Abstract
Background
Allopregnanolone (ALLO) is a physiologically-relevant neurosteroid modulator of GABAA receptors, and it exhibits a psychopharmacological profile that closely resembles the post-ingestive effects of ethanol. The 5α-reductase inhibitor finasteride (FIN), which inhibits biosynthesis of ALLO and structurally-related neurosteroids, was previously demonstrated to reduce the maintenance of limited-access ethanol consumption. The primary aim of the current work was to determine whether FIN would reduce the acquisition of drinking in ethanol-naïve mice.
Methods
Male C57BL/6J (B6) mice were acclimated to a reverse light/dark schedule, and were provided ad libitum access to chow and water. Following habituation to vehicle injections (VEH; 20% w/v β-cyclodextrin; i.p.) administered 22-hr prior to drinking sessions with water only, mice were divided into three treatment groups: vehicle control (VEH), 50 mg/kg FIN (FIN-50), and 100 mg/kg FIN (FIN-100). Twenty-two hrs after the first treatment, mice were permitted the inaugural 2-hr limited access to a 10% v/v ethanol solution (10E) and water. The acquisition of 10E consumption and underlying drinking patterns were assessed during FIN treatment (7 days) and subsequent FIN withdrawal (13 days) phases.
Results
FIN dose-dependently blocked the acquisition of 10E drinking and prevented the development of ethanol preference, thereby suggesting that GABAergic neurosteroids may be important in the establishment of stable drinking patterns. FIN-elicited reductions in 10E intake were primarily attributable to selective and marked reductions in bout frequency, as no changes were observed in bout size, duration, or lick rates following FIN treatment. FIN-treated mice continued to exhibit attenuated ethanol consumption after two weeks post-treatment, despite a full recovery in brain ALLO levels. A second study confirmed the rightward and downward shift in the acquisition of ethanol intake following 7 daily FIN injections. While there were no significant group differences in brain ALLO levels following the 7th day of ethanol drinking, ALLO levels were decreased by 28% in the FIN-50 group.
Conclusions
Although the exact mechanism is unclear, FIN and other pharmacological interventions that modulate the GABAergic system may prove useful in curbing ethanol intake acquisition in at-risk individuals.
Keywords: alcohol, drinking patterns, neurosteroid metabolism, finasteride, allopregnanolone, GABAA receptors
INTRODUCTION
Acquisition of ethanol intake is commonly discussed in terms of an ‘adaptive’ process. This adaptation purportedly involves a host of chemosensory cues (taste, smell, etc.) that are predictive of ethanol’s psychopharmacological (i.e., positive reinforcing) effects that accompany the gradual and incremental elevation in consumption levels to a point of pharmacological relevance (Colombo et al. 2002; Di Chiara, 2002; Samson & Hodge, 1996). Furthermore, taste properties of the ethanol solution made available are clearly important, and an alteration in taste sensitivity to ethanol following recurring exposure likely contributes to the behavioral processes associated with its acquisition (see Davidson & Amit, 1997). Regardless of the mechanism(s) underlying the adaptation that occurs during acquisition, a drug intervention that manipulates either the reinforcing properties of or the taste sensitivity to ethanol would predictably influence the pattern of ethanol drinking behavior. Consistent with this notion, additional investigation of neurobiological mechanisms underlying the acquisition of ethanol consumption will likely provide valuable insights to aid in circumventing the initiation of excessive drinking.
Recent research efforts have demonstrated that pharmacological manipulation of various neurotransmitter receptors can impact the normal time course of intake acquisition in a rat model of ethanol drinking. Cannabinoid CB1 receptor antagonism with SR141716 (Serra et al., 2001) and SR147778 (Gessa et al., 2005), opioid receptor antagonism with naltrexone (Davidson & Amit, 1997), and GABAB receptor activation with agonists baclofen and CGP44532 (Colombo et al., 2002) each significantly blunted the acquisition of ethanol consumption. However, the GABAB receptor influence on acquisition has led to disparate findings, as baclofen was reported in a separate study to augment the acquisition of voluntary ethanol intake (Smith et al., 1992). In contrast, GABAA receptor stimulation with the agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) culminated in an enhanced acquisition of ethanol intake when compared to vehicle-treated rats (Boyle et al., 1992; Smith et al., 1992). Consistent with the idea that combination therapy may be more effective at decreasing ethanol consumption, treatment with baclofen and naltrexone synergistically suppressed the acquisition of ethanol drinking when administered at doses that individually exhibited no efficacy (Colombo et al., 2005). These findings collectively advocate the involvement of multiple neurotransmitter systems in the regulation of ethanol consumption during the acquisition phase of drinking.
In the current study, neurobiological mechanisms underlying the acquisition of ethanol consumption were further examined by assessing the regulatory role of 5α-reduced neurosteroids (allopregnanolone, tetrahydrodeoxycorticosterone, etc.), which exhibit well-documented activity as positive modulators of GABAA receptors in vivo (Belelli & Lambert, 2005; Gasior et al., 1999). Previous work demonstrated that orally consumed ethanol elicits an elevation in brain allopregnanolone (ALLO) concentrations in male mice (Finn et al., 2004b) and plasma ALLO levels in adolescent male humans (Torres & Ortega, 2004). Furthermore, exogenous ALLO application stimulated limited-access ethanol drinking in male mice (Ford et al., 2005b). In contrast, administration of the 5α-reductase inhibitor finasteride (FIN), which decreases endogenous levels of 5α-reduced GABAergic steroids (including ALLO), significantly altered ethanol drinking and the subjective state commensurate to consumption in laboratory and clinical settings, respectively. Specifically, sub-chronic FIN treatment (7 days) significantly attenuated limited access ethanol preference drinking in male C57BL/6J (B6) mice (Ford et al., 2005a), whereas acute administration of FIN diminished multiple subjective measures following ethanol consumption in male social drinkers (Pierucci-Lagha et al., 2005). Based on these earlier observations with FIN and the accumulating evidence of GABAergic involvement in the adaptive processes underlying acquisition of ethanol consumption, it was hypothesized that inhibition of 5α-reduced neurosteroid biosynthesis would hinder the acquisition of ethanol intake, presumably by attenuating positive modulatory tone at GABAA receptors. Thus, the purpose of the present study was to examine the effect of sub-chronic (7-day) FIN administration on the acquisition of limited access ethanol preference drinking in male B6 mice.
MATERIALS AND METHODS
Animals
Six-week old male C57BL/6J (B6) mice were obtained from The Jackson Laboratory West (Davis, CA). Upon arriving at the Veterinary Medical Unit at the VA Medical Center, each mouse was individually housed and acclimated to a reverse light/dark schedule (12hr/12hr; lights off at 0800 hrs) for 2 weeks. All mice were provided ad libitum access to rodent chow and tap water in the home cage (i.e., lickometer chambers). Mice were weighed and handled daily throughout the last week of acclimation and during all experimental phases of the study. The local Institutional Animal Care and Use Committee approved all procedures in accordance with the guidelines described in the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council of the National Academies, 2003).
Apparatus
Custom-built lickometer chambers and drinking tubes were implemented as previously described (Ford et al., 2005a). Open electrical circuits wired between each metal sipper tube and the wire floors of the chambers were interfaced to an IBM compatible computer via a lickometer device (MED Associates, Inc., St. Albans, VT). This detection system independently recorded cumulative sipper contacts (i.e., licks) for each drinking tube, with data subsequently compiled with MED-PC IV software (MED Associates, Inc.). Cumulative licks were recorded in the 1st cohort of mice that were tested.
Preference Drinking Procedure, Habituation to Injection, and Drug Treatment
Prior to each drinking session, mice were removed from their lickometer chamber, weighed, and placed immediately back into their respective chamber. Mice were then provided 2-hr access (1000–1200 hrs; starting 2-hr after lights out) to two sipper tubes. During the initial 2 access periods, water baseline sessions were conducted in which both sippers contained tap water. With all subsequent sessions, one sipper tube contained a 10% v/v ethanol solution (10E) and a second sipper tube was filled with tap water. Consumed volumes were measured to the nearest 0.05 ml from the graduated sipper tubes. The 10E-containing sipper tubes were counterbalanced between the left and right sides across lickometer chambers to control for side preferences. Home cage water consumption between consecutive limited-access sessions was monitored by weighing fluid displacement during the 22-hr periods from 50-ml bottles similarly outfitted with metal sippers.
Since the aim of this study was to alter the acquisition of ethanol intake, commencement of finasteride (FIN) treatment occurred when the mice were still ethanol-naïve. To accomplish this, the 1st cohort of mice (n = 24) were habituated to vehicle injections (VEH; 20% w/v 2-hydroxypropyl-β-cyclodextrin; 0.01 ml/g; i.p.; Cerestar USA, Inc., Hammond, IN) administered 22-hrs prior to each water baseline session (see above). At the conclusion of the second baseline session, mice were assigned to one of three treatment groups that were balanced for water intake (summed total licks from both sippers): VEH (n = 8), 50 mg/kg FIN (FIN-50; n = 8), and 100 mg/kg FIN (FIN-100; n = 8). Similar to injection habituation, FIN pretreatments were given 22-hrs prior to the 2-hrs of limited-access to 10E, but were repeated once daily for a total of 7 days (sessions F1-F7). In order to assess subsequent FIN withdrawal effects, all mice received VEH pretreatments prior to the next 7 drinking sessions (sessions W1-W7). Because both FIN-treated groups exhibited a dampened acquisition of ethanol intake at 7-days post-treatment (see results below), an additional 6 drinking sessions (W8-W13) were conducted, during which no injections were administered. FIN doses and pretreatment time were selected based on previous studies that examined the effect of FIN on established ethanol drinking (Ford et al., 2005a) and a time course of brain ALLO concentrations that result from acute FIN treatment (Finn et al., 2004a). Mice were euthanized one day following the final limited-access drinking period (i.e., session W13) between 1000–1100 hrs to match the daily drinking session time. Mice were not permitted 10E access on this day.
An independent 2nd cohort of mice (n = 30; 10 mice per dose group) were tested to confirm the effect of 7 daily FIN injections on the acquisition of 10E intake and to measure brain ALLO levels at the conclusion of the 7th ethanol drinking session. Mice were treated as described above, with the exception that cumulative licks were not recorded. Mice were euthanized immediately following the 7th ethanol drinking session (session F7).
Blood Ethanol Concentration (BEC)
In the 1st cohort of mice, BECs were assayed twice; at the conclusion of the drinking sessions corresponding to the final day of FIN treatment (session F7) and early FIN withdrawal (session W7) phases, respectively. A 20-µl blood sample was acquired from the intra-orbital sinus. The blood samples were processed and assayed by gas chromatography as previously described (Ford et al., 2005a). In the 2nd cohort of mice, BECs were assayed at the conclusion of the 7th ethanol drinking session (session F7). A 20-µl blood sample, taken from the orbital sinus, was assayed by gas chromatography as described in Finn et al. (2007).
Brain Allopregnanolone (ALLO) Determination
Whole brains were rapidly removed, immediately frozen on dry ice, and stored at −80°C. ALLO was extracted from brain samples and subsequently measured by radioimmunoassay as previously published in detail (Finn et al., 2004b). Briefly, [3H]ALLO (New England Nuclear; Boston, MA) was added to each sample prior to three rounds of extraction with 10% (v/v) ethyl acetate in heptane in order to determine extraction efficiency. Resultant supernatants were applied to solid phase silica columns (VWR, Seattle, WA), eluted with 25% (v/v) acetone in pentane, dried under nitrogen gas, and reconstituted with 15% (v/v) isopropanol in assay buffer. The radioimmunoassay was performed with [3H]ALLO and a polyclonal antiserum that exhibits minimal cross-reactivity to other steroids (CoCensys; Irvine, CA). Counts per minute were normalized and fit to a least-square regression equation generated by a log-logit transformation of the standards (0.156–20 ng ALLO). Mass of the samples was calculated by interpolation of the standards and correction for extraction efficiency. The intra-assay coefficient of variation averaged 5.6%.
Drugs
The ethanol solution (10% v/v; Pharmco Products, Brookfield, CT) was prepared by dilution of a 200 proof stock in tap water. Finasteride [1,(5α)-androstan-4-aza-3-one-17β-(N-tert-butyl-carboxamide)] was purchased from Steraloids Inc (Newport, RI) and was solubilized in 20% w/v 2-hydroxypropyl-β-cyclodextrin at a stock concentration of 5 or 10 mg/ml, which permitted an injection volume of 0.01 ml/g body weight.
Statistical Analysis
Ethanol dose (g/kg/2-hrs) was calculated from the 10E volume (ml) displaced and body weight (g) recorded immediately prior to the session start. Ethanol preference ratio was derived from the total 10E licks divided by the summed total licks of 10E and water. A custom data analysis program was written (for R Project for Statistical Computing software; www.r-project.org) to determine multiple drinking pattern endpoints from the cumulative 10E lick records: bout frequency, bout size (licks), bout duration (min), lick rates (licks/min), and latency to first bout (min). Based upon our previous work in mice (Ford et al., 2005a, 2005b, 2007a, 2007b), an ethanol bout was defined as a minimum of 20 licks with no more than a 60-sec pause between successive licks. The reported lick rates were derived from the average rate of all bouts expressed, and did not encompass time elapsed between bouts. Because the volume of water intake during the limited access sessions was at or below the level of detection (≤ 0.05 ml), meaningful analyses of bout dynamics and consumption patterns for water were not possible.
All statistical analyses were performed with the SigmaStat version 2.03 software (SPSS Inc., Chicago, IL), and temporal distribution analysis of 10E licks was facilitated by SoftCR for Windows (MED Associates, Inc.). Consumption and bout pattern measures were assessed either as daily values (two-way repeated measures ANOVA; factors = FIN dose, session) or as treatment phase averages (two-way repeated measures ANOVA; factor = FIN dose, phase) for each experimental phase [i.e., FIN treatment (sessions F1-F7), early FIN withdrawal (sessions W1-W7), and late FIN withdrawal (sessions W8-W13)]. Based on earlier evidence that the cessation of FIN treatment resulted in a rapid reversal of effects on drinking maintenance (Ford et al., 2005a), our a priori hypothesis was that a dose-related effect of FIN on intake acquisition would only be apparent during the FIN treatment phase. Therefore, separate one-way ANOVAs were subsequently run on the phase averages as a factor of FIN dose for each treatment phase even in the absence of a significant factorial interaction. Two-way repeated measures ANOVA were separately conducted to identify possible factorial interactions [dose × session interval (20-min bins)] for the temporal distribution of 10E licks during the FIN treatment phase and the FIN withdrawal phase. A subsequent analysis of Simple Main effects for FIN dose within each 20-min interval was also conducted. When appropriate, pair-wise differences were determined by the Fisher’s least significant difference multiple comparisons procedure. For all statistical analyses, significance was set at P ≤ 0.05.
RESULTS
In the 1st cohort of mice, the total licks during the water baseline sessions for the VEH, FIN-50, and FIN-100 treatment groups were 204 ± 45, 206 ± 47, and 209 ± 53, respectively. One VEH-treated mouse was removed from the study because it was mistakenly administered 50 mg/kg FIN prior to the second 10E access period (i.e., session F2). In the 2nd cohort of mice, total fluid intake during the water baseline sessions were 0.77 ± 0.06 ml (VEH), 0.77 ± 0.05 ml (FIN-50), and 0.78 ±0.05 ml (FIN-100). One mouse in the FIN-50 group was removed from the study because it was mistakenly administered the 100 mg/kg FIN dose.
General Intake Measures
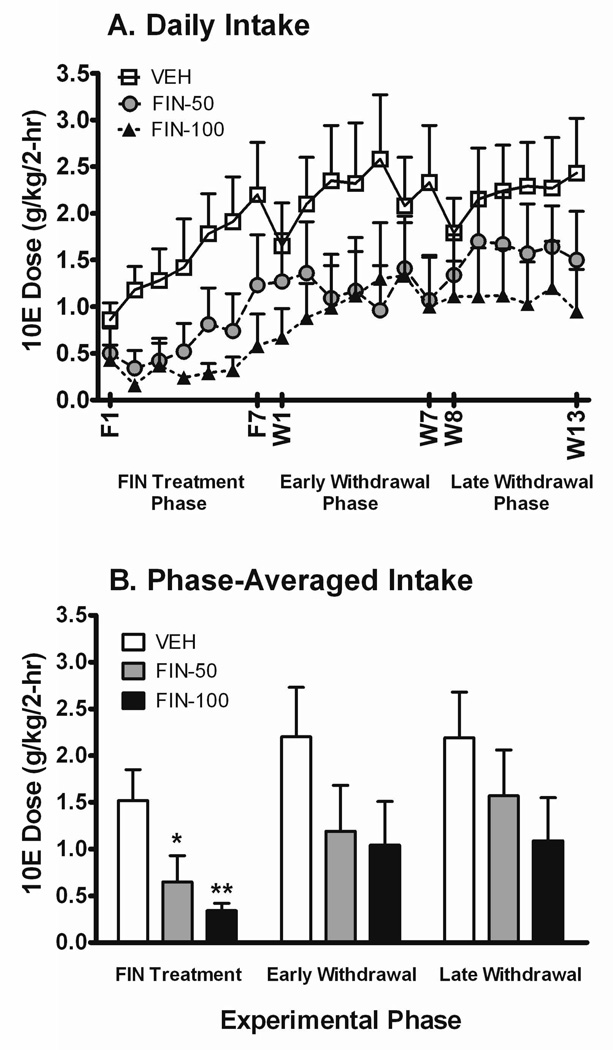
A two-way repeated measures ANOVA on the daily 10E dose consumed revealed a significant influence of treatment session in the 1st cohort of mice [F(19,380) = 6.60; P < 0.001], indicating that an escalation in 10E consumption occurred across successive acquisition sessions (Fig. 1A). Experimental phase averages of intakes were subsequently compiled and analyzed separately. A significant main effect of phase was similar detected for 10E dose [F(2,40) = 9.05; P < 0.001]. Consistent with our a priori hypothesis (stated in Methods section), 10E dose was significantly reduced as a product of FIN dose, but only during the FIN treatment phase [F(2,20) = 5.89; P < 0.01]. Decreases of 67% (P < 0.05) and 73% (P < 0.01) were observed in the 10E dose consumed by the FIN-50 and FIN-100 groups throughout the FIN treatment phase, respectively, when compared to the VEH group (Fig. 1B). Total 10E licks throughout the 2-hr session were positively correlated to the g/kg ethanol dose consumed (r = 0.962; P < 0.001; n = 529). Not surprisingly, the total session count of 10E licks (see Table 1) was similarly impacted by FIN dose, but only during the FIN treatment phase [F(2,20) = 4.72; P < 0.05].
Fig. 1. Effects of FIN treatment and its withdrawal on ethanol dose consumed during acquisition.
Daily intake values (panel A) for FIN treatment (sessions F1-F7), early FIN withdrawal (sessions W1-W7), and late FIN withdrawal (sessions W8-W13) phases are shown. A 3-day and a 2-day break elapsed between sessions W7 and W8 and between sessions W11 and W12, respectively. In panel B, treatment phase averages are depicted. All data points and bars represent the mean ± SEM ethanol intake for male B6 mice treated with VEH (n = 7), 50 mg/kg FIN (n = 8), and 100 mg/kg FIN (n = 8). *P ≤ 0.05 and **P ≤ 0.01 versus VEH-treated mice within respective treatment phase.
Table 1. Effects of FIN on general intake measures and bout dynamics during FIN treatment, early withdrawal, and late withdrawal phases.
All values represent the mean ± SEM of each treatment phase for n = 7 VEH-treated mice, n = 8 FIN-50 mice, and n = 8 FIN-100 mice.
| Experimental Phase | |||
|---|---|---|---|
| FIN Treatment |
Early Withdrawal |
Late Withdrawal |
|
| 10E Licks | |||
| VEH | 445 ± 107 | 698 ± 196 | 796 ± 180 |
| FIN-50 | 207 ± 91* | 396 ± 163 | 531 ± 160 |
| FIN-100 | 97 ± 29** | 344 ± 166 | 399 ± 173 |
| 10E Preference Ratio | |||
| VEH | 0.84 ± 0.05 | 0.87 ± 0.04 | 0.94 ± 0.01 |
| FIN-50 | 0.48 ± 0.09*** | 0.65 ± 0.13 | 0.83 ± 0.08 |
| FIN-100 | 0.41 ± 0.05*** | 0.70 ± 0.07 | 0.77 ± 0.10 |
| Total Fluid Intake (licks/2-hr) | |||
| VEH | 529 ± 127 | 821 ± 229 | 837 ± 186 |
| FIN-50 | 360 ± 139 | 513 ± 160 | 578 ± 166 |
| FIN-100 | 230 ± 81 | 432 ± 193 | 465 ± 176 |
| Inter-Session Water Intake (ml/22-hr) | |||
| VEH | 5.08 ± 0.25 | 4.92 ± 0.26 | 5.07 ± 0.24 |
| FIN-50 | 5.46 ± 0.03 | 5.23 ± 0.38 | 5.32 ± 0.33 |
| FIN-100 | 6.08 ± 0.29 | 5.62 ± 0.34 | 5.45 ± 0.31 |
| Bout Frequency | |||
| VEH | 6.1 ± 1.2 | 7.5 ± 1.8 | 8.3 ± 1.5 |
| FIN-50 | 2.6 ± 1.1* | 4.4 ± 1.8 | 5.5 ± 1.7 |
| FIN-100 | 1.3 ± 0.3** | 3.6 ± 1.5 | 3.7 ± 1.5 |
| Bout Size (licks) | |||
| VEH | 62.4 ± 8.0 | 85.0 ± 8.6 | 88.8 ± 13.9 |
| FIN-50 | 51.7 ± 7.4 | 87.4 ± 10.0 | 95.0 ± 13.9 |
| FIN-100 | 67.3 ± 10.1 | 85.6 ± 10.5 | 104.9 ± 15.3 |
P ≤ 0.05,
P ≤ 0.01
P ≤ 0.001 versus VEH group.
Ethanol preference was significantly influenced by FIN dose [F(2,20) = 4.23; P < 0.05] and by experimental phase [F(2,40) = 20.08; P < 0.001] in the 1st cohort of mice. Furthermore, a FIN dose × phase interaction was also present for 10E preference [F(4,40) = 2.60; P < 0.05]. The significant interaction was due to the fact that the effect of FIN on 10E preference was particularly robust during the FIN treatment phase [F(2,20) = 11.41; P < 0.001], during which time the FIN-50 and FIN-100 groups exhibited preference ratios that were 43% (P < 0.001) and 52% (P < 0.001) lower than VEH group values, respectively (Table 1). Ethanol preference ratios in FIN-treated mice partially recovered to VEH group levels during the early and late FIN withdrawal phases, a finding that was consistent with the profile of 10E g/kg consumed (Fig. 1B) and 10E licks observed (Table 1). Total fluid intake (TFI; measured as combined licks on 10E and water sippers) was significantly impacted by phase [F(2,40) = 8.39; P < 0.001], due to the marked increase in this measure throughout the acquisition of ethanol intake (Table 1). Although there was no statistical difference in TFI between treatment groups, there was a clear tendency for FIN-treated mice to exhibit a reduced TFI during each treatment phase (Table 1), a trend that followed the FIN-elicited decreases in 10E consumption. Notably, the TFI in the FIN-100 group during the FIN treatment phase (see Table 1; 230 ± 81 licks) was similar to the reported water baseline for this group (see text above; 209 ± 53 licks), thereby indicating that acquisition of 10E intake was severely blunted by 100 mg/kg FIN while baseline TFI levels during the 2-hr sessions were preserved. Furthermore, mice treated with 100 mg/kg FIN exhibited a tendency (P = 0.07) to consume greater quantities of inter-session water when compared to the VEH group (Table 1).
Results in the 2nd cohort of mice confirmed the findings described above for the effects of FIN treatment on ethanol intake. Analysis of daily ethanol intake (g/kg) indicated that FIN treatment produced a dose-dependent suppression of the acquisition of limited access 10E intake (main effect of dose: [F(2,25) = 8.12, P = 0.002], main effect of day: [F(6, 150) = 5.92, P < 0.001], dose × day interaction [F(12,150) = 2.55, P = 0.004]; data not shown). FIN treatment also produced a dose-dependent and significant decrease in averaged 10E intake [F(2,26) = 8.995, P = 0.001] (data not shown). Averaged 10E intake was decreased by 43% and 67% by pretreatment with the 50 mg/kg and 100 mg/kg FIN doses, respectively.
Ethanol Bout Micro-Architecture
Ethanol bout frequency was significantly influenced by experimental phase [F(2,40) = 6.87; P < 0.01], with augmented frequencies being found with each successive phase. The FIN-induced suppression of ethanol intake acquisition was primarily attributable to changes in bout frequency during the treatment phase [F(2,20) = 6.51; P < 0.01]. Frequencies were significantly decreased in the FIN-50 and FIN-100 groups by 58% (P < 0.05) and 78% (P < 0.01), respectively, versus VEH group values during the treatment phase (Table 1). Similar to 10E dose and preference ratio, bout frequencies in FIN-treated mice only partially recovered to VEH levels during the early and late FIN withdrawal phases. In contrast, although ethanol bout size progressively increased across successive experimental phases [F(2,37) = 22.35; P < 0.001], it was not affected as a factor of FIN dose during any experimental phase (Table 1). Additional analyses discerned no effect of FIN treatment on bout duration, lick rate, first bout size, or latency to first bout (data not shown).
Temporal Distribution of Licks
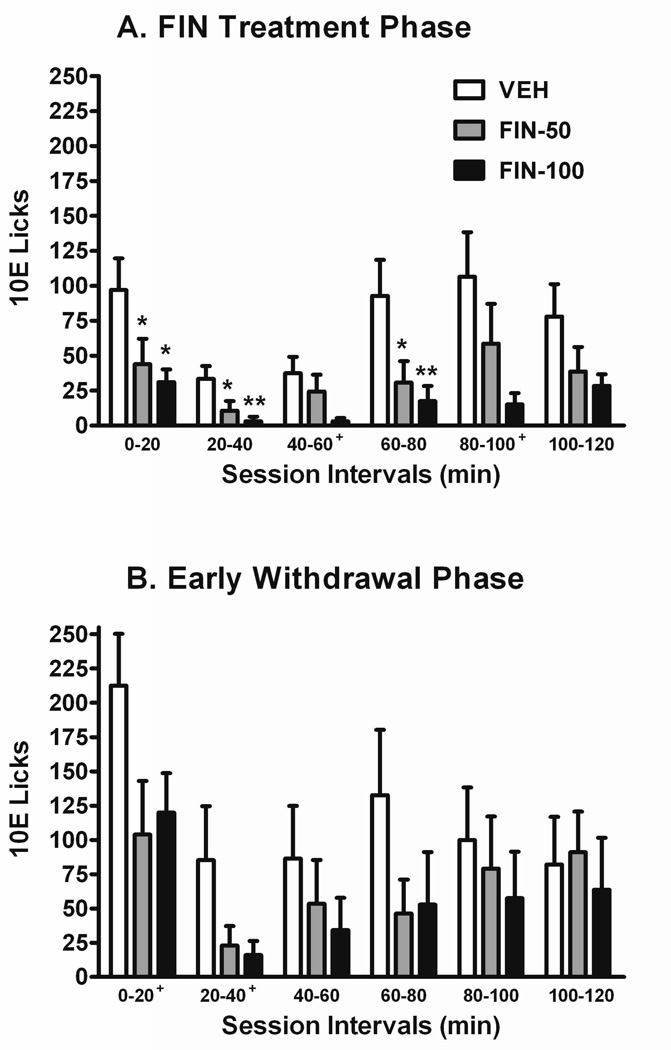
Cumulative lick records were analyzed and depicted as absolute values across 20-min time intervals for the FIN treatment (Fig. 2A) and subsequent early FIN withdrawal (Fig. 2B) phases. A 2-way repeated measures ANOVA for 10E licks throughout the FIN treatment phase determined significant main effects of FIN dose [F(2,20) = 4.67; P < 0.05] and session interval [F(5,100) = 9.66; P < 0.001]. The FIN-50 and FIN-100 groups exhibited significantly suppressed 10E licks during the 0–20, 20–40, and 60–80 min intervals [F(2,20) = 4.04 – 5.56; P < 0.05 for each), and trends toward decreased 10E licks during the 40–60 and 80–100 min intervals (P < 0.06) when compared to VEH group values (Fig. 2A). Despite the absence of a significant main effect of FIN dose, 10E lick patterns in FIN-treated mice remained blunted in the first half of the limited access session when compared to VEH-treated mice during the early FIN withdrawal phase (Fig. 2B). Trends towards significant decreases in 10E licks as a factor of FIN dose (P < 0.10) were noted in session intervals 0–20 and 20–40 (Fig. 2B).
Fig. 2. Effects of FIN treatment and its withdrawal on the temporal distribution of 10E licks during acquisition.
The absolute lick values recorded throughout the 2-hr drinking session are depicted in 20-min bins. Treatment phase (i.e., 7-day) averages of lick distributions during FIN treatment (panel A) and its subsequent early withdrawal (sessions W1-W7; panel B) are shown. Vertical bars represent the mean ± SEM licks for the VEH (n = 7), FIN-50 (n = 8), and FIN-100 (n = 8) groups. *P ≤ 0.05 and **P ≤ 0.01 versus VEH-treated mice within respective session interval. +P ≤ 0.10 denotes a trend towards a significant effect of FIN dose within the designated session intervals.
BECs and Brain ALLO Levels
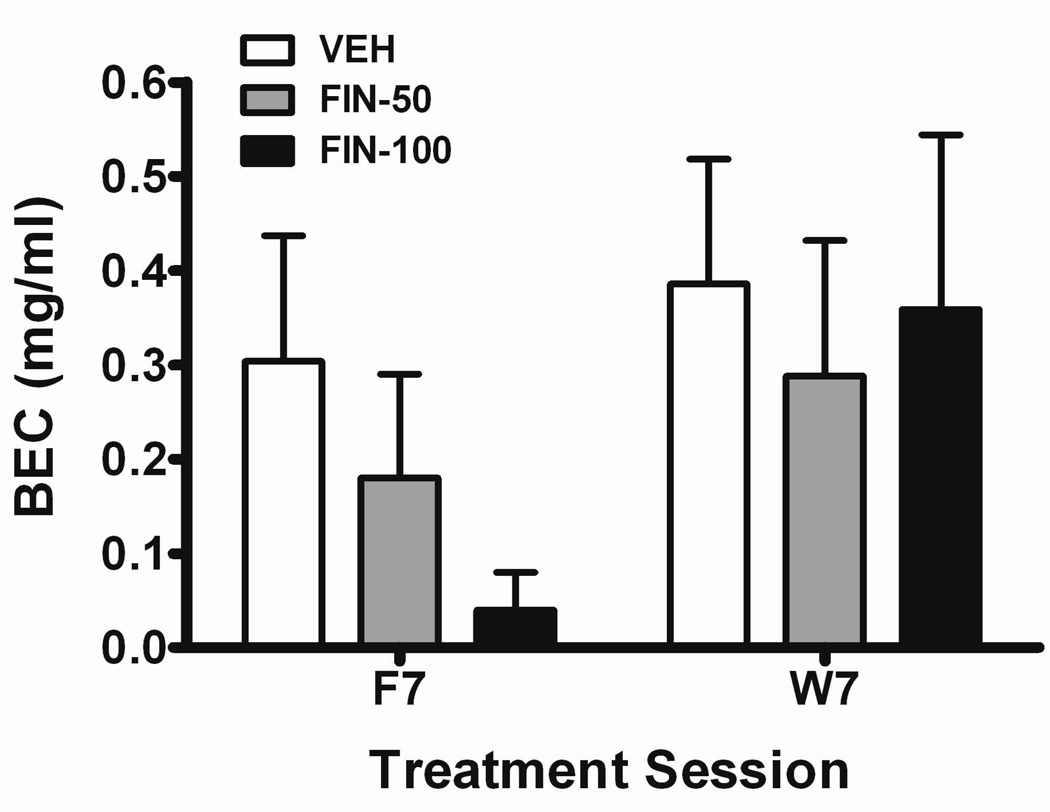
In the 1st cohort of mice, the BECs observed for the FIN-50 and FIN-100 groups at the conclusion of the treatment phase were 40% and 86% less than those reported for the VEH group (Fig. 3). However, no significant influence of dose group was detected. A strong correlation between g/kg dose consumed and BEC was noted during this time point (r = 0.88; P < 0.001; n = 23). Surprisingly, BECs taken at the conclusion of the withdrawal phase were similar for all groups (Fig. 3). This observation was despite a noticeable disparity in g/kg dose between VEH and the two FIN-treated groups during the FIN withdrawal phase (see Fig. 1B). Consistent with these differences, the correlation between g/kg dose and BEC during FIN withdrawal (r = 0.65; P < 0.001; n = 23) was weaker than previously observed during the FIN treatment phase.
Fig. 3. Effects of FIN treatment and its withdrawal on BEC during acquisition.
Blood ethanol concentrations (BECs) for VEH (n = 7), FIN-50 (n = 8), and FIN-100 (n = 8) groups are depicted. Blood samples were collected approximately 24-hr (F7 post-session) and 8 days (W7 post-session) following the final FIN injection.
In the 2nd cohort of mice, BECs at the conclusion of the FIN treatment phase were significantly decreased by 86 – 89% by both doses of FIN [F(2,26) = 11.80, P < 0.001]. Mean ± SEM BEC were 0.76 ± 0.11 mg/ml (VEH), 0.11 ± 0.12 mg/ml (FIN-50), and 0.09 ± 0.11 mg/ml (FIN-100). The corresponding 10E doses on the day that BEC was determined were 3.42 ± 0.40 g/kg (VEH), 1.48 ± 0.42 g/kg (FIN-50), and 1.03 ± 0.23 g/kg (FIN-100).
Brain ALLO levels were 2.87 ± 0.25, 2.85 ± 0.25, and 2.64 ± 0.44 ng/g tissue for the VEH, FIN-50, and FIN-100 groups, respectively, on the day following session W13 (i.e., 22-hrs after the final 10E access period). The absence of statistical difference between brain ALLO concentrations across the treatment groups suggested that 5α-reductase enzyme activity recovered during FIN withdrawal. In the 2nd cohort of mice, brain ALLO levels following the F7 drinking session were 1.53 ± 0.28 ng/g (VEH, n = 9), 1.10 ± 0.21 ng/g (FIN-50, n = 8), and 1.56 ± 0.36 ng/g (FIN-100, n = 10). Although ALLO levels were suppressed by 28% in the FIN-50 group, there were no significant group differences in ALLO levels.
DISCUSSION
Treatment with 50 and 100 mg/kg FIN for 7 days produced a rightward and downward shift in the acquisition of limited access, ethanol drinking in male B6 mice. This blunted acquisition was accompanied by a significant suppression of 10E preference, and appeared to be primarily attributable to a reduction in the bout frequency (as mean bout size was preserved). As evidenced by the temporal distribution of licks, FIN treatment decreased 10E licks almost uniformly throughout the 2-hr drinking session, and therefore influenced both the onset and maintenance of consumption. Although FIN-treated mice exhibited partially recovered intakes during subsequent early and late withdrawal phases, this recovery occurred mainly during the second half of the drinking session, as evidenced by the similarity in both the lick distributions (Fig. 2B) and the BEC measurements (Fig. 3) collected at the end of the session.
In an earlier report, our laboratory demonstrated the effects of 7-day FIN treatment (50 mg/kg) on established and stably-expressed limited access 10E consumption patterns in male B6 mice (Ford et al., 2005a). In this case, FIN treatment suppressed established drinking by 20% during a 2-hr limited access session. In a fashion similar to the current work on intake acquisition, FIN robustly attenuated the onset of consumption in ethanol-experienced mice, reducing the amount of licks exhibited in the initial 20-min by 40% (Ford et al., 2005a). However, a key difference was noted in FIN’s influence over drinking patterns when it was administered to mice during intake acquisition versus to mice with an established drinking routine. Chiefly, the blockade of ethanol intake acquisition was achieved through a reduction in bout frequency whereas the reduction of stable consumption was derived from selective decreases in bout size and duration (Ford et al., 2005a). Collectively, these findings suggest that 5α-reduced neurosteroids (and inhibition of their synthesis) might regulate drinking patterns differentially based upon the degree and length of ethanol experience. One explanation for this difference may be the way in which the mice perceived ethanol’s central effects (see below).
Several observations were noteworthy regarding the temporal distribution of licks during FIN withdrawal. The remaining discrepancy in g/kg intakes between the dose groups was primarily due to a more robust onset of drinking in the VEH group (Fig. 2B) when compared to the FIN-treated mice. However, the VEH-treated mice were still in the process of acquisition, since lick patterns had not fully stabilized between the treatment and withdrawal phases (see Fig. 1A). A clear example of this was the initial 20-min of the drinking session, during which time the VEH mice consumed approximately 100 and 200 licks, respectively, during the treatment (Fig. 2A) and early FIN withdrawal (Fig. 2B) phases. Notably, the 10E licks exhibited during the initial 20-min of the drinking session in the FIN-50 and FIN-100 groups during the early withdrawal phase was approximately 100 licks (Fig. 2B), which is closely matched to the beginning stages of acquisition observed in the VEH group (refer to Fig. 2A). Collectively, these findings suggest that FIN treatment might have delayed, but not permanently blocked, the acquisition of ethanol intake. It is possible that extension of the late withdrawal phase for an additional 2–3 weeks may have revealed an acquisition in FIN-treated mice comparable to that achieved by the VEH group.
BECs taken following the 7th drinking session (i.e., F7, conclusion of FIN treatment) were decreased by 40 – 86% (cohort 1) and by 86 – 89% (cohort 2) by FIN treatment. The strong correlation between BEC and ethanol dose consumed is consistent with the suppressive effect of FIN treatment on ethanol intake. However, BECs at the conclusion of the early withdrawal phase were equivalent among groups. The lack of group differences at this time point could be attributable to the similarity of lick distribution patterns in all treatment groups during the final 40 minutes of the drinking session (refer to Fig. 2B), the period of drinking access time in closest proximity to the collection of the BEC sample. If blood samples had been taken following the initial 20-min of the session, then group differences in BEC would likely have been more robust. Alterations in drinking patterns can have a pronounced influence on the resulting BEC, even if similar or different total intake levels throughout a limited access session are observed (Samson and Hodge, 1996). Thus, the lower overall intake but similar BECs in FIN- versus VEH-treated mice during FIN withdrawal would suggest a difference in bout patterns and their temporal distribution in relation to the conclusion of the session, at which time the BEC measurement was taken. The lower overall positive correlation between g/kg consumed and BEC during withdrawal is consistent with a shift in drinking patterns.
One concern regarding FIN treatment was that it would impact all ingestive behavior during both the 2-hr 10E drinking sessions and the intervening 22-hr periods between sessions. Several findings in the current study suggested that the influence of FIN was selective for ethanol. While the 2-hr total fluid intake (TFI) of the FIN-100 group tended to be as much as 60% lower than that observed in VEH-treated mice during the FIN treatment phase ( see Table1), it is important to note that this level of intake in the FIN-100 group (i.e., 230 ± 81 licks) was closely matched to the water baseline measures (i.e., 209 ± 53 licks) collected prior to the onset of FIN treatment and the introduction of the novel 10E solution. Taken together, these observations indicate that 100 mg/kg FIN did not necessarily decrease TFI, but rather prevented the elevation in TFI that was observed to accompany the introduction of the 10E solution in the VEH-treated mice. Furthermore, the FIN-100 group exhibited inter-session water consumption (i.e., ml/22-hr) that was 20% greater than the VEH group throughout the FIN treatment phase (Table 1).Thus, FIN-treated mice consumed similar levels of total fluid throughout a 24-hr period, but this intake was distributed differently between the 2-hr drinking session and the 22-hr inter-session period. Second, although food intake was not directly measured, a normal progression in body weight gain was observed in each treatment group, and FIN-50 and FIN-100 treated mice exhibited similar body weights to VEH-treated mice throughout all experimental phases (data not shown). Collectively, these findings suggested that FIN blunted ethanol acquisition without globally altering ingestive patterns.
Previous work has ascertained the importance of GABAergic neurotransmission on ethanol intake acquisition. In general, propagation of GABAergic tone with a GABAA receptor agonist enhances acquisition (Boyle et al., 1992; Smith et al., 1992) whereas alteration of GABAergic tone via GABAB receptor agonism supplants acquisition (Colombo et al., 2002). Consistent with these earlier findings, the current study demonstrated that biosynthetic blockade of 5α-reduced neurosteroids, which normally potentiate GABAA receptor-mediated inhibition, resulted in a blunted acquisition of ethanol intake. In addition, Boyle and colleagues (1992) observed that the GABAA agonist THIP enhanced consumption acquisition of a 10% ethanol solution primarily via an increased bout frequency when compared to saline-treated controls. In contrast, the FIN-elicited blockade of intake acquisition was chiefly due to a suppression of bout frequency (see Table 1). Collectively, these observations demonstrate a bimodal modulation of ethanol intake acquisition via manipulation of the GABAergic system, and further suggest that this regulation is mechanistically specific with regard to changes in 10E drinking patterns (i.e., alteration in bout frequency). The relevance of this specific regulation during drinking acquisition was made clear when the day to day shift in drinking patterns that occurred in the VEH-treated mice was analyzed during the first 7 days of ethanol access (overlapping the FIN treatment phase). In these mice, bout frequency incrementally increased up to 3.5-fold (i.e., from 2.6 to 9.0 bouts) between the first and seventh 10E drinking session, whereas mean bout size exhibited only a nominal increase of less than 25%. Thus, an elevation in bout frequency is the driving force for the acquisition of limited access 10E intake in male B6 mice, and FIN treatment prevented any increase in this measure across these initial drinking sessions.
Colombo and colleagues postulated that the pharmacological blockade of ethanol acquisition might involve a reduction in ethanol’s perceived effects that normally would promote continued consumption (Gessa et al., 2005). In particular, these investigators noted that acquisition of intake in Sardinian alcohol-preferring (sP) rats failed to reach control group levels even 2–3 weeks following the cessation of treatment with the CB1 receptor antagonist SR 141716 (Serra et al., 2001). The authors suggested that this pharmacological intervention interfered with the association between consumption and the concomitant reinforcing and/or subjective effects of ethanol. There was some evidence suggesting that a similar phenomenon occurred in the current work. Mainly, FIN-treated mice were incapable of acquiring ethanol intake at levels comparable to that following VEH treatment, even after more than 2 weeks post-treatment (refer to sessions W8-W13 in Fig. 1A). One possible interpretation of these findings is that ethanol intake levels in the FIN-50 and FIN-100 groups were maintained below a threshold at which pharmacological relevance and perceived neurobiological effects of orally consumed ethanol could be experienced. Consequently, the association between elevated consumption and ethanol’s subjective/reinforcing effects would likely never have been experienced and solidified during the FIN treatment phase (i.e., initial stages of acquisition). Consistent with this interpretation, ethanol consumption onset (initial 20-min of session) was blunted by more than 50% during the withdrawal phase in FIN-treated mice when compared to the VEH group (Fig. 2B) in the present study, and FIN pre-treatment significantly blunted several measures of ethanol’s subjective effects in social drinkers following ethanol consumption (Pierucci-Lagha et al., 2005). This explanation also is congruent with a series of rodent studies that purport the involvement of 5α-reduced neurosteroids in ethanol’s discriminative stimulus (Bowen et al., 1999; Hodge et al., 2001) and reinforcing (Ford et al., 2007b; Janak & Gill, 2003) properties.
Measurement of ALLO levels on the final day of FIN treatment (cohort 2) and one day following late FIN withdrawal (cohort 1) suggest that the putative involvement of GABAergic neurosteroids in the FIN-induced suppression of ethanol intake was more complex than simply being due to a concomitant suppression of ALLO levels following FIN treatment. ALLO levels assessed immediately after the 7th limited access ethanol session (session F7) were decreased by 28% by the 50 mg/kg dose of FIN, which corresponded to a 43% reduction in 10E intake. However, a similar relationship between ALLO levels and ethanol intake was unexpectedly absent in the animals pretreated with 100 mg/kg FIN. An important consideration with regard to the interpretation of these data is that limited access 10E consumption significantly increases brain ALLO levels in male B6 mice (Finn et al., 2004b) and that FIN pretreatment blunts (by 50%, but does not eliminate) the increase in brain ALLO levels following ethanol injection in rats (VanDoren et al., 2000). Thus, it is possible that limited access 10E intake normalized brain ALLO levels among groups (since FIN was administered 22 hrs prior to each limited access session), which would suggest another mechanism of action of FIN to decrease 10E intake. Another consideration is that ALLO levels were not measured prior to the 7th limited access ethanol session, so we do not know whether ALLO levels were suppressed prior to 10E access. It was presumed that this would be the case, based on previous work from our laboratory demonstrating that a single 50 mg/kg FIN injection suppressed brain ALLO by 80% at 24 hrs following this acute treatment (Finn et al., 2004a). Finally, on the day following session W13, all three treatment groups exhibited similar brain ALLO levels, suggesting that 5α-reductase enzyme activity recovered during FIN withdrawal. This finding is consistent with the idea that a protracted suppression of 5α-reductase enzyme activity following FIN withdrawal could not explain the continued discrepancy in g/kg ethanol dose consumed between treatment groups.
In summary, FIN blocked the acquisition of ethanol drinking in an animal model that typically exhibits a high propensity to consume large quantities of ethanol (e.g., Belknap et al., 1993). Given that orally consumed ethanol is associated with increases in brain concentrations of the 5α-reduced neurosteroid, ALLO, in male mice (Finn et al., 2004b) and plasma ALLO levels in adolescent human males (Torres & Ortega, 2004), a FIN-induced inhibition of ALLO synthesis may have blunted neurosteroid mediation of ethanol’s positive motivational or neurobehavioral effects (particularly in the FIN-50 group). Alternately, it also is possible that FIN produced an aversion to ethanol and/or an alteration in the discriminative stimulus (i.e., subjective) properties of ethanol. We are currently investigating these possibilities. Although further investigation is warranted, FIN and other drugs that alter the metabolism of 5α-reduced neurosteroids may be useful as a therapeutic intervention to blunt intake acquisition in at-risk individuals.
ACKNOWLEDGEMENTS
The current study was supported by a VA Merit Review grant from the Department of Veterans Affairs (to DAF). MMF is supported by KO1 grant AA016849. The authors would like to thank the laboratory of Dr. John C. Crabbe (cohort 1) and Mr. Christopher Snelling (cohort 2) for BEC measurements.
REFERENCES
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effect of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999;289:405–411. [PubMed] [Google Scholar]
- Boyle AE, Smith BR, Amit Z. Microstructural analysis of the effects of THIP, a GABAA agonist, on voluntary ethanol intake in laboratory rats. Pharmacol Biochem Behav. 1992;43:1121–1127. doi: 10.1016/0091-3057(92)90491-w. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Atzori G, Pani M, Vacca G, Addolorato G, Froestl W, Carai MA, Gessa GL. The GABAB receptor agonists baclofen and CGP 44532 prevent acquisition of alcohol drinking behaviour in alcohol-preferring rats. Alcohol Alcohol. 2002;37:499–503. doi: 10.1093/alcalc/37.5.499. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Effect of the combination of naltrexone and baclofen, on acquisition of alcohol drinking behavior in alcohol-preferring rats. Drug Alcohol Depend. 2005;77:87–91. doi: 10.1016/j.drugalcdep.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Davidson D, Amit Z. Naltrexone blocks acquisition of voluntary ethanol intake in rats. Alcohol Clin Exp Res. 1997;21:677–683. [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004a;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004b;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol. 2005a;37:23–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005b;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Mark GP, Finn DA. Influence of reinforcement schedule on ethanol consumption patterns in non-food restricted male C57BL/6J mice. Alcohol. 2007a;41:21–29. doi: 10.1016/j.alcohol.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007b;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK. Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2001;25:1441–1447. doi: 10.1097/00000374-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Janak PH, Gill MT. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge CW. In: Neurobehavioral regulation of ethanol intake, in Pharmacological Effects of Ethanol on the Nervous System. Deitrich RA, editor. New York: CRC Press; 1996. pp. 203–226. [Google Scholar]
- Serra S, Carai MA, Brunetti G, Gomez R, Melis S, Vacca G, Colombo G, Gessa GL. The cannabinoid receptor antagonist SR 141716 prevents acquisition of drinking behavior in alcohol-preferring rats. Eur J Pharmacol. 2001;430:369–371. doi: 10.1016/s0014-2999(01)01379-6. [DOI] [PubMed] [Google Scholar]
- Smith BR, Robidoux J, Amit Z. GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol Alcohol. 1992;27:227–231. [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology. 2004;172:352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]