SUMMARY
At excitatory glutamatergic synapses, postsynaptic endocytic zones (EZs), which are adjacent to the postsynaptic density (PSD), mediate clathrin-dependent endocytosis of surface AMPA Receptors (AMPAR) as a first step to receptor recycling or degradation. However, it remains unknown if receptor recycling influences AMPARs lateral diffusion, and if EZs are important for the expression of synaptic potentiation. Here we demonstrate that the presence of both EZs and AMPAR recycling maintain a large pool of mobile AMPARs at synapses. In addition, we find that synaptic potentiation is accompanied by an accumulation and immobilization of AMPARs at synapses resulting from both their exocytosis and stabilization at the PSD. Displacement of EZs from the postsynaptic region impairs the expression of synaptic potentiation by blocking AMPAR recycling. Thus receptor recycling is crucial for maintaining a mobile population of surface AMPARs which can be delivered to synapses for increases in synaptic strength.
INTRODUCTION
At glutamatergic synapses, changes in the number of AMPARs tunes synaptic efficacy. Such modifications are dependent on both the availability of receptor binding sites inside the PSD and on the equilibrium between receptor influx and efflux at synapses (Newpher and Ehlers, 2008; Shepherd and Huganir, 2007; Triller and Choquet, 2008). Moreover, the abundance of AMPARs available to enter into synapses is dependent on their relative rates of exocytosis and endocytosis at the postsynaptic membrane. These trafficking pathways were initially considered to be the main determinants of receptor density at synapses (Carroll et al., 1999a; Turrigiano, 2000). For example, AMPAR accumulation at synapses during synaptic potentiation involves enhanced receptor exocytosis and recycling (Hayashi et al., 2000; Lu et al., 2001; Park et al., 2004; Passafaro et al., 2001; Pickard et al., 2001; Shi et al., 1999). Conversely, the reduced number of synaptic AMPARs found during LTD correlates with receptor endocytosis (Carroll et al., 1999b; Man et al., 2000).
A few years ago, in addition to the intracellular trafficking, the lateral diffusion of glutamate receptors in the plasma membrane (Borgdorff and Choquet, 2002; Tardin et al., 2003) has been proposed as an essential process to drive receptor exchange to and from synapses (Ashby et al., 2004; Boehm et al., 2006; Groc et al., 2004; Oh et al., 2006; Tardin et al., 2003). Single particle tracking of glutamate receptors in the postsynaptic membrane has demonstrated that AMPARs rapidly alternate between periods of Brownian-like lateral mobility, often at extrasynaptic sites, and periods of confinement or immobility, mostly at synapses. The reduced mobility of AMPARs at synapses is thought to occur as a result of interactions with PSD scaffold proteins. For example, stabilization of AMPARs at synaptic sites requires the interaction between stargazin-like transmembrane AMPAR regulatory proteins (TARPs) and PSD-95 (Bats et al., 2007). The dynamic interactions between glutamate receptors and PSD scaffold proteins results in mixed population of AMPAR mobility, with more than half of synaptic receptors able to exchange with the extrasynaptic membrane (Heine et al., 2008; Tardin et al., 2003). Therefore, the fast exchange of mobile AMPARs at synapses is likely to be one major mechanism allowing for changes of synaptic strength (Heine et al., 2008).
A direct link between receptor lateral mobility and synaptic potentiation has not been established yet. One hypothesis is that AMPAR lateral diffusion provides a pool of mobile receptors available in the vicinity of synapse to be recruited upon appropriate stimulus during synaptic potentiation. A variety of stimuli have been shown to modulate AMPAR lateral mobility over a wide dynamic range. For example, global glutamate application (Tardin et al., 2003), neuronal depolarization (Groc et al., 2004) or long-term synapse-specific block of neuronal activity (Ehlers et al., 2007) increase AMPAR movements inside synapses, while a local rise of intracellular calcium (Borgdorff and Choquet, 2002) and high frequency neuronal activity (Heine et al., 2008) both rapidly immobilize AMPARs.
Given that synaptic activity controls both the lateral diffusion and the intracellular trafficking of AMPARs, it will be important to understand how these two processes are coupled to allow for the expression of synaptic plasticity. In addition, AMPAR endocytosis and exocytosis likely occur at membrane sites lateral to the PSD - either within or outside spines - (Ashby et al., 2004; Blanpied et al., 2002; Boehm et al., 2006; Park et al., 2006; Racz et al., 2004; Yudowski et al., 2007). This suggests that receptor lateral diffusion to endocytic zones (EZs) and from exocytic sites will affect receptor accumulation at synapses (Choquet and Triller, 2003; Turrigiano, 2000).
While the molecular mechanisms and precise locations of AMPAR exocytosis are still a matter of debate (Gerges et al., 2006; Park et al., 2006; Yudowski et al., 2007), recent studies indicate that AMPAR endocytosis occurs through a dynamin-dependent process involving many proteins including clathrin and AP-2 (Carroll et al., 1999a; Lee et al., 2004). The positioning of EZs in the proximity of the PSD (Blanpied et al., 2002; Petralia et al., 2003; Racz et al., 2004) depends on the direct interaction between the large GTPase dynamin-3 (Dyn3) (Gray et al., 2003) and the postsynaptic scaffold complex Homer/Shank (Lu et al., 2007). Importantly, disruption of this interaction leads to a displacement of EZs from the vicinity of the PSD, along with a decrease in the abundance of AMPARs at synapses. These findings suggest that spine-localized endocytosis may serve to capture AMPARs diffusing within the extrasynaptic membrane and allows their recycling to the plasma membrane for potential incorporation at synapses (Lu et al., 2007).
In the present study we test the influence of EZs on the lateral diffusion of AMPARs and the expression of synaptic potentiation. Using single-particle tracking and high resolution real-time fluorescence microscopy we find that both EZs and local receptor recycling are required to maintain a mobile pool of AMPARs at synapses. Furthermore, we demonstrate that this proximate pool of mobile AMPARs is essential for controlling the mobility and accumulation of synaptic receptors during synaptic potentiation.
RESULTS
AMPARs are transiently confined at synapses and endocytic zones
We first characterized the lateral mobility of surface GluR1-containing AMPARs at both synapses and EZs by combining single-particle tracking and live cell fluorescence imaging. Cultured hippocampal neurons were transfected with Dyn3, along with Homer∷GFP and clathrin∷DsRed (Figure 1A). Homer and clathrin were used to monitor synapses and clathrin-rich zones, respectively. Importantly, overexpression of these fluorescently-labeled proteins did not result in any detectable structural or functional effects on synapses as reported in Figure S1 and Supplementary Data. In addition, overexpression of Dyn3 did not alter either synapse density (Figure S1E) or GluR1 synaptic accumulation (Figure S1F). Consistent with previous studies (Blanpied et al., 2002; Lu et al., 2007; Petralia et al., 2003; Racz et al., 2004), Homer and clathrin puncta were observed to be in close proximity and partially overlapping (~80%), (Figure 1A, upper inset). To unequivocally define synapses and EZs, we performed object segmentation by wavelet transform [see Experimental Procedures and (Racine et al., 2007)] (Figure 1A, lower inset) on fluorescence images, thus overcoming the possible bias of selecting thresholds. Consequently AMPAR localization was distinguished as being either within synapses or clathrin puncta.
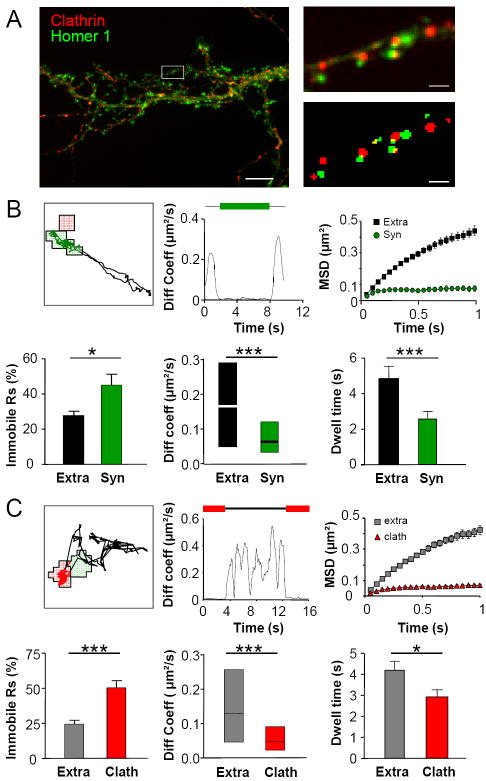
Figure 1. AMPA receptors are reversibly stabilized at synapses and Endocytic Zones.
(A) EZ and synapses localization. Left, example image of a hippocampal neuron expressing clathrin∷DsRed (red) and Homer∷GFP (green), along with Dyn3∷FLAG. Right, Higher magnification of the left inset (top); same dendritic segment as above, after signal segmentation (bottom). Scale bars 10μm and 1μm. (B) GluR1 surface diffusion synapses. Top: left, example trajectory of a GluR1-QD exploring extrasynaptic zones (black) and a synapse (green); middle, diffusion coefficient over time plot of the particle on the left. Above lines represent synaptic (green) and extrasynaptic (black) localization of the particle; right, Mean Square Displacement (MSD) versus time for GluR1-QD complexes at extrasynaptic and synaptic locations. Linear MSD indicates free diffusion; curved MSD shows confined diffusion. The MSD plateau value is indicative of the surface explored. Bottom: immobile receptor fraction (left), median diffusion coefficient (middle) and dwell time (right) of receptors exploring synapses (green) and extrasynaptic regions (black). n trajectories=325. (C) GluR1 can reversibly enter and exit clathrin puncta (clath). Top: left, example trajectory of GluR1 at extra-clath regions (black) and clath (red); middle, diffusion coefficient versus time plot of the GluR1-QD complex on the left, colour code as on the left; right, average MSD versus time plot of QDs at extra-clath and clath zones. GluR1 exhibits confined diffusion at clath. Bottom, Immobile receptor fraction (left), median diffusion coefficient (middle) and dwell time (right) of receptors exploring clath (red) and extra-clath regions (black). n trajectories=298 (Hereafter unless otherwise stated data are mean±SEM, p<0.05 (*), p<0.001 (***), Mann-Whitney test).
To monitor AMPAR mobility at synapses and clathrin-rich zones, surface AMPARs were tagged with quantum dots (QDs) and imaged over 60-seconds time periods. QD trajectories reconstructed with ~ 40 nm accuracy (see Experimental Procedures) revealed that GluR1-QD complexes alternate between periods of high and reduced mobility (Figure 1B-C). Consistent with previous reports (Bats et al., 2007; Ehlers et al., 2007; Heine et al., 2008; Tardin et al., 2003), synaptic AMPARs had slower mobility than the highly mobile extrasynaptic receptor population (Figure 1B and Movie S1). The mean square displacement (MSD, a measure of the surface explored by the receptors versus time) of AMPARs at extrasynaptic zones varied quasi-linearly with time, indicative of Brownian diffusion (Figure 1B upper right panel). In contrast, the MSD function of synaptic receptors saturated, indicating that receptors were moving in a confined space (Figure 1B, upper right panel). According to the diffusion coefficient threshold (0.0075 μm2/s) set to define immobile receptors (Tardin et al., 2003), we observed that the percentage of immobile receptors at synapses was much higher than at extrasynaptic compartments (Figure 1B, lower left panel). In addition, the instantaneous diffusion coefficients of mobile receptors were significantly lower at synapses relative to extrasynaptic sites (Figure 1B lower middle panel). Noteworthy, a large proportion of mobile synaptic receptors (80 ± 5 %) exchanged between synaptic and extrasynaptic compartments during the imaging period (1 min), indicating that most receptors were only transiently stabilized at synapses on the minute time scale, with a shorter dwell time at synapses (Figure 1B lower right panel) compared to extrasynaptic sites.
We then analyzed the dynamics of surface AMPARs on clathrin-rich zones. About half of clathrin puncta (55%) were positioned in close proximity to the PSD (hereafter referred to as EZs). The remaining population of clathrin puncta was distributed along dendritic shafts, likely representing non-synapse-associated clathrin coated pits (CCPs), as well as endosomal and trans-golgi network (TGN) organelles containing clathrin. Analogous to the observed receptor mobility at synapses, AMPAR lateral mobility at clathrin was reduced compared to extra-clathrin zones (Figure 1C and Movie S2). At clathrin puncta, the percentage of immobile receptors was doubled compared to extra-clathrin sites and the median diffusion coefficient of mobile receptors was smaller than that outside clathrin (Figure 1C lower panels). The overall dynamic behaviour of AMPARs at clathrin puncta was highly confined, as shown by the strongly curved MSD function (Figure 1C upper right panel). Interestingly, only half of the receptors found at clathrin-rich zones were permanently stabilized, while 48 ± 9 % exchanged location between clathrin puncta and extra-clathrin areas with a mean dwell time of 2.9 ± 0.3 s on clathrin and 4.1 ± 0.6 s outside (Figure 1C lower right panel). Importantly, GluR1 coupled to antibody-QD complexes could still undergo clathrin-mediated endocytosis indicating that the observed GluR1-QD complexes were present at the cell surface, and that the presence of QDs did not impair the trafficking functions of clathrin-rich zones (Figure S2 and Supplementary Data).
EZ-PSD proximity is important to maintain a mobile pool of AMPARs at synapses
A first analysis of the influence of synapse-EZ proximity on receptor dynamics revealed that there is a selective negative correlation between GluR1 mobility at synapses and the distance between synapses and clathrin-rich zones (Supplementary Data and Figure S3). GluR1 is more mobile at synapses next to an EZ than at synapses devoid of an EZ. In order to further investigate the role of EZ proximity to the PSD on AMPAR mobility, we used a point mutant of Dyn3 unable to bind Homer1 (Dyn3-PL), which was previously shown to displace EZs from the PSD, and impair receptor recycling (Lu et al., 2007). Similar to previous experiments (Lu et al., 2007), overexpression of Dyn3-PL reduced the percentage of PSDs associated with an EZ (Figure 2A), and reduced AMPAR accumulation at synapses. This effect was selective to synapses, as the number of synaptic AMPARs in Dyn3-PL-expressing neurons was not even half of that measured in Dyn3-Wt neurons, although the total surface expression was comparable under the two conditions (Figure 2B). Accordingly, expression of Dyn3-PL did not increase receptor number, either on clathrin puncta or at domains simultaneously excluding clathrin and synaptic staining (GluR1 fluorescence intensity/pixel: clath: Dyn3-Wt = 20.6±1.2 a.u.; Dyn3-PL = 24.0±1.0 a.u, p>0.05; extra: Dyn3-Wt = 16.5±1.2 a.u.; Dyn3-PL = 21.3±1.4 a.u, p>0.05, Student’s t test, n = 15 neurons in each condition, not shown). Interestingly, we observed that in Dyn3-PL neurons GluR1 mobility both at synapses and at clathrin-rich zones was not influenced by synapse-clathrin distance (Figure S4A-B and Supplementary Data).
Figure 2. Displacement of EZ from the PSD depletes the mobile pool of synaptic AMPARs.
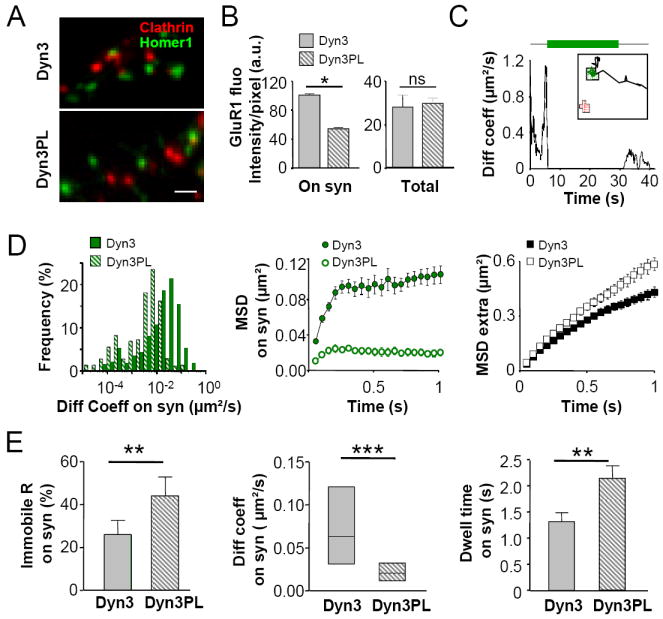
(A) Dyn3-PL expression, displaces EZs form the PSD vicinity. Left, Example image of clathrin and Homer fluorescence in Dyn3 (top) and Dyn3-PL (bottom) neurons. Scale bar 1μm. (B) Dyn3-PL expression selectively reduces synaptic GluR1. Quantification of synaptic (left) and total (right) surface GluR1 fluorescence in Dyn3 and Dyn3-PL neurons. (n=15 neurons in each condition, p<0.05 (*), Student’s t-test). (C) Example trajectory of GluR1-QD exploring a synapse in a Dyn3-PL neuron (inset) and its corresponding diffusion coefficient over time plot (compare with Figure 1B, same color code). (D) Lateral mobility of synaptic GluR1 in Dyn3 and Dyn3-PL neurons. Left, diffusion coefficient distribution of synaptic GluR1 in Dyn3 and Dyn3-PL neurons. Middle and right, MSD versus time plot of receptors exploring synaptic (middle) or extrasynaptic regions (right). (E) immobile fraction (left), diffusion coefficient of mobile synaptic receptors (median ± IQR, p< 0.001, Mann-Whitney test) (middle) and dwell time (right) of receptors exchanging between synaptic and extrasynaptic regions in Dyn3 and Dyn3-PL neurons. n trajectories=325 and 267, respectively. p<0.01 (**), p<0.001 (***), unless otherwise stated Student’s t test.
In the presence of a close EZ, wild-type synapses contain both mobile and immobile receptors (Figure 1). In order to test whether the presence of EZs influences AMPAR lateral diffusion at synapses, we uncoupled EZs from the PSD. Indeed, displacement of EZs, and the consequent impairment of receptor recycling with Dyn3-PL overexpression (Lu et al., 2007), highly reduced the mobility of synaptic GluR1 compared to Wt conditions (Figure 2C and Movie S4). Compared to Dyn3-Wt, in Dyn3-PL neurons the distribution of receptor diffusion coefficients at synapses was shifted to lower values (Figure 2D), and the immobile fraction of synaptic receptors doubled (Figure 2E). Accordingly, the mean MSD of synaptic trajectories in Dyn3-PL neurons reached a lower plateau compared to that in Dyn3-Wt neurons (Figure 2D middle panel), revealing strong receptor confinement. Importantly, the fraction of immobile receptors is relative to the total number of receptors tracked in each condition. Taking into account that synapses in Dyn3-Wt neurons exhibit approximately twice the number of receptors as Dyn3-PL synapses (see Figure 2B), this suggests that the absolute number of immobile receptors in Dyn3-Wt and Dyn3-PL neurons is similar, therefore the Dyn3-PL mutant had a specific reduction in the population of mobile synaptic AMPARs. In addition, Dyn3-PL neurons exhibited a lower rate of receptor exchange between synaptic and extrasynaptic sites (66 ± 6 % versus 79 ± 7 % exchanging receptors at synapses in Dyn3-PL and Dyn3-Wt, respectively) and a longer AMPAR dwell time at synapses (Figure 2E right panel). Importantly, knock down of endogenous Dyn3 by RNA interference had comparable effects to Dyn3-PL overexpression, thus indicating that endogenous Dyn3 is required for maintaining a mobile pool of receptors at synapses (Figure S5 and Supplementary Data). AMPAR lateral mobility at clathrin puncta and in membrane domains simultaneously excluding Homer∷EGFP and clathrin∷DsRed fluorescence was not significantly affected by the overexpression of any Dyn3 mutant (Figure S4C-G), as confirmed by the comparable MSD functions in Dyn3-Wt and Dyn3-PL expressing neurons (Figure 2D right panel). Altogether, we conclude that removing EZs from the vicinity of the PSD selectively affects AMPAR mobility at synapses by reducing the mobile pool of receptors, while leaving the immobile pool unaffected.
To establish whether Dyn3-Wt overexpression influences AMPAR mobility, we compared AMPAR lateral diffusion in these neurons to those untransfected with Dyn3. In the absence of Dyn3 overexpression, AMPAR mobility at synapses was intermediate between that observed in Dyn3-Wt and Dyn3-PL transfected neurons (Figure S6A), indicating that Dyn3-Wt and Dyn3-PL have opposite effects on synaptic receptor mobility compared to endogenous Dyn3. Interestingly, none of the expressed forms of Dyn3 altered the density or intensity of PSD-95 clusters compared to the endogenous case (Figure S6B). These data render unlikely the possibility that changes in receptor mobility at synapses are due to modifications in the availability of scaffold proteins following overexpression of Dyn3. Although surprising, these results support the hypothesis that impairment of receptor recycling (through displacement of EZs) depletes the mobile pool of synaptic AMPARs. In this scenario, in the absence of a nearby EZ, mobile AMPARs exit from synapses and diffuse in the extrasynaptic space without being recaptured.
Impairment of AMPAR interaction with the endocytic machinery reduces receptor trapping at EZs and depletes synapses of mobile receptors
The transient trapping of AMPARs at EZs reported here (Figure 1C) likely relies on the interaction of the GluR1 subunit with adaptor proteins present in clathrin-rich zones. Accordingly, the GluR1-R838A mutant unable to bind AP2 (Lee et al., 2002) displayed reduced internalization (Figure S7A-B) and mobility at synapses (Figure S7C-E) as described in the Supplementary Data. Thus, both the physical proximity of the EZs and interaction with clathrin adaptors is required to maintain a mobile pool of AMPARs at synapses.
The mobile pool of synaptic AMPARs is maintained by receptor recycling
To directly test whether Dyn3-PL effects on AMPAR mobility are consistent with impairment of AMPAR recycling, we compared the mobility of AMPARs in the Dyn3-PL mutant to a constitutively inactive form of the small GTPase Rab11a (Rab11a-S25N) which blocks receptor recycling and induces loss of synaptic receptors (Park et al., 2004). Total surface and synaptic GluR1 immunoreactivity was significantly decreased in Rab11a-S25N neurons (not shown and Figure 3A). Cell wide inhibition of receptor transport from recycling endosomes to the plasma membrane with Rab11a-S25N expression induced the loss of mobile surface AMPARs at synapses (Figure 3B-D). The distribution of diffusion coefficients of synaptic GluR1 was shifted to lower values in Rab11a-S25N compared to Rab11a-Wt neurons (Figure 3B), mirrored by a significant decrease in the median diffusion of synaptic mobile receptors (Figure 3D) and by a stronger confinement (Figure 3C). Mobility of GluR1 was also decreased at extrasynaptic sites, but to a lesser extent (Diff coeff: Rab11-Wt = 0.046 μm2/s, IQR=0.020-0.127; Rab11-S25N = 0.040 um2/s, IQR= 0.015-0.096, p<0.05 Mann Whitney). Altogether these data indicate that global impairment of receptor recycling reduces the population of mobile receptors at synapses, and suggest that recycled AMPARs comprise a mobile population that can readily exchange at synapses.
Figure 3. Impairment of recycling receptors exocytosis depletes mobile AMPARs at synapses.
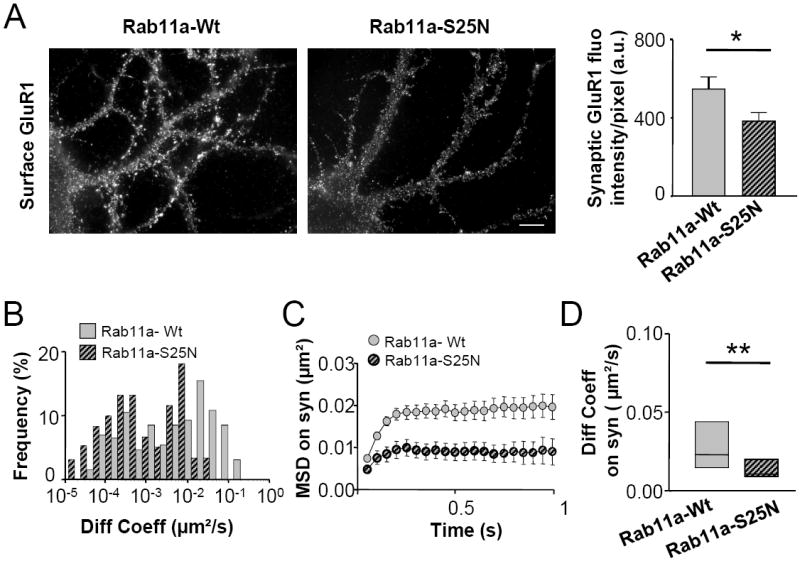
(A) Blockade of recycling receptor exocytosis reduces surface AMPARs. Left: Example images of surface GluR1 subunits in neurons expressing Rab11a-Wt (left) or Rab11a-S25N (right). Scalebar 10 μm. Right: Quantification of synaptic GluR1 immunoreactivity in the two configurations (mean fluorescence intensity/pixel ± SEM, n=18 in each condition, p<0.05(*), Student’s t test). (B-D) Synaptic AMPARs are less mobile in neurons exhibiting impaired receptor delivery from recycling endosomes to the surface. (B) Diffusion coefficient distributions of synaptic GluR1-QD complexes in neurons expressing either Rab11a Wt or the constitutively inactive form Rab11aS25N. Receptors with diffusion coefficients below 0.0075μm2/s are considered immobile. (C) MSD (mean±SEM) of synaptic receptors in Rab11a Wt and Rab11aS25N neurons. (D) Median diffusion coefficient (± IQR) of mobile receptors at synapses, n trajectories =178-159, p<0.05 (*), p<0.01(**), Mann-Whitney test.
Direct measure of receptor exocytosis upon displacement of EZs from the PSD
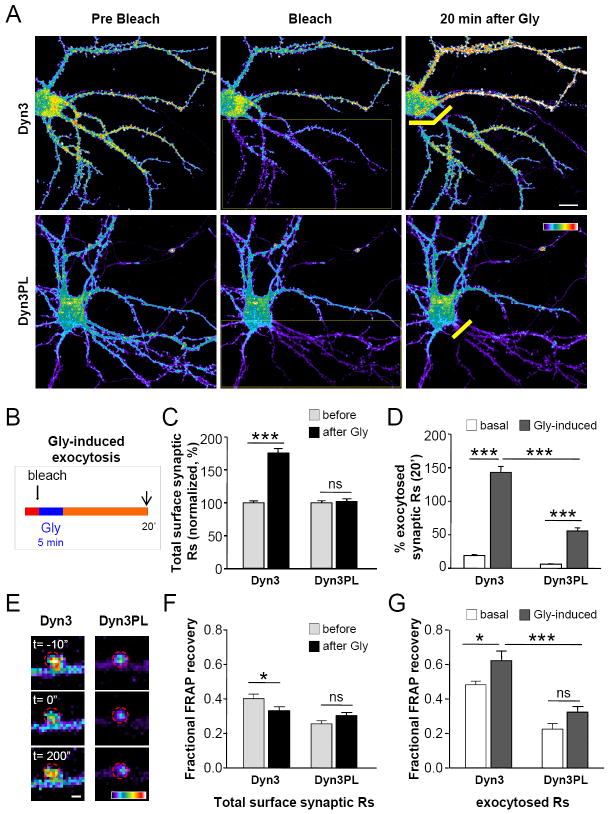
Disruption of endocytic recycling should also reduce the insertion of AMPARs into the postsynaptic membrane. To directly measure the amount and dynamic properties of newly exocytosed receptors in the presence and absence of endocytic recycling, we set up an experimental approach based on the pH sensitive pHluorin-tagged GluR1 receptors (SEP-GluR1). Relying on the possibility to selectively photobleach surface SEP-GluR1s (see Experimental Procedures) (Ashby et al., 2004; Heine et al., 2008), we bleached a large portion of dendrites (“large bleach”) and measured receptor exocytosis as the return of fluorescence due to unbleached intracellular receptors being delivered to the surface (Supplementary Methods and Figure S7A). After 20 min, GluR1 exocytosis was strongly reduced in Dyn3-PL neurons compared to Dyn3-Wt, both in synaptic and extrasynaptic regions (Figure 5A-C). This observation is fully consistent with the finding that receptor recycling is impaired upon displacement of EZs from the PSD. Interestingly, under all conditions exocytosed receptors accumulated at synapses. Altogether, those data strongly support the hypothesis that AMPAR endocytosis in close proximity to synapses is necessary to maintain a recycling pool of AMPARs, which ultimately regulates the number of synaptic receptors.
Figure 5. Anchoring of EZ close to PSD is required for Gly-induced increase in synaptic AMPAR number and potentiation of synaptic transmission.
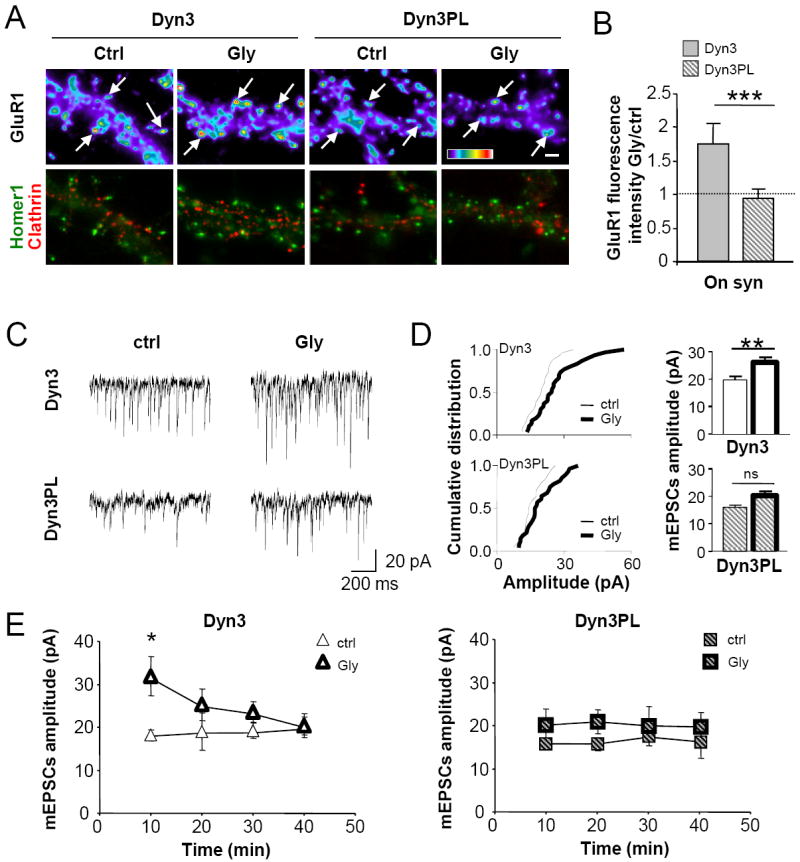
(A) Glycine application increases GluR1 synaptic abundance only in Dyn3 neurons. Representative pseudocolor images of GluR1 fluorescence (top) and overlay Homer∷GFP and clathrin∷DsRed fluorescence (bottom) in Dyn3 and Dyn3-PL neurons, exposed to Gly or control treatment as indicated (scale bar 2μm). Arrows indicate example synapses. (B) Quantification of surface synaptic GluR1 after Gly. Data are expressed as ratio of Glycine/control integrated fluorescence intensity of synaptic GluR1 immunoreactivity. n=20 in each condition, p<0.001 (***), Student’s t test. (C) Gly increases mEPSCs amplitude only in Dyn3 neurons. Example traces of mEPSCs recorded from Dyn3 or Dyn3-PL neurons incubated either with Gly or a control solution. (D) Gly-induced synaptic potentiation. Left: cumulative distribution of mEPSCs amplitude with or without Gly stimulation in Dyn3 (top) and Dyn3-PL (bottom) neurons. Gly-induced increase in mEPSCs amplitude was statistically significant in Dyn3 neurons and not in Dyn3-PL ones (p=0.02 and p=0.23, respectively, Kolmogorov Smirnov test). Right, Quantification of mEPSCs amplitude in the four types of experiments reported in C., n: Dyn3=24-30, Dyn3-PL=32-28, p<0.05 (*) and p<0.01 (**), one way ANOVA. (E) Transient potentiating effect of Gly on excitatory synaptic transmission in Dyn3 neurons. Mean mEPSCs amplitude over time in control conditions and after Gly in Dyn3 (left) and Dyn3-PL (right) neurons. p<0.05 (*), one way ANOVA (n= 6-8 cells/time point). All data are expressed as mean ± SEM
Mobility of newly exocytosed receptors
To determine if newly exocytosed receptors are mobile and enter into synapses, we monitored the surface dynamics of newly exocytosed SEP-GluR1. Fluorescence recovery after photobleaching (FRAP) was performed at the end of the above described protocol to isolate newly exocytosed receptors (Figure 4D). Specifically, we first bleached a large dendritic area and allowed 20 min for specific fluorescence return by exocytosis (Figure S8A). Next, we photobleached a diffraction limited area containing newly exocytosed receptors and assayed their lateral mobility by measuring the FRAP after 200 seconds (Figure 4D upper insets). The same FRAP performed in adjacent regions not exposed to the large bleach provided insight on the mobility of the total population of surface receptors (pre-existing + exocytosed) (Figure 4D lower insets). Strikingly, at Dyn3 synapses the mobility of newly exocytosed receptors was larger than mobility of the total population of surface receptors (Figure 4E), and was comparable to extrasynaptic receptor population (not shown). These findings provide direct evidence that newly exocytosed AMPARs can be incorporated into synapses and represent a pool of highly mobile and not yet stabilized receptors. This was not observed in Dyn3-PL neurons, where the mobility of newly exocytosed receptors at synapses was comparable to that of the total pool of SEP-GluR1 (Figure 4E). Therefore the relatively few exocytosed receptors incorporated at Dyn3-PL synapses did not exhibit increased mobility. Altogether, these data suggest that receptor recycling (requiring the interaction of GluR1 to AP2, the presence of EZs close to the PSD and efficient exocytic machinery) provides synapses with a pool of highly mobile exocytosed receptors.
Figure 4. Displacement of EZ reduces receptor exocytosis and prevents the increased mobility of newly exocytosed receptors.
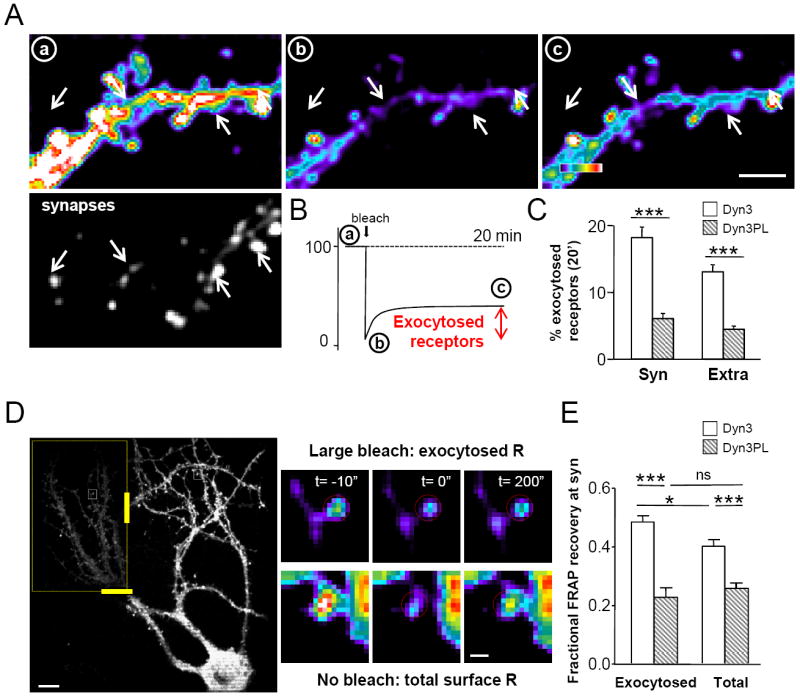
(A) Direct imaging of exocytosed receptors. Top: Pseudocolor images of SEP∷GluR1 fluorescence before (a), just after (b) and 20 minutes after (c) the “large bleach” to eliminate surface receptors fluorescence as explained in Figure S8. The same timepoints are reported in (B) Bottom: Homer∷DsRed fluorescence from the same cotransfected neuron to identify synapses. Arrows indicate example synapses. Scale bar=5μm. (B) Quantification SEP∷GluR1 exocytosis 20 minutes after “large bleach” as described in Supplementary Methods. (C) Quantitative analysis of SEP∷GluR1 exocytosis in Dyn3-Wt and Dyn3-PL neurons at synapses (n=106 and 96) and extrasynaptic zones (n=107 and 109), Student’s t test. (D) Study of exocytosed GluR1 mobility by FRAP. Left: Example neuron transfected with SEP∷GluR1 (left) exposed to the large bleach in the dotted region, before performing FRAP. Scalebar 10 μm. Optical barriers (see Experimental Procedures and Figure S8) are indicated with yellow bars. White squares (in the bleached and non-bleached regions) contain two examples magnified on the right. On the right: pseudocolor images of synapses on the left insets, at - 10”, 0” and 200” of the FRAP protocol to measure receptor mobility. Inset from the large bleached region (top) involves only newly exocytosed receptors; Inset from the non-bleached region includes total surface receptors (bottom). Scalebar 1μm. (E) Quantification by FRAP of receptor mobility. In Dyn3 neurons newly exocytosed receptors are more mobile than total surface receptors at synapses (*, p<0.05). In Dyn3-PL neurons the poor mobility of newly exocytosed receptors is comparable to that of total surface ones (ns). The reduced mobility of total AMPARs in Dyn3-PL synapses compared to Dyn3-Wt ones confirms data obtained by single particle tracking (***, p<0.001). n: Dyn3=88-72; Dyn3PL=63-68. Student’s t test. All data are expressed as mean ± SEM.
Uncoupling EZs from PSDs renders synapses insensitive to Glycine-induced potentiation
Our finding that the EZ and endocytic recycling are required to maintain a mobile population of AMPARs, suggests that the EZ may also be important for increases in synaptic strength. To assess the contribution of local receptor recycling to synaptic potentiation, we used an established protocol to enhance the number of synaptic AMPARs and increase the amplitude of mEPSCs by activating NMDA receptors (Glycine 200 μM + Picrotoxin 1μM for 5 minutes (“Gly stimulation”) (Lu et al., 2001; Park et al., 2004; Passafaro et al., 2001). Quantitative immunocytochemical analysis of surface GluR1 fluorescence intensity revealed that in Dyn3 neurons, Gly induced a doubling of GluR1 accumulation at synapses compared to a control treatment with no drugs. On the contrary, in Dyn3-PL neurons, synaptic GluR1 average intensity was comparable between Gly and control (Figure 5A-B). Furthermore, Gly stimulation induced a significant increase (p<0.05, Student’s t test) in mEPSC amplitudes in Dyn3 neurons, consistent with the observed increase in synaptic GluR1. On the contrary, in Dyn3-PL neurons excitatory synaptic transmission was modestly increased (p=0.09, n=32 and 28, Student’s t test (Figure 5C-E and Supplementary Data)). Therefore, EZ localization is important for synaptic potentiation.
Blocking receptor recycling impairs Glycine-induced AMPAR exocytosis
Gly-induced enhancement of synaptic AMPAR content requires postsynaptic exocytosis (Lu et al., 2001; Park et al., 2004; Passafaro et al., 2001). To test whether the lack of Gly effects on Dyn3-PL neurons was due to impaired receptor exocytosis (and recycling), we monitored SEP∷GluR1 exocytosis during synaptic potentiation. The protocol included “Gly stimulation” immediately after the “large bleach” (Figure 6B). Gly-induced exocytosis was measured at synaptic and extrasynaptic compartments in both unbleached and bleached regions 20 min after the stimulating protocol. Consistent with the results obtained by antibody labelling (Figure 5A-B), Gly treatment nearly doubled total surface synaptic receptors in Dyn3 neurons, while this effect was completely absent in Dyn3-PL neurons (Figure 6C). The measurement of receptor exocytosis in large bleached regions (as measured in Figure 4B) revealed that, compared to basal conditions, Gly stimulation strongly promoted GluR1 exocytosis in Dyn3 neurons and to a lesser extent in Dyn3-PL neurons (Figure 6D). Those data confirm that Gly stimulation induces AMPARs exocytosis.
Figure 6. During synaptic potentiation receptor recycling is essential to allow increased receptor exocytosis and enhanced mobility of newly exocytosed receptors at synapses.
(A) Gly-induced promoted exocytosis is lacking in Dyn3-PL neurons. Representative pseudocolor images of neurons expressing SEP∷GluR1 along with either Dyn3 (top) or Dyn3-PL (bottom) before the “large bleach” (left), right after the bleach (dotted region, middle) and 20 minutes after Gly stimulation (right). Yellow bars represent optical barriers. Scalebar 10μm (B) Exemplification of the protocol applied to measure exocytosed receptors in (A,C,D). Gly was applied immediately after the large bleach (thick arrow). Newly exocytosed receptors were imaged in the bleached region 20 minutes after Gly stimulation (thin arrow). SEP fluorescence in the non-bleached region indicated total surface receptors. (C) Quantification of Gly effect on total surface synaptic GluR1. SEP∷GluR1 fluorescence intensity at synapses in the non-bleached region 20 minutes after Gly is normalized to that before Gly in Dyn3 and Dyn3-PL neurons. n: Dyn3=86; Dyn3-PL=98, p<0.001(***), non significant (ns), Student’s t test. (D) Gly-induced exocytosis. SEP∷GluR1 fluorescence of exocytosed receptors at synapses in the large bleached region after Gly is expressed as a percentage of the fluorescence in the same synapses before the large bleach. The values measured during basal activity and after synaptic stimulation are reported. n=106-115; 96-127, p<0.001 (***), non significant (ns), one way ANOVA. (E) Mobility of exocytosed receptors, measured by adding FRAP at the end of the protocol in (B). Pseudocolor images of synapses (dotted red region) from Dyn3 (right) and Dyn3-PL (right) neurons at different time points of the FRAP protocol, as indicated. Scale bar 1μm. (F) Mobility of total surface receptors at synapses expressed as FRAP after 200”, normalized to the fluorescence before, setting to zero the residual fluorescence at the bleach. The values of synaptic receptors in Dyn3 and Dyn3-PL neurons are reported. n=88-99; 63-92, p<0.05 (*), non significant (ns), one way ANOVA. (G) Mobility of newly exocytosed receptors at synapses from Dyn3 and Dyn3-PL neurons during basal activity and after Gly. n=75-81; 58-67, p<0.05 (*), p<0.001 (***), non significant (ns), one way ANOVA. All data are presented as mean ± SEM.
The reduced Gly potentiation observed in Dyn3-PL neurons might be due to impaired filling of recycling vesicles following EZ displacement. Therefore, to measure the amount of receptors in the reserve pool, we performed the “large bleach” protocol 3 minutes after Gly stimulation (Figure S9A) in order to immediately photobleach newly exocytosed receptors. The reserve pool of receptors available for exocytosis was quantified 20 min after the large bleach as increased surface SEP-GluR1 fluorescence. Interestingly, the values measured for both Dyn3-Wt and Dyn3-PL neurons were modest compared to Gly-induced or basal exocytosis (Figure S9B). This indicates that the Gly stimulation empties the reserve pool of receptors.
Receptor recycling during synaptic potentiation provides a major source of mobile receptors at synapses
We next investigated AMPAR surface mobility during synaptic potentiation. The effects of Gly treatment were tested 20 minutes after stimulation by performing FRAP on small synaptic and extrasynaptic regions expressing SEP-GluR1 (Figure 7E). Interestingly, Gly stimulation reduced the mobility of total (pre-existing + exocytosed) surface receptors at synapses in Dyn3 neurons, but had no effect on synapses in Dyn3-PL neurons (Figure 7F). In order to dissect out the dynamic behavior of newly exocytosed receptors from total surface receptors, the “large bleach” protocol was performed immediately before Gly treatment (Figure 7B). In Dyn3 neurons we observed a larger GluR1 fractional FRAP recovery 20 min after Gly stimulation, indicating that exocytosed GluR1 receptors at synapses were more mobile than in basal conditions (Figure 7G). This effect was not observed in Dyn3-PL synapses, indicating that during synaptic potentiation receptor recycling represents a major source of mobile exocytosed receptors at synapses.
Figure 7. Impairment of receptor recycling prevents Glycine-induced accumulation and global immobilization of AMPARs at synapses.
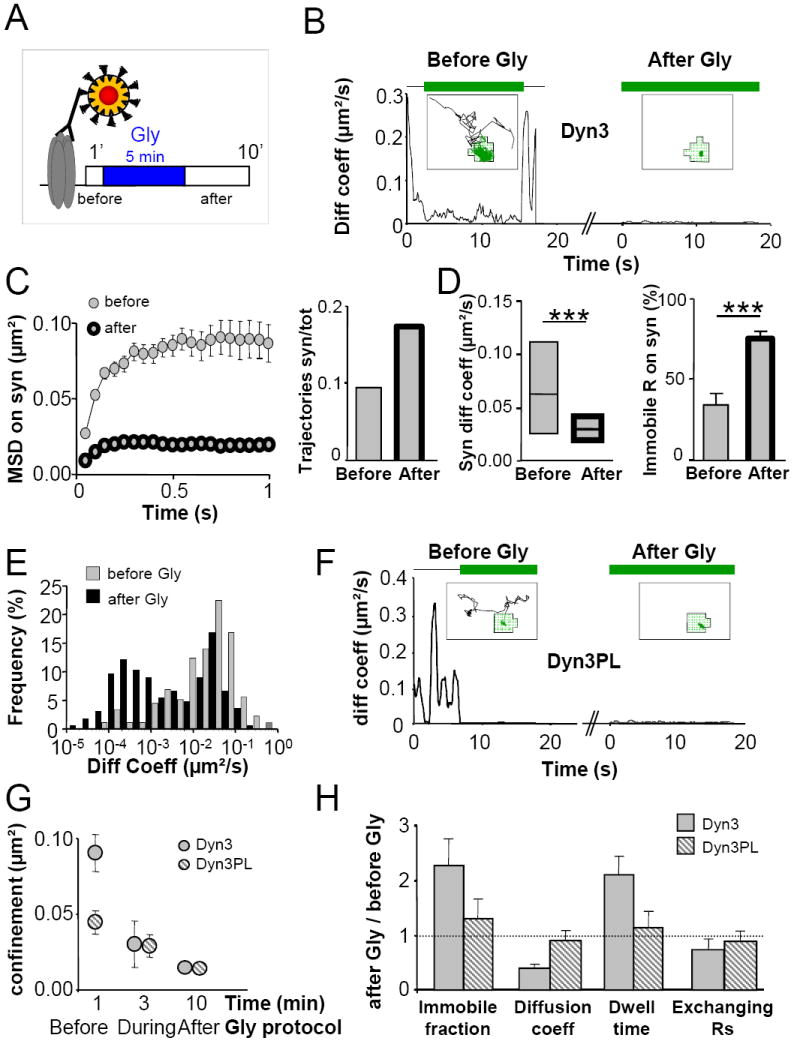
(A) Schematic diagram of the protocol used to follow the influence of Gly on AMPAR present at the neuronal surface before stimulation. Receptors were first coupled to QDs and then their mobility was recorded 1 min before Gly and 10 min after Gly. (B) In Dyn3 neurons Gly promotes synaptic accumulation and stabilization of GluR1 present at the surface before stimulation. Instantaneous diffusion coefficient over time of a representative GluR1-QD complex before and after Gly. Above lines represent synaptic (green) and extrasynaptic (black) localization of the particle. Insets: Corresponding trajectories of the same receptor-QD complex exploring the synapse (green) and the extrasynaptic compartment (black) before and after Gly. (C) Gly induces strong receptor accumulation and confinement at synapses. Left, MSD versus time plot of synaptic receptors before and after Gly. Right, normalized number of trajectories (at synapse / total) observed in the same dendritic region before and after Gly stimulation (n=991-955). (D) Gly reduces GluR1 mobility at synapses. Median (± IQR) diffusion coefficient of mobile receptors and percentage of immobile receptors at synapses, before and after Gly (p<0.001, Mann-Whitney test). (E) Both the mobile and the immobile pools of synaptic receptors are affected by Gly stimulation in Dyn3 neurons. Diffusion coefficient distribution of synaptic GluR1 before and after Gly. Receptors with diffusion coefficients below 0.0075μm2/s are considered immobile. (F) EZ displacement prevents Gly effects on GluR1 lateral mobility. Instantaneous diffusion coefficient of a GluR1-QD complex exploring a synapse before (left) and after (right) Gly in a Dyn3-PL neuron. Color codes as in (B). (G) The poor mobility of synaptic GluR1 in Dyn3-PL neurons prevents the immobilizing effect of Gly. Confinement (plateau of the MSD versus time curve) of GluR1-QD complexes before, during and after Glycine application in Dyn3 and Dyn3-PL neurons. (H) Normalized values of immobile fraction, diffusion coefficient, dwell time and exchanging fraction of synaptic receptors in Dyn3 and Dyn3-PL neurons (after Gly/before Gly). The dotted lines represent no changes after Gly. Unless otherwise stated, data are expressed as mean ± SEM.
Surface receptors are immobilized at synapses following glycine stimulation
Our FRAP data from total and newly exocytosed receptors at synapses (Figure 6F-G) suggested an immobilizing effect of synaptic potentiation on pre-existing surface AMPARs. We tested this hypothesis by single-particle tracking. QD labelling was performed before Gly treatment in order to selectively track receptors present at the neuronal membrane before synaptic potentiation (Figure 7A). Following Gly treatment, we found more receptors at synapses in Dyn3 neurons, but with a smaller mobility (Figure 7B-E). The proportion of observed GluR1-QD trajectories at synapses doubled after the stimulating protocol (Figure 8C). This was accompanied by a reduced fraction of exchanging receptors (74 ± 6 % before, 55 ± 10 % after Gly, p<0.05, not shown) and prolonged receptor dwell time (1.5 ± 0.2 s before, to 2.8 ± 0.4 s after Gly, p<0.05, not shown), indicating that after Gly stimulation there is an increased number of receptor-QD complexes at synapses. Thus, in addition to the previously demonstrated increase in AMPAR insertion in the membrane (Park et al., 2004; Passafaro et al., 2001; Yudowski et al., 2007), Gly treatment promotes accumulation and stabilization of pre-existing extrasynaptic surface AMPARs at synapses. Control experiments, performed by applying vehicle instead of Gly, excluded possible bias due to drug application (Figure S10).
Figure 8. Proposed role of receptor recycling during basal activity and synaptic potentiation.
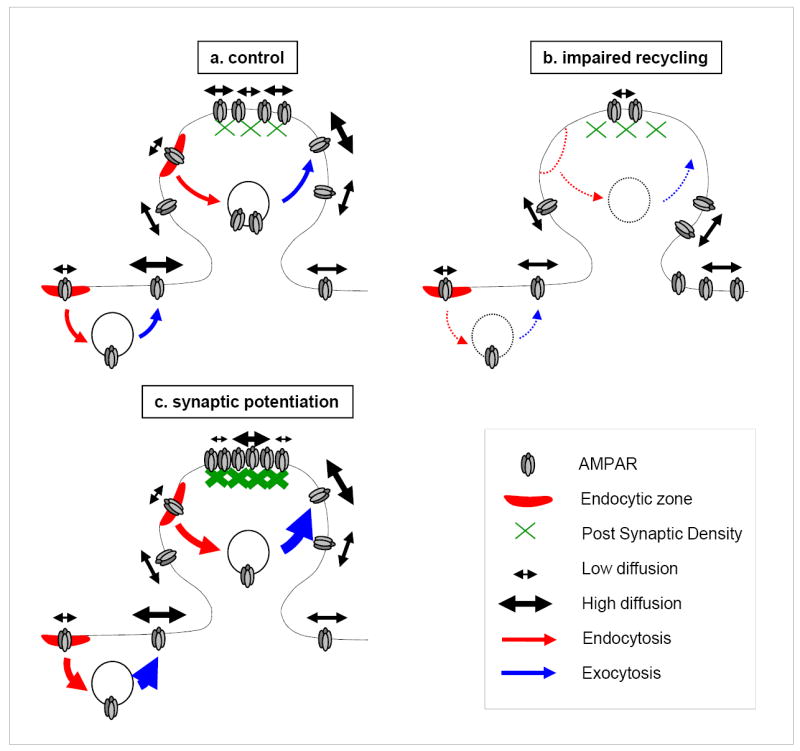
(a) During basal synaptic activity the presence of intact receptor recycling (control) provides a constant source of mobile receptors to synapses. At synapses (green), there are mobile and immobile pools of AMPARs. Mobile receptors leaving synapses can be trapped at EZs (red) either for transient stabilization or for endocytosis (red arrow) and recycling (blue arrow). Newly exocytosed receptors exhibit high mobility and accumulate at synapses. The presence of EZs near PSDs prolongs the time mobile receptors spend close to synapses. The location of exocytic sites is being still debated, the model thus includes exocytosis both at spines and at dendritic shafts. (b) Impairment of receptor recycling leaves synapses with few receptors mainly by reducing receptor exocytosis and by allowing mobile receptors to diffuse away. During impaired recycling (by displacement of EZs, by impaired receptor interaction with the endocytic machinery or by reduced exocytosis of recycling receptors, dotted lines and arrows), the poor mobility of synaptic receptors can be explained by the presence of more free docking sites available at the PSD (green), since the density of postsynaptic scaffold proteins is unchanged and the number of receptors reduced. (c) During synaptic potentiation the increased receptor accumulation at synapses is due to: i) enhanced receptor recycling, ii) stabilization at synapses of receptors previously present at the neuronal surface.
Impairment of receptor recycling prevents Glycine-induced immobilization of synaptic GluR1 through depletion of the mobile receptor pool
We next tested the importance of AMPAR recycling for the expression of synaptic potentiation. As expected, the lower mobility of synaptic GluR1 at synapses lacking EZs (Figure 2) was little affected after Gly treatment (Figure 7F and H). Therefore, in order to study Gly-stimulation induced changes in AMPAR dynamics over time, we monitored receptor confinement at synapses during synaptic potentiation (Figure 7G). In Dyn3 neurons, the confinement of surface synaptic receptors was significantly reduced during Gly application (3 min) as compared to the control period before Gly application, thus indicating an immediate effect on AMPAR mobility during the induction of synaptic potentiation. A further minor reduction was observed after Gly application (10 min). In contrast, receptor confinement in Dyn3-PL neurons was greater than in Dyn3-Wt neurons before Gly treatment (Figure 7G, first time point) and only a moderate further immobilization of AMPARs was observed after Gly application. Altogether, these experiments establish that the immobilizing effect of Gly cannot be observed in Dyn3-PL expressing neurons. Furthermore, they suggest that impairment of receptor recycling prevents the expression of synaptic potentiation by inducing the loss of the mobile pool of synaptic receptors.
DISCUSSION
Through multiple independent manipulations, we have found that AMPAR recycling is important to supply a mobile pool of AMPARs to synapses. Supporting this model are our findings that EZ displacement and recycling endosome inhibition reduce receptor exocytosis and deplete the mobile population of postsynaptic AMPARs. Based on these finding, we propose a model in which the pool of mobile AMPARs observed at synapses arises from recycled receptors initially captured at EZs (Figure 8). The reduced number and mobility of synaptic receptors following EZ displacement is due to impaired filling of the recycling receptor pool and subsequent decreased receptor exocytosis. During synaptic potentiation, accumulation of AMPARs at synapses relies on synaptic trapping of a mobile pool of surface receptors and exocytosis of intracellular receptors that become mobile on the surface. Both mechanisms require intact recycling sustained by the presence of an EZ adjacent to the PSD and imply global AMPAR stabilization at synapses. Altogether these data point towards a pivotal role for EZs and receptor recycling in controlling AMPAR accumulation and mobility at synapses both during basal activity and synaptic potentiation.
AMPA receptor reversible stabilization at EZs
Transient interactions with transmembrane, intracellular and extracellular proteins (Bats et al., 2007; Choquet and Triller, 2003; Elias et al., 2006) influence the lateral diffusion of AMPARs in the neuronal membrane (Borgdorff and Choquet, 2002; Groc et al., 2004; Tardin et al., 2003), resulting in a dynamic equilibrium between diffusive and stabilized receptor states that regulates receptor accumulation at specific compartments. In the present study, we provide the first direct measurement of AMPARs lateral mobility at clathrin-rich zones. GluR1 dynamic behaviour at EZs was similar to that at synapses, namely slow mobility, reversible confinement and the ability to exit the compartment (Movies S2 and S3). Analogous to their behaviour at synapses, this is likely due to reversible interactions of AMPARs with proteins of the clathrin endocytic machinery such as the AP2 adaptor complex (Lee et al., 2002). Reduced GluR1 mobility and confinement at EZs is not associated with detectable receptor accumulation, as is the case at synapses, and likely originates from the recurrent endocytosis of surface AMPARs stabilized at EZs. A similar cargo dependent stabilization and commitment to endocytosis of CCPs has been demonstrated in non-neuronal cells (Ehrlich et al., 2004).
Role of EZ and receptor recycling in maintaining a pool of mobile receptors at synapses
Although counterintuitive, EZ displacement depletes synapses of mobile AMPARs. We propose that this defect arises from impairment of receptor recycling that decreases AMPAR exocytosis and thus enrichment of synapses with newly exocytosed mobile receptors. In addition, the reduced trapping of mobile receptors at clathrin-rich zones due to EZ displacement allows them to diffuse away from synapses to join the mobile pool of extrasynaptic receptors. The evidence that receptor endocytosis still occurs at displaced EZ can account for the unaffected AMPAR extrasynaptic content in Dyn3 mutants.
As EZ displacement does not alter synaptic scaffold composition (Figure S5B), the strong reduction in synaptic receptor content in Dyn3 mutants (Figure 2B) likely leaves available free docking sites or slots at synapses (see the Supplementary Discussion on the absence of detectable side effects of Dyn3 mutants).
We have demonstrated that AMPAR exocytosis from recycling endosomes represents a constant source of mobile receptors. In Dyn3 mutants, newly exocytosed mobile receptors could thus immediately be immobilized at free slots in the PSD. Although we cannot formally exclude the possibility that EZ displacement affects the molecular mechanisms of AMPAR stabilization at synapses, we favor a model whereby Dyn3-PL neurons maintain a large number of free PSD slots which can readily capture freely diffusing exocytosed receptors, and thereby, prevent the enrichment of AMPARs mobile pool at synapses.
Synaptic potentiation and mobile AMPARs
Activity-dependent regulation of AMPAR number at synapses is one of the major postsynaptic effectors of synaptic plasticity (Malenka and Bear, 2004; Shepherd and Huganir, 2007). Here, using FRAP of SEP∷GluR1 and tracking QD-tagged receptors, we were able to monitor changes in the mobility of different pools of AMPAR during synaptic potentiation as considered in the Supplementary Discussion. Yudowski et al., (2007) reported that the Gly-induced increase in the frequency of SEP-GluR1 exocytosis events was often associated with rapid lateral diffusion of inserted receptors to neighbouring spines. Beyond confirming this data, our results show that Gly-induced GluR1 exocytosis provides a larger pool of mobile receptors at synapses than basal receptor exocytosis. It is worth noting that we never observed any directed movement towards or away from the synapse that could be indicative of an active transport on the neuronal surface. The higher number of AMPAR at synapses following Gly stimulation results from synaptic incorporation of newly exocytosed receptors and previously existing receptors diffusing in the synapse vicinity (Boehm et al., 2006; Oh et al., 2006).
Global AMPAR stabilization at synapses following Gly stimulation is formally different from that observed upon impairment of receptor recycling as discussed above. In fact during synaptic potentiation AMPARs are likely stabilized through a yet to be characterized modification of an interaction of the AMPAR complex with scaffold proteins, presumably through activation of a calcium-dependent signalling cascade (Borgdorff and Choquet, 2002; Heine et al., 2008).
Synaptic potentiation and receptor recycling
AMPAR recycling plays a key role during synaptic plasticity as it tunes receptor abundance at synapses by setting the equilibrium between receptor endocytosis, reinsertion to the plasma membrane and degradation (Ehlers, 2000; Park et al., 2004; Turrigiano, 2000). Here we provide the first evidence that postsynaptic endocytosis is important for the expression of synaptic potentiation. Specifically, we demonstrate that the presence of EZ close to the PSD, allows Gly-induced potentiation of synaptic transmission and modulates receptor mobility by sustaining correct receptor recycling. It is likely that removal of EZs from the proximity of the PSD prevents filling of the receptor recycling pool, thus reducing the availability of newly exocytosed receptors to be accumulated at synapses during synaptic potentiation (Figure 8). Indeed, Gly stimulation induces a nearly complete depletion of the intracellular recycling pool (Figure S8) and correlates with increased delivery of recycling endosomes to spines (Park et al., 2006; Wang et al., 2008) and activity-dependent activation of endocytosis-related proteins such as Arc/Arg3.1 (Chowdhury et al., 2006), CPG2 (Cottrell et al., 2004) and Rab5 (Brown et al., 2005). The lack of Gly-induced potentiation in Dyn3-PL neurons is not due to prior depletion of an extrasynaptic receptor pool required for cLTP as the density of extrasynaptic GluR1 is rather slightly increased upon EZ displacement in basal conditions. Recent results demonstrating Ca2+-dependent mobilization of recycling endosomes into spines by myosin Vb and the endosomal adaptor Rab11-FIP2 (Wang et al., 2008) suggest that, as with the EZ, the positioning of endosomes in spines contributes to the local pool of activity-regulated cycling receptors (Correia et al., 2008; Lu et al., 2007; Wang et al., 2008).
In conclusion, our work reveals that the regulation of AMPAR accumulation at synapses occurs via a complex equilibrium of receptor fluxes between specialized membrane zones (the PSD and the EZ) and intracellular compartments, notably recycling endosomes. The precise spatial arrangement of these subcellular domains around synapses appears to have important functional consequences for the control of synaptic transmission.
EXPERIMENTAL PROCEDURES
Plasmid constructs, primary neuronal cultures and transfection, antibodies and drugs, acid strip, QD labeling and live cell imaging, confocal imaging, immunocytochemistry methods, electrophysiology and statistics are included in the supplementary methods.
Single Particle Tracking
Single QDs, recognized by their diffraction-limited fluorescence spot size and characteristic blinking, were identified with 2D object segmentation by wavelet transform (Groc et al., 2007; Racine et al., 2007) and tracked with subwavelenght accuracy (~40 nm) in each frame as in Heine et al., (2008). QD coordinates were compared to those of synapses and clathrin puncta, identified as sets of connected pixels obtained using 2D object segmentation by wavelet transform from Homer∷GFP and clathrin∷DsRed fluorescence spots. The exchanging fraction was calculated as the fraction of total GluR1-QD complexes that moved at least once into and away from a given compartment during the acquisition time. The comparison between Dyn3-PL and Dyn3-Wt neurons was focused on EZ-negative and EZ-positive synapses, respectively, while endogenous EZ-negative synapses in Wt neurons and EZ-positive synapses still present in mutant neurons were removed from the analysis.
Imaging of newly exocytosed receptors
Neurons cotransfected with DsRed∷Homer and SEP-GluR1 were imaged on an inverted microscope with 473 nm and 532 nm lasers. Photobleach was performed with a Saphire laser 488nm (200mW) at 70% power. The laser was coupled to the microscope via galvometric mirrors, which allowed rapid photobleaching of several regions. At the beginning of each experiment controls were performed to check for the presence of intracellular quenched receptors with NH4Cl (50mM) and to confirm the pH-sensitivity of surface SEP-GluR1 as the disappearance of SEP fluorescence upon application of acidic solution (pH=5.5, same solution used in electrophysiology, with MOPS replacing HEPES). Since photobleach affects only fluorescent (surface) receptors and not quenched ones (intracellular), the photobleach of a large portion of dendrites (“large bleach”) was performed in order to ablate SEP∷GluR1 surface fluorescence. Exocytosis was observed after 20 min as the increased fluorescence in the large bleached region due to quenched intracellular receptors reaching the neuronal surface. As represented in Figure S8A panel c, we applied an optical barrier at the edge of the bleached region in order to avoid the lateral diffusion of non-bleached surface receptors. Exocytosed receptors at synapses were measured as SEP fluorescence in small regions of interest (800nm diameter) colocalizing with synaptic marker. Experiments were conducted in an open chamber at 37°C.
Fluorescence recovery after photobleaching (FRAP)
FRAP of SEP-GluR1 was used to measure receptor mobility. Diffraction-limited regions expressing SEP-GluR1 were photobleached for 5ms. Recovery from photobleach was monitored by 200s consecutive acquisitions at 0.5Hz and normalized to the fluorescence measured before the photobleach. Residual fluorescence right after the photobleach was set to zero. Recovery curves were corrected for continous photobleach and background noise. When the FRAP protocol was performed in the large bleached regions, only newly exocytosed receptors were imaged.
Supplementary Material
Acknowledgments
We thank C. Breillat, D. Bouchet and B. Tessier for help in molecular biology and cell cultures, C. Poujol and P. Legros from the PICIN for support in confocal imaging. We thank R. Huganir for generous gift of the anti-GluR1 antibody. We thank T. Newpher for carefully editing the manuscript. This work was supported by grants from the Centre National de la Recherche Scientifique, the Conseil Régional d’Aquitaine, the Ministère de la Recherche, the Fondation pour la Recherche Médicale, The Human Frontier Science Program to D.C., and the European Community’s Sixth Framework Programme (FP6) through an EIF Fellowship awarded to E.M.P., and by grants from the NIH to M.D.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24:5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999a;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999b;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci. 2003;4:251–265. doi: 10.1038/nrn1077. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- Cottrell JR, Borok E, Horvath TL, Nedivi E. CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron. 2004;44:677–690. doi: 10.1016/j.neuron.2004.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. Embo J. 2006;25:1623–1634. doi: 10.1038/sj.emboj.7601065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NW, Fourgeaud L, Huang B, Chen J, Cao H, Oswald BJ, Hemar A, McNiven MA. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol. 2003;13:510–515. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- Groc L, Lafourcade M, Heine M, Renner M, Racine V, Sibarita JB, Lounis B, Choquet D, Cognet L. Surface trafficking of neurotransmitter receptor: comparison between single-molecule/quantum dot strategies. J Neurosci. 2007;27:12433–12437. doi: 10.1523/JNEUROSCI.3349-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, Ehlers MD. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Internalization at glutamatergic synapses during development. Eur J Neurosci. 2003;18:3207–3217. doi: 10.1111/j.1460-9568.2003.03074.x. [DOI] [PubMed] [Google Scholar]
- Pickard L, Noel J, Duckworth JK, Fitzjohn SM, Henley JM, Collingridge GL, Molnar E. Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors at hippocampal neuronal plasma membranes. Neuropharmacology. 2001;41:700–713. doi: 10.1016/s0028-3908(01)00127-7. [DOI] [PubMed] [Google Scholar]
- Racine V, Sachse M, Salamero J, Fraisier V, Trubuil A, Sibarita JB. Visualization and quantification of vesicle trafficking on a three-dimensional cytoskeleton network in living cells. J Microsc. 2007;225:214–228. doi: 10.1111/j.1365-2818.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. Embo J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron. 2008;59:359–374. doi: 10.1016/j.neuron.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. AMPA receptors unbound: membrane cycling and synaptic plasticity. Neuron. 2000;26:5–8. doi: 10.1016/s0896-6273(00)81131-9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.