions >5cm were at significantly lower odds of receiving RFA (OR 0.57 [0.39-0.85]). Patients with >1 lesion were at significantly lower odds of undergoing resection compared to other therapies (OR 0.55 [0.47-0.65]). Metastatic disease was not associated with the receipt of any intervention except other ablative modalities (OR 1.67; [1.07-2.63]).
Table 3.
Multivariate Logistic Regression Analysis: Treated Patients
| Ablation (n=976) | RFA (n=805) | Resection (n=1,847) | Transplant (n=1,205) | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI (p value) | OR | 95% CI (p value) | OR | 95% CI (p value) | OR | 95% CI (p value) | |
| Year of diagnosis | 0.98 | 0.89-1.08 (0.66) | 1.36 | 1.24-1.49 (<0.001) | 0.83 | 0.78-0.88 (<0.001) | 1.02 | 0.97-1.07 (0.49) |
| Age, y | ||||||||
| <55 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 55-64 | 1.32 | 1.11-1.58 (0.002) | 1.29 | 1.08-1.53 (0.005) | 0.92 | 0.84-1.00 (0.05) | 0.78 | 0.70-0.88 (<0.001) |
| 65-74 | 1.50 | 1.18-1.90 (0.001) | 1.81 | 1.39-2.34 (<0.001) | 1.42 | 1.23-1.63 (<0.001) | 0.27 | 0.23-0.32 (<0.001) |
| ≥75 | 1.80 | 1.30-2.49 (<0.001) | 2.17 | 1.69-2.79 (<0.001) | 1.64 | 1.20-2.26 (0.002) | 0.007 | 0.002-0.02 (<0.001) |
| Female gender | 0.75 | 0.67-0.85 (<0.001) | 1.15 | 0.97-1.35 (0.11) | 1.21 | 1.00-1.48 (0.06) | 0.94 | 0.77-1.15 (0.55) |
| Race | ||||||||
| White | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| African American | 1.01 | 0.72-1.40 (0.98) | 1.06 | 0.84-1.34 (0.62) | 1.44 | 1.00-2.08 (0.05) | 0.51 | 0.36-0.72 (<0.001) |
| Other | 0.88 | 0.65-1.19 (0.41) | 1.20 | 1.01-1.41 (0.03) | 1.70 | 1.39-2.10 (<0.001) | 0.47 | 0.37-0.59 (<0.001) |
| Marital status—Married | 0.79 | 0.68-0.92 (0.003) | 0.77 | 0.62-0.97 (0.03) | 0.95 | 0.82-1.09 (0.44) | 1.69 | 1.47-1.94 (<0.001) |
| Tumor size, cm | ||||||||
| ≤2 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 2.1-5 | 1.65 | 1.36-2.01 (<0.001) | 2.03 | 1.50-2.74 (<0.001) | 1.52 | 1.17-1.98 (0.002) | 0.33 | 0.26-0.42 (<0.001) |
| >5 | 1.59 | 1.13-2.23 (0.008) | 0.57 | 0.39-0.85 (0.006) | 6.99 | 5.17-9.44 (<0.001) | 0.07 | 0.05-0.08 (<0.001) |
| >1 lesion | 1.33 | 1.14-1.54 (<0.001) | 0.95 | 0.77-1.17 (0.61) | 0.55 | 0.47-0.65 (<0.001) | 1.70 | 1.38-2.09 (<0.001) |
| Prior malignancy | 1.10 | 0.86-1.41 (0.44) | 0.92 | 0.77-1.11 (0.41) | 1.19 | 0.95-1.48 (0.13) | 0.67 | 0.50-0.90 (0.007) |
| Metastases | 1.67 | 1.07-2.63 (0.03) | 0.86 | 0.37-1.99 (0.72) | 1.28 | 0.80-2.03 (0.30) | 0.16 | 0.07-0.35 (<0.001) |
Survival
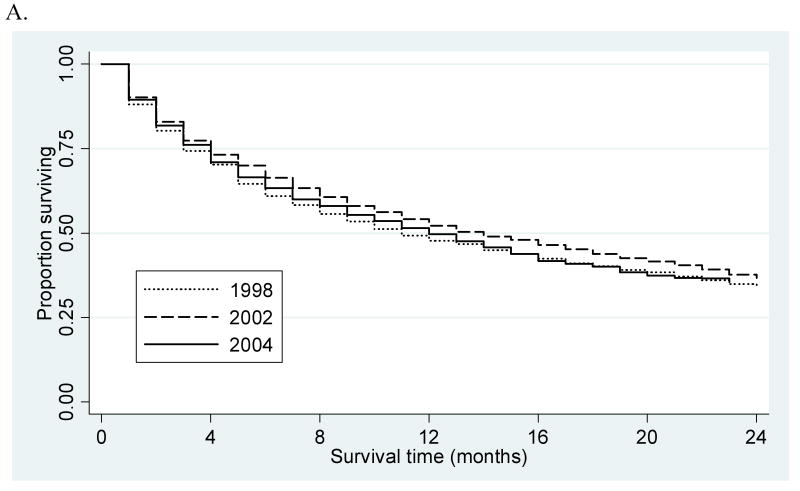
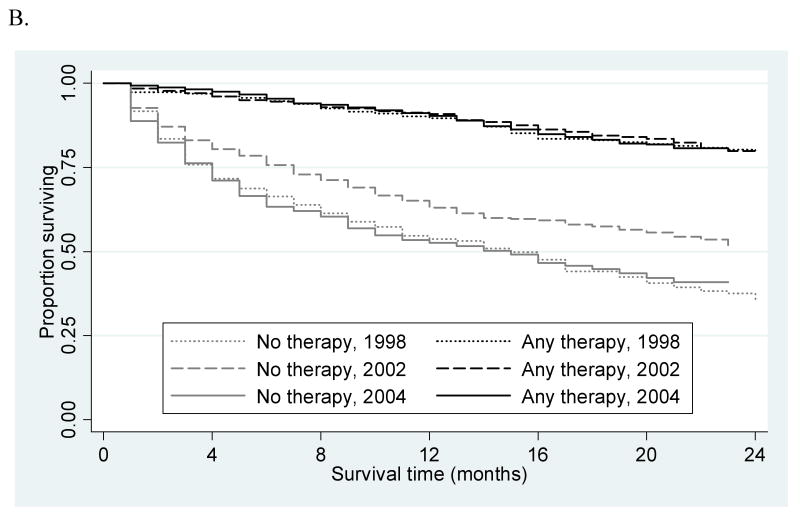
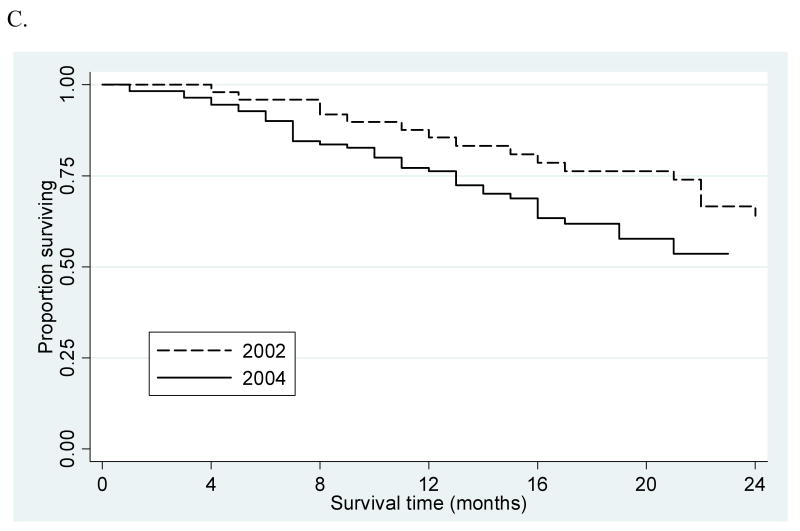
Overall one- and two-year survival (Figure 1A) remained stable over the course of the study period for all patients (48% and 34%, 52% and 37%, 50% and 36%; in 1998, 2002, and 2004, respectively; p=0.31) and patients with solitary lesions (59% and 41%, 66% and 50%, 57% and 41%, p=0.67). Among patients with solitary tumors, one- and two-year survival was significantly different (p<0.001) between those who did (90% and 80%, 91% and 79%, 90% and 81%) and did not undergo a therapeutic intervention (54% and 36%, 63% and 51%, 53% and 41%), but remained stable (p=0.83) over time among untreated patients (Figure 1B). Survival remained stable for patients who underwent transplant (97% and 94%, 95% and 89%, 94% and 86%; p=0.99) and improved after resection (83% and 71%, 91% and 84%, 97% and 94%; p=0.03). There were too few observations in the early years of the study to provide meaningful survival estimates for patients who underwent RFA (3 cases each in 1998 and 1999). However, survival remained stable for patients receiving RFA (Figure 1C) in 2002 as compared to 2004 (86% and 64%, 76% and 54%; p=0.97).
Figure 1.
(A) Overall two-year survival by year of diagnosis. (B) Two-year survival among treated and untreated patients with solitary lesions by year of diagnosis. (C) RFA two-year survival among patients with solitary lesions by year of diagnosis.
Discussion
RFA was approved by the Food and Drug Administration in 2001 for general use in patients with unresectable primary and metastatic liver tumors.22, 23 Approval occurred after an assessment of RFA's ability to safely create a zone of ablation in the liver, not as an effective treatment of tumors within the liver.23 At that time, market data already demonstrated dramatic worldwide increases in the use of RFA.24 Recently, liberalization of the indications for RFA as a potentially curative treatment for patients with small HCC lesions has been suggested by several authors. In addition to liberalized indications, there are a number of reasons to expect continued growth in the use of RFA in the US—can be performed less invasively, potentially minimizes perceived surgical risk in patients with underlying liver disease, can be performed by non-surgical and/or non-oncologic practitioners at non-referral centers, limited surgeons and institutions with resources to offer resection or transplant, a complex disease process, and limited donor organs.
For a number of reasons (patient choice, institutional financial interests, enthusiasm for new technology, etc), there may be a desire to expand the indications of RFA to include curative treatment, regardless of resectability. Yet, few studies provide high-level evidence to support RFA use as a curative treatment in patients with resectable or transplantable HCC. The only randomized trial in the English literature is a small, single-center study12 demonstrating similar 4-year overall and disease-free survival between RFA and resection. However, this trial did not employ a non-inferiority design. Other studies, although providing encouraging results, may be of limited value because they were retrospective, single referral center series. In addition, there remains uncertainty regarding RFA's effectiveness as a curative therapy as several studies have correlated histopathologic exam of explanted organs from patients who underwent pre-transplant RFA with incomplete response in 20-90% of patients.25-27
Most of the available data regarding RFA effectiveness come from case series performed at institutions in Asia where etiologic differences for cirrhosis may raise questions about the generalizability of these result to the US. In the Eastern hemisphere, the predominant etiology of cirrhosis and subsequent HCC is hepatitis B, not HCV as in the United States—an important consideration because HCV has a greater association with recurrence, multifocal disease at presentation, and a higher yearly de novo incidence rate.28-30 Therefore, outcomes using RFA to treat hepatitis B associated HCC may not be generalizable to patients with tumors arising in the setting of HCV.
Since being introduced as a treatment for patients with HCC, growth in the use of RFA has been assumed. However, the extent of RFA growth in the US has remained unclear. In this population-based cohort, we observed a dramatic increase in the utilization of all therapeutic interventions for HCC—which was largely attributable to increased RFA. In 2005, ablative interventions were the most frequently performed procedures and, among these, RFA was utilized most. Not surprisingly, the most rapid uptake in RFA occurred around the time a CPT billing code was created for the procedure (in 2002). However, around this time period newer generation RFA probes and generators were also introduced which may be easier to use and have lower rates of margin recurrence when used in patients with hepatic colorectal metastases.31
With each advancing study year, the likelihood of a patient undergoing RFA increased with an associated decrease in the odds of undergoing resection. Notably, RFA use increased with a coincident decrease in resection among patients with solitary lesions and, in particular, solitary lesions 2.1-5cm. This, along with the finding overall resection rate remained stable over time, raises the possibility of a replacement phenomenon (RFA replacing the use of resection) among certain subgroups of HCC patients. However, the relationship between RFA use and the utilization of resection requires further evaluation because SEER does not contain specific information on severity of cirrhosis, comorbid conditions, tumor location, or procedural indication. As a result, we could not ascertain whether RFA was intended as primary therapy in an otherwise resectable patient, as a primary treatment in a patient unable to undergo resection due to severe cirrhosis or comorbid illness, or as a bridge to transplantation. We are therefore unable to discern whether the observed increase in utilization was or was not appropriate. For instance, if RFA utilization were increasing solely among patients with liver disease or tumor location(s) precluding resection, this may simply represent diffusion of this therapeutic modality into a prior untreatable group of patients. However, if this were the case, we would have expected survival among the remaining, untreated patients (who presumably had comorbid conditions or tumors so severe as to preclude any therapy and qualifying them as patients at highest surgical risk) to decrease over time rather than remain stable.
One- and two-year survival remained stable or improved over time among patients with solitary lesions who underwent transplantation or resection. Earlier detection because of increasing use of improved imaging, improvements in critical care and operative technique, and changes in recipient and donor selection are all likely influential factors. Similarly, survival among patients with solitary lesions who underwent RFA was statistically unchanged. However, this study was underpowered to detect a significant survival difference among patients undergoing RFA—we needed at least 261 patients in each year (we had 42 and 126 patients in 2002 and 2004, respectively) to demonstrate a statistically significant survival difference of the magnitude observed (at the α=0.05 level with 80% power) at one-year (400 patients would have been necessary for two-year survival). Nonetheless, the absolute 10% survival decrease over a two year period was unexpected, is not reassuring of the benefits of RFA or proper selection of patients undergoing this procedure, and is worthy of further scrutiny.
A significant amount of healthcare spending has been shown to occur in the final year of a patient's life.32 The observed growth in RFA as well as other ablative therapies, particularly in the more recent study years, may have been among patients with poor prognosis, little expected survival benefit, or may simply indicate futile care among patients not expected to benefit from any intervention. More clinical data would be valuable to further explore this possibility. As compared to the indications for resection and transplantation, which have remained fairly well-defined, these findings could also be indicative of more liberal RFA use in treatable, but higher risk patients (i.e.: marginal liver function, hyperbilirubinemia, difficult tumor location, etc.). Provider training and experience, which have been shown to be associated with patient outcomes for lung cancer, may also play a role.33 Because of the relative ease with which RFA can be performed, many providers without advanced specialty training (in hepatobiliary, oncologic, or laparoscopic surgery) or who are untrained in the use of intraoperative ultrasound have access to RFA. Differences in the risk of recurrence between resection and RFA34 as well as difference between the operative (laparoscopic or open) and percutaneous RFA approaches (with higher local recurrence after the latter) have been described.35 Since, the majority of non-referral hospitals are unlikely to offer transplantation or specialized hepatobiliary services capable of performing RFA via a surgical approach, a possible explanation may be that many RFA procedures are being done percutaneously outside referral centers. As yet, no study has evaluated use of the percutaneous and operative approaches in the general community. Unfortunately, SEER does not offer information on surgical risk factors, surgical approach, or provider specialty to evaluate these possibilities. This study has several other important limitations. There was a high proportion of missing data relating to tumor characteristics. However, the degree of missing data was substantially less among patients included in our main survival analyses. The biggest limitation is SEER does not provide data on important comorbid conditions, in particular underlying cirrhosis and the presence of factors denoting advanced liver disease such as ascites, coagulopathy, and jaundice.
These factors play an important role in surgical decision-making and may have lead to confounding by severity or simply residual confounding because of our inability to adjust for these conditions using SEER data. Although we acknowledge the possibility of residual confounding in our analysis, it is also possible that with liberalized RFA use over time patients treated in more recent years may have had less severe disease, meaning these survival estimates are conservatively biased. SEER does not contain information on sequential procedures and therefore our data likely underestimate the true rate of use of ablative therapy. For example, RFA used for bridging to transplantation would not be captured in this dataset (the patient would be recorded as having undergone transplant). Information on sequential procedures would have also been useful to identify intent and potential indications for RFA and ablative therapy. Misclassification of RFA was also possible because RFA was identified in this dataset as “ablation by heat.” Microwave coagulation therapy (MCT) is another ablative technique that causes thermal tumor necrosis. However, bias introduced by this coding scheme is likely minimal because commercial MCT generators were not widely available during the study period. Evaluation of HCC-specific rather than overall survival would have also been valuable. Although SEER contains information on cause of death, the validity of this variable is debated because it is based on death certificate data.18 Evaluation of recurrence after treatment would have also been desirable; however, SEER does not provide such data. Finally, the most recent study year contained incident cancers from 2005. More current data would have been desirable, but there is a time lag for updates to SEER data which is the only dataset allowing a population-based analysis of all cancer care (inpatient and outpatient) among the entire US cohort without age restriction.
Despite these limitations, SEER permits a population-based assessment of care and outcomes among patients with HCC, allowing this study to offer a more representative sample of surgical practice and outcomes in the general community as compared to that derived from referral center experiences. As pressure increases to liberalize the indications for RFA, continued reassessment of the relative benefits as well as patient survival will be important. While a prospective, randomized, non-inferiority trial would be most appropriate to address whether survival after RFA is not meaningfully worse than after resection or transplantation, such a study is unlikely to be performed in the near future. As such, additional studies utilizing secondary data sources richer in clinical variables or prospective studies evaluating the potential benefits of RFA among specific, higher risk patient subgroups may be useful to inform patient selection criteria. For example, Molinari et al used Markov modeling to demonstrate no overall improvement in quality-adjusted survival for RFA when compared to resection, except among patients older than 75 years.36 At present, because of a lack of data identifying patients who benefit most from RFA, we recommend patients with HCC should be offered a multidisciplinary assessment which considers and has the potential to offer all possible treatment options.
Acknowledgments
This work was supported by the National Institutes of Health under a Ruth L Kirschstein National Research Service Award 1F32CA136135-01 (Dr Massarweh) and an Established Investigator Award in Cancer Prevention & Control 1K05CA124911 (Dr Vaughan) from the National Cancer Institute.
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the American College of Surgeons 95th Annual Clinical Congress, Chicago, IL, October 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donckier V, Van Laethem JL, Van Gansbeke D, et al. New considerations for an overall approach to treat hepatocellular carcinoma in cirrhotic patients. J Surg Oncol. 2003;84:36–44. doi: 10.1002/jso.10281. discussion 44. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 9.Chitturi S, Abeygunasekera S, Farrell GC, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 10.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 11.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533. viii. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:21–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi S, Kudo M, Chung H, et al. Initial treatment response is essential to improve survival in patients with hepatocellular carcinoma who underwent curative radiofrequency ablation therapy. Oncology. 2007;72:98–103. doi: 10.1159/000111714. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi S, Kudo M, Chung H, et al. Outcomes of nontransplant potentially curative therapy for early-stage hepatocellular carcinoma in Child-Pugh stage A cirrhosis is comparable with liver transplantation. Dig Dis. 2007;25:303–309. doi: 10.1159/000106909. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenday CJ, Dimick JB, Schulick RD, Choti MA. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. J Gastrointest Surg. 2007;11:1636–1646. doi: 10.1007/s11605-007-0315-8. discussion 1646. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz RE, Smith DD. Trends in local therapy for hepatocellular carcinoma and survival outcomes in the US population. Am J Surg. 2008;195:829–836. doi: 10.1016/j.amjsurg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Bach PB, Guadagnoli E, Schrag D, et al. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40:IV-19–25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 19.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 20.Farinati F, Sergio A, Baldan A, et al. Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study BMC Cancer. 2009;9:33. doi: 10.1186/1471-2407-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci Publ; 1980. pp. 5–338. [PubMed] [Google Scholar]
- 22.Curley SA. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol. 2008;15:11–13. doi: 10.1245/s10434-007-9668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strasberg SM, Ludbrook PA. Who oversees innovative practice? Is there a structure that meets the monitoring needs of new techniques? J Am Coll Surg. 2003;196:938–948. doi: 10.1016/S1072-7515(03)00112-1. [DOI] [PubMed] [Google Scholar]
- 24.MedTech. Analysis of RFA. 2003:1–2. [Google Scholar]
- 25.Johnson EW, Holck PS, Levy AE, Yeh MM, Yeung RS. The role of tumor ablation in bridging patients to liver transplantation. Arch Surg. 2004;139:825–829. doi: 10.1001/archsurg.139.8.825. discussion 829-830. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Sanjuan JC, Gonzalez F, Juanco C, et al. Radiological and pathological assessment of hepatocellular carcinoma response to radiofrequency. A study on removed liver after transplantation. World J Surg. 2008;32:1489–1494. doi: 10.1007/s00268-008-9559-z. [DOI] [PubMed] [Google Scholar]
- 27.Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 28.Yamanaka N, Tanaka T, Tanaka W, et al. Correlation of hepatitis virus serologic status with clinicopathologic features in patients undergoing hepatectomy for hepatocellular carcinoma. Cancer. 1997;79:1509–1515. doi: 10.1002/(sici)1097-0142(19970415)79:8<1509::aid-cncr10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Huang YH, Wu JC, Chen CH, et al. Comparison of recurrence after hepatic resection in patients with hepatitis B vs. hepatitis C-related small hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int. 2005;25:236–241. doi: 10.1111/j.1478-3231.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 30.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad A, Chen SL, Kavanagh MA, et al. Radiofrequency ablation of hepatic metastases from colorectal cancer: are newer generation probes better? Am Surg. 2006;72:875–879. [PubMed] [Google Scholar]
- 32.Hogan C, Lunney J, Gabel J, Lynn J. Medicare beneficiaries' costs of care in the last year of life. Health Aff (Millwood) 2001;20(4):188–195. doi: 10.1377/hlthaff.20.4.188. [DOI] [PubMed] [Google Scholar]
- 33.Farjah F, Flum DR, Varghese TK, Jr, et al. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg. 2009;87:995–1004. doi: 10.1016/j.athoracsur.2008.12.030. discussion 1005-1006. [DOI] [PubMed] [Google Scholar]
- 34.Abu-Hilal M, Primrose JN, Casaril A, et al. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg. 2008;12:1521–1526. doi: 10.1007/s11605-008-0553-4. [DOI] [PubMed] [Google Scholar]
- 35.Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molinari M, Helton S. Hepatic resection versus radiofrequency ablation for hepatocellular carcinoma in cirrhotic individuals not candidates for liver transplantation: a Markov model decision analysis. Am J Surg. 2009;198:396–406. doi: 10.1016/j.amjsurg.2009.01.016. [DOI] [PubMed] [Google Scholar]