Abstract
Objective: To assess the efficacy of ultrasound treatment for mild to moderate idiopathic carpal tunnel syndrome.
Design: Randomised, double blind, “sham” controlled trial with assessments at baseline, after 2 weeks’ and 7 weeks’ treatment, and at a follow up assessment 6 months later (8 months after baseline evaluation).
Setting: Outpatient clinic of a university department of physical medicine and rehabilitation in Vienna.
Subjects: 45 patients with mild to moderate bilateral carpal tunnel syndrome as verified by electroneurography.
Intervention: 20 sessions of ultrasound (active) treatment (1 MHz, 1.0 W/cm2, pulsed mode 1:4, 15 minutes per session) applied to the area over the carpal tunnel of one wrist, and indistinguishable sham ultrasound treatment applied to the other. The first 10 treatments were performed daily (5 sessions/week); 10 further treatments were twice weekly for 5 weeks.
Main outcome measures: Score of subjective symptom ratings assessed by visual analogue scale; electroneurographic measures (for example, motor distal latency and sensory antidromic nerve conduction velocity).
Results: Improvement was significantly more pronounced in actively treated than in sham treated wrists for both subjective symptoms (P<0.001, paired t test) and electroneurographic variables (motor distal latency P<0.001, paired t test; sensory antidromic nerve conduction velocity P<0.001, paired t test). Effects were sustained at 6 months’ follow up.
Conclusion: Results suggest there are satisfying short to medium term effects due to ultrasound treatment in patients with mild to moderate idiopathic carpal tunnel syndrome. Findings need to be confirmed, and ultrasound treatment will have to be compared with standard conservative and invasive treatment options.
Key messages
Chronic entrapment of the median nerve at the wrist (the carpal tunnel syndrome) is probably the most common peripheral nerve lesion
No satisfactory conservative treatment is available at present
Twenty sessions of ultrasound treatment show good short and medium term efficacy in patients with bilateral, mild to moderate forms of the carpal tunnel syndrome
Optimal treatment schedules of ultrasound treatment alone or in combination with other non-surgical treatments awaitelucidation
Introduction
The carpal tunnel syndrome, caused by compression of the median nerve at the wrist, is considered the most common entrapment neuropathy.1 Patients complain of paraesthesia (with or without numbness or pain) involving the fingers innervated by the median nerve, and a weakness of thumb abduction. Symptoms are worst at night and often wake the patient. Standard treatments include splints, local injection of corticosteroids, and surgical decompression. Benefit from non-surgical treatment, however, seems to be limited,2 and not all patients respond to surgery.3,4
Ultrasound treatment within an intensity range of 0.5-2.0 W/cm2 may have the potential to induce various biophysical effects within tissue.5,6 Experiments on the stimulation of nerve regeneration7 and on nerve conduction by ultrasound treatment8,9 and findings of an anti-inflammatory effect of such treatment10 support the concept that ultrasound treatment might facilitate recovery from nerve compression.7 However, few studies report a benefit of ultrasound treatment in the carpal tunnel syndrome under clinical conditions.11,12 We sought to investigate the clinical efficacy of pulsed ultrasound in the treatment of idiopathic carpal tunnel syndrome by means of a rigorous, controlled clinical trial.
Material and methods
Patients
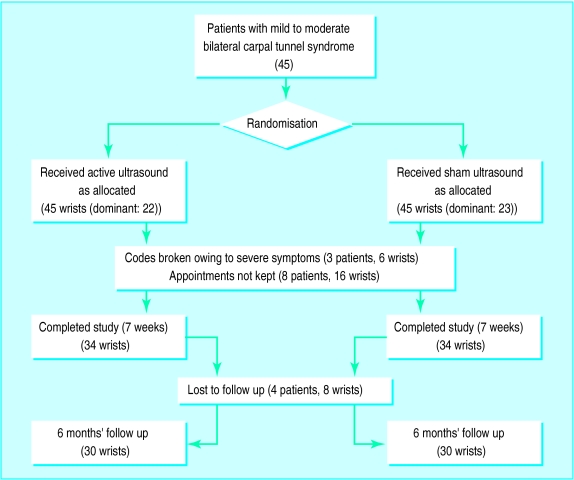
Over two years patients with clinically suspected carpal tunnel syndrome referred to the outpatient clinic of the department of physical medicine and rehabilitation of the University of Vienna were invited to take part in this randomised, double blind study of ultrasound treatment versus “sham” ultrasound treatment (fig 1).
Figure 1.
Trial profile
We diagnosed the carpal tunnel syndrome by using standard electrophysiological criteria.13,14 Criteria for inclusion in the study were bilateral, idiopathic carpal tunnel syndrome; mild to moderate pain lasting more than three months; and written informed consent. Patients were excluded if they had secondary entrapment neuropathies, systemic diseases with increased risk of the carpal tunnel syndrome, or electroneurographic and clinical signs for axonal degeneration of the median nerve; had gained surgical relief of the syndrome; had been treated with ultrasound for the syndrome; had a history of steroid injections into the carpal tunnel; or had required regular analgesic or anti-inflammatory drugs.
Intervention
Ultrasound treatment was administered as monotherapy for 15 minutes per session to the area over the carpal tunnel at a frequency of 1 MHz and an intensity of 1.0 W/cm2, pulsed mode 1:4, with a transducer of 5 cm2 (Sonodyn, Siemens) and with aquasonic gel as couplant. The machine was standardised initially, and the output was controlled regularly on a simple underwater radiation balance. An on/off key introduced into the transducer circuit allowed mock insonation to be given to a sham group without affecting the normal ultrasonic output when the key was turned to the “on” position. The first 10 treatments of a total of 20 ultrasound treatments were performed daily 5 times a week for 2 weeks, and the second 10 treatments twice a week for another 5 weeks.
For occasional pain relief, analgesics (usually tramadol) were allowed, but not non-steroidal or steroidal antirheumatics.
Outcome measures
Primary
Primary outcome measures for each wrist comprised (a) a sum score of subjective symptoms consisting of ratings of main complaints and sensory loss and (b) quantification of electroneurographic measurements. Main complaints were defined as complaints related to pain or paraesthesia, or to both, which the patient considered the most important ones at baseline. Severity of complaints at the clinical examination, and the worst complaints experienced within 3 days before the consultation were quantified by the study physician (GRE) by means of a coloured visual analogue scale, on which the patients could indicate their assessment along a distance of 10 cm, ranging from white (“no complaints at all”) to red (“the most intense complaints I can imagine”). Sensory loss (hypalgesia or hyperpathia, or both) was assessed by means of a sharp pin wheel and compared with “normal” sensation in the fifth digit. Quantification was again by coloured visual analogue scale (“no difference at all” to “greatest possible difference”).
All electroneurographic measurements were performed with a Viking II Nicolet (EMS, Madison, USA) electromyography device. Briefly, median motor nerve conduction was measured at the wrist and elbow with bipolar surface disc electrodes. Median distal motor latency was recorded with cathodes 6.5 cm apart. Antidromic sensory nerve action potentials were recorded from the wrist to the second digit, with ring electrodes placed around the proximal and distal interphalangeal joints. At least 15 sensory nerve action potentials were averaged, and antidromic sensory nerve conduction velocity was calculated as appropriate. The skin temperature of the forearm was kept constant at 32-33°C during all treatments.15
Secondary
Secondary outcome measures comprised (a) quantification of physical functioning and (b) the patients’ general improvement. Tests of physical functioning comprised dynamometric measurements (dynamometer by Preston, New York) of hand grip and finger pinch strength. The patients’ positioning was standardised, and the average force of three consecutive trials was calculated. The patients rated their overall change at the end of the treatment series on a five point ordinal scale (1=free of symptoms, 5=much worse).
Other factors
At each appointment the patients rated their main complaint without being reminded of the ratings they had made at previous appointments. Drugs taken for pain relief were registered and side effects of the ultrasound treatment reported.
Electrophysiological measurements and clinical examinations were performed before the first treatment session, after 10 sessions (week 2), and after the last session (week 7). A follow up was performed six months later (8 months after baseline evaluation). After the follow up examination the treatment code was broken, and patients were either discharged or offered an alternative treatment.
Sample size
A sample size calculation was performed based on the assumptions that the main outcome measurement (changes in sum score between baseline and end of treatment on visual analogue scale) is continuous in nature, fairly normally distributed, and that an additional improvement in the intervention side of 10 percentage points (standard deviation=15 percentage points) is considered clinically relevant. If the incidence of the carpal tunnel syndrome on one wrist could be considered completely independent from the incidence on the other wrist, 36 independent observations in each group would be necessary to detect that difference at the 5% level (α=0.05) with an 80% chance (β=0.2). Synchronicity of the carpal tunnel syndrome in both wrists happens in about one third of all cases, but to our knowledge no evidence exists that the natural course of symptoms goes strictly in parallel in these cases. In addition, systemic interventions that would probably affect both wrists, such as pain killers, were among the exclusion criteria. Taken together, 45 to 50 independent observations in each group might be a sensible estimate.
Statistics
Longitudinal changes between wrists were compared, with two tailed t tests for paired samples for fairly normally distributed variables (visual analogue scores and force measurements) and Wilcoxon tests for skewed data. Subsequently, a χ2 analysis was performed on dichotomised data of the mean score of subjective symptoms, with an overall improvement of more than 35 percentage points from baseline values as cut off point.
Assignment
A randomisation list was produced with a random number generator of a popular spreadsheet program (Lotus Symphony). After the eligible patients had been enrolled, an ultrasound therapist not involved in the treatment allocated the dominant wrist of each consecutive patient to ultrasound or sham treatment (the patient’s other wrist received the other treatment) by means of sequentially numbered sealed opaque envelopes containing the group allocation (active or sham). This therapist was the only person aware of treatment allocation during the trial.
Blinding
The patients, GRE, and the therapists who delivered the ultrasound treatment were all unaware of the treatment allocation. Only the therapist who was in charge of group allocation switched the ultrasonic generator to the respective modes before each treatment session (see above). This procedure allowed blinding of both the patients and the therapists delivering the treatment. Intensity of ultrasound treatment was below sensitivity threshold.
Results
Baseline evaluation
Forty five patients with bilateral carpal tunnel syndrome (90 wrists) fulfilled all inclusion criteria; 11 (24%) of these patients discontinued treatment after randomisation (8 patients early after randomisation because of non-compliance in keeping appointments, and 3 patients because of excessive pain requiring additional therapeutic measures). Thus 34 patients—that is, 34 actively treated and 34 sham treated wrists—completed the study. Their characteristics did not differ from the original 45 patients in the study. Thirty of them (67% of the initial 45 patients) completed a follow up at 6 months.
The wrists were similar in terms of the duration of current episodes of main complaints regardless of randomisation group (table 1). There were slight group imbalances at baseline. Most complaints in the actively treated group were significantly more severe (P=0.05, Wilcoxon test) when rated on the visual analogue scale. Baseline differences were also present in the mean score of physical functioning and strength of hand grip, whereas finger pinch was comparable.
Table 1.
Demographic data and baseline characteristics of patients who completed study, according to which group (active or sham ultrasound) their dominant wrist was randomised to. Values are means (SD) unless stated otherwise
| Variable | Treatment
|
|
|---|---|---|
| Active | Sham | |
| No of subjects who completed the study | 34 | |
| Age (years) | 51 (15) | |
| Body mass index (kg/m2) | 25.9 (5.1) | |
| No of wrists with complaints | 29 | 27 |
| No of wrists with sensory loss | 25 | 19 |
| Duration of current episode of main complaints (months) | 7.8 (6.7) | 7.2 (6.5) |
| Subjective symptoms: | ||
| Score of all subjective symptoms | 4.1 (2.1) | 3.3 (1.5) |
| Main complaint (cm)* | 3.3 (2.8) | 2.0 (1.9) |
| Worst complaint (cm)* | 6.5 (2.6) | 5.8 (2.8) |
| Sensory loss (cm)* | 2.4 (2.4) | 2.0 (2.4) |
| Physical functioning: | ||
| Score of physical functioning | 21.3 (11.9) | 25.5 (11.3) |
| Handgrip strength (kg) | 15.8 (10.9) | 19.8 (10.0) |
| Finger pinch (×0.2 kg) | 5.5 (1.8) | 5.8 (1.8) |
| Electroneurography: | ||
| Motor distal latency (ms) | 5.2 (1.0) | 5.2 (1.2) |
| Peak to peak amplitude | 14.5 (3.4) | 14.6 (3.7) |
| Antidromic sensory nerve conduction velocity wrist-digit II (m/s) | 40.0 (7.2) | 42.1 (7.2) |
Distance along a coloured visual analogue scale, on which the patients indicated their assessment (white, 0=minimum complaint; red, 10=maximum complaint). See methods section for further details.
Other subjective symptoms—for example, scores of main complaints, sensory loss, and the mean score of all subjective symptoms—were similar at baseline. Electroneurography, motor distal latency, peak to peak amplitude, and antidromic sensory nerve conduction velocity did not differ significantly between wrists.
Effect of treatment
Subjective symptoms
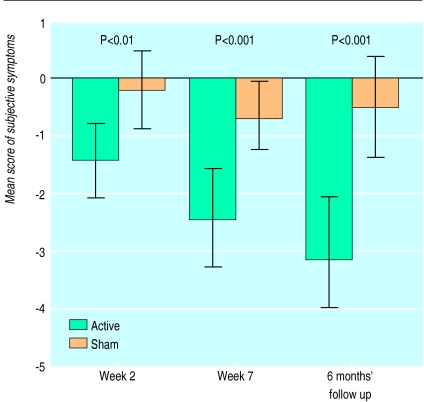
Table 2 and figure 2 show longitudinal changes of subjective symptoms. Improvement in the mean score of all ratings of subjective symptoms was significantly more pronounced in the actively treated wrists at week 2 (P<0.008), at the end of treatment (P<0.0001), and at the 6 month follow up (P<0.0001).
Table 2.
Mean change (95% confidence interval) from baseline values for outcome measures at week 2, at end of treatment (week 7), and 6 months later
| Outcome measure | Week 2 | End of therapy | 6 months’ follow up |
|---|---|---|---|
| Subjective symptoms | |||
| Mean change in main complaints (cm)*: | |||
| Sham | 0.05 (−0.48 to 0.58) | −0.17 (−0.92 to 0.57) | −0.08 (−1.06 to 0.90) |
| Active | −1.05 (−1.91 to −0.19) | −2.14 (−3.15 to −1.12) | −2.76 (−3.79 to −1.73) |
| Paired difference (t test) | 1.1 (0.23 to 1.98) | 1.96 (0.91 to 3.01) | 2.26 (1.49 to 3.88) |
| P value (2 tailed) | 0.015 | 0.001 | <0.0005 |
| Mean change in worst complaints (cm)*: | |||
| Sham | −0.90 (−2.24 to 0.43) | −1.56 (−2.58 to −0.54) | −0.95 (−2.43 to 0.54) |
| Active | −2.20 (−3.16 to −1.25) | −3.91 (−5.07 to −2.75) | −4.78 (−5.85 to −3.70) |
| Paired difference (t test) | 1.30 (−0.38 to 2.98) | 2.35 (0.92 to 3.78) | 3.83 (2.32 to 5.34) |
| P value (2 tailed) | 0.125 | 0.002 | <0.0005 |
| Mean change in sensory loss (cm)*: | |||
| Sham | 0.42 (−0.29 to 1.13) | −0.07 (−0.86 to 0.72) | −0.08 (−0.91 to 0.76) |
| Active | −0.82 (−1.69 to 0.05) | −1.14 (−1.99 to −0.29) | −1.60 (−2.55 to −0.65) |
| Paired difference (t test) | 1.24 (0.33 to 2.15) | 1.07 (0.31 to 1.83) | 1.53 (0.85 to 2.20) |
| P value (2 tailed) | 0.009 | 0.007 | <0.0005 |
| Physical functioning | |||
| Mean change in hand grip strength (kg): | |||
| Sham | −0.61 (−1.88 to 0.66) | −0.09 (−2.04 to 1.85) | −1.99 (−4.08 to 0.09) |
| Active | 0.71 (−1.35 to 2.77) | 3.87 (2.06 to 5.67) | 5.44 (2.91 to 7.96) |
| Paired difference (t test) | −1.32 (0.35 to −2.99) | −3.96 (−2.01 to −5.90) | −7.43 (−5.22 to −9.64) |
| P value (2 tailed) | 0.118 | <0.0005 | <0.0005 |
| Mean change in pinch strength (kg): | |||
| Sham | −0.20 (−0.25 to −0.15) | 0.06 (−0.26 to 0.38) | −0.22 (−0.38 to −0.06) |
| Active | −0.01 (−0.13 to 0.12) | 0.33 (0.17 to 0.50) | 0.49 (0.28 to 0.70) |
| Paired difference (t test) | −0.19 (0.43 to −0.81) | −0.27 (0.37 to −0.91) | −0.71 (−0.15 to −1.27) |
| P value (2 tailed) | 0.537 | 0.392 | 0.014 |
| Electroneurography | |||
| Mean change in motor distal latency (ms): | |||
| Sham | 0.04 (−0.08 to 0.15) | 0.06 (−0.08 to 0.21) | 0.04 (−0.10 to 0.19) |
| Active | −0.23 (−0.37 to −0.10) | −0.55 (−0.71 to −0.39) | −0.31 (−0.45 to −0.18) |
| Paired difference (t test) | 0.27 (0.11 to 0.42) | 0.61 (0.43 to 0.79) | 0.36 (0.18 to 0.54) |
| P value (2 tailed) | 0.001 | <0.0005 | <0.0005 |
| Mean change in antidromic sensory nerve conduction velocity (m/s): | |||
| Sham | −0.84 (−1.07 to −0.62) | −0.89 (−1.11 to −0.66) | −0.27 (−0.51 to −0.03) |
| Active | 4.50 (4.34 to 4.66) | 7.35 (6.98 to 7.71) | 2.69 (2.39 to 2.99) |
| Paired difference (t test) | −5.34 (−3.58 to −7.11) | −8.23 (−6.22 to −10.24) | −2.96 (−1.66 to −4.66) |
| P value (2 tailed) | <0.0005 | p<0.0005 | 0.001 |
Distance along a coloured visual analogue scale, on which the patients indicated their assessment (white, 0=minimum complaint; red, 10=maximum complaint). See methods section for further details.
Figure 2.
Mean change (and 95% confidence intervals) from baseline score for all subjective symptoms (active versus sham treatment) at week 2, end of treatment, and 6 months’ follow up (paired t test)
Satisfactory improvement or complete remission of symptoms was observed in 68% (23/34) of the wrists receiving active treatment versus 38% (13/34) of those receiving sham treatment (P<0.001; relative risk reduction 48%) at the end of the treatment series, and in 74% (22/30) versus 20% (6/30) (P<0.001; 67%) at 6 months’ follow up.
Electroneurography
The results of electroneurography are shown in table 2. Motor distal latency decreased with active treatment and remained unchanged with sham treatment both at the end of treatment and at 6 months’ follow up (end of treatment: active −0.55 ms (95% confidence interval −0.71 to −0.39) and sham 0.06 ms (−0.08 to 0.21); at follow up: −0.31 ms (−0.45 to −0.18) and 0.04 ms (−0.10 to 0.19); P<0.001 for both time periods).
Similar significant changes in the velocity of sensory nerve conduction were observed at the end of treatment and at 6 months’ follow up with active treatment, whereas velocity remained unchanged with sham treatment (P<0.0001 between groups).
Physical functioning
Hand grip and finger pinch strength had improved significantly with active treatment at the end of treatment and at 6 months’ follow up (table 2).
Other measurements
Patients’ ratings of overall improvement at the end of treatment significantly favoured active over sham treatment (Mann-Whitney U test P=0.002). Good or excellent treatment results were stated by 76% (26/34) of the patients for actively treated wrists versus 32% (11/34) for sham treated wrists.
At 6 months’ follow up 28 patients showed an unsatisfactory outcome (9 actively treated and 19 sham treated wrists) and were offered further treatment. Subsequently 13 patients were offered ultrasound treatment and splints for their sham treated wrists, and 10 wrists (3 sham treated) were injected with steroids. Surgical relief of the carpal tunnel syndrome was planned for 5 patients (3 sham treated wrists).
Average consumption of analgesics during treatment and follow up phase was low: 8 out of the 34 patients occasionally took analgesics, and three patients were off work. No side effects due to ultrasound treatment were reported.
Discussion
An increase in pressure in the carpal tunnel is usually caused by non-specific flexor tenosynovitis.16 Chronic focal compression of a nerve trunk can cause focal demyelination by mechanical stress deforming the myelin lamellae. Ischaemia also plays a pathogenic role in the carpal tunnel syndrome. It could account for intermittent paraesthesia that occurs at night or with wrist flexion.2 The carpal tunnel syndrome is often observed bilaterally. Symptoms are usually markedly worse on one (mostly the dominant) side.
Conservative treatment approaches seem to offer clear advantages over surgical treatment of the carpal tunnel syndrome. Recent studies have confirmed short term effects of steroid injections into the carpal tunnel, with modest or complete pain relief in up to 92% of the patients, although long term recurrence rates seem variable.17–19 Potential adverse effects to nerves and tendons with repeated injections have limited the value of this treatment.20,21 Palmar wrist splints worn at night seem suitable only when symptoms are mainly nocturnal,22 and ergonomic strategies have not yet been evaluated.
The findings of the present study confirm preliminary data that ultrasound treatment may facilitate recovery from the carpal tunnel syndrome.11,12 Given the favourable response rate of 68% of patients at the end of treatment, ultrasound treatment may be similar in effectiveness to steroid injections or wrist splinting; improvements persisting for at least 6 months in most patients might even suggest the potential superiority of ultrasound treatment.
Serial ratings by patients of overall improvement suggest that ultrasound treatment would be best administered every day. Frequent treatment, however, is time consuming (as seen by the relatively high drop out rate in our study), but ultrasound treatment could be performed by compliant patients at home.
According to the pathophysiology of the carpal tunnel syndrome, ultrasonography might elicit anti-inflammatory and tissue stimulating effects, as already shown experimentally23 and in recent clinical trials.10,24
Conclusion
Our trial suggests that ultrasound treatment has good short term effectiveness and even yields satisfying medium term effects in patients with mild to moderate idiopathic carpal tunnel syndrome. Further research is required to confirm independently these findings, to evaluate optimal treatment schedules with this method, and to investigate whether ultrasound treatment or one of the non-surgical treatments alone or in combination is superior, or whether early decompression may provide better long term results with fewer eventual neurological deficits.
Acknowledgments
We thank the therapists Helene Göttel, Beate Grabner, Renate Humenberger, Elfriede Jöbstel, Brigitte Kalleitner, Ilse Kirchweger, Constantin Ofner, and Renate Sedlacek for their help in the study, and Professor Brigitte Stanek for helpful review of the manuscript.
Footnotes
Funding: None.
Conflict of interest: None.
References
- 1.Diagnosis of the carpal tunnel syndrome [editorial]. Lancet 1985;i:854-8. [PubMed]
- 2.Dawson DM. Entrapment neuropathies of the upper extremities. N Engl J Med. 1993;329:2013–2018. doi: 10.1056/NEJM199312303292707. [DOI] [PubMed] [Google Scholar]
- 3.Cotton P. Symptoms may return after carpal tunnel surgery. JAMA. 1991;265:1921–1922. doi: 10.1001/jama.265.15.1922b. [DOI] [PubMed] [Google Scholar]
- 4.O’Malley MJ, Evanoff M, Terrono AL, Millender LH. Factors that determine reexploration treatment of carpal tunnel syndrome. J Hand Surg (Am) 1992;17:638–641. doi: 10.1016/0363-5023(92)90307-b. [DOI] [PubMed] [Google Scholar]
- 5.Barnett SB, Ter Haar GR, Ziskin MC, Nyborg WL, Maeda K, Bang J. Current status of research on biophysical effects of ultrasound. Ultrasound Med Biol. 1994;20:205–218. doi: 10.1016/0301-5629(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 6.Dyson M. Mechanisms involved in therapeutic ultrasound. Physiotherapy. 1987;73:116–120. [Google Scholar]
- 7.Hong CZ, Liu HH, Yu J. Ultrasound thermotherapy effect on the recovery of nerve conduction in experimental compression neuropathy. Arch Phys Med Rehabil. 1988;69:410–414. [PubMed] [Google Scholar]
- 8.Currier DP, Greathouse D, Swift T. Sensory nerve conduction: effect of ultrasound. Arch Phys Med Rehabil. 1978;59:181–185. [PubMed] [Google Scholar]
- 9.Kramer JF. Effect of therapeutic ultrasound intensity on subcutaneous tissue temperature and ulnar nerve conduction velocity. Am J Phys Med. 1989;64:1–9. [PubMed] [Google Scholar]
- 10.El Hag M, Coghlan K, Christmas P, Harvey W, Harris M. The anti-inflammatory effects of dexamethasone and therapeutic ultrasound in oral surgery. Br J Oral Maxillofac Surg. 1985;23:17–23. doi: 10.1016/0266-4356(85)90074-9. [DOI] [PubMed] [Google Scholar]
- 11.Edel H, Bergmann P. Studies on the effect of ultrasonics in different dosage on the neural conduction velocity in man. Arch Phys Ther Leipz. 1970;22:255–259. [PubMed] [Google Scholar]
- 12.Mayr H, Ammer K. Impulsgalvanisation und Ultraschall zur Therapie des Carpaltunnelsyndromes. Österr Z Phys Med. 1994;4:95–99. [Google Scholar]
- 13.Stevens JC. AAEE minimonograph #26: the electrodiagnosis of the carpal tunnel syndrome. Muscle Nerve. 1987;10:99–113. doi: 10.1002/mus.880100202. [DOI] [PubMed] [Google Scholar]
- 14.Ludin HP. Praktische Elektromyographie. 4th ed. Stuttgart: Enke; 1993. [Google Scholar]
- 15.Baysal AI, Chang CW, Oh SJ. Temperature effects on nerve conduction studies in patients with carpal tunnel syndrome. Acta Neurol Scand. 1993;88:213–216. doi: 10.1111/j.1600-0404.1993.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 16.Gelberman RH, Hergenroeder PT, Hargens AR, Lundborg GN, Akeson WH. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg (Am) 1981;63:380–383. [PubMed] [Google Scholar]
- 17.Gelberman RH, Aronson D, Weisman MH. Carpal tunnel result of a prospective trial of steroid injection and splinting. J Bone Joint Surg (Am) 1980;62:1181–1184. [PubMed] [Google Scholar]
- 18.Giannini F, Passero S, Cioni R, Paradiso C, Battistini N, Giordano N, et al. Electrophysiologic evaluation of local steroid injection in carpal tunnel syndrome. Arch Phys Med Rehabil. 1991;72:738–742. [PubMed] [Google Scholar]
- 19.Girlanda P, Dattola R, Venuto C, Mangiapane R, Nicolosi C, Messina C. Local steroid treatment in idiopathic carpal tunnel syndrome: short and long term efficacy. J Neurol. 1993;240:187–190. doi: 10.1007/BF00857526. [DOI] [PubMed] [Google Scholar]
- 20.McConnell JR, Bush DC. Intraneural steroid injection as a complication in the management of carpal tunnel syndrome. Clin Orthop. 1990;250:181–184. [PubMed] [Google Scholar]
- 21.Burton RI, Littler J. Entrapment syndromes of the retinacular or restraining systems of the hand: carpal tunnel syndrome. Curr Probl Surg. 1975;12(suppl 7):17. [Google Scholar]
- 22.Burke DT, McHale Burke M, Stewart GW, Cambre A. Splinting in carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241–1244. doi: 10.1016/0003-9993(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 23.Byl NN, McKenzie AL, West JM, Whitney JAD, Hunt T, Scheuenstuhl H. Low-dose ultrasound effects on wound healing. A controlled study with Yucatan pigs. Arch Phys Med Rehabil. 1992;3:656–664. [PubMed] [Google Scholar]
- 24.Binder A, Hodge G, Greenwood AM, Hazelman BL, Page Thomas DP. Is therapeutic ultrasound effective in treating soft tissue lesions? BMJ. 1985;290:512–514. doi: 10.1136/bmj.290.6467.512. [DOI] [PMC free article] [PubMed] [Google Scholar]