Abstract
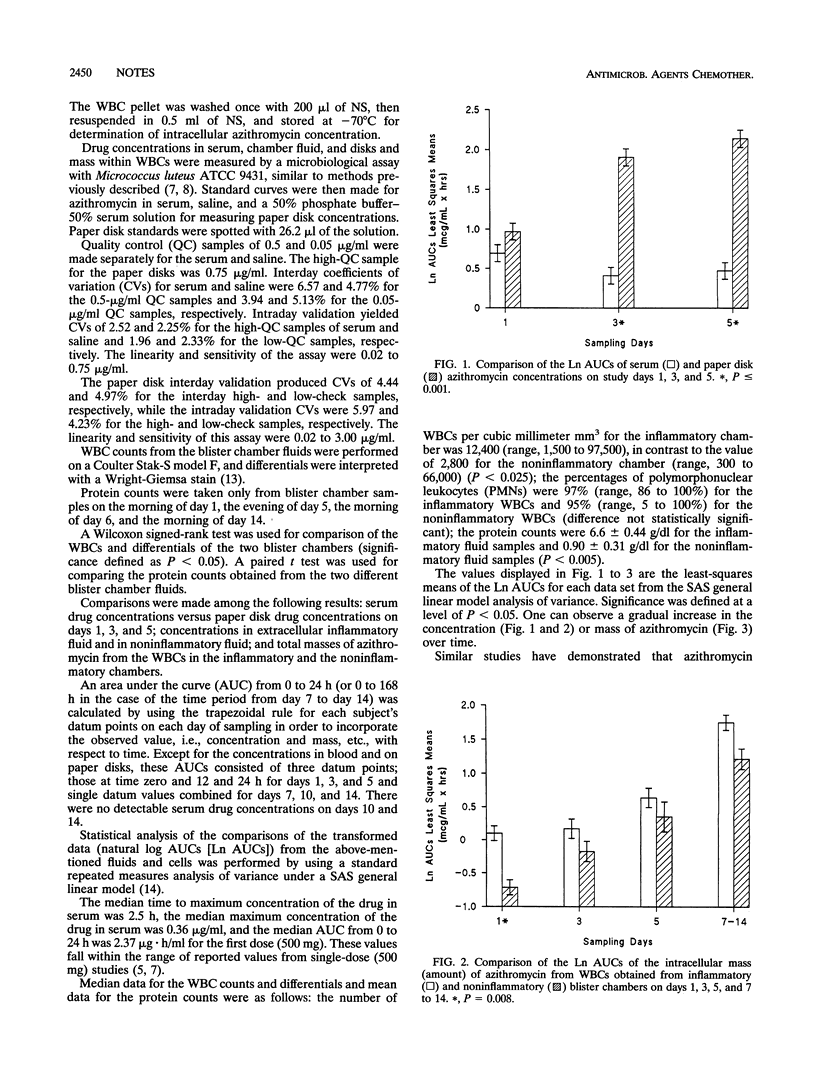
The penetration of azithromycin into the blister fluids of six volunteers was analyzed after a 5-day regimen (total of 1.5 g). Differences in drug concentrations in a paper disk and serum and in the mass of azithromycin from inflammatory blister chamber leukocytes and noninflammatory blister chamber leukocytes were significant (P < 0.05).
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez L. E., Inderlied C., Young L. S. Stimulation with cytokines enhances penetration of azithromycin into human macrophages. Antimicrob Agents Chemother. 1991 Dec;35(12):2625–2629. doi: 10.1128/aac.35.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet M., Van der Auwera P. In vitro and in vivo intraleukocytic accumulation of azithromycin (CP-62, 993) and its influence on ex vivo leukocyte chemiluminescence. Antimicrob Agents Chemother. 1992 Jun;36(6):1302–1309. doi: 10.1128/aac.36.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. A., Nye K., Andrews J. M., Wise R. The pharmacokinetics and inflammatory fluid penetration of orally administered azithromycin. J Antimicrob Chemother. 1990 Oct;26(4):533–538. doi: 10.1093/jac/26.4.533. [DOI] [PubMed] [Google Scholar]
- Dubertret L., Lebreton C., Touraine R. Neutrophil studies in psoriatics: in vivo migration, phagocytosis and bacterial killing. J Invest Dermatol. 1982 Aug;79(2):74–78. doi: 10.1111/1523-1747.ep12500028. [DOI] [PubMed] [Google Scholar]
- Foulds G., Shepard R. M., Johnson R. B. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R. P., Bright G. M., Isaacson R. E., Newborg M. F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989 Mar;33(3):277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R. P., Snider M. E. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob Agents Chemother. 1990 Jun;34(6):1056–1060. doi: 10.1128/aac.34.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellum K. B., Solberg C. O. Human leucocyte migration: studies with an improved skin chamber technique. Acta Pathol Microbiol Scand C. 1977 Dec;85C(6):413–423. doi: 10.1111/j.1699-0463.1977.tb03663.x. [DOI] [PubMed] [Google Scholar]
- Kuhns D. B., DeCarlo E., Hawk D. M., Gallin J. I. Dynamics of the cellular and humoral components of the inflammatory response elicited in skin blisters in humans. J Clin Invest. 1992 Jun;89(6):1734–1740. doi: 10.1172/JCI115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellado J. M., Christou N. V. Critically ill anergic patients demonstrate polymorphonuclear neutrophil activation in the intravascular compartment with decreased cell delivery to inflammatory focci. J Leukoc Biol. 1991 Dec;50(6):547–553. doi: 10.1002/jlb.50.6.547. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Seligmann B., Gallin J. I. Exudation primes human and guinea pig neutrophils for subsequent responsiveness to the chemotactic peptide N-formylmethionylleucylphenylalanine and increases complement component C3bi receptor expression. J Clin Invest. 1986 Mar;77(3):925–933. doi: 10.1172/JCI112391. [DOI] [PMC free article] [PubMed] [Google Scholar]


