Abstract
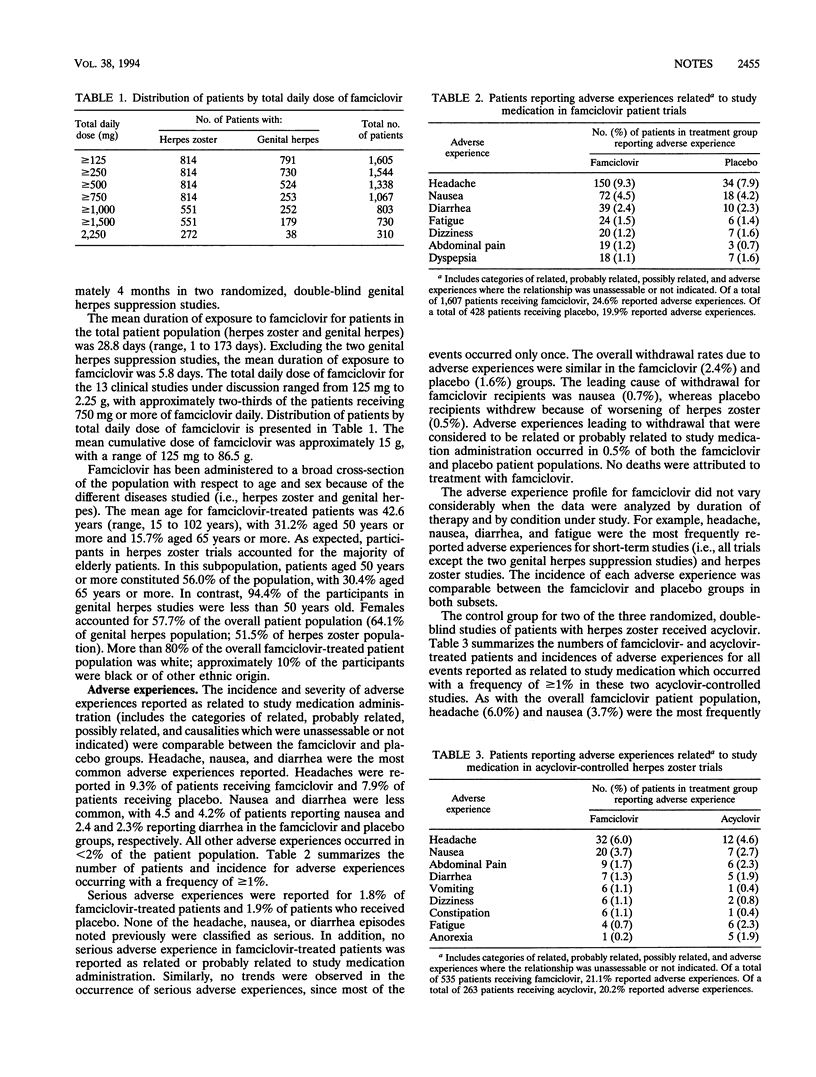
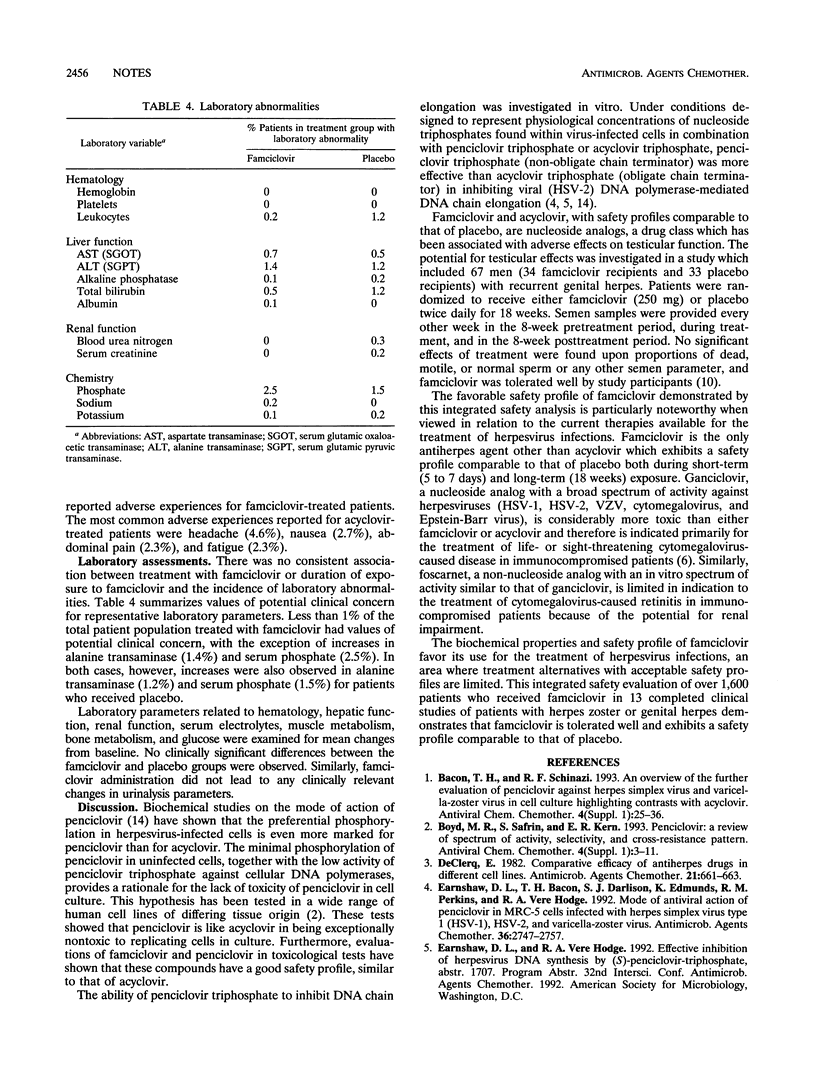
Safety reporting from individual ongoing and completed clinical studies has demonstrated that famciclovir, the well-absorbed oral form of the antiherpesvirus agent penciclovir, has been well tolerated by more than 3,000 individuals worldwide. An integrated safety evaluation has been performed and includes over 1,600 patients from 11 completed, randomized, double-blind clinical trials and 2 open trials. The famciclovir population consisted of 816 herpes zoster patients (four trials), 409 patients with acute genital herpesvirus infections (seven trials), and 382 patients from two genital herpes suppression studies. Overall, the famciclovir-treated patient population was 57.7% female and ranged in age from 15 to 102 years (mean, 42.6 years), with 31.2% aged 50 years or more and 15.7% aged 65 years or more. The mean duration of exposure to famciclovir was 28.8 days (5.8 days excluding suppression studies). The total daily doses ranged from 125 mg to 2.25 g. The most common adverse experiences reported as related to study medication (famciclovir and placebo) were headache, nausea, and diarrhea. The frequencies of adverse experiences and laboratory abnormalities (hematology, clinical chemistry, and urinalysis parameters) were similar in both famciclovir and placebo recipients. Thus, safety data from the analysis of 13 completed clinical studies demonstrate that famciclovir is tolerated well by patients with either herpes zoster or genital and has a safety profile comparable to that of placebo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Clercq E. Comparative efficacy of antiherpes drugs in different cell lines. Antimicrob Agents Chemother. 1982 Apr;21(4):661–663. doi: 10.1128/aac.21.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw D. L., Bacon T. H., Darlison S. J., Edmonds K., Perkins R. M., Vere Hodge R. A. Mode of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. Antimicrob Agents Chemother. 1992 Dec;36(12):2747–2757. doi: 10.1128/aac.36.12.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulds D., Heel R. C. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs. 1990 Apr;39(4):597–638. doi: 10.2165/00003495-199039040-00008. [DOI] [PubMed] [Google Scholar]
- Hodge R. A., Perkins R. M. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrob Agents Chemother. 1989 Feb;33(2):223–229. doi: 10.1128/aac.33.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz G. J., Eron L., Kaufman R., Goldberg L., Raab B., Conant M., Mills J., Kurtz T., Davis L. G. Prolonged continuous versus intermittent oral acyclovir treatment in normal adults with frequently recurring genital herpes simplex virus infection. Am J Med. 1988 Aug 29;85(2A):14–19. [PubMed] [Google Scholar]
- Morton P., Thomson A. N. Oral acyclovir in the treatment of herpes zoster in general practice. N Z Med J. 1989 Mar 8;102(863):93–95. [PubMed] [Google Scholar]
- Weinberg A., Bate B. J., Masters H. B., Schneider S. A., Clark J. C., Wren C. G., Allaman J. A., Levin M. J. In vitro activities of penciclovir and acyclovir against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1992 Sep;36(9):2037–2038. doi: 10.1128/aac.36.9.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]


