Abstract
Background
Intermittent antegrade cold-blood cardioplegia followed by terminal warm-blood cardioplegic reperfusion or hot-shot is reported to reduce myocardial injury in the setting of coronary surgery. The efficacy of this cardioplegic technique in patients with left ventricular hypertrophy secondary to aortic stenosis remains uncertain.
Methods
Thirty-six patients with left ventricular hypertrophy undergoing aortic valve replacement were prospectively randomized to cold-blood cardioplegia either alone (cold-blood cardioplegia group) or with retrograde hot-shot (hot-shot group). Reperfusion injury was assessed by measuring myocardial levels of adenosine triphosphate and lactate in left and right ventricular biopsies taken 5 minutes after institution of cardiopulmonary bypass and 20 minutes after removal of cross-clamp using high-performance liquid chromatography and enzymatic techniques. Myocardial injury was assessed by serial release of troponin I up to 48 hours postoperatively. Overall clinical outcome was prospectively collected.
Results
Baseline and intraoperative characteristics were similar between groups. In the hot-shot group, there were no significant changes in the myocardial concentration of adenosine triphosphate and lactate in both left and right ventricular biopsies after reperfusion. In the cold-blood cardioplegia group, there was a trend to a fall in adenosine triphosphate levels in the left and right ventricular biopsies after reperfusion, but this reached statistical significance only in the right ventricle. Troponin I release was raised in both groups at 4 and 12 hours after surgery (p < 0.05), but did not reach levels of myocardial infarction.
Conclusions
The terminal retrograde hot-shot reperfusion does not add any extra benefit to antegrade cold-blood cardioplegia in preventing myocardial injury in patients with left ventricular hypertrophy undergoing aortic valve replacement. Nevertheless, it appears to reduce ischemic stress in the right ventricle. There was no difference in clinical outcome between groups.
Left ventricular (LV) hypertrophy increases end-diastolic volume and pressure, myocardial work, and oxygen demand [1]. Its metabolic state is anaerobic [2], with increased vulnerability to ischemia and reperfusion injury. Surgically induced ischemia and reperfusion continues to be a major contributor to morbidity and mortality after cardiac surgery in such patients and has been attributed to inadequate myocardial protection [3, 4]. Nevertheless, little work has been carried out on myocardial protection in patients with LV hypertrophy in whom the choice of optimal cardioplegia remains controversial [5-8]. We have previously shown that the metabolic state of hypertrophic hearts is different from that with ischemic disease [9]. Therefore, cardioprotective strategies developed for ischemic hearts should not be extrapolated uncritically to hypertrophic hearts.
Postischemic recovery might be improved by using a terminal warm-blood cardioplegic reperfusate or hot-shot (HS), which allows cellular energy stores to be regenerated and channeled into repairing reversibly injured myocardium during a period of electromechanical quiescence [10, 11]. This has been shown to improve metabolic and short-term functional recovery and to decrease mortality in cardiac surgery operations [12, 13].
The aim of this prospective study was to compare the efficacy of intermittent antegrade cold-blood (CB) cardioplegia with or without retrograde terminal warm-blood cardioplegic reperfusion HS on the intracellular concentrations of biochemical markers of ischemic and metabolic stress and on clinical outcome in patients with LV hypertrophy secondary to aortic stenosis undergoing aortic valve replacement. Our rationale of using a retrograde delivery for the HS is mostly to optimize coronary artery and aortic root removal of air and to shorten ischemic time as the retrograde HS can be delivered while closing the transverse aortotomy.
Material and Methods
Adult patients undergoing elective aortic valve replacement surgery for aortic stenosis between October 2001 and September 2003 at the Bristol Royal Infirmary were prospectively randomized to receive CB cardioplegia either with terminal HS (HS group; n = 16) or without (CB group; n = 20).
Exclusion criteria included coronary artery disease, concomitant aortic regurgitation, LV ejection fraction of less than 0.30, history of congestive heart failure, diabetes mellitus, and reoperation, emergency, or salvage procedures. Eligibility for surgery was based on the medical history, echocardiography, and the most recent angiogram.
Simple random treatment allocations (ie, not blocked or stratified) were generated in advance of starting the study and were concealed in sequentially numbered and sealed opaque envelopes. After written informed consent was obtained, a patient was randomized by opening the next numbered envelope. The study was approved by the United Bristol Healthcare Trust Ethics Committee, and all patients gave informed consent.
Operative Procedures
Anesthetic and surgical techniques have been reported previously [14, 15]. Briefly, a standard cardiopulmonary bypass circuit was primed with 1,000 mL of Hartmann’s solution, 500 mL of colloidal solution, 0.5 g/kg mannitol, 7 mL of 10% calcium gluconate, and 6,000 IU of heparin. Nonpulsatile flow rates throughout bypass were 2.4 L/m2 per minute. Systemic temperature was actively cooled down to 32°C. The LV was vented in all patients through the right superior pulmonary vein. Myocardial protection was achieved with antegrade CB (6° to 8°C) cardioplegia, with added potassium and magnesium to give a final concentration of 20 mmol/L potassium and 5 mmol/L magnesium. The CB cardioplegic solution was similar in the two groups; it was a mixture of the patient’s blood withdrawn from the cardiopulmonary bypass circuit and St. Thomas’s I cardioplegic solution (4 parts blood to 1 part St. Thomas’s I) [7, 11]. The delivery of CB cardioplegia was similar between the two groups. After cross-clamping and opening of the ascending aorta, the cardioplegia was administered directly into the coronary ostia as a 1-L bolus (700 mL in the left and 300 mL in the right ostia) at a pressure of 120 mm Hg (total delivery time approximately 3 minutes). Infusions of 200 mL for each ostium were repeated at 15-minute intervals. In the CB with HS group, CB cardioplegia was also delivered directly in the coronary ostia, and the final HS was delivered by means of the retrograde route (to optimize coronary and aortic root air removal and to save 6 to 8 minutes of ischemic time required for aortotomy suturing). A retrograde coronary sinus cardioplegic catheter (Edwards LifeSciences, Irvine, CA) was introduced through a pursestring suture into the right atrium and guided into the coronary sinus. The catheter was inserted for 1.5 cm in the coronary sinus, and its position was checked by manual palpation and pressure, and by the type of peculiar wave pressure. The HS solution was identical in composition to the induction and maintenance doses and was administered at 300 mL for 2 minutes at 37°C immediately before unclamping the aorta.
Postoperative Management and Assessment of Clinical Outcome
Patients were admitted to the intensive care unit after the operation and managed by intensivists. Decisions regarding inotropic support and ventilation were based on unit protocols, hemodynamic status, and clinical judgment [14, 15]. Intraoperative and postoperative clinical variables were prospectively recorded. Heart rate and rhythm were continuously monitored and displayed on a monitor inclusive of an automated detector of arrhythmia during the first 72 hours postoperatively. Twelve-lead electrocardiographic recordings were performed preoperatively, 2 hours postoperatively, and then daily thereafter until discharge. Clinical diagnostic criteria for perioperative myocardial infarction were new Q waves of greater than 0.04 ms or a reduction in R waves greater than 25% in at least two leads. Biochemical diagnostic criteria for perioperative myocardial infarction were peak troponin I concentrations higher than 3.7 g/L and a troponin I concentration greater than 3.1 g/L 12 hours postoperatively or greater than 2.5 g/L 24 hours postoperatively [16, 17].
Collection of Ventricular Biopsy Specimens
Transmural biopsies of the LV apical or anterolateral free wall and the right ventricular (RV) free wall (4 to 12 mg wet weight) were taken using a Trucut needle (Baxter Healthcare Corporation, Northbrook, IL). Two biopsies were collected from each ventricle; the first biopsy 5 minutes after institution of cardiopulmonary bypass before aortic cross-clamping (control), the second after 20 minutes of reperfusion after removal of the aortic cross-clamp. Each specimen was immediately frozen in liquid nitrogen until processing for analysis of adenosine triphosphate (ATP) and lactate, as previously reported [9, 14, 18]. A research technician blind to the operative technique performed the analyses.
Measurement of Cardiac Troponin I
Serum concentrations of cardiac troponin I were determined before surgical intervention and at 1, 4, 12, 24, and 48 hours postoperatively by using the ACCESS Immunoassay System (Beckman Instruments, Fullerton, CA).
Statistical Analysis
Data were expressed as mean ± standard error of the mean unless stated otherwise. Categorical variables were analyzed using either the Fisher’s exact test or the χ2 test as appropriate. Comparison between continuous variables within and between groups was tested using a nonparametric test (Wilcoxon’s signed rank test). Because ATP measurements were carried out using enzymatic and high-performance liquid chromatography techniques, the actual values are given in the results section but are expressed as percentage of change at reperfusion versus baseline values in the relevant figure. All statistical analyses mentioned were performed with the aid of a computerized software package (Statview for Windows; SAS Institute Inc, Cary, NC).
Results
Clinical Outcome
A total of 36 patients were recruited into the study. Preoperative characteristics are shown in Table 1. Intraoperative and postoperative data are shown in Table 2. There were neither in-hospital deaths nor perioperative myocardial infarction as per predefined troponin I levels or serial postoperative electrocardiographs. The total incidence of postoperative arrhythmias including requirement for temporary pacing, permanent pacemaker, and atrial fibrillation did not differ between groups. No differences were observed between groups with regard to postoperative incidence of renal, respiratory, and neurologic complications (data not shown).
Table 1. Preoperative Dataa.
| Variable | CB (n = 20) |
CB + HS (n = 16) |
|---|---|---|
| Male | 12 (60%) | 10 (62.5%) |
| Age (y) | 65.3 ± 9.6 | 64.8 ± 7.3 |
| BSA (m2) | 1.91 ± 0.22 | 1.87 ± 0.19 |
| History of hypertension | 13 (65%) | 10 (62.5%) |
| Ejection fraction | ||
| Good (>0.49) | 16 (80%) | 12 (75%) |
| Fair (0.30–0.49) | 4 (20%) | 4 (25%) |
| Ventricular mass index (g/m2) | 181 ± 42 | 179 ± 37 |
| Transvalvular peak gradient (mm Hg) | 75.7 ± 21.4 | 79.1 ± 18.2 |
| NYHA class | ||
| I–II | 12 (60%) | 10 (62.5%) |
| III–IV | 8 (40%) | 6 (37.5%) |
| Euro score | 4.1 ± 0.8 | 4.0 ± 1.1 |
Data are presented as mean ± standard deviation or as number with percentage in parenthesis.
BSA = body surface area; CB = cold-blood cardioplegia; HS = retrograde hot-shot cardioplegia; NYHA = New York Heart Association.
Table 2. Intraoperative and Postoperative Dataa.
| CB | CB + HS | |
|---|---|---|
| Variable | (n = 20) | (n = 16) |
| CPB time (min) | 112.5 ± 23.8 | 108.9 ± 27.3 |
| Cross-clamp time (min) | 68.7 ± 21.7 | 73.2 ± 19.8 |
| Mean valve size inserted (mm) | 21.6 ± 3.1 | 21.8 ± 2.9 |
| Rhythm after cross-clamp removal | ||
| Sinus | 15 (75%) | 12 (75%) |
| Pacing | 3 (15%) | 4 (25%) |
| VF | 1 (5%) | 0 |
| Perioperative MI | 0 | 0 |
| Low cardiac output | 0 | 0 |
| Postoperative arrhythmia | 5 (25%) | 2 (12.5%) |
| Reopening for bleeding | 0 | 0 |
Data are presented as mean ± standard deviation or as number with percentage in parenthesis.
CB = cold-blood cardioplegia; CPB = cardiopulmonary bypass; HS = retrograde hot-shot cardioplegia; MI = myocardial infarction; VF = ventricular fibrillation.
Myocardial Metabolic Changes
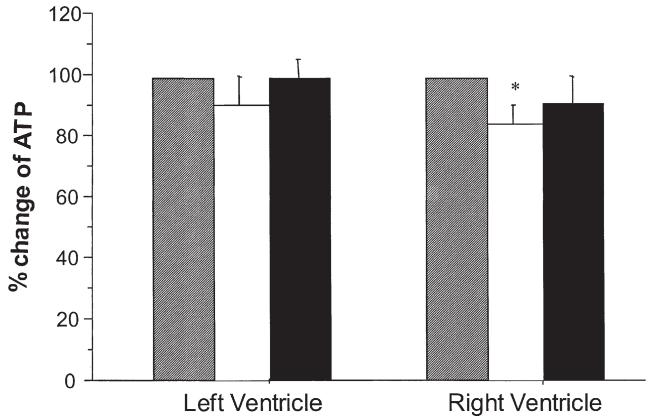
Percent changes between control and reperfusion in ATP levels for both LV and RV are shown in Figure 1. In the HS group there were no significant differences in levels of ATP as a result of reperfusion in LV biopsies (from 25 ± 2 to 25 ± 2 nmol/mg protein), but there was a small insignificant decrease in ATP in RV biopsies (from 27 ± 3 to 23 ± 3 nmol/mg protein; p = 0.13). On the other hand, the changes in the CB group showed a small significant fall in ATP in the RV biopsy (39 ± 3 to 32 ± 2 nmol/mg protein; p < 0.05) but not in the LV biopsy (34 ± 2 to 30 ± 3 nmol/mg protein; p = 0.17).
Fig 1.
Changes in adenosine triphosphate (ATP; percent change compared with preischemia; gray bars) in left and right ventricles during cold-blood cardioplegia with (HS; black bars) or without (CB; white bars) terminal hot-shot. Biopsies were collected from left and right ventricle before cross-clamping the aorta (control = 100%) and 20 minutes after reperfusion. *p < 0.05.
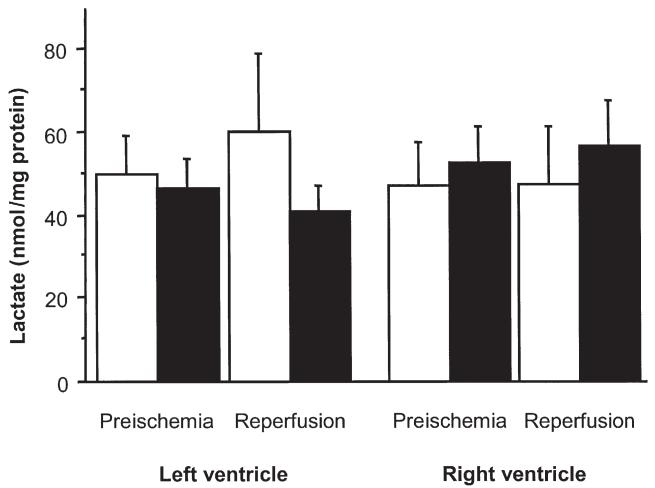
Changes in lactate are shown in Figure 2. In the CB group there was a trend for a higher release of lactate at reperfusion in the LV biopsy, but this did not reach statistical significance (from 42 ± 8 to 50 ± 13 nmol/mg protein and from 46 ± 7 to 41 ± 6 nmol/mg protein, CB versus HS groups, respectively; p = 0.1).
Fig 2.
Changes in myocardial lactate concentration in left and right ventricles during cold-blood cardioplegia with (HS; black bars) or without (CB; white bars) terminal hot-shot. Biopsies were collected from the left and right ventricles before cross-clamping the aorta (preischemia) and 20 minutes after reperfusion.
Myocardial Injury
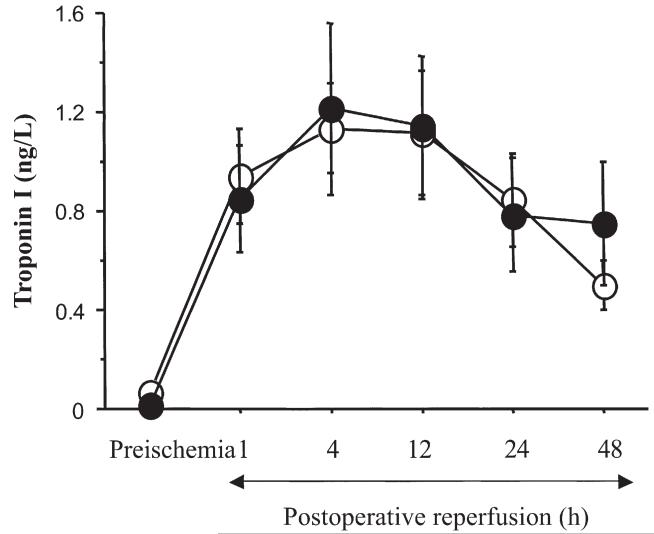
Troponin I release is shown in Figure 3. Postoperative release increased 1 hour after surgery, reaching a maximum at 4 hours with subsequent gradual decline up to 48 hours after surgery. However, comparisons between groups did not show any difference at any of the times investigated.
Fig 3.
Myocardial troponin I release shown at different times postoperatively after cold-blood cardioplegia with (HS; black circles) or without (CB; white circles) terminal hot-shot. Data are presented as mean ± standard error of the mean and expressed as nanograms per milliliter.
None of the patients in either group had a postoperative myocardial infarction as per predefined criteria of troponin I release.
Comment
Reperfusion after cardioplegic arrest–induced myocardial ischemia is known to cause irreversible cellular changes [19, 20]. The potential for reperfusion damage exists in all cardiac operations requiring temporary interruption of the coronary circulation to perform surgery. This may contribute to the impaired cardiac performance that develops during the postoperative period, and to the eventual myocardial fibrosis that may result after surgical correction of congenital or acquired cardiac diseases [19-22].
Previous studies in adults have shown that the fate of the myocardium jeopardized by global ischemia might be determined by careful control of the conditions of reperfusion and the composition of the reperfusate [19, 20, 23]. As result, the terminal HS is now used in most adult cardiac surgical procedures even if this method of myocardial protection has been tested mostly in the setting of coronary surgery. The rationale of using HS is to actively resuscitate the ischemically damaged, energy- and substrate-depleted heart by maximizing the kinetics of repair and minimizing oxygen demands by maintaining arrest.
Our study investigates the efficacy of terminal HS in patients with LV hypertrophy, following other studies we have carried out at our institution on this topic. We have demonstrated that CB cardioplegia is associated with less ischemic stress and myocardial injury compared with warm-blood cardioplegia in hypertrophic hearts [15], and that when using CB cardioplegia the route of delivery (ie, retrograde or antegrade) does not affect the degree of injury [14]. However, with CB cardioplegia alone the myocardial protection of hypertrophic hearts was still suboptimal, and this prompted us to undertake the present study.
The data suggest that for an average cardioplegic arrest time of 70 minutes the use of retrograde HS is associated with full metabolic recovery in both LV and RV biopsies. This is supported by the complete preservation of ATP and by the lack of increase of lactate. However, the use of CB cardioplegia alone, although seeming to provide protection for the LV, was associated with a small drop in ATP in the RV biopsy. This drop in ATP was not observed in the HS group, suggesting that retrograde delivery of HS might be protective for the RV. We could not find any metabolic explanation for this finding. Nevertheless, one possible explanation might be the air removal effect of the retrograde delivery of HS on the right coronary artery as, owing to its anatomic position on the anterior wall of the ascending aorta, it is more exposed than the left coronary artery to air microembolization.
Our finding also confirms the value of retrograde delivery in protecting the RV as some investigators are concerned that this route of delivery may affect distribution of cardioplegia to the RV free wall [24].
It is worthy noting that the mean cardioplegic arrest time in our groups was relatively short. It is possible that for complex operations requiring prolonged cardioplegic arrest time, the use of terminal HS might prove to be more beneficial when compared with CB, but this hypothesis should be tested in another study. Nomura and coworkers [25] reported a clear metabolic advantage of terminal HS in neonatal lamb hearts. However, in their study the hearts were more stressed as a result of almost double the duration of global ischemia (120 minutes).
The increase of troponin I after surgery was observed to an equal extent in both groups, and was statistically significant as compared with baseline values. This finding is consistent with the occurrence of a degree of myocardial injury and suggests that both these techniques provide suboptimal myocardial protection. A possible explanation of the lack of beneficial effect of HS on troponin I release might be that we used a protocol with 2 minutes of retrograde delivery, which was adapted from our previous experience in coronary artery bypass grafting surgery [13] and might have been insufficient to protect these hypertrophic hearts. Nevertheless, the release of troponin I at no time of observation reached the predefined level suggesting occurrence of myocardial infarction [16, 17]. Also, there were no episodes of low cardiac output in either group. Levels of ATP have been shown to correlate with function, and indeed ATP levels in LV biopsies were preserved with both cardioplegic techniques at reperfusion.
In summary, the terminal retrograde HS does not add any extra benefit to antegrade CB cardioplegia in preventing LV reperfusion and myocardial injury in patients with LV hypertrophy undergoing aortic valve replacement with relatively short cardioplegic arrest time. Nevertheless, it appears to reduce metabolic stress in the RV. Both cardioplegic techniques are associated with a minor degree of myocardial injury, which does not reach levels of clinical relevance.
Acknowledgments
This study was supported by the British Heart Foundation. Doctor Ascione is also supported by the Garfield Weston Trust. We thank colleagues in the unit, especially Attilio A. Lotto, MD, and Massimo Caputo, MD, for help with collecting some biopsies.
References
- 1.Hensley FA, Martin DE. A practical approach to cardiac anesthesia. 2nd ed. Little Brown; 1995. pp. 296–325. [Google Scholar]
- 2.Zhu Y-C, Zhu Y-Z, Spitznagel H, Gohlke P, Unger T. Substrate metabolism, hormone interaction, and angiotensin-converting enzyme inhibitors in left ventricular hypertrophy. Diabetes. 1996;45:S59–65. doi: 10.2337/diab.45.1.s59. [DOI] [PubMed] [Google Scholar]
- 3.Bull C, Cooper J, Stark J. Cardioplegic protection of the child’s heart. J Thorac Cardiovasc Surg. 1984;88:287–93. [PubMed] [Google Scholar]
- 4.Hammon JW., Jr. Myocardial protection in the immature heart. Ann Thorac Surg. 1995;60:839–42. doi: 10.1016/0003-4975(95)00573-4. [DOI] [PubMed] [Google Scholar]
- 5.Calafiore AM, Teodori G, Bosco G, et al. Intermittent antegrade warm blood cardioplegia in aortic valve replacement. J Cardiac Surg. 1996;11:348–54. doi: 10.1111/j.1540-8191.1996.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson WA, Berrizbeitia LD, Ilkowski DA, et al. Normothermic retrograde cardioplegia is effective in patients with left ventricular hypertrophy—a prospective randomised study. J Cardiothorac Surg. 1995;36:17–24. [PubMed] [Google Scholar]
- 7.Dorman BH, Hebbar L, Clair MJ, Hinton RB, Roy RC, Spinale FG. Potassium channel opener augmented cardioplegia: protection of myocyte contractility with chronic left ventricular dysfunction. Circulation. 1997;96(Suppl):II-253–9. [PubMed] [Google Scholar]
- 8.Jin XY, Gibson DG, Pepper JR. Early changes in regional and global left ventricular function after aortic valve replacement: comparison of crystalloid, cold blood and warm blood cardioplegia. Circulation. 1995;92(Suppl):II-155–62. doi: 10.1161/01.cir.92.9.155. [DOI] [PubMed] [Google Scholar]
- 9.Suleiman M-S, Caputo M, Ascione R, et al. Metabolic differences between hearts of patients with aortic disease and hearts of patients with ischaemic disease. J Mol Cell Cardiol. 1998;30:2519–23. doi: 10.1006/jmcc.1998.0814. [DOI] [PubMed] [Google Scholar]
- 10.Follette DM, Steed DL, Foglia RP, Fey KH, Buckberg GD. Reduction of postischemic myocardial damage by maintaining arrest during initial reperfusion. Surg Forum. 1977;28:281–3. [PubMed] [Google Scholar]
- 11.Follette DM, Fey KH, Steed DL, Foglia RP, Buckberg GD. Reducing reperfusion injury with hypothermic, hyperkalaemic, alkalotic blood during reoxygenation. Surg Forum. 1978;29:284–6. [PubMed] [Google Scholar]
- 12.Kirklin JW, Blackstone EH, Tchervenkov CI, Castaneda AR. Clinical outcomes after the arterial switch operation for transposition: patient, support, procedural, and institutional risk factors. Circulation. 1992;86:1501–15. doi: 10.1161/01.cir.86.5.1501. [DOI] [PubMed] [Google Scholar]
- 13.Caputo M, Dihmis WC, Bryan AJ, Suleiman MS, Angelini GD. Warm blood hyperkalaemic reperfusion (hot shot) prevents myocardial substrate derangement in patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg. 1998;13:559–64. doi: 10.1016/s1010-7940(98)00056-6. [DOI] [PubMed] [Google Scholar]
- 14.Lotto AA, Ascione R, Caputo M, Bryan AJ, Angelini GD, Suleiman MS. Myocardial protection with intermittent cold blood during aortic valve operation: antegrade versus retrograde delivery. Ann Thorac Surg. 2003;76:1227–33. doi: 10.1016/s0003-4975(03)00840-3. [DOI] [PubMed] [Google Scholar]
- 15.Ascione R, Caputo M, Gomes WJ, et al. Myocardial injury in hypertrophic hearts of patients undergoing aortic valve surgery using cold or warm blood cardioplegia. Eur J Cardiothorac Surg. 2002;21:440–6. doi: 10.1016/s1010-7940(01)01168-x. [DOI] [PubMed] [Google Scholar]
- 16.Logeais Y, Langanay T, Roussin R, et al. Surgery for aortic stenosis in elderly patients: a study of surgical risk and predictive factors. Circulation. 1994;90:2891–8. doi: 10.1161/01.cir.90.6.2891. [DOI] [PubMed] [Google Scholar]
- 17.Mair J, Larue C, Mair P, Balogh D, Calzolari C, Puschendorf B. Use of cardiac troponin I to diagnose perioperative myocardial infarction in coronary artery bypass grafting. Clin Chem. 1994;40:2066–70. [PubMed] [Google Scholar]
- 18.Suleiman M-S, Dihmis WC, Caputo M, Angelini GD, Bryan AJ. Changes in the intracellular concentration of glutamate and aspartate in hearts of patients undergoing coronary artery surgery. Am J Physiol. 1997;272:H1063–9. doi: 10.1152/ajpheart.1997.272.3.H1063. [DOI] [PubMed] [Google Scholar]
- 19.Buckberg GD, Allen BS. Myocardial protection management during adult cardiac operations. In: Baue AE, Geha AS, Hammond GL, Laks H, Naunheim KS, editors. Glenn’s thoracic and cardiovascular surgery. 6th ed. Appleton & Lange; Stamford, CT: 1995. pp. 1653–87. [Google Scholar]
- 20.Follette DM, Fey K, Buckberg GD, et al. Reducing postischemic damage by temporary modification of reperfusate calcium, potassium, pH, and osmolarity. J Thorac Cardiovasc Surg. 1981;82:221–38. [PubMed] [Google Scholar]
- 21.Castañeda AR, Jonas RA, Mayer JE, Jr, Hanley FL. Myocardial preservation in the immature heart. In: Castañeda AR, Jonas RA, Mayer JE Jr, Hanley FL, editors. Cardiac surgery of the neonate and infant. WB Saunders; Philadelphia: 1994. pp. 41–54. [Google Scholar]
- 22.Kirklin J, Barratt-Boyes B. Myocardial management during cardiac surgery with cardiopulmonary bypass. In: Kirklin J, Barratt-Boyes B, editors. Cardiac surgery. 2nd ed. Churchill Livingstone; New York: 1993. pp. 129–66. [Google Scholar]
- 23.Allen BS, Okamoto F, Buckberg GD, et al. Studies of controlled reperfusion after ischemia. XV: immediate functional recovery after 6 hours of regional ischemia by careful control of conditions of reperfusion and composition of reperfusate. J Thorac Cardiovasc Surg. 1986;92:621–35. [PubMed] [Google Scholar]
- 24.Allen BS, Winkelmann JW, Hanafy H, et al. Retrograde cardioplegia does not adequately perfuse the right ventricle. J Thorac Cardiovasc Surg. 1995;109:1116–24. doi: 10.1016/S0022-5223(95)70195-8. [DOI] [PubMed] [Google Scholar]
- 25.Nomura F, Forbess JM, Mayer EJ. Effects of hot shot on recovery after hypothermic ischemia in neonatal lamb heart. J Cardiovasc Surg (Torino) 2001;42:1–7. [PubMed] [Google Scholar]