Abstract
The development of smart anti-cancer drugs that can selectively kill cancer cells while sparing the surrounding healthy tissues/cells unharmed is of paramount importance for safe and effective cancer therapy. We report a novel class of bifunctional compounds based on diarylidenylpiperidone (DAP) conjugated with an N-hydroxypyrroline (NOH, a nitroxide precursor) group. We hypothesized that the DAP would have cytotoxic (anti-cancer) activity, while the NOH moiety would function as a tissue-specific modulator (anti-oxidant) of cytotoxicity. The study used four DAPs, namely H-4073 and H-4318 without NOH and HO-3867 and HO-4200 with NOH substitution. The goal of the study was to evaluate the ‘proof-of-concept’ anticancer-versus-antioxidant efficacy of the DAPs using a number of cancerous (breast, colon, head and neck, liver, lung, ovarian, and prostate cancer) and noncancerous (smooth muscle, aortic endothelial, and ovarian surface epithelial cells) human cell lines. Cytotoxicity was determined using an MTT-based cell viability assay. All four compounds induced significant loss of cell viability in cancer cells, while HO-3867 and HO-4200 showed significantly less cytotoxicity in noncancerous cells. EPR measurements showed a metabolic conversion of the N-hydroxylamine function to nitroxide with significantly higher levels of the metabolite and superoxide radical-scavenging (antioxidant) activity in noncancerous cells when compared to cancer cells. Western-blot analysis showed that the DAP-induced growth arrest and apoptosis in cancer cells were mediated by inhibition of STAT3 phosphorylation at Tyr705 and Ser727 residues and induction of apoptotic markers of cleaved caspase-3 and PARP. The results suggest that the antioxidant-conjugated DAPs will be useful as a safe and effective anticancer agent for cancer therapy.
Keywords: human cancer cell line, ovarian cancer, diarylidenylpiperidone, STAT3, curcumin, nitroxide
INTRODUCTION
Curcumin, a beta-diketone constituent of turmeric derived from the rhizome of the plant Curcuma longa, has been shown to inhibit many cellular signaling pathways and downregulate the expression of many tumor-promoting proteins [1–3]. Curcumin has been shown to have antiproliferative and antiangiogenic activities in several tumors, including ovarian cancer [4–7]. However, the clinical use of curcumin has been limited due to its low anticancer activity and poor absorption. Recently, a novel class of curcumin analogs, diarylidenylpiperidones (DAPs), has been developed by incorporating a piperidone link to the beta-diketone structure and fluoro substitutions on the phenyl groups [8, 9]. The DAP compounds, in general, were more effective than curcumin in inhibiting the proliferation of a variety of cancer cell lines [10]. EF24, one of the DAP compounds with ortho-fluorinated phenyl group exhibited potent anticancer efficacy in vitro when tested using breast cancer [10], colon cancer [11], and ovarian epithelial cancer [12] cell lines. Subsequently, we observed that H-4073, a para-fluorinated variant, was more potent than EF24 in inducing cytotoxicity to ovarian cancer cells [12, 13].
A nonspecific cytotoxic compound may have side effects caused by damage to normal cells. For example, many chemotherapeutic agents act by producing free radicals, which may increase oxidative stress in normal cells [14, 15]. To minimize this toxicity, there is a need to use detoxicants, such as antioxidants, that can differentiate between healthy and cancerous cells and selectively protect the healthy cells by scavenging free radicals [16, 17]. It has been shown that nitroxides, a class of small-molecular-weight heterocyclic molecules containing “>NO”, and hydroxylamines, the one-electron-reduced form of nitroxides characterized by “>NOH”, preferentially scavenge oxygen radicals in cells that have normal oxygenation or redox status [18, 19]. Hydroxylamines are referred to as “pro-nitroxide”, as the hydroxylamine form of the molecules exist in equilibrium with the nitroxide form in well-oxygenated tissues [18, 19]. Most solid tumors are hypoxic in nature, and their cellular environment is more reducing (for example, thiol-rich) when compared to healthy cells [20, 21]. The difference in the redox environment between normal and cancerous tissues has led us to the design of a new class of anticancer compounds based on DAP backbone, but with a pro-nitroxide addendum (Figure 1). The rationale behind the design was to confer target-dependent functionality - anticancer and antioxidant properties - to the same molecule. The goal of the present study was to evaluate the ‘proof-of-concept’ anticancer-versus-antioxidant efficacy of the new class of compounds (DAPs) using a number of cancerous as well as noncancerous human cell lines. The study showed that the DAPs induce potential cytotoxicity in cancer cells while sparing noncancerous cells. The differential cytotoxicity is shown to be mediated through inhibition of STAT3 activation in cancer cells while providing antioxidant protection to the healthy cells. The results suggest that the antioxidant-conjugated DAPs will be useful as a safe and effective anticancer agent for cancer therapy.
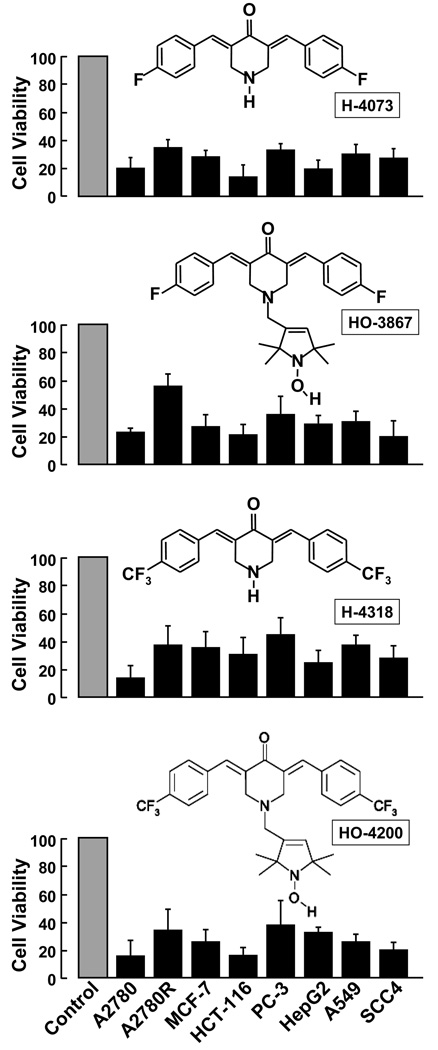
Figure 1. Cytotoxicity of DAPs to cancer cells.
A number of established human cancer cell lines, namely A2780 (ovarian), A2780R (cisplatin-resistant ovarian), MCF-7 (breast), HCT-116 (colon), PC-3 (prostate), HepG2 (liver), A549 (lung), SCC4 (squamous cell carcinoma), were exposed to 10-µM DAP (H-4073, HO-3867, H-4318, HO-4200) for 24 h followed by measurement of cell viability by MTT assay. The viable cells were quantified as mean±SE (N=6) and expressed as percent of respective untreated controls. The data show that DAPs induced substantial loss cell viability in all cancer cell lines tested.
MATERIALS AND METHODS
Chemicals
Superoxide dismutase (SOD), 6-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, diacetoxymethyl ester (H2DCF-DA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and antibodies against actin were obtained from Sigma (St. Louis, MO). 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DEPMPO) was from Radical Vision (Jerome-Marseille, France). Cell-culture medium (RPMI 1640), fetal-bovine serum (FBS), antibiotics, sodium pyruvate, trypsin, and phosphate-buffered saline (PBS) were purchased from Gibco (Grand Island, NY). Polyvinylidene fluoride (PVDF) membrane and molecular-weight markers were obtained from Bio-Rad (Hercules, CA). Antibodies against poly-adenosine diphosphate ribose polymerase (PARP), cleaved caspase-3, STAT3, phospho-STAT3 (Tyr705), were purchased from Cell Signaling Technology (Beverly, MA). Enhanced chemiluminescence (ECL) reagents were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). The DAPs used in this study, namely, H-4073 ((3E,5E)-3,5-bis(4-fluorobenzylidene)piperidin-4-one), HO-3867 (1-[(1-oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-yl)methyl]-(3E,5E)-3,5-bis(4-fluorobenzylidene)piperidin-4-one), H-4318 ((3E,5E)-3,5-bis(4-trifluoromethylbenzylidene)-piperidin-4-one), and HO-4200 (1-[(1-oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-yl)-methyl]-(3E,5E)-3,5-bis(4-trifluoromethylbenzylidene)piperidin-4-one) were synthesized as described [22]. Stock solutions of the compounds were freshly prepared in dimethylsulfoxide (DMSO). All other reagents, of analytical grade or higher, were purchased from Sigma-Aldrich, unless otherwise noted.
Cell lines and cultures
The A2780 human epithelial ovarian cancer cell line and human aortic smooth muscle cell line (HSMC) were used for most of the study reported in this work. Other cancer cell lines used are: A2780R (cisplatin-resistant human ovarian cell line), A549 (human lung cancer cell line), HepG2 (human liver cancer cell line), HCT-116 (human colon cancer cell line), PC3 (human prostate cancer cell line), MCF-7 (human breast cancer cell line), and SCC4 (human squamous cell carcinoma cell line). The study also used the following noncancerous (healthy) cell lines: human ovarian surface epithelial cells (hOSE; ScienCell Ovarian Cell System) and human aortic endothelial cells (HAEC).
The cells were grown in the following medium: cancer cells in RPMI 1640 or DMEM; HSMC and HAEC in SmBM; hOSE cells in OEPiCM). The medium was supplemented with 10% FBS, 2% sodium pyruvate, 1% penicillin and 1% streptomycin. Cells were grown in a 75-mm flask to 70% confluence at 37°C in an atmosphere of 5% CO2 and 95% air. Cells were routinely trypsinized (0.05% trypsin/EDTA) and counted using an automated counter (NucleoCounter, New Brunswick Scientific, Edison, NJ).
Cell counting
Untreated and DAP compounds-treated cells were counted using a NucleoCounter (New Brunswick Scientific).
Electron paramagnetic resonance spectroscopy
Electron paramagnetic resonance (EPR) spectroscopy was used to quantitatively determine the relative superoxide-scavenging ability of the DAPs in comparison with DEPMPO, a known superoxide scavenger [23]. Superoxide radicals were generated using an aerated solution of PBS (pH 7.4) containing xanthine (0.2 mM), xanthine oxidase (0.02 U/ml) and diethylenetriamine-pentaacetic acid (DTPA, 0.1 mM). The superoxide radicals (O2⋅−) generated by the xanthine-xanthine oxidase reaction were reacted with DEPMPO (1 mM) to form a paramagnetic adduct (DEPMPO-OOH), which was detected using X-band (9.8 GHz) EPR spectroscopy. In separate experimental groups, H-4073 (100 µM), HO-3867 (100 µM), H-4318 (100 µM), HO-4318 (100 µM) or SOD (500 U/ml) were added to the reaction mixture. If the DAPs could scavenge superoxide radicals, then there would be a competition between these compounds and DEPMPO for reaction with superoxide, and hence the EPR intensity of DEPMPO-OOH adduct could serve as a measure of the superoxide-scavenging ability of the test compounds. The generation of the DEPMPO-OOH adduct was measured at 10 min after initiation of the reaction.
Cell viability by MTT assay
Cell viability was determined by a colorimetric assay using MTT. In the mitochondria of living cells, yellow MTT undergoes a reductive conversion to formazan, giving a purple color. Cells, grown to ~80% confluence in 75-mm flasks, were trypsinized, counted, seeded in 96-well plates with an average population of 7,000 cells/well, incubated overnight, and then treated with the DAPs (H-4073, HO-3867, H-4318 or HO-4200; 10 µM) for 24 h. The dose and time of incubation were determined from a set of preliminary experiments as reported [13, 24]. All experiments were done using 8 replicates and repeated at least three times. Cell viability was expressed as a percent MTT viability of untreated cells.
Measurement of intracellular ROS
The ROS levels in cells treated with DAPs were determined using H2DCF-DA, a membrane-permeable fluorogenic probe. The acetate and acetoxymethyl ester groups of this probe are enzymatically cleaved inside living cells. The probe can then be oxidized by intracellular oxidants (ROS) to give a product, DCF, which emits a strong, green fluorescence (λex=504 nm; λem=529 nm). The fluorescence intensity is proportional to the level of cellular oxidants. Cells, grown to 80% confluence on 6-mm glass cover-slips, were treated with 10-µM H-4073, HO-3867, H-4318 or HO-4200 for 12 h, followed by incubation with H2DCF-DA. The cells were further incubated in the dark for 20 min, washed with protein-free medium and then fluorescence images were immediately captured with a Nikon Eclipse TE2000-U camera system using excitation/emission at 495/520 nm. The captured images were then analyzed using MetaMorph image analysis software.
Western-blot assay
Cells were grown in RPMI 1640 medium and treated with DMSO (control) or HO-3867 (10 µM). Equal volumes of DMSO (0.1% v/v) were present in both groups. Following treatment, cell lysates were prepared in nondenaturing lysis buffer (10-mM Tris-HCl (pH 7.4), 150-mM NaCl, 1% Triton X-100, 1-mM EDTA, 1-mM EGTA, 0.3-mM phenylmethylsulfonyl fluoride, 0.2-mM sodium orthovanadate, 0.5% NP40, 1-µg/ml aprotinin and 1-µg/ml leupetin. Cell lysates were centrifuged at 10,000×g for 20 min at 4°C, and the supernatant was separated. The protein concentration in the lysates was determined using a Pierce detergent-compatible protein assay kit. For Western blotting, 25 to 50 µg of protein lysate per sample was denatured in 2× sample buffer and subjected to SDS-PAGE on a 10% or 12% tris-glycine gel. The separated proteins were transferred to a PVDF membrane and then the membrane was blocked with 5% nonfat milk powder (w/v) in TBST (10-mM Tris, 100-mM NaCl, 0.1% Tween 20) for 1 h at room temperature or overnight at 4°C. The membranes were incubated with the primary antibodies described above. The bound antibodies were detected with horseradish peroxidase (HRP)-labeled sheep anti-mouse IgG or HRP-labeled donkey anti-rabbit IgG (Amersham) using an enhanced chemiluminescence detection system (ECL Advanced kit). Protein expressions were determined using Image Gauge version 3.45.
Statistical analysis
Data were expressed as mean±SEM. Comparisons among groups were performed using the Student’s t test. The significance level was set at p < 0.05.
RESULTS
Cytotoxicity of DAPs to cancer cells
The cytotoxicity of DAPs (H-4073, HO-3867, H-4318, HO-4200) to established human cancer cell lines, namely A27820, A2780R, MCF-7, HCT-116, PC-3, HepG2, A549, and SCC4, was evaluated by exposing the cells to 10-µM concentration of the compound for 24 h. All four compounds induced a substantial loss of cell viability in all the human cancer cell lines tested (Figure 1). In particular, H-4073 and H-4318 exhibited higher toxicity when compared to HO-3867 or HO-4200. The results further indicated that the DAPs were more cytotoxic to ovarian (A2780) and colon (HCT-116) cancer cells when compared to other cancer cells tested.
Cytotoxicity of DAPs to noncancerous cells
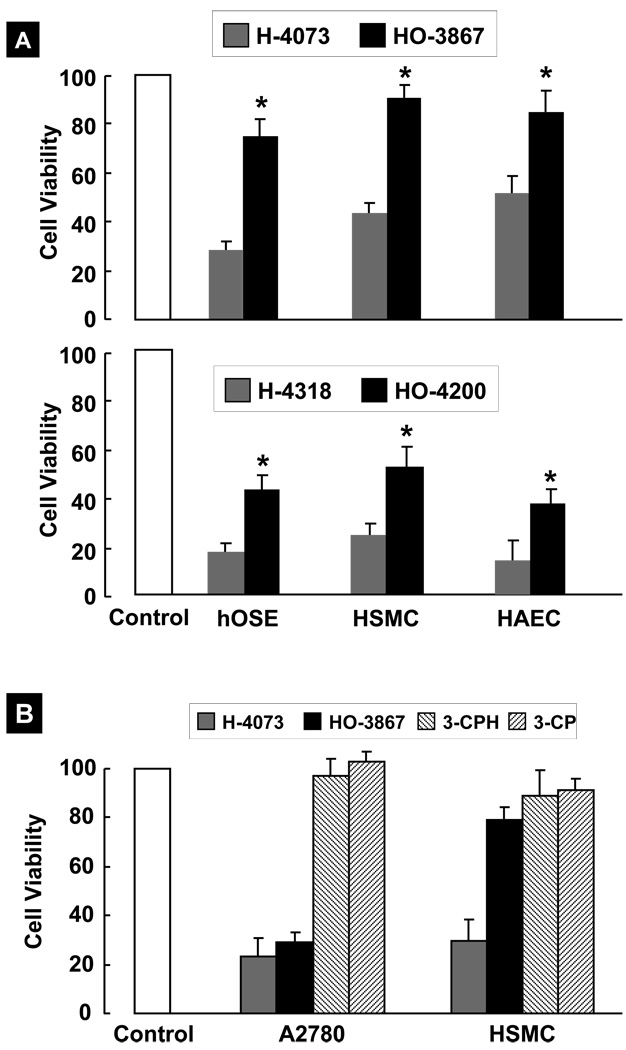
We next compared the effect of DAPs (10-µM; 24-h incubation) on the viability of noncancerous (healthy) human cell lines, namely human ovarian surface epithelial (hOSE) cells, human smooth muscle cells (HSMC), and human aortic endothelial cells (HAEC). All four compounds, in general, induced a substantial loss of cell viability to the cells tested, although to different extents (Figure 2A). The N-hydroxypyrroline-appended DAPs, HO-3867 and HO-4200, were significantly less toxic to the healthy cells when compared to H-4073 and H-4318, respectively. In particular, the results of HO-3867 seem to suggest a strikingly differential effect on cancer versus noncancerous cells. We hypothesized that this differential effect could stem from the N-hydroxypyrroline function. In order to test this hypothesis, and to determine the role of N-hydroxypyrroline function in the cytotoxicity, we additionally evaluated the effect of 3-CPH (a stand-alone analog of N-hydroxypyrroline) and 3-CP (a nitroxide version 3-CPH) on A27180 and HSMC cells. The results did not show any significant effect of 3-CPH or 3-CP on the cell viability (Figure 2B) suggesting that the N-hydroxypyrroline or its nitroxide form are not cytotoxic to either type of cells under the conditions used. Overall the viability results seem to implicate the diarylidenylpiperidone group in inducing cytotoxicity and N-hydroxypyrroline group in protecting noncancerous cells.
Figure 2. Cytotoxicity of DAPs to noncancerous human cells.
Cells were exposed to 10-µM DAPs for 24 hours followed by measurement of cell viability by MTT assay. The viable cells were quantified as mean±SE (N=6) and expressed as percent of respective (untreated) controls. (A) Viability of noncancerous cells, namely hOSE (human ovarian surface epithelial), HSMC (human smooth muscle cell), and HAEC (human aortic endothelial cell). The data show that DAPs were cytotoxic to all three noncancerous cell lines tested; however, the cytotoxicity was significantly less in the case of HO-3867 and HO-4200 when compared to H-4073 and H-4318, respectively (*p<0.05). (B) Viability of A2780 and HSMC cells exposed to 10-µM H-4073, HO-3867, 3-CPH (N-hydroxy-3-carbamoyl proxyl), or 3-CP (3-carbamoyl proxyl) for 24 h. The results did not show any significant effect of 3-CPH or 3-CP on the cell viability suggesting that the N-hydroxypyrroline or its nitroxide form are not cytotoxic to either type of cells under the conditions used.
Metabolic conversion of DAPs in cells
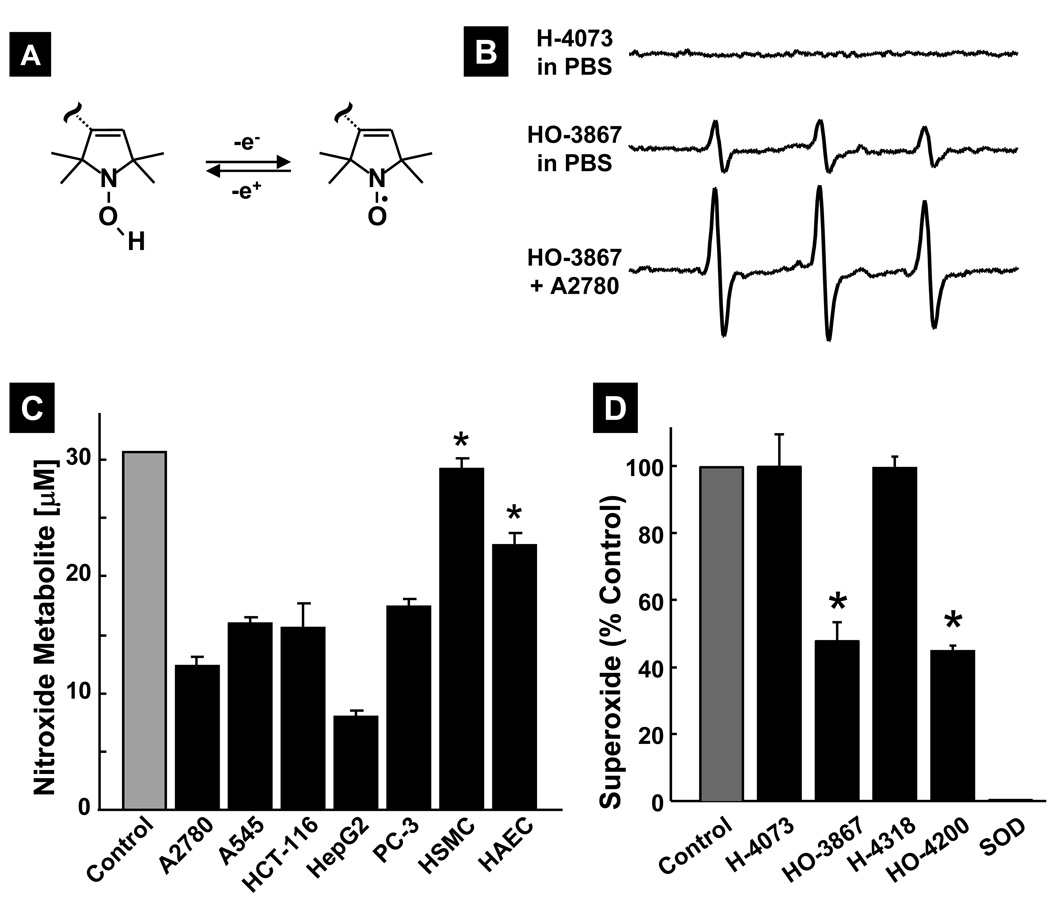
The N-hydroxypyrroline (>NOH) moiety is capable of undergoing a reversible one-electron oxidation to its nitroxide form (>NO; Figure 3A), which is paramagnetic and detectable by EPR spectroscopy. Hence, we next determined whether HO-3867 and HO-4200 are converted to their corresponding nitroxide form (>NO) in cells. The EPR spectrum measured from a 100-µM solution of HO-3867 incubated with A2780 cells showed a characteristic triplet feature (Figure 3B) attributable to nitroxide, as verified by using an authentic nitroxide form of HO-3867 (data not shown). A 5-fold increase in the EPR signal intensity of the nitroxide metabolite was observed in HO-3867 incubated with A2870 cells when compared to PBS. Similar results were obtained with HO-4200 (data not shown). Under these conditions, H-4073 or HO-4318 did not show any EPR signal suggesting that the N-hydroxypyrroline moiety is the source of the observed EPR signal. Figure 3C shows the nitroxide metabolite levels upon incubation of cells with 100-µM HO-3867 at 37°C for 6 hours. The results showed the presence of a significant level of the nitroxide form in cells tested, and that the metabolite level was significantly higher (25–30%) in noncancerous cells when compared to cancer cells (7–16%).
Figure 3. Metabolic conversion and superoxide-scavenging of DAPs in cells.
(A) Reversible, one-electron oxidation of the >NOH moiety to nitroxide (>NO). which is paramagnetic and can be detected by EPR spectroscopy. (B) EPR spectra of 10-µM H-4073 in PBS, 10-µM HO-3867 in PBS, and 10-µM HO-3867after 6-h incubation with A2780 cells at 37°C. The three-line pattern is characteristic of the nitroxide (>NO) metabolite in solution. (C) Amounts of nitroxide upon incubation of 100-µM HO-3867 with different cancer and noncancerous cells for 6 h at 37°C. Control represents the measure of nitroxide in the culture medium (without cells) incubated for 6 h at 37°C. Data represent mean±SD (N=5). The data show the presence of substantial portion of HO-3867 in the nitroxide form in cellular incubations and further that the nitroxide levels in the noncancerous cells are significantly higher (*p<0.05) when compared to each of the cancer cells. (D) Superoxide radical-scavenging ability of DAPs. Superoxide radicals were generated using an aerobic solution of xanthine and xanthine oxidase and detected as DEPMPO-OOH adduct by EPR spectroscopy. The DAP compounds (100 µM) were used to compete with 1-mM DEPMPO for the superoxide ions. Superoxide dismutase (SOD, 4.2 µM) was used as a positive control. Data represent mean±SD (N=5), *p<0.05 versus Control. The results show that the N-hydroxypyrroline-modified DAPs, HO-3867 and HO-4200, are capable of scavenging superoxide radicals.
Superoxide radical-scavenging activity of DAPs
Both the N-hydroxypyrroline and nitroxide are generally known to have antioxidant properties including superoxide dismutase (SOD)- and catalase-mimetic activity [25, 26]. Therefore, we determined the superoxide radical-scavenging ability of DAPs using a competitive reaction in presence of DEPEMPO [23]. Superoxide radicals were generated using an aerobic solution of xanthine and xanthine oxidase and detected as DEPMPO-OOH adduct by EPR spectroscopy. The DAP compounds (100 µM) were used to compete with 1-mM DEPMPO for the superoxide ions. Superoxide dismutase (SOD, 4.2 µM) was used as a positive control. HO-3867 and HO-4200 showed substantial diminution (~50%) of the DEPMPO-OOH concentration, indicative of the scavenging of superoxide radicals (Figure 3D). On the contrary, H-4073 or H-4318 did not show any significant effect on the superoxide adduct level. The EPR studies clearly demonstrated that the N-hydroxypyrroline-modified DAPs are capable of scavenging superoxide radicals.
ROS levels in cells treated with DAPs
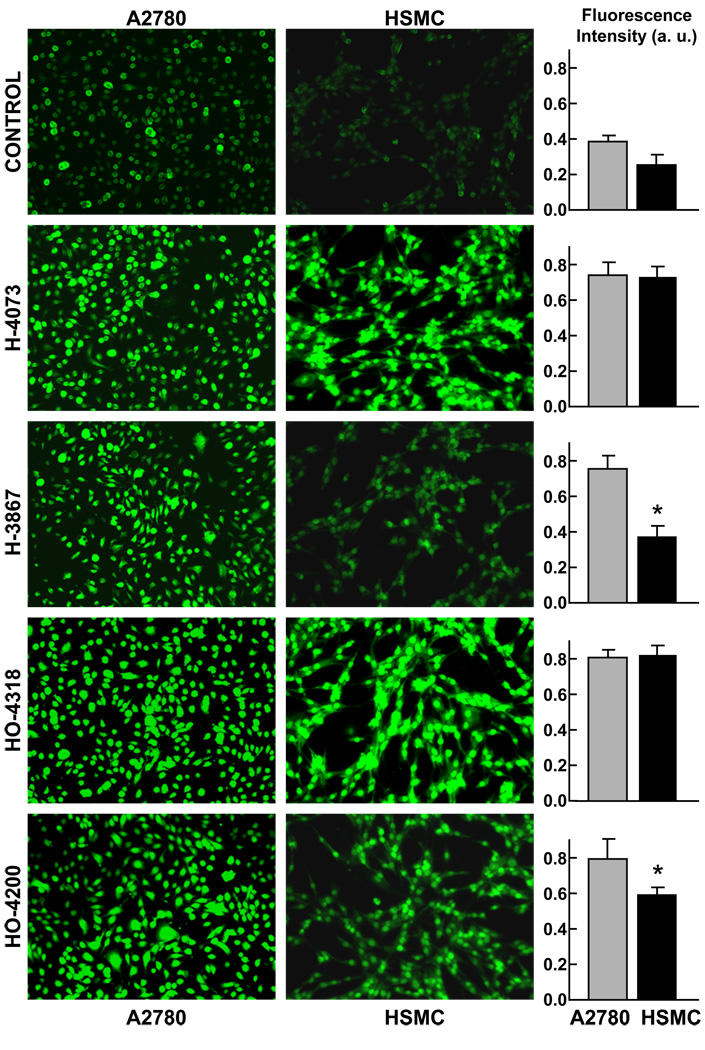
Previous studies have shown that the cytotoxicity of diarylidenyl ketones, such as curcumin and its analogs, is associated with ROS generation in cells [27, 28]. Therefore, we sought to determine whether the DAPs could have a similar effect upon cancer cells. A2780 cells were incubated with 5- or 10-µM DAPs for 12 h and intracellular ROS generation was measured by DCF fluorescence. The fluorescence intensity observed in A2780 cells was significantly higher in the cells treated with DAPs when compared to untreated controls (Figure 4). In contrast, the DCF-fluorescence intensity in HSMC cells treated with HO-3867 was not significantly different from the untreated cells. H-4073, HO-4318 and HO-4200 induced significant ROS generation in both A2780 and HSMC cells. These results suggested that both H-4073 and HO-3867 were comparable in inducing ROS generation in A2780 cells. However, in HSMC cells, HO-3867 generated significantly less ROS level when compared to H-4073 and HO-4318. Taken together, the results implied that HO-3867 was capable of inducing oxidative stress in cancer cells while sparing healthy cells.
Figure 4. Intracellular ROS-generation by DAPs.
A2870 and HSMC cells were incubated with 10-µM DAP (H-4073, HO-2867, H-4318, HO-4200) for 12 h. ROS formation was assessed using fluorescence dye CM-H2DCFDA (10 µM). Representative fluorescence images and quantitation (mean±SE; N=5) of ROS production in A2780 and HSMC cells are shown. Control refers to untreated cells. *p<0.05 vs respective A2870 cells. The results demonstrate that the N-hydroxypyrroline-conjugated DAPs, HO-3867 and HO-4200, show significantly lower amounts of ROS in HSMC when compared to A2780 cells.
Effect of HO-3867 on markers of cell proliferation and apoptosis
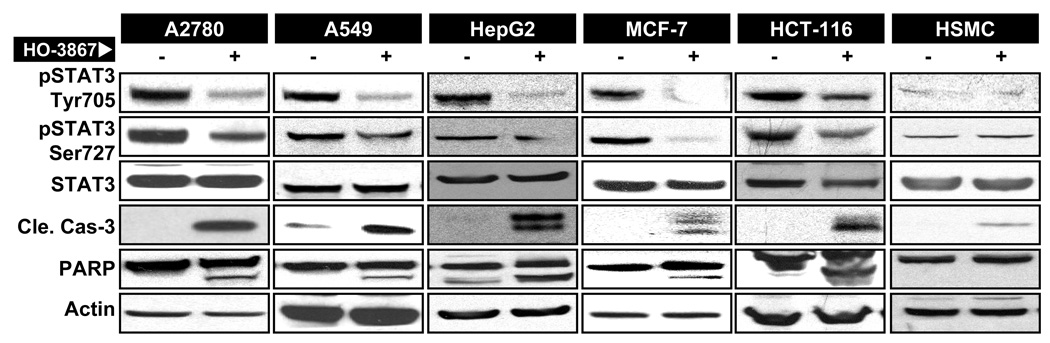
Constitutive activation of signal transducer and activator of transcription 3 (STAT3) has been shown to regulate the expression of genes implicated in the proliferation, survival and inhibition of apoptosis in cancer cells [29, 30]. Inhibition of STAT3-phosphorylation appears to be a key in the anticancer activity of diarylidenyl ketones. To determine whether the DAP-induced loss of cell viability in the present study was mediated by STAT3, we determined the level of phosphorylated STAT3 (Tyr705 and Ser727) by Western-blot analysis. The data (Figure 5) showed that both the Tyr705- and Ser727-pSTAT3 levels were significantly attenuated in cancer cells treated with 10-µM HO-3867 for 24 hours. We also observed significant elevation of activated caspases-3 and PARP in the treated cells suggesting the induction of apoptosis by inhibition of STAT3-signaling in the HO-3867-exposed cancer cells. It should be noted that HO-3867 did not induce any significant change in the activation of STAT3 or apoptotic markers in HSMC cells.
Figure 5. Inhibition of STAT3-signaling by HO-3867.
Cells were treated with HO-3867 for 24 h and subjected to Western-blot analysis. (A) Representative immunoblot images of total and phosphorylated (Tyr705 and Ser727) STAT3, and apoptotic markers of cleaved caspase-3 and PARP are shown for A2780, A-549, HepG2, MCF-7, HCT-116 and HSMC cells. Note the decreased levels of both pSTAT3 Tyr705 and Ser727 and corresponding increased levels of cleaved caspase-3 and cleaved PARP in the treated cells compared to untreated cells.
DISCUSSION
The development of smart anti-cancer agents that selectively destroy cancer cells while sparing the surrounding healthy tissues/cells unharmed is the main goal of cancer therapy. The results of the present study showed that all four DAPs induce potential cytotoxicity in cancer cells. The N-hydroxypyrroline-conjugated DAPs, HO-3867 and HO-4200, while equally toxic to cancer cells, they are significantly less toxic to noncancerous (healthy) cells. The differential cytotoxicity is shown to be mediated through inhibition of STAT3 activation in cancer cells while providing antioxidant protection to healthy cells. The results suggest that the antioxidant-conjugated DAPs will be useful as a safe and effective anticancer agent for cancer therapy.
In biological tissues, nitroxides are reduced to their corresponding hydroxylamine forms (Figure 3A). It is well established that these two forms of nitroxide coexist in tissues [19–21]. Nitroxides can be reduced to the corresponding hydroxylamines by reductants such as ascorbate, glutathione, semiquinone radicals, and also by intercepting reducing equivalents from the electron-transport chain. The hydroxylamines, on the other hand, can be oxidized to nitroxides in the presence of hydrogen peroxide and other oxidants such as transition metal complexes. Previous studies have shown that the redox transformation of the nitroxide/hydroxylamine is tissue specific [20, 21]. Studies have shown that nitroxides confer greater protection to normal tissues than to tumor tissues [31, 32]. This tissue specificity may be due to the fact that the nitroxide remains in the oxidized form in healthy, well-oxygenated tissues but is reduced to its hydroxylamine form in hypoxic tissues, such as those in tumors.
In the present study, we observed by using EPR measurements that HO-3867 and HO-4200 were apparently able to undergo redox-cycling to its corresponding nitroxide forms in vitro. We examined the role of the nitroxide moiety by comparing HO-3867 and HO-4200, with their parent forms H-4073 and H-4318, respectively. We observed that treatment with HO-3867 and HO-4200 induced a significant loss of cell viability in all cancer cells tested. The decrease in the viability of cancer cells upon HO-3867 treatment was comparable to that of H-4073. It should be noted that the cytotoxic effects of H-4073 and HO-3867 on A2780 cells were significantly higher when compared to curcumin under similar conditions [13, 24]. Further, HO-3867 had very little effect on the viability of hOSE cells, whereas H-4073, at the same dose, significantly reduced the viability of hOSE cells.
Low-molecular-weight nitroxides are used in clinical trials of cancer treatment [33]. The nitroxides have no anticancer efficacy, but they are used as protectors of normal tissue against chemo- or radiation induced cytotoxicity [34–36]. Our results using low-molecular-weight nitroxides, namely 3-CP and 3-CPH did not show any cytotoxicity toward A2780 or HSMC cells suggesting that the anticancer efficacy of HO-3867 or HO-4200 is not due to the pyrroline function. On the other hand, the decreased cytotoxicity of these compounds in the healthy cells could be due to the protective (antioxidant) nature of the nitroxide metabolite, which is present in significantly higher levels in noncancerous cells than in cancer cells (Figure 3C). The SOD-mimetic activity of these compounds is comparable to the manganese complexes of diacetylcurcumin that have been shown to have similar radical-scavenging properties [37]. Both HO-3867 and HO-4200 induced a significantly higher level of ROS in A2780 cells when compared to HSMC cells (Figure 4). Although the mechanism of induction of ROS in these cells by DAPS is not known, the relatively lower levels of ROS in the noncancerous cells appears to be due to the elevated levels of nitroxide metabolite. Overall, the differential cytotoxicity of HO-3867 observed in the cancer and noncancerous cells appears to be due the nitroxide metabolite levels.
The Western-blot analyses show that HO-3867 induces apoptosis in cancer cells. STAT3 activation plays a key role in the regulation of many genes implicated in the proliferation, survival and inhibition of apoptosis in cancer cells [29, 30, 38]. STAT3 is persistently activated in several primary human cancers, including all the major carcinomas as well as some hematologic tumors [29, 39–46]. In addition, a constitutively-active mutant form of STAT3 is sufficient to induce oncogenic transformation of cells, which form tumors in vivo. For these reasons, inhibition of STAT3-activation is an attractive strategy drug target. It has been reported that many diarylidenyl diketones, collectively known as curcumin analogs, are inhibitors of STAT3 activation [1, 3, 47–51]. Our results showed that HO-3867, whose structure has close similarity to the diketones, inhibited pSTAT3 Tyr705 and Ser727 in all five cancer cell lines tested (Figure 5). The results further showed that HO-3867 activated cleaved caspase-3 and induction apoptotic markers of PARP in the cancer cell lines suggesting that HO-3867 induces apoptosis in human cancer cells by targeting STAT3 proteins. Many reports showed that blockage of constitutive STAT3-signaling results in growth inhibition and induction of apoptosis in tumor cells in vitro and in vivo [30, 48, 52–56]. In future, we will perform in-depth studies to understand how this compound inhibits pSTAT3 activation
In summary, the study showed that the DAPs induce potential cytotoxicity in cancer cells while sparing noncancerous cells. The differential cytotoxicity is shown to be mediated through inhibition of STAT3 activation in cancer cells while providing antioxidant protection to the healthy cells. The results suggest that the antioxidant-conjugated DAPs will be useful as a safe and effective anticancer agent for cancer therapy.
Acknowledgements
This work was supported by National Institutes of Health Grant CA102264 (PK), Kaleidoscope of Hope Foundation Grant (KS), and Hungarian Research Fund Grant OTKA T048334 (KH).
ABBREVIATIONS
- DAP
diarylidenyl piperidone
- DEPMPO
5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide
- EPR
electron paramagnetic resonance
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STAT3
signal transducer and activator of transcription 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karuppaiyah Selvendiran, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Shabnam Ahmed, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Alex Dayton, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
M. Lakshmi Kuppusamy, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Mia Tazi, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Anna Bratasz, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Liyue Tong, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Brian K. Rivera, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA
Tamás Kálai, Institute of Organic and Medicinal Chemistry, University of Pécs, Pécs, Hungary.
Kálmán Hideg, Institute of Organic and Medicinal Chemistry, University of Pécs, Pécs, Hungary.
Periannan Kuppusamy, Department of Internal Medicine, Davis Heart and Lung Research Institute, and Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
REFERENCES
- 1.Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, Estrov Z, Talpaz M, Aggarwal BB. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 2.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- 3.Bill MA, Bakan C, Benson DM, Jr, Fuchs J, Young G, Lesinski GB. Curcumin induces proapoptotic effects against human melanoma cells and modulates the cellular response to immunotherapeutic cytokines. Mol Cancer Ther. 2009;8:2726–2735. doi: 10.1158/1535-7163.MCT-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther. 2007;6:178–184. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK, Aggarwal BB, Sood AK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan-Coyne G, O'Sullivan GC, O'Donovan TR, Piwocka K, McKenna SL. Curcumin induces apoptosis-independent death in oesophageal cancer cells. Br J Cancer. 2009;101:1585–1595. doi: 10.1038/sj.bjc.6605308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu S, Shen G, Khor TO, Kim JH, Kong AN. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008;7:2609–2620. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Selvendiran K, Kuppusamy ML, Bratasz A, Tong L, Rivera BK, Rink C, Sen CK, Kalai T, Hideg K, Kuppusamy P. Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by HO-3867, a novel synthetic curcuminoid, through up-regulation of PTEN expression. J Pharmacol Exp Ther. 2009;329:959–966. doi: 10.1124/jpet.108.150367. [DOI] [PubMed] [Google Scholar]
- 10.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, Ramanujam RP, Hideg K, Dieckgraefe BK, Houchen CW, Anant S. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008;68:1962–1969. doi: 10.1158/0008-5472.CAN-07-6011. [DOI] [PubMed] [Google Scholar]
- 12.Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK, Hideg K, Kuppusamy P. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282:28609–28618. doi: 10.1074/jbc.M703796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tazi MF, Selvendiran K, Kuppusamy ML, Tong L, Rivera BK, K K, Kuppusamy P. Evaluation of a novel class of fluorinated curcumin analogs for safe and targeted anticancer therapy (STAT) Free Radic Biol Med. 2008;45:S56–S57. [Google Scholar]
- 14.Injac R, Strukelj B. Recent Advances in Protection Against Doxorubicin-induced Toxicity. Technol Cancer Res Treat. 2008;7:497–516. doi: 10.1177/153303460800700611. [DOI] [PubMed] [Google Scholar]
- 15.Santos NA, Bezerra CS, Martins NM, Curti C, Bianchi ML, Santos AC. Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemother Pharmacol. 2008;61:145–155. doi: 10.1007/s00280-007-0459-y. [DOI] [PubMed] [Google Scholar]
- 16.Maliakel DM, Kagiya TV, Nair CK. Prevention of cisplatin-induced nephrotoxicity by glucosides of ascorbic acid and alpha-tocopherol. Exp Toxicol Pathol. 2008;60:521–527. doi: 10.1016/j.etp.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer. 2000;37:1–18. doi: 10.1207/S15327914NC3701_1. [DOI] [PubMed] [Google Scholar]
- 18.Samuni Y, Gamson J, Samuni A, Yamada K, Russo A, Krishna MC, Mitchell JB. Factors influencing nitroxide reduction and cytotoxicity in vitro. Antioxid Redox Signal. 2004;6:587–595. doi: 10.1089/152308604773934341. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JB, Krishna MC, Kuppusamy P, Cook JA, Russo A. Protection against oxidative stress by nitroxides. Exp Biol Med (Maywood) 2001;226:620–621. doi: 10.1177/153537020222600703. [DOI] [PubMed] [Google Scholar]
- 20.Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002;62:307–312. [PubMed] [Google Scholar]
- 21.Kuppusamy P, Wang P, Shankar RA, Ma L, Trimble CE, Hsia CJ, Zweier JL. In vivo topical EPR spectroscopy and imaging of nitroxide free radicals and polynitroxyl-albumin. Magn Reson Med. 1998;40:806–811. doi: 10.1002/mrm.1910400604. [DOI] [PubMed] [Google Scholar]
- 22.Kalai T, Kuppusamy P, Hideg K. Synthesis, characterization and structure-activity studies with a novel class of diarylidenylpiperidones. (to be published) [Google Scholar]
- 23.Mohan IK, Khan M, Wisel S, Selvendiran K, Sridhar A, Carnes CA, Bognar B, Kalai T, Hideg K, Kuppusamy P. Cardioprotection by HO-4038, a novel verapamil derivative, targeted against ischemia and reperfusion-mediated acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H140–H151. doi: 10.1152/ajpheart.00687.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvendiran K, Tong L, Bratasz A, Kuppusamy ML, Ahmed S, Ravi Y, Trigg NJ, Rivera BK, Kalai T, Hideg K, Kuppusamy P. Anticancer efficacy of a difluorodiarylidenyl piperidone (HO-3867) in human ovarian cancer cells and tumor xenografts. Molecular Cancer Therapeutics. 2010 doi: 10.1158/1535-7163.MCT-09-1207. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishna MC, Samuni A, Taira J, Goldstein S, Mitchell JB, Russo A. Stimulation by nitroxides of catalase-like activity of hemeproteins. Kinetics and mechanism. J Biol Chem. 1996;271:26018–26025. doi: 10.1074/jbc.271.42.26018. [DOI] [PubMed] [Google Scholar]
- 26.Krishna MC, Russo A, Mitchell JB, Goldstein S, Dafni H, Samuni A. Do nitroxide antioxidants act as scavengers of O2-. or as SOD mimics? J Biol Chem. 1996;271:26026–26031. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- 27.Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- 28.Su CC, Lin JG, Li TM, Chung JG, Yang JS, Ip SW, Lin WC, Chen GW. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379–4389. [PubMed] [Google Scholar]
- 29.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 30.Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, Lin J. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 31.Hahn SM, Sullivan FJ, DeLuca AM, Krishna CM, Wersto N, Venzon D, Russo A, Mitchell JB. Evaluation of tempol radioprotection in a murine tumor model. Free Radic Biol Med. 1997;22:1211–1216. doi: 10.1016/s0891-5849(96)00556-4. [DOI] [PubMed] [Google Scholar]
- 32.Hahn SM, Wilson L, Krishna CM, Liebmann J, DeGraff W, Gamson J, Samuni A, Venzon D, Mitchell JB. Identification of nitroxide radioprotectors. Radiat Res. 1992;132:87–93. [PubMed] [Google Scholar]
- 33.Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, Glatstein E, Hahn SM. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res. 2004;10:6411–6417. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]
- 34.Vitolo JM, Cotrim AP, Sowers AL, Russo A, Wellner RB, Pillemer SR, Mitchell JB, Baum BJ. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res. 2004;10:1807–1812. doi: 10.1158/1078-0432.ccr-03-0194. [DOI] [PubMed] [Google Scholar]
- 35.Hahn SM, Krishna MC, DeLuca AM, Coffin D, Mitchell JB. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic Biol Med. 2000;28:953–958. doi: 10.1016/s0891-5849(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 36.Hahn SM, Krishna CM, Samuni A, DeGraff W, Cuscela DO, Johnstone P, Mitchell JB. Potential use of nitroxides in radiation oncology. Cancer Res. 1994;54:2006s–2010s. [PubMed] [Google Scholar]
- 37.Vajragupta O, Boonchoong P, Berliner LJ. Manganese complexes of curcumin analogues: evaluation of hydroxyl radical scavenging ability, superoxide dismutase activity and stability towards hydrolysis. Free Radic Res. 2004;38:303–314. doi: 10.1080/10715760310001643339. [DOI] [PubMed] [Google Scholar]
- 38.Klampfer L. Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets. 2006;6:107–121. doi: 10.2174/156800906776056491. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A. Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med. 2002;196:641–653. doi: 10.1084/jem.20012127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 41.Weaver AM, Silva CM. Signal transducer and activator of transcription 5b: a new target of breast tumor kinase/protein tyrosine kinase 6. Breast Cancer Res. 2007;9:R79. doi: 10.1186/bcr1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen DG, Mercado-Uribe I, Yang G, Bast RC, Jr, Amin HM, Lai R, Liu J. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer. 2006;107:2730–2740. doi: 10.1002/cncr.22293. [DOI] [PubMed] [Google Scholar]
- 43.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 44.Spiekermann K, Bagrintseva K, Schwab R, Schmieja K, Hiddemann W. Overexpression and constitutive activation of FLT3 induces STAT5 activation in primary acute myeloid leukemia blast cells. Clin Cancer Res. 2003;9:2140–2150. [PubMed] [Google Scholar]
- 45.Zhou J, Bi C, Janakakumara JV, Liu SC, Chng WJ, Tay KG, Poon LF, Xie Z, Palaniyandi S, Yu H, Glaser KB, Albert DH, Davidsen SK, Chen CS. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 2009;113:4052–4062. doi: 10.1182/blood-2008-05-156422. [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee M, Jain S, Stuhmer T, Andrulis M, Ungethum U, Kuban RJ, Lorentz H, Bommert K, Topp M, Kramer D, Muller-Hermelink HK, Einsele H, Greiner A, Bargou RC. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007;109:720–728. doi: 10.1182/blood-2006-05-024372. [DOI] [PubMed] [Google Scholar]
- 47.Seo JH, Jeong KJ, Oh WJ, Sul HJ, Sohn JS, Kim YK, Cho DY, Kang JK, Park CG, Lee HY. Lysophosphatidic acid induces STAT3 phosphorylation and ovarian cancer cell motility: Their inhibition by curcumin. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Shen Y, Song R, Sun Y, Xu J, Xu Q. An anticancer effect of curcumin mediated by down-regulating phosphatase of regenerating liver-3 expression on highly metastatic melanoma cells. Mol Pharmacol. 2009;76:1238–1245. doi: 10.1124/mol.109.059105. [DOI] [PubMed] [Google Scholar]
- 49.Hutzen B, Friedman L, Sobo M, Lin L, Cen L, De Angelis S, Yamakoshi H, Shibata H, Iwabuchi Y, Lin J. Curcumin analogue GO-Y030 inhibits STAT3 activity and cell growth in breast and pancreatic carcinomas. Int J Oncol. 2009;35:867–872. doi: 10.3892/ijo_00000401. [DOI] [PubMed] [Google Scholar]
- 50.Lin L, Hutzen B, Ball S, Foust E, Sobo M, Deangelis S, Pandit B, Friedman L, Li C, Li PK, Fuchs J, Lin J. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit AKT and signal transducer and activator of transcription 3 phosphorylation in breast and prostate cancer cells. Cancer Sci. 2009;100:1719–1727. doi: 10.1111/j.1349-7006.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moser C, Lang SA, Mori A, Hellerbrand C, Schlitt HJ, Geissler EK, Fogler WE, Stoeltzing O. ENMD-1198, a novel tubulin-binding agent reduces HIF-1alpha and STAT3 activity in human hepatocellular carcinoma(HCC) cells, and inhibits growth and vascularization in vivo. BMC Cancer. 2008;8:206. doi: 10.1186/1471-2407-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, Yano H, Kojiro M, Sata M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66:4826–4834. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 53.Selvendiran K, Bratasz A, Tong L, Ignarro LJ, Kuppusamy P. NCX-4016, a nitro-derivative of aspirin, inhibits EGFR and STAT3 signaling and modulates Bcl-2 proteins in cisplatin-resistant human ovarian cancer cells and xenografts. Cell Cycle. 2008;7:81–88. doi: 10.4161/cc.7.1.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selvendiran K, Bratasz A, Kuppusamy ML, Tazi MF, Rivera BK, Kuppusamy P. Hypoxia induces chemoresistance in ovarian cancer cells by activation of signal transducer and activator of transcription 3. Int J Cancer. 2009;125:2198–2204. doi: 10.1002/ijc.24601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 56.Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ, Lu R, Chen YX, Fang JY. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]