Summary
During signal transduction by two-component regulatory systems, sensor kinases detect and encode input information while response regulators control output. Most receiver domains function as phosphorylation-mediated switches within response regulators, but some transfer phosphoryl groups in multistep phosphorelays. Conserved features of receiver domain amino acid sequence correlate with structure and hence function. Receiver domains catalyze their own phosphorylation and dephosphorylation in reactions requiring a divalent cation. Molecular dynamics simulations are supplementing structural investigation of the conformational changes that underlie receiver domain switch function. As understanding of features shared by all receiver domains matures, factors conferring differences (e.g. in reaction rate or specificity) are receiving increased attention. Numerous examples of atypical- or pseudo-receiver domains that function without phosphorylation have recently been characterized.
Introduction
Response regulator (RR) proteins, the second component in two-component signal transduction systems, are defined by the presence of a receiver domain. The term “receiver module” was proposed by Kofoid and Parkinson to emphasize both partnership with the “transmitter” function of sensor kinases (SKs) and the modular design of two-component systems [1]. “Receiver domain” came into prominence after structural studies revealed that receiver modules, originally defined on the basis of amino acid sequence similarity, are independently folded protein domains. In the language of bioinformatics, receiver domains are designated REC in the SMART protein domain database or Response_reg in the Pfam protein family database. As of January 2010, public databases contained ~30,000 nonredundant amino acid sequences of receiver domains and three-dimensional structures of ~200 receiver domains. However, only a tiny fraction of known receiver domains have been experimentally examined. Indeed, most knowledge concerning receiver domains arises from investigation of just a few RRs. This review summarizes what is known about receiver domain structure and function. Recent progress is highlighted and some prominent areas of incomplete understanding are noted.
The primary known function of most receiver domains is to act as phosphorylation-mediated switches within RRs. In addition to a receiver domain, most RRs contain one or more output domains, the majority of which regulate transcription (reviewed in [2]). Receiver domains are remarkably versatile in that the same basic structure can be paired with and apparently regulates more than 60 different output domains. Although an individual receiver domain is a binary logic element (existing in either phosphorylated or unphosphorylated states), two-component systems are not restricted to simple on/off outputs. Connecting the elements of two-component systems in various network architectures can convert the phosphorylation state information of receiver molecule populations into a diversity of response types, including a graded output (reviewed in [3]).
Some receiver domains are not attached to an output domain. About 15% of RRs in Bacteria and ~50% of RRs in Archaea consist of a single receiver domain. Many single receiver domains function as RRs and presumably exert their influence by binding to other proteins instead of interacting with an attached output domain (reviewed in [4]). However, another group of receiver domains carry phosphoryl groups between SKs and histidine phosphotransferases (HPts) in multistep phosphorelays rather than acting as switches. Receiver domains whose primary function is phosphotransfer are often attached to SKs to form “hybrid kinases”, but may also occur as single domain proteins. Determining whether the primary function of an isolated receiver domain is switching or phosphotransfer cannot currently be predicted from amino acid sequence alone and requires further elucidation of the signaling pathway.
Receiver domain amino acid sequence and structure
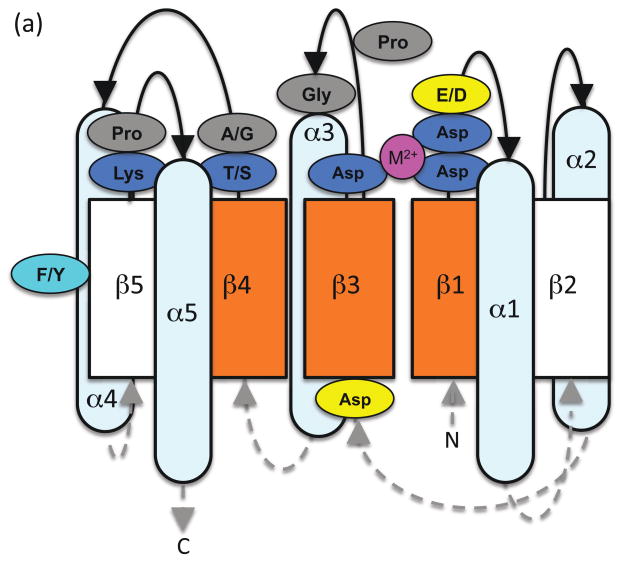
Receiver domains typically adopt a (βα)5 topology. Alternating β-strands and α-helices in the primary structure fold into a central five-stranded parallel β-sheet surrounded by two α-helices on one side and three on the other (Figure 1). The overall structure reflects patterns within receiver domain amino acid sequences that are sufficiently conserved to be manually recognized with some practice [5]. Three runs of four consecutive hydrophobic residues correspond to the three central β-strands in the core of the receiver domain. Conserved active site residues are located at the C-terminal ends of these three strands. β1, located near the N-terminus, is followed by two highly conserved Asp residues (the first of which is sometimes a Glu) that are involved in metal ion binding. The pair is often followed by a third acidic (Asp or Glu) residue. β3 is often preceded by an Asp, and ends in the highly conserved Asp site of phosphorylation. The loop between β3 and α3 includes a 180° turn in the space of three residues, initiated by a strongly conserved Pro located four residues to the C-terminal side of the Asp phosphorylation site. α3 begins with a strongly conserved Gly located another four residues to the C-terminal side of the Pro. β4 ends in a highly conserved Thr/Ser, which is immediately followed by a small residue (typically Ala or Gly but sometimes Ser or Thr). The conserved Thr/Ser interacts with the phosphoryl group and the subsequent small residue allows access to the phosphorylation site. β5, on the edge of the sheet, is harder to recognize in the amino acid sequence. A moderately conserved Phe/Tyr residue in the middle of β5 and a highly conserved Lys at the end of β5 are important for phosphorylation-mediated conformational changes. The Lys is often joined to a subsequent Pro in a cis-peptide bond. Series of hydrophobic resides spaced three or four residues (a helical turn) apart correspond to amphipathic α-helices 1, 3, and 5.
Figure 1.
Schematic diagram of the relationship between receiver domain amino acid sequence and basic structural elements as described in the text, with the active site viewed from the (a) side or (b) top. Five α-helices surround a parallel five-stranded β-sheet. Loops connecting strands and helices are shown as black solid lines on the active site side of the domain and gray dashed lines on the opposite side, with arrowheads indicating N- to C-terminal direction. Orange indicates pattern of conserved hydrophobic residues on the central β-strands and faces of three α-helices. The highly conserved residues of the active site quintet are in blue, with the moderately conserved aromatic residue in cyan. Divalent metal ion is magenta. Residues presumed to be strongly conserved for structural reasons are in gray. Frequently conserved acidic residues of unknown function are in yellow.
Thus, the receiver domain active site consists of a characteristic quintet of highly conserved residues. Three Asp residues bind a divalent metal ion that is essential for all phosphoryl group chemistry. One of the Asps is also the phosphorylation site. The Lys [6] and Thr/Ser [7] residues are critical for signal transduction, as is a more distant Phe/Tyr residue. Specific roles of these six residues are discussed in later sections.
Inspection of 205 receiver domain structures showed few departures from the typical architecture depicted in Figure 1. The most striking is Mycobacterium tuberculosis DosR, which adopts a (βα)4 fold with α4 on the same side of the β-sheet as α1 (pdb 3c3w) [8•]. The amino acids that would normally form β5 are incorporated into α5, which extends away from the rest of the domain. Thus, the key conserved Thr/Ser, Phe/Tyr, and Lys residues are far from the DosR phosphorylation site. Another conspicuous case is Thermotoga maritima DrrD, in which α4 is roughly perpendicular rather than parallel to the other helices (pdb 1kgs) [9]. In some receiver domain structures (pdb 1yio, 2b4a, 3c97, 3cg4, 3h5i, 3a0u), the α4 helix is missing or very short. Finally, receiver domain dimers can be formed by swapping secondary structural elements from different monomers. There are examples of swappingα5 (pdb 1dz3, 3c3m), α4β4α5 (pdb 2oqr, 3cg0), or α3β4α4β5α5 (pdb 3c97). It is not known if domain swapping is physiologically relevant.
A metal ion is essential for phosphoryl group chemistry
A divalent cation adjacent to the phosphorylation site is necessary to add or remove phosphoryl groups in the receiver domain, whether the reactions are mediated by the receiver alone or also involve a SK, HPt, or phosphatase. The six coordination positions of the metal ion are occupied by the three conserved Asp residues (one acting through a water molecule), a backbone carbonyl group, and two water molecules. Because multiple coordination positions are occupied by water and hence not restricted in space, the binding site can accommodate metal ions of different sizes. For many receivers, the preferred metal ion is Mg2+, but other divalent cations (particularly Mn2+) support different reactions to varying extents [10,11]. Trivalent cations bind ~1,000 times more tightly to the CheY RR than divalent cations [11], but are not known to be physiologically relevant. It is also not known if trivalent cations can support RR phosphorylation and dephosphorylation reactions.
Metal binding has been measured for few RRs, so the range of affinities that might exist amongst RRs is unknown. The 50-fold range of binding affinities reported for Mg2+ [10,12] thus represents a lower bound. Because the ~1 mM concentration of free Mg2+ in the cytoplasm of Escherichia coli is at the low end of this Kd range [13], the fraction of a RR population that is active in vivo may be influenced by intracellular metal ion concentration. However, NMR measurements indicate that CheY, with a reported Kd of 0.5–1 mM, exists predominantly in the metal bound form in vivo [14]. During phosphorylation, an oxygen atom in the phosphoryl group replaces one of the waters in the metal ion coordination sphere. Metal binding affinity presumably increases upon phosphorylation, but to the best of my knowledge has not been measured for a phosphorylated RR. The determinants responsible for variation between receiver domains in metal ion binding affinity and specificity have not been characterized. However, hydrophobic amino acids in the position immediately C-terminal to the conserved pair of metal-binding acidic residues have been proposed to strengthen nearby electrostatic interactions with the metal ion, whereas hydrophilic residues may weaken binding [15].
Receiver domains catalyze autophosphorylation
Although RRs typically receive phosphoryl groups from SKs in vivo, receiver domains can catalyze their own phosphorylation in vitro using suitable small molecule phosphodonors [16]. Thus, receiver domains are active participants in the phosphorylation reaction rather than passive substrates. Small molecule phosphodonors for RRs fall into two categories: phosphoramidates (R2N-PO32−, e.g. phosphoramidate, monophosphoimidazole) and acyl phosphates (R-COO-PO32−, e.g. acetyl phosphate, carbamoyl phosphate). The phospho-His of SKs is a phosphoramidate, so chemically similar small molecule phosphoramidates are useful tools for investigation of RR autophosphorylation, but are not known to be physiologically relevant themselves. In contrast, autophosphorylation with acyl phosphates may be a physiologically relevant means to connect metabolic state to two-component signal transduction (reviewed in [17]).
The autophosphorylation reaction is believed to involve nucleophilic attack on the phosphorus atom by the Asp carboxylate oxygen that is not bound to metal ion. The attack is oriented in line with the bond linking the phosphorus to the leaving group of the phosphodonor (an amine for phosphoramidates or a carboxylic acid for acylphosphates). For phosphoramidates, the nitrogen must be protonated to make a good leaving group, so the reaction rate is pH dependent [18]. In contrast, autophosphorylation with acyl phosphates is largely pH independent. The autophosphorylation transition state has not been observed experimentally, but based on analogy to the structurally related haloacid dehalogenase (HAD) superfamily of proteins [19], probably involves a trigonal bipyrimidal structure consisting of a planar PO3 with attacking and leaving groups axial to the phosphorus. The oxygen atoms of the phosphoryl group would be coordinated by the metal ion, the conserved Thr/Ser, or the conserved Lys. Consistent with such a transition state, the rate of CheY autophosphorylation is substantially reduced by replacement of the conserved Thr/Ser or Lys [20]. In the proposed mechanism, autophosphorylation would proceed with inversion of phosphoryl group stereochemistry.
Autophosphorylation of the CheY RR is inhibited by increasing ionic strength [21]. Given that both the receiver domain active site and small molecule phosphodonors are highly charged, an ionic strength effect that perhaps disrupts interactions between the two is not surprising. When measured at constant ionic strength, the rate of CheY autophosphorylation exhibits the remarkable property of direct proportionality to phosphodonor substrate concentration and does not display the saturation expected for Michaelis-Menton kinetics [22]. The implication of nonsaturable kinetics is that affinity of the receiver domain active site for the phosphodonor substrate is extraordinarily weak. Weak binding between phosphodonor and receiver domain is consistent with the analogy to HAD proteins, in which substrate binding and chemical catalysis are delegated to different domains (reviewed in [23•]). Receiver domains correspond structurally to the core catalytic portion of HAD proteins and lack the cap domain that confers substrate specificity. To the best of my knowledge, neither the effect of ionic strength on autophosphorylation nor the kinetics of autophosphorylation as a function of phosphodonor concentration at constant ionic strength have been reported for any RR other than CheY. However, it would be surprising if the autophosphorylation characteristics described above are unique to CheY.
HAD phosphatases first transfer the phosphoryl group from the substrate to the conserved Asp and then rapidly hydrolyze the HAD-P intermediate to complete the reaction cycle. In comparison, the RR-P “intermediate” is more stable. The analogy between RR autophosphorylation and HAD phosphatase activity is further emphasized by the observation that 22 HAD phosphatases from E. coli could all hydrolyze phosphoramidates and acyl phosphates, whereas seven non-HAD phosphatases from the same organism could not [24]. Interestingly, the HAD phosphatases exhibited Michaelis-Menton kinetics towards acetyl phosphate and imidodiphosphate, but the reaction with carbamoyl phosphate did not saturate.
The fundamental reaction chemistry is presumably similar whether a RR catalyzes phosphorylation using a small molecule, a SK, or an HPt. In the few cases that have been measured, phosphotransfer from a SK or HPt to a RR is faster than RR autophosphorylation [25,26]. The rate enhancement by a partner phosphoprotein can be partly explained by an increased phospho-His concentration in the vicinity of the Asp phosphorylation site as a result of protein-protein binding [27]. Furthermore, rapid formation of specific complexes between RR and SK-P or HPt-P means that the rate-limiting step for phosphotransfer is the reaction chemistry itself rather than binding of the reactants [28]. However, the mechanistic basis of any catalytic contribution of SK or HPt residues to phosphotransfer has not been determined.
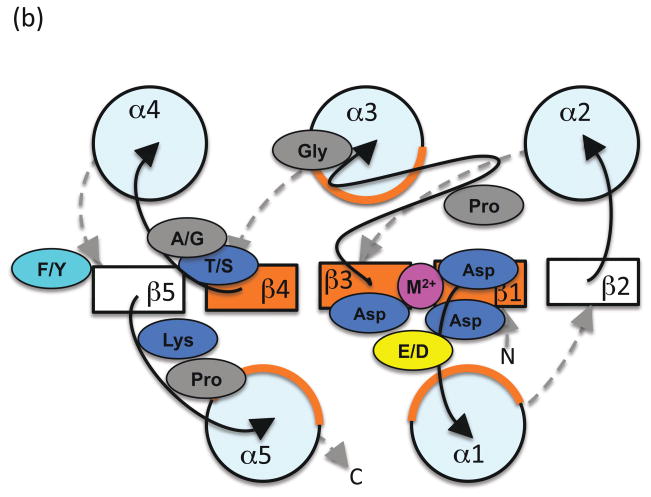
Phosphorylation-mediated conformational change
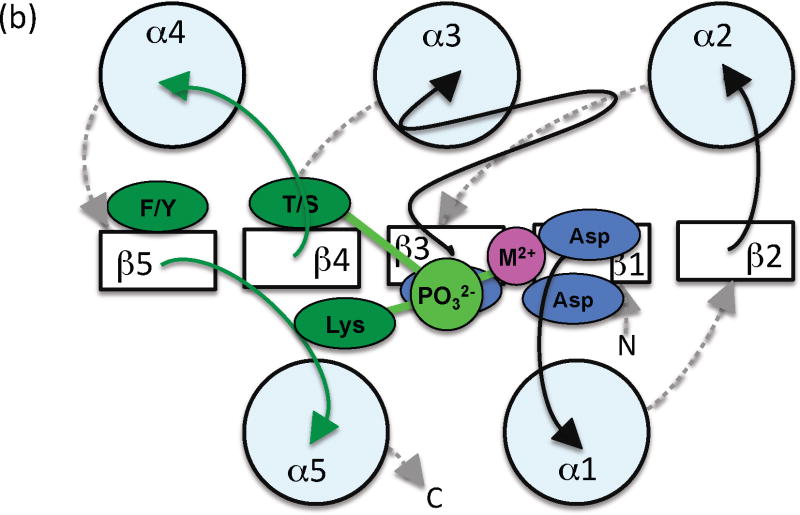
Comparison of the structures of receiver domains that are phosphorylated or bound to BeF3− (a phosphoryl group analog) with their nonphosphorylated counterparts reveals key differences between the active and inactive conformations ([29]). The three phosphoryl group oxygens each interact specifically with a conserved element of the receiver domain active site to form a hydrogen bond with the Thr/Ser, a salt bridge with the Lys, and coordinate the metal ion (Figure 2). Repositioning the Thr/Ser involves moving the β4α4 loop several Å and also stabilizes a rotomeric conformation of the Phe/Tyr within a hydrophobic cavity. Repositioning the Lys involves more modest changes in the β5α5 loop. Thus, the largest differences between the phosphorylated and nonphosphorylated conformations typically occur on the α4β5α5 face of the receiver domain, which is often exploited to control output function through interaction with other domains or proteins (reviewed in [30]).
Figure 2.
Schematic diagram indicating key differences between (a) inactive and (b) active conformations. View and color coding as in Figure 1b, with some features removed for clarity. Residues and loops that undergo the most significant changes are shown in red (inactive) or dark green (active). Phosphoryl group and lines indicating key hydrogen bonds are in light green.
One way to think of the structural differences is as an allosteric conformational change induced by phosphorylation that propagates from the active site to the α4β5α5 face of the receiver domain. Indeed, there is evidence that conformational change can also travel in the opposite direction so that output function affects active site structure [20,31]. However, it is important to recognize that phosphorylation state and receiver domain conformation are not perfectly coupled. Thus, another possibility is that phosphorylation might stabilize a pre-existing fraction of the receiver domain population that samples the active conformation in the absence of phosphorylation and so shift an equilibrium between inactive and active conformations [32]. X-ray crystallography cannot distinguish between various possible dynamic processes, but has revealed some potential intermediate conformations between the inactive and active states [33,34].
Molecular dynamics and other types of computer simulations allow investigation of the mechanism of conformational change at time scales and spatial resolutions that are not easily accessed experimentally. Simulations of both CheY [35•] and NtrC [36] suggest that the characteristic changes between the inactive and active orientations of the Thr/Ser and Phe/Tyr residues can occur independently of one another. NtrC provides a rich opportunity for exploration because the changes between inactive and active conformations also involve α3, β4, α4, β5 plus the loops connecting these elements and so are more extensive than seen in other receiver domains. Different simulations apparently do not agree on the key mechanistic features of NtrC conformational change upon phosphorylation. One model suggests movement of α4 is followed by movement of α2 and then a flip of the β3α3 loop [37]. Another simulation indicates the key feature is stabilization of the α3β4 and α4β5 loops on the opposite side of the receiver domain from the active site [38]. A third simulation proposes a tilt of α4 is followed by rotation of α4 and then a flip of the α4β5 loop [39].
Atypical- or pseudo-receiver domains do not use phosphorylation
Multiple reports over the past decade describe atypical- or pseudo-receiver domains that have amino acid sequences and three-dimensional structures similar to receiver domains, yet lack one or more residues of the highly conserved active site quintet. Pseudo-receiver domains are believed to function in a phosphorylation-independent manner whether or not the Asp phosphorylation site is present, but the mechanisms of action are poorly understood.
Myxococcus xanthus FrzS lacks the Asp phosphorylation site and the conserved Thr/Ser, and does not bind Mg2+ or BeF3−. Nevertheless, the Phe/Tyr that would participate in phosphorylation-mediated conformational change in typical receiver domains (Figure 2) is important for FrzS function [40]. Helicobacter pylori HP1021 and HP1043 also lack the Asp phosphorylation site [41], but HP1043 forms dimers using the same α4β5α5 interface as phosphorylation-mediated dimerization in other RRs [42]. Synechococcus NblR and Streptomyces coelicor RamR both contain the putative Asp phosphorylation site. A NblR mutant in which the Asp is replaced by a non-phosphorylatable Ala remains functional [43]. RamR lacks one of the metal-binding Asp residues, cannot autophosphorylate with phosphoramidate, and does not require the other metal-binding Asp or the conserved Thr/Ser for function [44•], again consistent with a mechanism that does not involve phosphorylation.
Some pseudo-receiver domains appear to regulate activity through ligand binding. Synechococcus elongatus CikA binds quinone on the α1β2 face [45]. Streptomyces venezuelae JadR1 binds the end product of the biosynthetic pathway that it regulates [46•].
Receiver domains catalyze autodephosphorylation
Receiver domain autodephosphorylation is generally presumed to proceed by a mechanism similar to that previously outlined for autophosphorylation, but in reverse. A water molecule would execute a nucleophilic in-line attack on the phosphorus, again resulting in a planar PO3 transition state coordinated by the conserved Thr/Ser, conserved Lys, and metal ion.
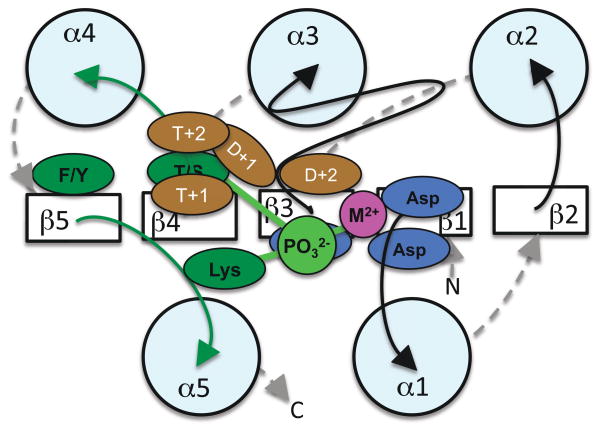
Removal of the phosphoryl group from the receiver domain to terminate a response is a critical aspect of signal transduction. Although receiver domains share extensive structural and chemical similarities, a wide range of RR autodephosphorylation rates have been reported (0.28 sec−1 for Rhodobacter sphaeroides CheY6 [47] to 0.25 week−1 for M. xanthus RedF [48]). The reaction rates presumably reflect the different timescales of the biological processes (e.g. chemotaxis for CheY6, multicellular development for RedF) controlled by various two-component systems. Combinations of amino acids that vary between different receiver domain active sites influence the autodephosphorylation rate. In particular, varying the amino acids two positions to the C-terminal sides of the Asp phosphorylation site and the conserved Thr/Ser (residues “D+2” and “T+2” respectively) can cause a ≥100-fold change in autodephosphorylation rate [49•] (Figure 3). Structural analysis of receiver domains bearing various combinations of D+2 and T+2 residues revealed that altered accessibility of the attacking water molecule to the phosphoryl group was responsible for some differences in autodephosphorylation rates [50•]. In other cases, interactions between T+2 (located on the mobile β4α4 loop) and D+1 raise the possibility that retarding the change from active to inactive conformation might impede the autodephosphorylation reaction, if the two processes are coupled. However, the putative influence of residue D+1 on autodephosphorylation rate has not been established experimentally. Similarly, Volz’s suggestion that residue T+1 affects access to the phosphorylation site [5], and hence might influence autodephosphorylation kinetics, has not been tested.
Figure 3.
Variable active site residues affect autodephosphorylation kinetics. View and color coding as in Figure 2b. The amino acids located one and two positions to the C-terminal sides of the Asp phosphorylation site and the highly conserved Thr/Ser are shown in brown. Established and putative roles are described in the text.
In many two-component systems, the primary route of RR dephosphorylation is through the phosphatase activity of other proteins. The available evidence indicates that auxiliary phosphatases function by stimulating intrinsic RR autodephosphorylation activity, rather than utilizing a distinct mechanism [51–53]. In particular, a conserved active site amide residue of the CheZ [51] or CheX [53] phosphatase extends into the RR active site and helps position a water molecule for nucleophilic attack. In the case of CheX, the RR D+2 residue assists in orientation of the water molecule. This is reminiscent of the HAD enzymes, in which residue D+2 acts as a general base to deprotonate as well as orient the attacking water molecule [54]. In the case of CheZ, the RR T+2 residue is critical for phosphatase activity, although the mechanistic role of T+2 has not been determined. It is noteworthy that the receiver domain D+2 and T+2 residues, which are known to influence autodephosphorylation rate, also affect the ability of auxiliary phosphatases to stimulate the same reaction.
Conclusions
Although many characteristics, particularly the conserved features, of receiver domains are now reasonably well understood, the variable aspects that presumably lead to important functional differences between RRs have only recently begun to be investigated. Also, some of what is known about receiver domains is based on analysis of a limited subset of RRs (or only one), so general applicability has not been confirmed. Several key unanswered questions for future research are listed in Box 1.
Box 1.
Some unanswered questions about receiver domain structure and function
Is there a simple way to predict whether a protein consisting of just a receiver domain primarily functions as a switch or in phosphotransfer?
What determines the metal ion specificity and affinity of receiver domains? What range of receiver domain metal binding affinities exists in nature?
What determines the extent to which various divalent cations support receiver domain autophosphorylaiton or autodephosphorylation? Do trivalent metal ions support receiver domain phosphorylation chemistry?
Are non-saturable reaction kinetics a general property of response regulator autophosphorylation?
How do some response regulators exclude acetyl phosphate as a phosphodonor, particularly in light of weak substrate binding?
What range of response regulator autophosphorylation rates exists in nature?
What do the transition states of receiver domain autophosphorylation and autodephosphorylation reactions look like?
Do changes in phosphoryl group linkage and receiver domain conformation occur simultaneously or sequentially? If sequentially, does phosphorylation induce change from the inactive to the active conformation or stabilize a subset of the population that attains the active conformation without phosphorylation? Is dephosphorylation essentially the reverse of phosphorylation or are the pathways for the two reactions significantly different?
How do pseudo-receiver domains function?
How is the ≥700,000-fold range of response regulator autodephosphorylation rates achieved?
Acknowledgments
National Institutes of Health grant GM050860 supports research on receiver domains in my laboratory. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kofoid EC, Parkinson JS. Transmitter and receiver modules in bacterial signaling proteins. Proc Natl Acad Sci U S A. 1988;85:4981–4985. doi: 10.1073/pnas.85.14.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galperin MY. Diversity of structure and function of response regulator output domains. Curr Opin Microbiol. 2010;13:???–???. doi: 10.1016/j.mib.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulian M. Two-component signaling circuit structure and properties. Curr Opin Microbiol. 2010;13:???–???. doi: 10.1016/j.mib.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenal U, Galperin MY. Single domain response regulators: molecular switches with emerging roles in cell organization and dynamics. Curr Opin Microbiol. 2009;12:152–160. doi: 10.1016/j.mib.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volz K. Structural conservation in the CheY superfamily. Biochemistry. 1993;32:11741–11753. doi: 10.1021/bi00095a001. [DOI] [PubMed] [Google Scholar]
- 6.Lukat GS, Lee BH, Mottonen JM, Stock AM, Stock JB. Roles of the highly conserved aspartate and lysine residues in the response regulator of bacterial chemotaxis. J Biol Chem. 1991;266:8348–8354. [PubMed] [Google Scholar]
- 7.Appleby JL, Bourret RB. Proposed signal transduction role for conserved CheY residue Thr87, a member of the response regulator active site quintet. J Bacteriol. 1998;180:3563–3569. doi: 10.1128/jb.180.14.3563-3569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 •.Wisedchaisri G, Wu M, Sherman DR, Hol WG. Crystal structures of the response regulator DosR from Mycobacterium tuberculosis suggest a helix rearrangement mechanism for phosphorylation activation. J Mol Biol. 2008;378:227–242. doi: 10.1016/j.jmb.2008.02.029. Remarkable as a description of the only known receiver domain that does not adopt a (βα)5 fold. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckler DR, Zhou Y, Stock AM. Evidence of intradomain and interdomain flexibility in an OmpR/PhoB homolog from Thermotoga maritima. Structure. 2002;10:153–164. doi: 10.1016/s0969-2126(01)00706-7. [DOI] [PubMed] [Google Scholar]
- 10.Lukat GS, Stock AM, Stock JB. Divalent metal ion binding to the CheY protein and its significance to phosphotransfer in bacterial chemotaxis. Biochemistry. 1990;29:5436–5442. doi: 10.1021/bi00475a004. [DOI] [PubMed] [Google Scholar]
- 11.Needham JV, Chen TY, Falke JJ. Novel ion specificity of a carboxylate cluster Mg(II) binding site: strong charge selectivity and weak size selectivity. Biochemistry. 1993;32:3363–3367. doi: 10.1021/bi00064a020. [DOI] [PubMed] [Google Scholar]
- 12.Guillet V, Ohta N, Cabantous S, Newton A, Samama JP. Crystallographic and biochemical studies of DivK reveal novel features of an essential response regulator in Caulobacter crescentus. J Biol Chem. 2002;277:42003–42010. doi: 10.1074/jbc.M204789200. [DOI] [PubMed] [Google Scholar]
- 13.Alatossava T, Jutte H, Kuhn A, Kellenberger E. Manipulation of intracellular magnesium content in polymyxin B nonapeptide-sensitized Escherichia coli by ionophore A23187. J Bacteriol. 1985;162:413–419. doi: 10.1128/jb.162.1.413-419.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard JA, MacLachlan LK, King GW, Jones JJ, Fosberry AP. Nuclear magnetic resonance spectroscopy reveals the functional state of the signalling protein CheY in vivo in Escherichia coli. Mol Microbiol. 2003;49:1191–1200. doi: 10.1046/j.1365-2958.2003.03628.x. [DOI] [PubMed] [Google Scholar]
- 15.Feher VA, Zapf JW, Hoch JA, Dahlquist FW, Whiteley JM, Cavanagh J. 1H, 15N, and 13C backbone chemical shift assignments, secondary structure, and magnesium-binding characteristics of the Bacillus subtilis response regulator, Spo0F, determined by heteronuclear high-resolution NMR. Protein Sci. 1995;4:1801–1814. doi: 10.1002/pro.5560040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukat GS, McCleary WR, Stock AM, Stock JB. Phosphorylation of bacterial response regulator proteins by low molecular weight phosphodonors. Proc Natl Acad Sci U S A. 1992;89:718–722. doi: 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe AJ. Physiologically relevant small phosphodonors link metabolism to signal transduction. Curr Opin Microbiol. 2010;13:???–???. doi: 10.1016/j.mib.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silversmith RE, Appleby JL, Bourret RB. Catalytic mechanism of phosphorylation and dephosphorylation of CheY: Kinetic characterization of imidazole phosphates as phosphodonors and the role of acid catalysis. Biochemistry. 1997;36:14965–14974. doi: 10.1021/bi9715573. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Cho HS, Kim R, Jancarik J, Yokota H, Nguyen HT, Grigoriev IV, Wemmer DE, Kim SH. Structural characterization of the reaction pathway in phosphoserine phosphatase: crystallographic “snapshots” of intermediate states. J Mol Biol. 2002;319:421–431. doi: 10.1016/S0022-2836(02)00324-8. [DOI] [PubMed] [Google Scholar]
- 20.Schuster M, Silversmith RE, Bourret RB. Conformational coupling in the chemotaxis response regulator CheY. Proc Natl Acad Sci U S A. 2001;98:6003–6008. doi: 10.1073/pnas.101571298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayover TL, Halkides CJ, Stewart RC. Kinetic characterization of CheY phosphorylation reactions: Comparison of P-CheA and small-molecule phosphodonors. Biochemistry. 1999;38:2259–2271. doi: 10.1021/bi981707p. [DOI] [PubMed] [Google Scholar]
- 22.Da Re SS, Deville-Bonne D, Tolstykh T, Veron M, Stock JB. Kinetics of CheY phosphorylation by small molecule phosphodonors. FEBS Lett. 1999;457:323–326. doi: 10.1016/s0014-5793(99)01057-1. [DOI] [PubMed] [Google Scholar]
- 23 •.Allen KN, Dunaway-Mariano D. Markers of fitness in a successful enzyme superfamily. Curr Opin Struct Biol. 2009;19:658–665. doi: 10.1016/j.sbi.2009.09.008. Excellent review summarizing the HAD superfamily, which provides a perspective relevant to understanding receiver domain structure and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuznetsova E, Proudfoot M, Gonzalez CF, Brown G, Omelchenko MV, Borozan I, Carmel L, Wolf YI, Mori H, Savchenko AV, et al. Genome-wide analysis of substrate specificities of the Escherichia coli haloacid dehalogenase-like phosphatase family. J Biol Chem. 2006;281:36149–36161. doi: 10.1074/jbc.M605449200. [DOI] [PubMed] [Google Scholar]
- 25.Stewart RC, VanBruggen R. Phosphorylation and binding interactions of CheY studied by use of Badan-labeled protein. Biochemistry. 2004;43:8766–8777. doi: 10.1021/bi0495735. [DOI] [PubMed] [Google Scholar]
- 26.Janiak-Spens F, Cook PF, West AH. Kinetic analysis of YPD1-dependent phosphotransfer reactions in the yeast osmoregulatory phosphorelay system. Biochemistry. 2005;44:377–386. doi: 10.1021/bi048433s. [DOI] [PubMed] [Google Scholar]
- 27.Stewart RC, Jahreis K, Parkinson JS. Rapid phosphotransfer to CheY from a CheA protein lacking the CheY-binding domain. Biochemistry. 2000;39:13157–13165. doi: 10.1021/bi001100k. [DOI] [PubMed] [Google Scholar]
- 28.Fisher SL, Kim SK, Wanner BL, Walsh CT. Kinetic comparison of the specificity of the vancomycin resistance kinase VanS for two response regulators, VanR and PhoB. Biochemistry. 1996;35:4732–4740. doi: 10.1021/bi9525435. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Cho HS, Pelton JG, Yan D, Berry EA, Wemmer DE. Crystal structure of activated CheY. Comparison with other activated receiver domains. J Biol Chem. 2001;276:16425–16431. doi: 10.1074/jbc.M101002200. [DOI] [PubMed] [Google Scholar]
- 30.Gao R, Stock AM. Molecular strategies for phosphorylation-mediated regulation of response regulator activity. Curr Opin Microbiol. 2010;13:???–???. doi: 10.1016/j.mib.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ames SK, Frankema N, Kenney LJ. C-terminal DNA binding stimulates N-terminal phosphorylation of the outer membrane protein regulator OmpR from Escherichia coli. Proc Natl Acad Sci U S A. 1999;96:11792–11797. doi: 10.1073/pnas.96.21.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkman BF, Lipson D, Wemmer DE, Kern D. Two-state allosteric behavior in a single-domain signaling protein. Science. 2001;291:2429–2433. doi: 10.1126/science.291.5512.2429. [DOI] [PubMed] [Google Scholar]
- 33.Simonovic M, Volz K. A distinct meta-active conformation in the 1.1 Å resolution structure of wild-type apoCheY. J Biol Chem. 2001;276:28637–28640. doi: 10.1074/jbc.C100295200. [DOI] [PubMed] [Google Scholar]
- 34.Dyer CM, Dahlquist FW. Switched or not?: the structure of unphosphorylated CheY bound to the N terminus of FliM. J Bacteriol. 2006;188:7354–7363. doi: 10.1128/JB.00637-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35 •.Ma L, Cui Q. Activation mechanism of a signaling protein at atomic resolution from advanced computations. J Am Chem Soc. 2007;129:10261–10268. doi: 10.1021/ja073059f. A nice study combining computer simulations with a thorough consideration of available experimental evidence to try to understand the mechanism of phosphorylation-mediated conformational change in CheY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damjanovic A, Garcia-Moreno EB, Brooks BR. Self-guided Langevin dynamics study of regulatory interactions in NtrC. Proteins. 2009;76:1007–1019. doi: 10.1002/prot.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalili M, Wales DJ. Pathways for conformational change in nitrogen regulatory protein C from discrete path sampling. J Phys Chem B. 2008;112:2456–2465. doi: 10.1021/jp076628e. [DOI] [PubMed] [Google Scholar]
- 38.Liu MS, Todd BD, Yao S, Feng ZP, Norton RS, Sadus RJ. Coarse-grained dynamics of the receiver domain of NtrC: fluctuations, correlations and implications for allosteric cooperativity. Proteins. 2008;73:218–227. doi: 10.1002/prot.22056. [DOI] [PubMed] [Google Scholar]
- 39.Lei M, Velos J, Gardino A, Kivenson A, Karplus M, Kern D. Segmented transition pathway of the signaling protein nitrogen regulatory protein C. J Mol Biol. 2009;392:823–836. doi: 10.1016/j.jmb.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraser JS, Merlie JP, Jr, Echols N, Weisfield SR, Mignot T, Wemmer DE, Zusman DR, Alber T. An atypical receiver domain controls the dynamic polar localization of the Myxococcus xanthus social motility protein FrzS. Mol Microbiol. 2007;65:319–332. doi: 10.1111/j.1365-2958.2007.05785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schar J, Sickmann A, Beier D. Phosphorylation-independent activity of atypical response regulators of Helicobacter pylori. J Bacteriol. 2005;187:3100–3109. doi: 10.1128/JB.187.9.3100-3109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong E, Lee HM, Ko H, Kim DU, Jeon BY, Jung J, Shin J, Lee SA, Kim Y, Jeon YH, et al. Structure of an atypical orphan response regulator protein supports a new phosphorylation-independent regulatory mechanism. J Biol Chem. 2007;282:20667–20675. doi: 10.1074/jbc.M609104200. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz D, Salinas P, Lopez-Redondo ML, Cayuela ML, Marina A, Contreras A. Phosphorylation-independent activation of the atypical response regulator NblR. Microbiology. 2008;154:3002–3015. doi: 10.1099/mic.0.2008/020677-0. [DOI] [PubMed] [Google Scholar]
- 44 •.O’Connor TJ, Nodwell JR. Pivotal roles for the receiver domain in the mechanism of action of the response regulator RamR of Streptomyces coelicolor. J Mol Biol. 2005;351:1030–1047. doi: 10.1016/j.jmb.2005.06.053. Interesting study of a pseudo-receiver domain that lacks only one residue of the active site quintet yet apparently functions without phosphorylation. [DOI] [PubMed] [Google Scholar]
- 45.Gao T, Zhang X, Ivleva NB, Golden SS, LiWang A. NMR structure of the pseudo-receiver domain of CikA. Protein Sci. 2007;16:465–475. doi: 10.1110/ps.062532007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46 •.Wang L, Tian X, Wang J, Yang H, Fan K, Xu G, Yang K, Tan H. Autoregulation of antibiotic biosynthesis by binding of the end product to an atypical response regulator. Proc Natl Acad Sci U S A. 2009;106:8617–8622. doi: 10.1073/pnas.0900592106. A compelling example of a pseudo-receiver domain that appears to be regulated by ligand binding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter SL, Armitage JP. Phosphotransfer in Rhodobacter sphaeroides chemotaxis. J Mol Biol. 2002;324:35–45. doi: 10.1016/s0022-2836(02)01031-8. [DOI] [PubMed] [Google Scholar]
- 48.Jagadeesan S, Mann P, Schink CW, Higgs PI. A novel “four-component” two-component signal transduction mechanism regulates developmental progression in Myxococcus xanthus. J Biol Chem. 2009;284:21435–21445. doi: 10.1074/jbc.M109.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49 •.Thomas SA, Brewster JA, Bourret RB. Two variable active site residues modulate response regulator phosphoryl group stability. Mol Microbiol. 2008;69:453–465. doi: 10.1111/j.1365-2958.2008.06296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50 •.Pazy Y, Wollish AC, Thomas SA, Miller PJ, Collins EJ, Bourret RB, Silversmith RE. Matching biochemical reaction kinetics to the timescales of life: Structural determinants that influence the autodephosphorylation rate of response regulator proteins. J Mol Biol. 2009;392:1205–1220. doi: 10.1016/j.jmb.2009.07.064. References 49 & 50 begin to reveal the factors that control receiver domain autodephosphorylation rate and suggest mechanistic explanations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao R, Collins EJ, Bourret RB, Silversmith RE. Structure and catalytic mechanism of the E. coli chemotaxis phosphatase CheZ. Nat Struct Biol. 2002;9:570–575. doi: 10.1038/nsb816. [DOI] [PubMed] [Google Scholar]
- 52.Pioszak AA, Ninfa AJ. Mutations altering the N-terminal receiver domain of NRI (NtrC) that prevent dephosphorylation by the NRII-PII complex in Escherichia coli. J Bacteriol. 2004;186:5730–5740. doi: 10.1128/JB.186.17.5730-5740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pazy Y, Motaleb MA, Guarnieri MT, Charon NW, Zhao R, Silversmith RE. Identical phosphatase mechanisms achieved through distinct modes of binding phosphoprotein substrate. Proc Natl Acad Sci U S A. 2010;107:???–???. doi: 10.1073/pnas.0911185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Z, Dunaway-Mariano D, Allen KN. The catalytic scaffold of the haloalkanoic acid dehalogenase enzyme superfamily acts as a mold for the trigonal bipyramidal transition state. Proc Natl Acad Sci U S A. 2008;105:5687–5692. doi: 10.1073/pnas.0710800105. [DOI] [PMC free article] [PubMed] [Google Scholar]