Abstract
The Asn-Gly-Arg (NGR) motif in both cyclic and linear form has previously been shown to specifically bind to CD13/aminopeptidase N that is selectively overexpressed in tumor vasculature and some tumor cells. However, previous versions of cyclic NGR used a liable disulfide bridge between cysteine residues that may be problematic for liposome targeting due to disulfide bond formation between adjacent peptides on the liposomal surface. In this study, we report the design, synthesis, and characterization of a novel cyclic NGR containing peptide, cKNGRE, which does not contain a disulfide bridge. cKNGRE was synthesized in good yield and purity and attached to the fluorescent reporter Oregon Green (cKNGRE-OG) and lysolipid-containing temperature sensitive liposome (LTSLs). The identity of cKNGRE was verified with NMR and mass spectral techniques. In vitro fluorescence microscopy evaluation of cKNGRE-OG demonstrated binding and active uptake by CD13+ cancer cells and minimal binding to CD13− cancer cells. The cKNGRE-OG ligand displayed 3.6-fold greater affinity for CD13+ cancer cells than a linear NGR containing peptide. Affinity for CD13+ cancer cells was similarly improved 10-fold for both the cyclic and linear NGR when presented in a multivalent fashion on the surface of an LTSL. cKNGRE-targeted LTSLs rapidly released (>75% in <4 sec) doxorubicin at 41.3°C with minimal release at 37°C. These results demonstrate the ability to synthesize a cKNGRE-targeted temperature sensitive liposome that lacks a disulfide bridge and has sufficient binding affinity for biological applications.
Keywords: cNGR, angiogenesis, liposome, tumor, CD13/APN
1. Introduction
The targeting of tumor-specific or tumor-associated antigens [1] is a widely used paradigm for broad applications including anticancer therapy, site-specific drug delivery, and molecular imaging. Often, these antigens are either entirely absent or barely present on normal cells and tissues [2]. The use of various tumor-associated antigens for targeted cancer therapies is well documented and includes leukocyte differentiation antigen (CD33) for acute myeloid leukemia [3], GD2 for neuroblastoma [4, 5], and the folate receptor for a wide variety of human tumors [6–8]. Antigens expressed on angiogenic tumor vasculature are particularly attractive tumor-associated targets because they have intimate contact with the blood and are therefore geographically accessible immediately following intravenous injection of a targeted agent [9]. Widely used tumor vascular targets include integrins [10, 11], vascular endothelial growth factor receptor (VEGFR) [12, 13], platelet-derived growth factor receptor (PDGFR) [14, 15], and CD13/aminopeptidase N (APN, referred to as CD13 throughout) [2, 16–21]. CD13 is the focus of this study.
Angiogenic tumor vessels are important elements for tumor growth and metastasis. They are essential for transporting metabolically crucial materials to and from the tumor cells and also provide a route for the dissemination of tumor cells to distal sites. The Asn-Gly-Arg (NGR) peptide motif has been used to target drugs and drug-containing liposomes to the tumor vascular antigen CD13, resulting in improved biodistribution and tumor therapy [21–24]. Although linear NGR peptides have demonstrated suitable biodistribution and efficacy [21–28], the antitumor activity of drug associated with a cyclic form of NGR was reported to be 10-fold greater [22]. Despite the higher affinity of cyclic NGR peptides [22], there has been a preference to use linear NGR-containing motifs to target liposomes to avoid the formation of disulfide bridges between adjacent peptides on the liposome surface that may render the ligand ineffective [29].
The objectives of this study were to design and synthesize a novel cyclic NGR peptide that does not contain a disulfide bridge and to evaluate this peptide for specificity and affinity to CD13+ cancer cells. A linear NGR control peptide was synthesized for comparison. Our goal is to synthesize targeted lysolipid-containing temperature sensitive liposomes (LTSLs) for image-guided, heat-activated delivery of chemotherapeutics to solid tumors. LTSLs mainly composed of 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPC; Tm = 41.5 °C) [30] rapidly release their contents at clinically relevant hyperthermic temperatures (40–42 °C) [31] when a small fraction (~10 %) of lysolipid (e.g. 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine [MSPC]) is incorporated into the lipid bilayer [32]. LTSLs may be combined with focal hyperthermia or thermal ablation to selectively deliver encapsulated drugs to a heated region [33, 34]. To this end, we have synthesized an NGR-targeted LTSL and evaluated the binding of the targeted LTSL to CD13+ cells as well as release of encapsulated Doxorubicin (Dox) as a function of temperature. NGR-targeted LTSLs have the potential to improve therapeutic efficacy by: 1) slowing the transit time of liposomes in the tumor vasculature to improve drug release, 2) improving total drug accumulation in the tumor, and 3) treating metastatic tumors not subjected to hyperthermia.
2. Materials and Methods
2.1. Materials
All reagents and solvents (anhydrous) were obtained from Sigma-Aldrich (MO) and used as received unless otherwise indicated. Fmoc-Arg(Pbf)-OH was supplied by either Novabiochem (CA) or Chem-Impex International Inc. (IL). NovaPEG Rink Amide Resin, N,N,N′,N′-Tetramethyl-O-(7-azabenzotriazol-1-yl) uronium hexafluorophosphate (HATU), and all other amino acids used in this study were purchased from Novabiochem. Fmoc-Gly-Rink-Amide MBHA Resin was purchased from Peptide International (KY). 1-Hydroxybenzotriazole hydrate (HOBt) was purchased from AnaSpec (CA). Oregon Green 488 (OG) and 3, 3’-dioctadecyloxacarbocyanine perchlorate (DiO) were purchased from Invitrogen (OR). MSPC, DPPC, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(Polyethylene Glycol)2000] ammonium salt (DSPE-PEG2000CH2COOH) were obtained from Avanti Polar Lipids (AL). 1H NMR and 13C NMR spectra were recorded using Bruker 600 and 300 MHz spectrometers operating at 600 MHz for 1H and 75 MHz for 13C, respectively. Mass spectral data were recorded on PE/SCIEX API 2000 (Applied Biosystems Inc., CA) and UltraFlex TOF-TOF (Bruker Daltonics Inc., MA) instruments. Purification of peptides was performed using preparative reverse phase HPLC on a Varian ProStar model 330 PDA detector with a C-18 Microsorb column (250 × 21.4 mm, 100Å/5 µm). Analytical HPLC was performed using the same instrument and with a C-18 Microsorb column (4.6 × 250 mm, 100 Å/5 µm).
2.2. Cell lines
Human fibrosarcoma (HT-1080) and human adenocarcinoma (MCF7) cells were purchased from American Type Culture Collection (ATCC, VA). HT-1080 cells were grown in MEME (ATCC) supplemented with 10% fetal bovine serum (ATCC) and 100 IU/ml of penicillin and 100 µg/ml streptomycin (Invitrogen). MCF7 cells were grown in the same culture medium with the addition of 0.01 mg/mL bovine insulin (Sigma-Aldrich). Both cell lines were maintained in a 5% CO2 incubator at 37°C.
2.3. Peptide synthesis
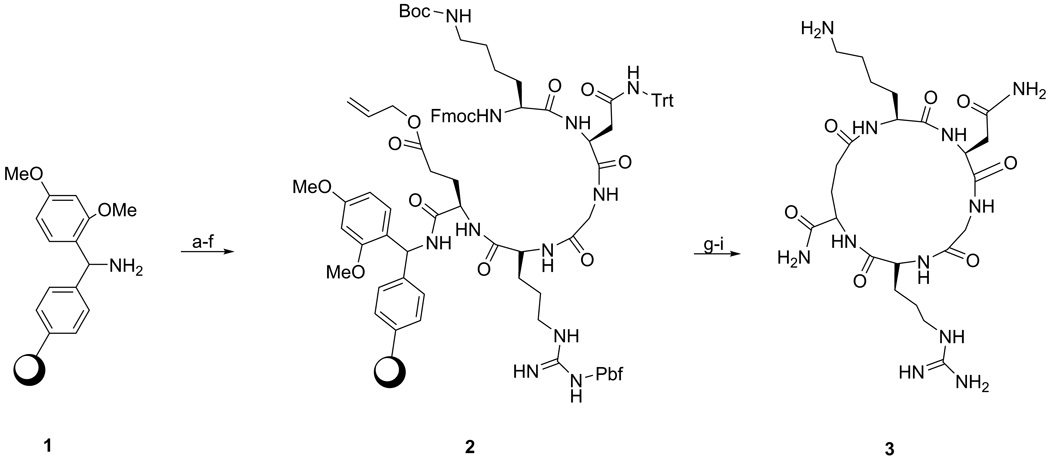
Cyclic KNGRE (cKNGRE) 3
NovaPEG Rink Amide Resin 1 (0.50–0.80 mmol/g loading) was washed with dichloromethane (DCM) and 1-methyl-2-pyrrolidinone (NMP) and swollen with DCM for 2 h. The resin was then washed with NMP and coupled with glutamic acid - via its Cα;-carboxylic acid - by agitating the resin with a solution of Fmoc-Glu(OAll)-OH (2.5 equiv), HATU (2 equiv), and N,N-Diisopropylethylamine (DIPEA; 4 equiv) in NMP. The resin was capped by washing with a solution of CH2Cl2-Acetic anhydride-DIPEA (20:4:1). The Fmoc protecting group was removed by treating the resin-attached peptide with a piperidine (20%) in NMP for 5 min (2X). The linear precursor peptides were constructed using Fmoc chemistry by adding the respective protected amino acid (2 equiv), HATU (2 equiv), and DIPEA (4 equiv) in NMP to give the linear penta peptide-resin 2. The Cω-terminal allyl ester of Glu was removed by treating the resulting penta-peptide with Pd(PPh3)4 (2 equiv) in CHCl3-AcOH-N-methylmorpholine (37:2:1) under argon atmosphere by gentle shaking for 2 h and then washing with DIPEA-NMP (5:95) followed by 0.5 % (w/v) of sodium diethyldithiocarbamate trihydrate in NMP (2 × 2 min a total of 100 mL). On-resin cyclization was performed by removing the N-Fmoc group from the α-amine of Lys and activating the Cω-carboxylic acid of Glu with HATU (2 equiv) and DIPEA (4 equiv). Cleavage of the peptide from the resin and removal of all the protecting groups was performed by agitating the resin-peptide with trifluoroacetic acid (TFA): DCM (1:1) for 2 h and washing with TFA-DCM (1:9). The acid wash was concentrated and Et2O was added until a white precipitate separated. The precipitate was freed from the solvent, dissolved in water, purified by preparative reverse phase HPLC using a gradient of MeCN-H2O (5–30% containing 0.1% TFA, 10 mL/min, 20 min run time), and lyophilized to give compound 3 as a white powder (193 mg, 33% based on estimated loading of peptide-resin). 1H NMR (600 MHz, D2O): δ = 1.45-1.54 (m, 2H), 1.57-1.81 (m, 8H), 2.04-2.10 (m, 2H), 2.17-2.23 (m, 1H), 2.36-2.41 (m, 1H), 2.78-2.80 (dd, 1H, J = 7.8, 17.7 Hz), 2.83-2.87 (m, 1H), 3.01(t, 2H, J = 7.0 Hz), 3.05-3.09 (dd, 1H, J = 4.8, 15.6 Hz), 3.22 (t, 2H, J = 7.0 Hz), 3.9 (d, 1H, J = 17.7 Hz), 4.14-4.23 (m, 3H), 4.46-4.48 (m, 2H). 13C NMR (75 MHz, D2O) δ = 22.3, 23.8, 24.6, 26.4, 27.9, 30.5, 31.5, 34.5, 39.1, 40.4, 42.9, 51.3, 52.8, 54.5, 55.5, 156.7, 172.4, 172.7, 174.0, 175.3, 175.3, 175.8, 176.2. Theoretical mass calculated for cKNGRE (C23H41N11O7) was 583.319; found MALDI-TOF-MS: m/z 584.24 [M+H]+, ESI-MS: m/z 584.3 [M+H]+. Analytical HPLC revealed a purity of 98% at 210 nm, tR = 10.05 min, using a gradient of MeCN-H2O (5–30 % containing 0.1% TFA, 1 ml/min, 20 min run time).
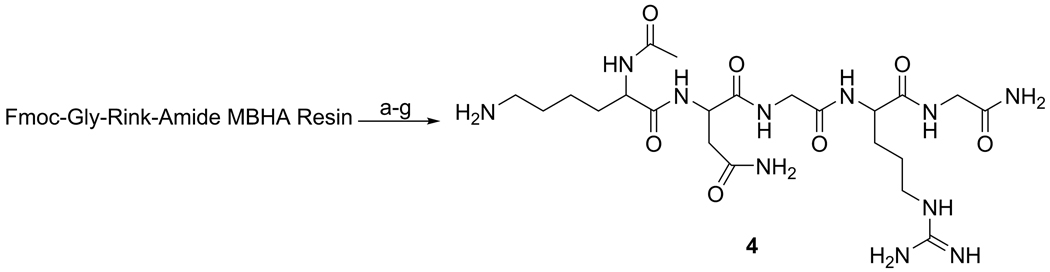
Linear KNGRG (KNGRG) 4
Synthesized using the same protocol as described above except Gly preloaded Rink amid MBHA resin was used instead of Glu to avoid the accompanying reactive functional group. After assembling the last amino acid, the Fmoc group was removed, the α-amine of Lys was acetylated, and the linear peptide was cleaved from the resin as described above. The peptide was then purified with preparative reverse phase HPLC using a gradient of MeCN-H2O (15–100% containing 0.1% TFA, 5mL/min, 20 min run time) and lyophilized to give compound 4 as a white powder (197 mg, 34.5 %). 1H NMR (600 MHz, D2O) δ = 1.39-1.50 (m, 2H), 1.60-1.94 (m, 8H), 2.04 (s, 3H, Me), 2.79 (dd, 1H, J = 8.0, 15.7 Hz), 2.88 (dd, 1H, J = 5.6, 15.7 Hz), 2.99 (t, 2H, J = 7.6 Hz), 3.22 (t, 2H, J = 7.0 Hz), 3.9 (d, 2H, J = 6.5 Hz), 3.97 (s, 2H), 4.25 (dd, 1H, J = 5.6, 8.8 Hz), 4.36 (dd, 1H, J = 5.3, 8.9 Hz), 4.72 (dd, 1H, J = 5.6, 7.9 Hz). 13C NMR (75 MHz, D2O) δ = 21.7, 22.0, 24.3, 26.2, 27.8, 30.1, 35.9, 39.2, 40.5, 42.1, 42.6, 50.5, 53.6, 54.0, 156.8, 171.6, 173.0, 174.0, 174.1, 174.3, 174.6, 174.8. Theoretical mass calculated for KNGRG (C22H41N11O7) was 571.319; found MALDI-TOF-MS: m/z 572.21 [M+H]+, ESI-MS: 572.3 [M+H]+. Analytical HPLC revealed a purity of 99% at 210 nm, tR = 6.85 min, using a gradient of MeCN-H2O (15–100 % containing 0.1% TFA, 0.5 ml/min, 10 min run time).
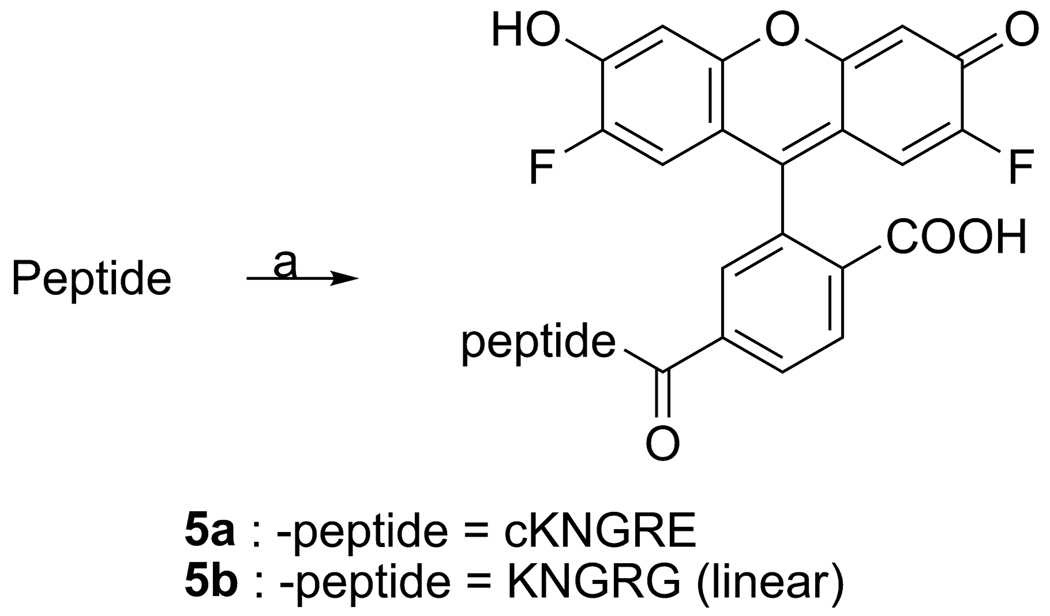
2.4 Conjugation of peptides to Oregon Green
General procedure
DIPEA (3 µL) was added to a solution of NGR peptide (1.0 equiv) and Oregon Green 488 carboxylic acid, succinimidyl ester, 6 isomer (5 mg, 0.0098 mmol, 1.1 equiv) in NMP and the resulting reaction mixture was stirred for 5 h at room temperature. The reaction mixture was precipitated by pouring it into 20 mL of diethylether and then filtering and washing it with diethylether. The resulting ether-free precipitate was dissolved in water and purified with preparative reverse phase HPLC.
cKNGRE-OG (5a)
Purified by preparative HPLC using a gradient of MeCN-H2O (20–40% containing 0.1% TFA, 5 mL/min, 30 min run time) and lyophilized to yield the desired Oregon Green coupled peptide 5a as a yellow powder (6.3 mg, 72%). 1H NMR (600 MHz, D2O): δ = 1.31-1.64 (m, 10H), 1.88-2.05 (m, 3H), 2.19-2.28 (m, 1H), 2.50-2.59 (m, 1H), 2.71- 2.75 (m, 1H), 2.94-2.96 (m, 1H), 3.11 (t, 2H, J = 7 Hz), 3.19-3.24 (m, 2H), 3.82 (d, 1H, J = 17.6 Hz), 3.94 (brs, 1H), 4.04-4.06 (m, 1H), 4.15 (d, 1H, J = 17.6 Hz), 4.34-4.37 (m, 1H), 4.38-4.40 (m, 1H), 6.56 (s, 2H), 6.74 (s, 2H), 7.58 (s, 1H), 7.97 (brs, 1H), 8.12 (brs, 1H). Theoretical mass calculated for cKNGRE-OG (C44H49F2N11O13) was 977.348; found MALDI-TOF-MS: m/z 978.36 (M +H)+, ESI-MS: m/z 978.3 [M+H]+. Purity was determined by analytical HPLC to be 99.5% at 254 nm, tR = 5.39 min, using a gradient of MeCN-H2O (30–40 % containing 0.1% TFA, 1 ml/min, 15 min run time).
KNGRG-OG (5b)
Purified by preparative HPLC using a gradient of MeCN-H2O (30–80% containing 0.1% TFA, 5 mL/min, 25 min run time) and lyophilized to give the desired Oregon Green coupled peptide 5b as a yellow powder (6.5 mg, 67.5 %). Theoretical mass calculated for KNGRG-OG (C43H49F2N11O13) was 965.348; found MALDI-TOF-MS: m/z 966.28 [M +H]+, ESI-MS: m/z 988.2 [M+Na]+, 966.0 [M+H]+. Analytical HPLC revealed a purity of 98.5% at 254 nm, tR = 7.04 min, using a gradient of MeCN-H2O (30–100 % containing 0.1% TFA, 0.5 ml/min, 10 min run time).
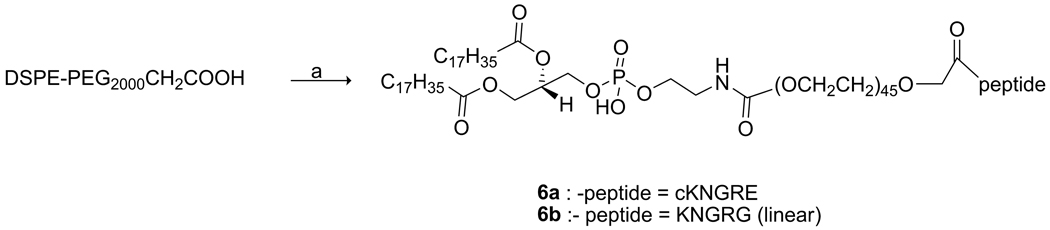
2.5. Coupling of the peptides onto DSPE-PEG2000CH2COOH
General Procedure
Average MW of DSPE-PEG2000CH2COOH was 2788.84 ± 44n (n = 0–8) g/mol. To a solution of DSPE-PEG2000CH2COOH (50 mg, 0.018 mmol), N,N′-Dicyclohexylcarbodiimide (DCC; 4.4 mg, 0.021 mmol), and HOBt (2.8 mg, 0.021 mmol) in NMP; DIPEA (10 µL) was added and stirred for 30 min at room temperature. Peptide 3 or 4 (1.2 equiv) was then added, and the resulting reaction mixture was allowed to stir overnight at ambient temperature. The mixture was powderized by pouring into diethylether (20 mL), and the precipitate was washed with diethylether and dried. The dried powder was dissolved with MeOH: CHCl3 (1:2) and purified with Sephadex-LH20 (CHCl3: MeOH, 2:1). The eluent was concentrated and Et2O was added until a white precipitate as DSPE-PEG2000CH2CO-cKNGRE (6a) or DSPE-PEG2000CH2CO-KNGRG (6b) was separated.
DSPE-PEG2000CH2CO-cKNGRE 6a
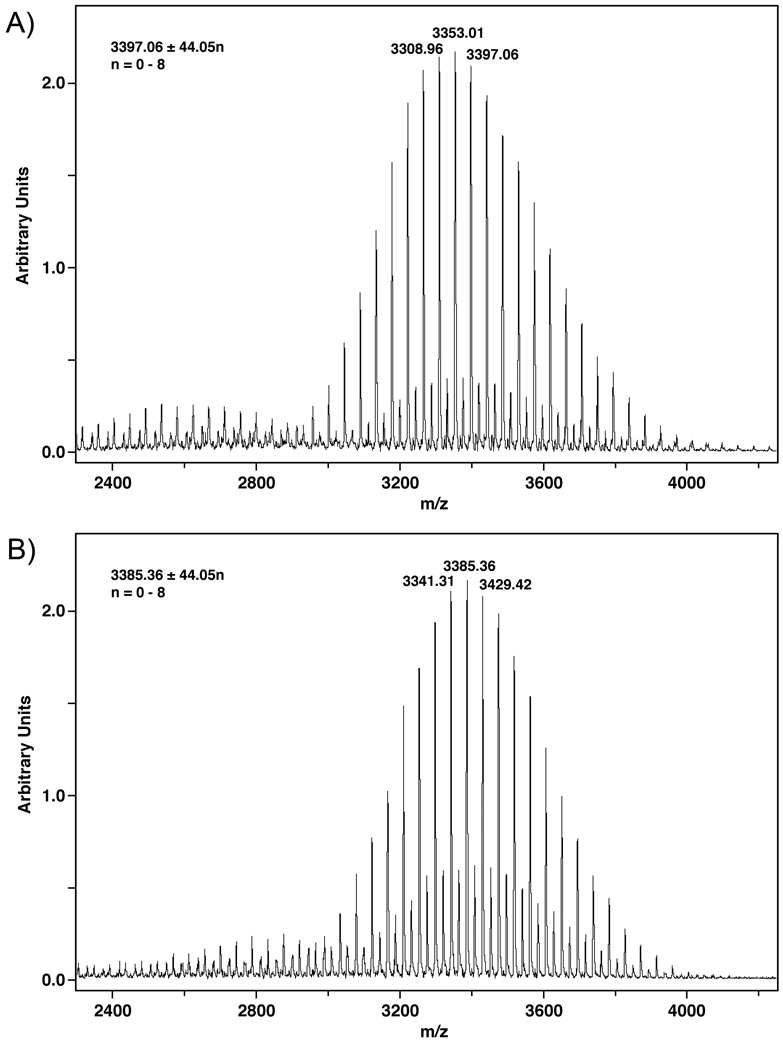
(40 mg, 65%), theoretical mass calculated for C157H303N12O63P was 3396.07 (average); found MALDI-TOF-MS: m/z 3397.06 ± 44n (n = 0–8) [M+H]+.
DSPE-PEG2000CH2CO-KNGRG 6b
48.8 mg, 80 %), theoretical mass calculated for C156H303N12O63P was 3385.06 (average); found MALDI-TOF-MS: m/z 3385.36 ± 44n (n = 0–8) [M+H]+.
2.6. Liposome preparation
NGR-targeted liposomes
Fluorescently labeled NGR-targeted liposomes were prepared as follows. DPPC: MSPC: DSPE-PEG2000-NGR: DiO in molar percent ratio of 85.2: 9.7: 5: 0.1 were dissolved in chloroform, mixed, dried by solvent evaporation, and left overnight in a vacuum desiccator. The dried film was hydrated with 2.5 mL of HEPES buffer (pH 7.4, 10 mM HEPES, 140 mM NaCl, 280 mOsm) at 55 °C for 1 h to yield a final lipid concentration of 10 mg/mL. The resulting multilamellar liposomes were sized by extrusion (10X) with a LIPEX™ Extruder (Northern Lipids Inc., Canada) at 55 °C through two stacked Nuclepore® polycarbonate membrane filters (Whatman plc, United Kingdom) with a pore size of 100 nm. The particle size of the liposome was determined by dynamic light scattering (ZetaPALS, Brookhaven Instruments Corporation, NY) and reported as the mean diameter ± standard deviation. DiO was included to monitor the liposome by this fluorescent label with flow cytometry.
Doxorubicin encapsulation
Dox loaded NGR-targeted liposomes were prepared as follows. DPPC: MSPC: DSPE-PEG2000-cNGR in molar percent ratio of 85.3: 9.7: 5 were prepared as described above. The dried film was hydrated with 300 mM citric acid (pH 4.0) at 60 °C for 15 minute to yield a final lipid concentration of 50 mg/mL. The resulting multilamellar preparation was sized and its particle size was determined as described above. Encapsulation of Dox into the extruded liposomes was carried out using the pH-gradient loading protocol as described by Mayer et al. [35] with slight modification. Briefly, the exterior pH of the extruded liposomes was titrated to 7.4 with sodium carbonate solution (500 mM) creating a pH gradient (acidic inside). The liposomes were incubated with Dox (at Dox: lipid wt ratio of 5:100) at 37 °C for 1h and passed through Sephadex-G50 (fine) spin column. Liposome-entrapped Dox was determined using UV-Vis spectrophotometer [36]. Dox loading efficiency is consistently >98% for LTSLs using this method.
2.7. Temperature triggered release of Dox from cNGR-LTSLs in vitro
The release of encapsulated Dox from cNGR-LTSLs as a function of temperature (25, and 37–41.3 °C) was determined by measuring the dequenching of Dox fluorescence as it was released from a liposome over a period of 15 minutes using Cary Eclipse spectrofluorimeter equipped with Eclipse multicell peltier, temperature controller, and Eclipse Kinetic Software (Varian Inc.) at an excitation and emission wavelength of 498 and 593 nm, respectively. A 10-µL sample of liposome was added into a cuvette containing 2 mL of HEPES buffer equilibrated to the desired temperature and the fluorescent intensity was measured at 2 sec intervals for the first 300 seconds and 5 second interval for the remainder. Then Triton®X-100 (10 µL, 10% w/w in 0.9 % saline) was added to completely disrupt the liposomal bi-layer for complete release of the entrapped Dox. Percent release is calculated by assuming 100% release with Triton® X-100 and 0% release at 25 °C in a HEPES buffer. Data are presented as the mean percent release (n = 3).
2.8. In vitro imaging studies
Cellular binding of the linear and cyclic forms of NGR-OG to CD13 was assessed by plating HT-1080 (CD13+) and MCF7 (CD13−) cells in eight-chambered slides (LabTekII, Nunc, NY) at a concentration of 15,000 cells/well. After 24 h, cells were washed once with phosphate-buffered saline (PBS) and incubated in complete culture media (including serum) at 37 °C for 30 min with cKNGRE-OG (20 µM), KNGRG-OG (20 µM), or a 1:20 dilution of anti-CD13 antibody (WM15, Alexa Fluor 488-conjugated, obtained from AbD Serotec, NC, final concentration 2.5 µg/mL). Slides were washed twice with PBS for 5 min, fixed in 4% paraformaldehyde for 20 min, mounted with Prolong Gold antifade reagent with DAPI (Invitrogen), and imaged with an epifluorescent microscope (Zeiss, Axio Imager.M1, NY). This dose of NGR (20µM) provided the best contrast between CD13 positive and negative cells for a range of examined doses (5, 10, 20, 40, 60, and 80 µM).
To examine internalization, cells were incubated with 20 µM cKNGRE-OG in complete culture media for 30 minutes at 37 °C or 4 °C, washed twice with PBS, and placed in complete media for 30 min at 37 °C. Slides were fixed and mounted as above. Cells were imaged using the same methods as above for epifluorescent microscopy or with a LSM-710 laser scanning confocal fluorescence microscope (Zeiss) using a 63x objective. Emission and excitation spectra were set to manufacturer’s recommended settings for all fluorophores.
2.9. In vitro binding studies
The influence of cyclization of the NGR peptide on its affinity for both the free peptide and the peptide conjugated to an LTSL was studied by a FACSCalibur flow cytometer (Becton Dickinson, Ca) with a 488 nm laser. Samples were read on the FITC channel and analyzed using Cell Quest Pro Software on a Mac G3. CD13+ HT-1080 cells were incubated on ice with cKNGRE-OG, KNGRG-OG, DiO-modified cKNGRE-LTSL, or DiO-modified KNGRG-LTSL at final NGR concentrations of 400, 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.625, 0.75, and 0.375 µM. The anti-CD13 antibody was used as a positive control. Fluorescence, for gated cell populations, was analyzed for 15,000 events (counts) per sample with consistent gating for the free peptide group and for the LTSL group. The % positive cells were fit with the Hill equation to determine the half maximal effective concentration (EC50) for binding.
3. Results
3.1. NGR peptide synthesis
The cKNGRE peptide was assembled using manual solid phase Fmoc peptide chemistry [37] as outlined in Scheme 1. N-Fmoc-Glu(OAll)-OH was selected as a anchoring site for the cyclization using its ω-carboxylic acid. HATU, 2 equiv, based on amino acids was used for peptide coupling in N-Methyl-2-pyrrolidinone. Piperidine in NMP was used for deprotection of the Fmoc group of the N-terminus and orthogonal deprotection of the Cω-allyl ester of the Glu using Pd(PPh3)4 in CHCl3-AcOH-N-Methylmorpholine [38]. On the resin cyclization was carried out by coupling the ω-carboxylic acid of the Glu to the α-amine of the Lys with HATU at high dilution [37]. Removal of the cyclic peptide from the resin and deprotection was performed using TFA in dichloromethane. The crude peptide was concentrated and purified by preparative reverse phase HPLC on a C18 column, obtaining 3 as a white powder that was 98% pure and had 33% yield. The linear KNGRG 4 was synthesized using the same methodology except Fmoc-Gly preloaded Rink Amide Resin (Scheme 2) was used instead of Glu to avoid the side chain reactive functional group. The overall product yield obtained was 34.5% with a purity of 99%. The free amines of the Lys residues of 3 and 4 were used to introduce the fluorescent label OG (1.1 equiv) to obtain compounds 5a and 5b, respectively (Scheme 3). Compounds 5a and 5b were purified with preparative reverse phase HPLC using a C18 column to give a yellow powder in 72 and 67.5% yield, respectively.
Scheme 1.
Reagents and conditions: a) N-Fmoc-Glu-(OAll)-OH (2.5 equiv), HATU (2 equiv), DIPEA (4 equiv), NMP, rt, 30 min.; b) Piperidine-DMF (1:4), rt, 5 min (X 2); c) N-Fmoc-Arg-(Pbf)-OH (2 equiv), HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min; d) N-Fmoc-Gly-OH, HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min; e) N-Fmoc-Asn-(Trt)-OH, HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min; f) N-Fmoc-Lys-(Boc)-OH, HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min; g) Pd(PPh3)4 (2 equiv), CHCl3-AcOH-N-Methylmorpholine (37:2:1), rt, 2 h; h) HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min;17 h; i) TFA-CH2Cl2 (1:1), rt, 2 h.
Scheme 2.
Reagents and conditions: a) Piperidine-DMF (1:4), rt, 5 min (X 2); b) N-Fmoc-Arg-(Pbf)-OH (2 equiv), HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min; c) N-Fmoc-Gly-OH, HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min; d) N-Fmoc-Asn-(Trt)-OH, HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min; e) N-Fmoc-Lys-(Boc)-OH, HATU, (2 equiv), DIPEA (4 equiv), rt, 30 min; f) CH2Cl2: Ac2O: DIPEA (20: 40: 1); g) TFA-CH2Cl2 (1:1), rt, 2 h.
Scheme 3.
Reagents and conditions: a) Oregon Green 488 carboxylic acid, succinimidyl ester, 6-isomer (1.1 equiv), DIPEA (2 equiv), NMP (1 mL), rt, 5 hr.
3.2 NGR targeted LTSL synthesis
NGR peptides were conjugated to DSPE-PEG2000CH2COOH to give compounds 6a and 6b (Scheme 4). In both cases the progress of the reaction was monitored by MALDI-TOF-MS until almost no DSPE-PEG2000CH2COOH was observed. Compounds 6a and 6b were purified by size exclusion chromatography using Sephadex LH-20 that was swollen in and eluted with CHCl3: MeOH (2:1). Peaks of the expected m/z ratios for compounds 6a (65 % yield) and 6b (80 % yield) were confirmed by MALDI-TOF-MS experiments (Figure 1). Minor peaks consistent with sodium salts of 6a and 6b (m/z +22) are seen between the major peaks in both spectra. NGR-targeted LTSLs were synthesized with a particle size of 99.0 ± 0.4 nm for KNGRG-LTSL and 105.6 ± 1.1 nm for cKNGRE-LTSL. Dox encapsulated cKNGRE-targeted LTSLs liposome had a particle size of 107.8 ± 1.5 nm.
Scheme 4.
Reagents and conditions: a) HOBt (1.1 equiv), DCC (1.1 equiv.), DIPEA (3 equiv), NMP (1 mL), 30 min.), 3 or 4 (1.2 equiv), rt, overnight.
Figure 1.
MALDI-TOF-MS Spectra for compounds 6a (A) & b (B).
3.3 In vitro binding of NGR peptide and NGR targeted LTSL to CD13
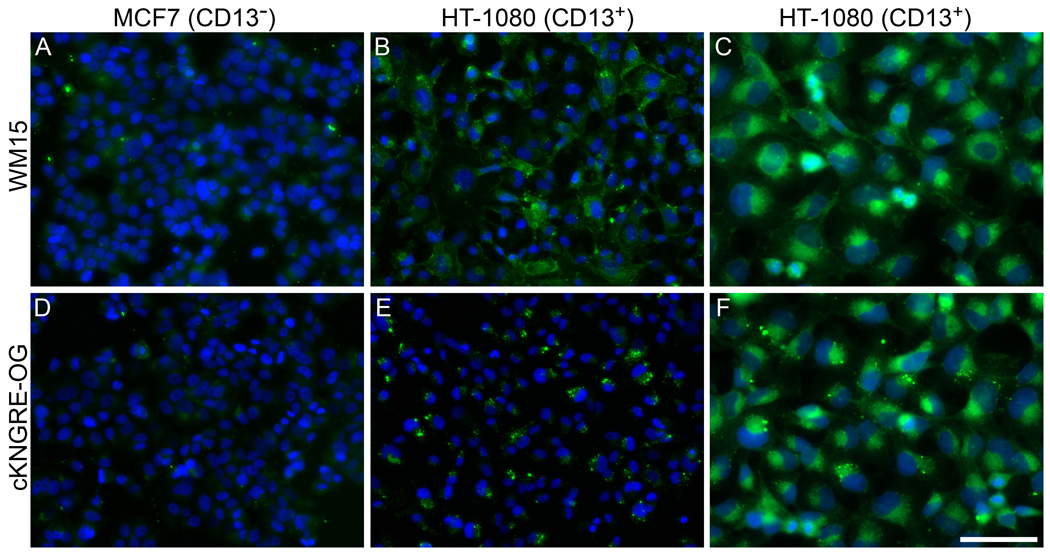
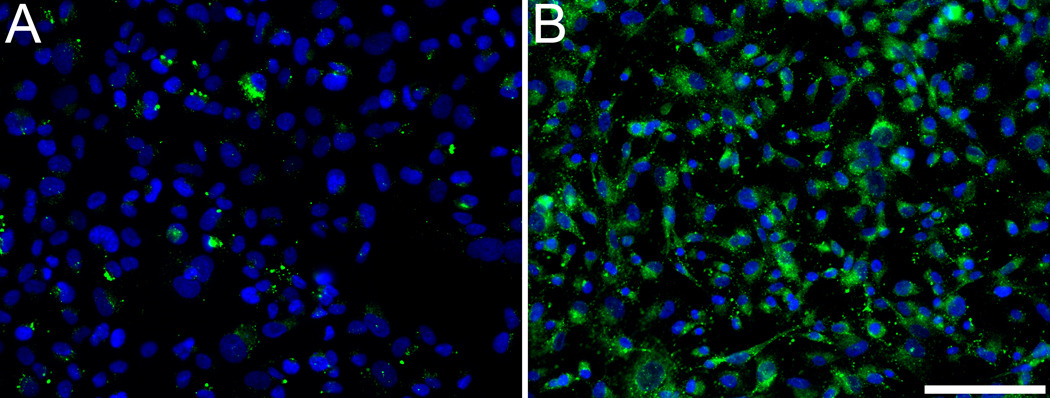
To evaluate in vitro binding of NGR peptide to human carcinoma cells, cKNGRE was conjugated to the fluorophore OG for visualization via epifluorescent microscopy. cKNGRE-OG and the anti-CD13 antibody, WM15, were incubated with HT-1080 (CD13+) and MCF7 (CD13−) cells. cKNGRE-OG and WM15 clearly demonstrated binding to HT-1080 cells (Figure 2B,E), whereas these agents displayed minimal binding to MCF7 cells (Figure 2A,D). Antibody fluorescence was membrane-associated and dispersed across the cell surface. In addition to membrane fluorescence, intense punctuate fluorescence was observed in HT-1080 cells incubated with cKNGRE-OG (Figure 2E), suggesting internalization of the peptide. At higher magnification (Figure 2C, F), the localization of cKNGRE-OG and WM15 appeared similar with the exception of the bright punctuate signal associated with cKNGRE-OG that was more intense than membrane associated fluorescence. The cellular distribution was similar when longer incubation periods up to 2 hours were investigated. Linear KNGRG-OG (Figure 3A) displayed a similar cellular distribution to cKNGRE-OG (Figure 3B) with punctuate fluorescence albeit at a lower overall fluorescent intensity than cKNGRE-OG. This distribution was similar to images of live cells.
Figure 2.
Fluorescence microscopy to evaluate in vitro binding of anti-CD13 antibody WM15 (top row: A, B, and C) and cKNGRE-OG (bottom row: D, E, and F) to CD13+ HT-1080 cells (B, C, E, and F) and CD13− MCF7 cells (A and D). Green, signal from cKNGRE-OG or WM15 antibody. Blue, signal from the nuclear staining agent DAPI. Images AB and DE were acquired with identical exposure times and displayed consistently to compare binding between MCF7 and HT-1080 cells. Bar equals 100 µm for A, B, D, and E and 50 µm for C and F
Figure 3.
Epifluorescence microscopy to evaluate in vitro binding of linear KNGRG-OG (A) and cKNGRE-OG (B) by HT-1080 cells at 37 °C. Green signal from KNGRG-OG or cKNGRE-OG and Blue signal from the nuclear staining agent DAPI. Images were acquired with identical exposure times and displayed consistent window and level. Bar equals 100 µm.
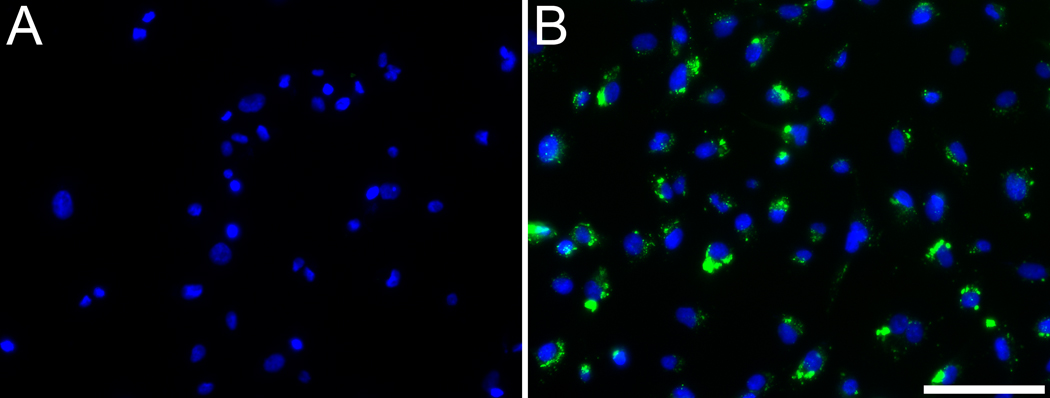
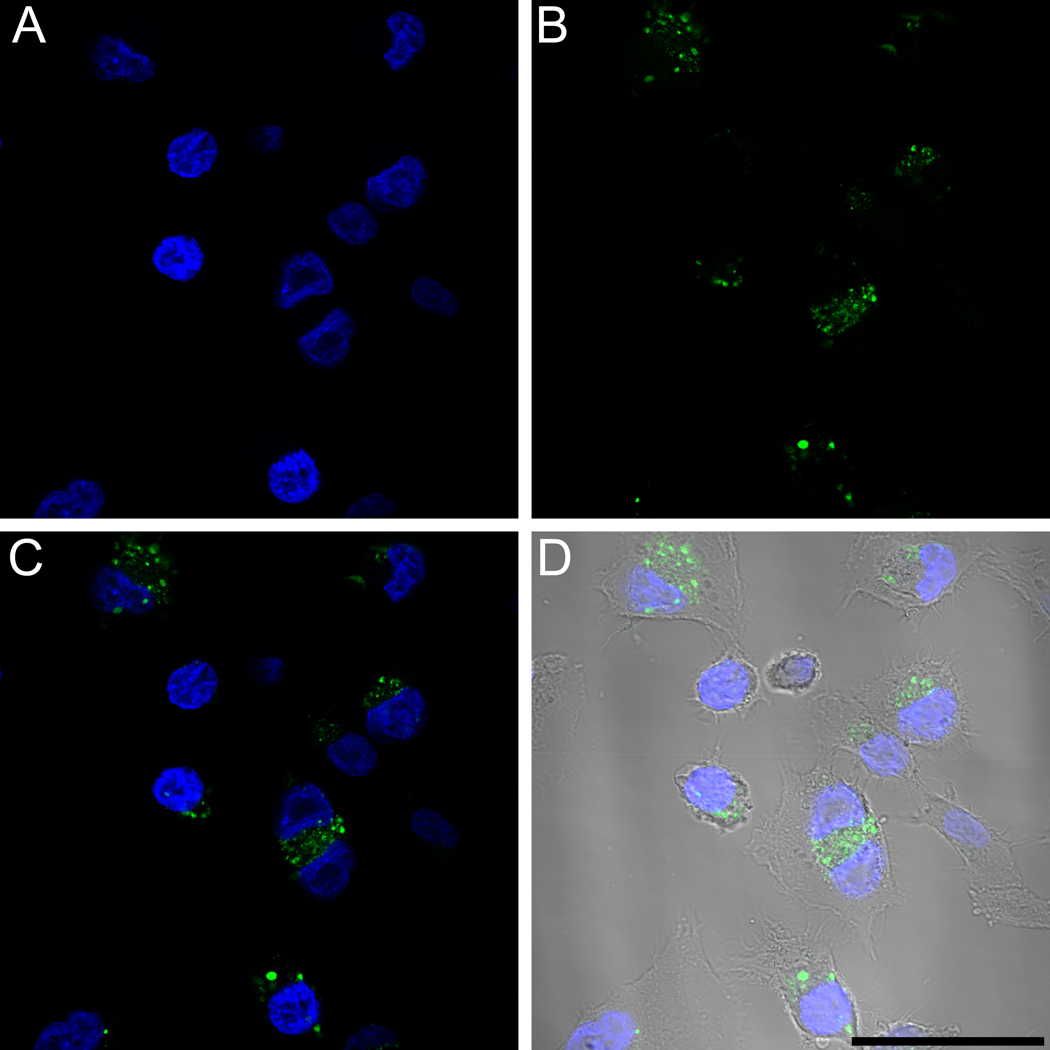
In order to elucidate the cause of punctuate fluorescence for cKNGRE-OG, HT-1080 cells were incubated with cKNGRE-OG for 30 minutes at 4 °C or 37 °C. Incubation at 4 °C reduces endocytosis and accumulation in cytoplasmic vesicles. In contrast to marked cellular internalization at 37 °C (Figure 4B), at 4 °C (Figure 4A) there was minimal uptake of cKNGRE-OG. Furthermore, the punctuate fluorescence at 37 °C was inside the cells as determined by confocal microscopy shown in Figure 5. This internalized cKNGRE-OG demonstrated a pattern consistent with endosomal uptake [39, 40].
Figure 4.
Epifluorescence microscopy to evaluate in vitro internalization of cKNGRE-OG by HT-1080 cells at 4 °C (A) or 37 °C (B). Green signal from cKNGRE-OG and Blue signal from the nuclear staining agent DAPI. Images were acquired with identical exposure times and displayed consistent window and level. Bar equals 100 µm.
Figure 5.
Confocal microscopy to evaluate internalization of cKNGRE-OG in HT-1080 cells at 37°C. Blue signal from the nuclear staining agent DAPI (A), Green signal from cKNGRE-OG (B), merged image of A and B (C), and merged image of A and B with Dodt contrast to visualize cell boundaries (D). Bar equals 50 µm.
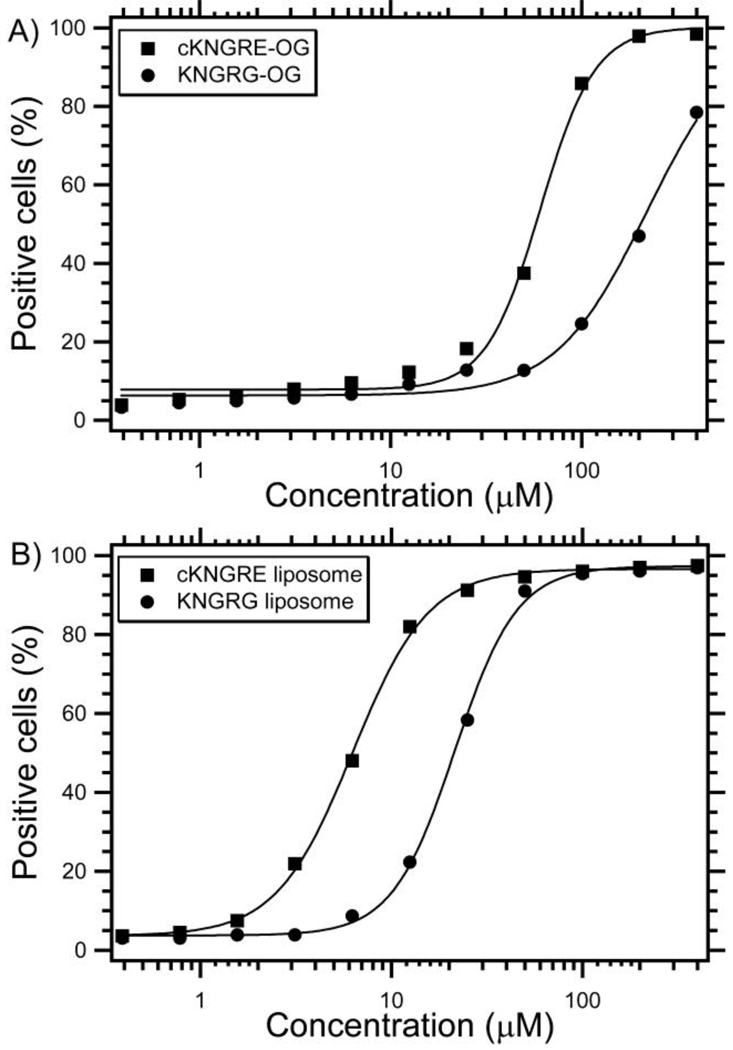
To investigate the binding affinity of the free NGR peptides and NGR-targeted LTSLs, EC50 values were determined using flow cytometry with CD13+ HT-1080 cells. Approximately 94% of the HT-1080 cells were positive for the WM15 antibody, indicating the presence of CD13. As shown in Figure 6A, cKNGRE-OG demonstrated 3.6-fold greater affinity for HT-1080 cells (EC50 = 61.0µM) than did the linear KNGRG-OG (EC50 = 219.9µM). Similarly, as shown in Figure 6B, LTSLs conjugated to cKNGRE (EC50 = 6.2µM) had a 3.5-fold greater avidity than did LTSLs conjugated to linear KNGRG (EC50 = 21.5µM). These experiments were repeated twice with similar results. These data demonstrated that the cyclic NGR had much greater affinity than the linear NGR peptide and their binding constants are sufficient for biological applications. Furthermore, the multivalent presentation of NGR peptides on the surface of liposomes increased the affinity ~10-fold over free peptides based on the concentration of NGR. Assuming a 100nm liposome, there would be ~2100 NGR ligands on the outer surface of the NGR-LTSL[41, 42]. This corresponds to ~4,200 total NGR ligands per liposome. Since there are many NGRs per liposome, the affinity based on a particle basis actually increased 41,000-fold (61µM for cKNGRE /1.5nM for cKNGRE-targeted LTSL) due to the multivalent presentation of cNGR[43].
Figure 6.
In vitro binding expressed as % positive HT-1080 cells (CD13+) versus concentration of cKNGRE and linear KNGRG conjugated to OG (A) and LTSL (B). The half maximal effective concentration (EC50) was determined from the Hill equation (shown as a solid line).
3.4 In vitro Dox release characteristics of cNGR-LTSLs
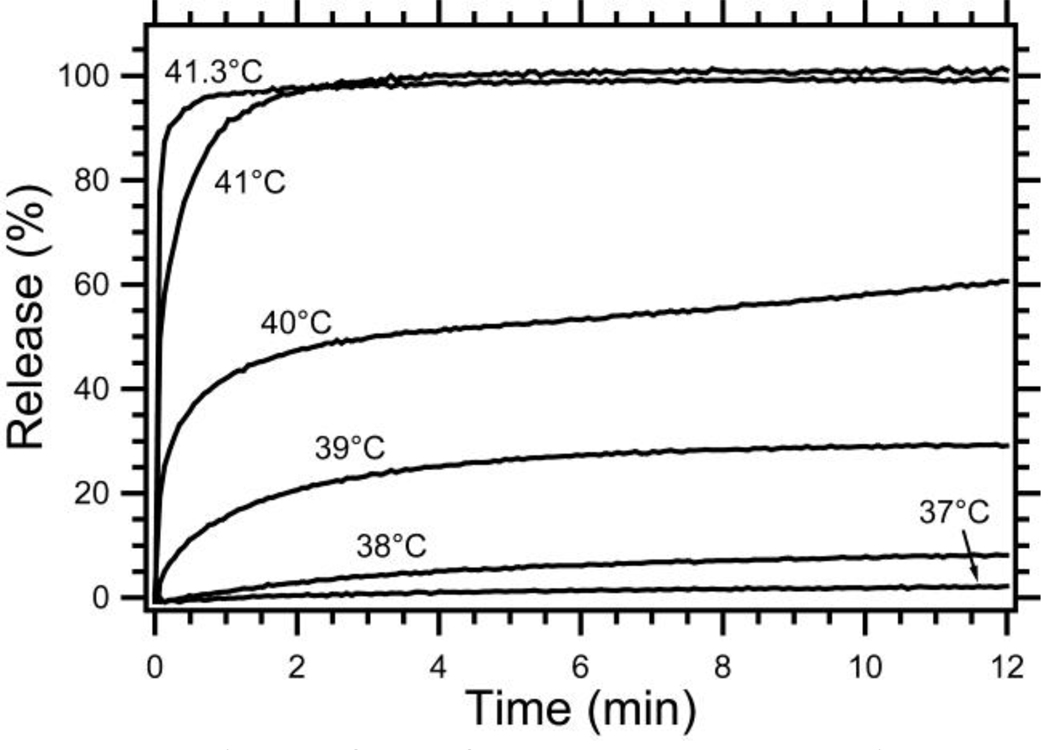
The temperature dependent release of Dox from cKNGRE-targeted LTSLs was investigated with a fluorescence dequenching assay. As shown in Figure 7, Dox is rapidly released at 41.3 °C (>75% release in < 4 sec) with minimal release at 37 °C. These release kinetics are similar to published reports of unmodified LTSLs (slightly different formulation) [32] as well as untargeted LTSLs with an identical composition in both HEPES buffer and plasma.
Figure 7.
Release of Dox from cKNGRE-targeted LTSLs. Percent release is calculated by assuming 100% release with Triton® X-100 and 0% release at 25 °C in a HEPES buffer.
4. Discussion
A novel permanently cyclized NGR peptide was synthesized and evaluated for binding to CD13+ cancer cells. The synthetic strategy resulted in a sufficient yield and purity (33% and 98%, respectively) of cKNGRE. The permanently cyclized NGR peptide had ~3.5-fold greater affinity than the linear form both as a free peptide and on the surface of a liposome. This increase in affinity due to cyclization [22] is similar to other peptides that exhibit improved affinity when conformationally constrained [44–46]. The NGR-LTSL had greater avidity than the free NGR-OG as would be expected due to the multivalent presentation of the NGR ligand on the surface of the liposome [43]. In the cyclized peptide design, we employed the Cω-carboxylic acid of Glu in the ring closure process with the α-amine of Lys so as to obtain the same number of chemical bonds in the ring as that of the originally identified and most common cyclic CNGRC peptide [2, 22], which is cyclized through a disulfide bridge. Additional modifications of the ring size and NGR flanking residues may further improve the binding affinity and specificity of cyclic NGR peptides.
Previous reports have demonstrated the utility of NGR peptides for both drug delivery [23, 28] and molecular imaging [47–49], but these works have employed a linear or disulfide bridge form of the peptide. On the surface of a liposome or other targeting vehicle, cyclic NGR peptides formed through disulfide bridges may develop additional disulfide bridges between adjacent peptides that render the ligand ineffective [29]. The new cyclized version of NGR reported herein avoided this potential pitfall while retaining sufficient binding avidity (EC50 = 6.2 µM) to target CD13 in vitro. In contrast to the method of attaching linear NGR peptides to liposomes reported by Pastorino et al [28] that involved chemical attachment of the peptide to the pre-synthesized liposome, we first synthesized the PEG-lipid with the cyclic NGR targeting moiety and then synthesized the liposome. Although the previously reported method had the advantages of simplicity and an overall faster synthesis, our method offers more favorable control over liposome composition, reduced manipulations after drug loading, and reproducibility. To our knowledge, this may be the first report of a tumor vascular targeted LTSL, but tumor targeted temperature sensitive liposomes have been reported [50]. This targeted liposome may also be combined with emerging heat sources, such as high intensity focused ultrasound[51], or imaging technologies for monitoring drug delivery[52–54].
The combination of tumor vascular targeting and temperature triggered drug release from liposomes has the potential to improve therapeutic efficacy by: 1) slowing the transit time of liposomes in the tumor vasculature to improve drug release, 2) improving total drug accumulation in the tumor, and 3) treating metastatic tumors not subjected to hyperthermia. The targeting of tumor vasculature with liposomes has the advantage over traditional tumor cell targeted immunoliposomes [55] of not requiring the slow process of extravasation and subsequent penetration before binding and cellular uptake can occur. In contrast to tumor cell antigens, tumor vascular antigens are immediately available for binding directly after intravenous administration. Furthermore, targeting angiogenic tumor vasculature is a more ubiquitous approach applicable to most solid tumors and does not require the overexpression of a tumor cell specific antigen that is often limited to a particular subtype of tumors such as HER2 [56]. Temperature triggered drug release from LTSLs has demonstrated excellent tumor control in preclinical models [33, 51] but this local-regional therapy is limited in its ability to treat widespread metastatic disease. The promising preclinical results of NGR-targeted non-thermally sensitive liposomes in metastatic models [28] suggests that the NGR-targeted thermally sensitive formulation reported herein may be able to provide excellent local-regional control with tumor targeted hyperthermia as well as improved therapy through NGR-targeting of unheated metastatic disease.
5. Conclusion
We report the synthesis of a novel cyclic NGR ligand, cKNGRE, and evaluation of its in vitro binding to CD13+ cancer cells. cKNGRE synthesis was verified with NMR and mass spectral techniques and resulted in high yield and purity. In vitro fluorescence microscopy studies revealed binding of cKNGRE-OG to CD13+ HT-1080 cells and minimal binding to CD13− MCF7 cells. The membrane localization of cKNGRE-OG was similar to that of the anti-CD13 WM15 antibody with the exception of a bright punctuate signal associated with active internalization of cKNGRE-OG. The cKNGRE ligand displayed 3.6-fold greater affinity for CD13+ cancer cells than did linear KNGRG. This affinity was similarly improved 10-fold for both the cyclic and linear NGR peptides when attached to the surface of an LTSL. cKNGRE-targeted LTSLs rapidly released (>75% in <4 sec) doxorubicin at 41.3 °C with minimal release at 37 °C. The results of this study are significant because they demonstrate improved avidity of an NGR-targeted LTSL without the limitation of a disulfide bridge.
Acknowledgements
This research supported in part by the Intramural Research Program of the National Institutes of Health. We thank Dr. Edyta Pawelczyk and Dr. Elaine Kay Jordan for assistance with flow cytometry, and Dr. Herman Yeh and Mr. Wesley White for 1H-NMR (600 MHz) experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevanovic S. Identification of tumour-associated T-CELL epitopes for vaccine development. Nature Reviews Cancer. 2002;2(7):514–520. doi: 10.1038/nrc841. [DOI] [PubMed] [Google Scholar]
- 2.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 3.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nature Reviews Cancer. 2002;2(10):750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 4.Brignole C, Marimpietri D, Gambini C, Allen TM, Ponzoni M, Pastorino F. Development of Fab' fragments of anti-GD(2) immunoliposomes entrapping doxorubicin for experimental therapy of human neuroblastoma. Cancer Letters. 2003;197(1–2):199–204. doi: 10.1016/s0304-3835(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 5.Pagnan G, Montaldo PC, Pastorino F, Raffaghello L, Kirchmeier M, Allen TM, Ponzoni M. GD2-mediated melanoma cell targeting and cytotoxicity of liposome-entrapped fenretinide. International Journal of Cancer. 1999;81(2):268–274. doi: 10.1002/(sici)1097-0215(19990412)81:2<268::aid-ijc17>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Gabizon A, Horowitz AT, Goren D, Tzemach D, Mandelbaum-Shavit F, Qazen MM, Zalipsky S. Targeting folate receptor with folate linked to extremities of poly(ethylene glycol)-grafted liposomes: In vitro studies. Bioconjugate Chemistry. 1999;10(2):289–298. doi: 10.1021/bc9801124. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani LT, Miotti S, Menard S, Canevari S, Raspagliesi F, Bottini C, Bottero F, Colnaghi MI. Folate Binding-Protein Distribution in Normal-Tissues and Biological-Fluids from Ovarian-Carcinoma Patients as Detected by the Monoclonal-Antibodies Mov18 and Mov19. European Journal of Cancer. 1994;30A(3):363–369. doi: 10.1016/0959-8049(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 8.Campbell IG, Jones TA, Foulkes WD, Trowsdale J. Folate-Binding Protein Is a Marker for Ovarian-Cancer. Cancer Research. 1991;51(19):5329–5338. [PubMed] [Google Scholar]
- 9.Neri D, Bicknell R. Tumour vascular targeting. Nature Reviews Cancer. 2005;5(6):436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 10.Kumar CC, Armstrong L, Yin Z, Malkowski M, Maxwell E, Ling H, Yaremko B, Liu M, Varner J, Smith EM, Neustadt B, Nechuta T. Targeting integrins alpha(v)beta(3) and alpha(v)beta(5) for blocking tumor-induced angiogenesis. Angiogenesis: From the Molecular to Integrative Pharmacology. 2000;476:169–180. [PubMed] [Google Scholar]
- 11.Curley GP, Blum H, Humphries MJ. Integrin antagonists. Cellular and Molecular Life Sciences. 1999;56(5–6):427–441. doi: 10.1007/s000180050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. Journal of Clinical Oncology. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Medicine. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 14.Yu JH, Ustach C, Kim HRC. Platelet-derived growth factor signaling and human cancer. Journal of Biochemistry and Molecular Biology. 2003;36(1):49–59. doi: 10.5483/bmbrep.2003.36.1.049. [DOI] [PubMed] [Google Scholar]
- 15.Pietras K, Sjoblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3(5):439–443. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 16.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis CJ, Pasqualini R. Steps toward mapping the human vasculature by phage display. Nature Medicine. 2002;8(2):121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 17.Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A. Enhancement of tumor necrosis factor a antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nature Biotechnology. 2000;18(11):1185–1190. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 18.Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. Journal of Clinical Investigation. 2002;110(4):475–482. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Del Rio G, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R. Anti-cancer activity of targeted pro-apoptotic peptides. Nature Medicine. 1999;5(9):1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 20.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296(5577):2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 21.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Research. 2000;60(3):722–727. [PMC free article] [PubMed] [Google Scholar]
- 22.Colombo G, Curnis F, De Mori GMS, Gasparri A, Longoni C, Sacchi A, Longhi R, Corti A. Structure-activity relationships of linear and cyclic peptides containing the NGR tumor-homing motif. Journal of Biological Chemistry. 2002;277(49):47891–47897. doi: 10.1074/jbc.M207500200. [DOI] [PubMed] [Google Scholar]
- 23.Garde SV, Forte AJ, Ge M, Lepekhin EA, Panchal CJ, Rabbani SA, Wu JJ. Binding and internalization of NGR-peptide-targeted liposomal doxorubicin (TVT-DOX) in CD13-expressing cells and its antitumor effects. Anti-Cancer Drugs. 2007;18(10):1189–1200. doi: 10.1097/CAD.0b013e3282a213ce. [DOI] [PubMed] [Google Scholar]
- 24.Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. Vascular damage and anti-angiogenic effects of tumor vesseltargeted liposomal chemotherapy. Cancer Research. 2003;63(21):7400–7409. [PubMed] [Google Scholar]
- 25.Fidler IJ, Ellis LM. The Implications of Angiogenesis for the Biology and Therapy of Cancer Metastasis. Cell. 1994;79(2):185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 26.Majhen D, Gabrilovac J, Eloit M, Richardson J, Ambriovic-Ristov A. Disulfide bond formation in NGR fiber-modified adenovirus is essential for retargeting to aminopeptidase N. Biochemical and Biophysical Research Communications. 2006;348(1):278–287. doi: 10.1016/j.bbrc.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Moffatt S, Papasakelariou C, Wiehle S, Cristiano R. Successful in vivo tumor targeting of prostate-specific membrane antigen with a highly efficient J591/PEI/DNA molecular conjugate. Gene Therapy. 2006;13(9):761–772. doi: 10.1038/sj.gt.3302721. [DOI] [PubMed] [Google Scholar]
- 28.Pastorino F, Brignole C, Di Paolo D, Nico B, Pezzolo A, Marimpietri D, Pagnan G, Piccardi F, Cilli N, Longhi R, Ribatti D, Corti A, Allen TM, Ponzoni M. Targeting liposomal chemotherapy via both tumor cell-specific and tumor vasculature-specific ligands potentiates therapeutic efficacy. Cancer Research. 2006;66(20):10073–10082. doi: 10.1158/0008-5472.CAN-06-2117. [DOI] [PubMed] [Google Scholar]
- 29.Corti A, Ponzoni M. Tumor vascular targeting with tumor necrosis factor alpha and chemotherapeutic drugs. Signal Transduction and Communication in Cancer Cells. 2004;1028:104–112. doi: 10.1196/annals.1322.011. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhry BZ, Lipka G, Dalziel AW, Sturtevant JM. Multicomponent Phase-Transitions of Diacylphosphatidylethanolamine Dispersions. Biophysical Journal. 1984;45(5):901–904. doi: 10.1016/S0006-3495(84)84236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong G, Dewhirst MW. Hyperthermia and liposomes. International Journal of Hyperthermia. 1999;15(5):345–370. doi: 10.1080/026567399285558. [DOI] [PubMed] [Google Scholar]
- 32.Mills JK, Needham D. Lysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transition. Biochimica. Biophysica Acta-Biomembranes. 2005;1716(2):77–96. doi: 10.1016/j.bbamem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Research. 2000;60(16):4440–4445. [PubMed] [Google Scholar]
- 34.Ferrara K. Driving delivery vehicles with ultrasound. ADVANCED DRUG DELIVERY REVIEWS. 2008;60(10):1097–1102. doi: 10.1016/j.addr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer LD, Tai LCL, Ko DSC, Masin D, Ginsberg RS, Cullis PR, Bally MB. Influence of Vesicle Size, Lipid-Composition, and Drug-to-Lipid Ratio on the Biological-Activity of Liposomal Doxorubicin in Mice. Cancer Research. 1989;49(21):5922–5930. [PubMed] [Google Scholar]
- 36.Fenske DB, Maurer N, Cullis P. In: Liposomes. 2nd Edition. Torchilin VP, Weissig V, editors. Vol. Oxford University Press; 2003. pp. 167–191. [Google Scholar]
- 37.Kates SA, Sole NA, Johnson CR, Hudson D, Barany G, Albericio F. A Novel, Convenient, 3-Dimensional Orthogonal Strategy for Solid-Phase Synthesis of Cyclic-Peptides. Tetrahedron Letters. 1993;34(10):1549–1552. [Google Scholar]
- 38.Kates SA, Delatorre BG, Eritja R, Albericio F. Solid-Phase N-Glycopeptide Synthesis Using Allyl Side-Chain Protected Fmoc-Amino Acids. Tetrahedron Letters. 1994;35(7):1033–1034. [Google Scholar]
- 39.Wang X, Wang YG, Chen XM, Wang JC, Zhang X, Zhang Q. NGR-modified micelles enhance their interaction with CD13-overexpressing tumor and endothelial cells. Journal of Controlled Release. 2009;139(1):56–62. doi: 10.1016/j.jconrel.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 40.Hansen GH, Delmas B, Besnardeau L, Vogel LK, Laude H, Sjostrom H, Noren O. The coronavirus transmissible gastroenteritis virus causes infection after receptor-mediated endocytosis and acid-dependent fusion with an intracellular compartment. Journal of Virology. 1998;72(1):527–534. doi: 10.1128/jvi.72.1.527-534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirpotin D, Park JW, Hong K, Zalipsky S, Li WL, Carter P, Benz CC, Papahadjopoulos D. Sterically stabilized Anti-HER2 immunoliposomes: Design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36(1):66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 42.Huang C, Mason JT. GEOMETRIC PACKING CONSTRAINTS IN EGG PHOSPHATIDYLCHOLINE VESICLES. Proceedings of the National Academy of Sciences of the United States of America. 1978;75(1):308–310. doi: 10.1073/pnas.75.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angewandte Chemie-International Edition. 1998;37(20):2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Lee G, Chan W, Hurle MR, Desjarlais RL, Watson F, Sathe GM, Wetzel R. Strong Inhibition of Fibrinogen Binding to Platelet Receptor -Alpha-Iib-Beta-3 by Rgd Sequences Installed into a Presentation Scaffold. Protein Engineering. 1993;6(7):745–754. doi: 10.1093/protein/6.7.745. [DOI] [PubMed] [Google Scholar]
- 45.Mousa SA, Bozarth JM, Naik UP, Slee A. Platelet GPIIb/IIIa binding characteristics of small molecule RGD mimetic: distinct binding profile for Roxifiban. British Journal of Pharmacology. 2001;133(3):331–336. doi: 10.1038/sj.bjp.0703943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang GF, Zhou ZM, Srinivasan R, Penn MS, Kottke-Marchant K, Marchant RE, Gupta AS. Affinity manipulation of surface-conjugated RGD peptide to modulate binding of liposomes to activated platelets. Biomaterials. 2008;29(11):1676–1685. doi: 10.1016/j.biomaterials.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Wallbrunn A, Waldeck J, Holtke C, Zuehlsdorf M, Mesters R, Heindel W, Schafers M, Bremer C. In vivo optical imaging of CD13/APN-expression in tumor xenografts. Journal of Biomedical Optics. 2008;13(1):9. doi: 10.1117/1.2839046. [DOI] [PubMed] [Google Scholar]
- 48.Dirksen A, Langereis S, de Waal BFM, van Genderen MHP, Meijer EW, de Lussanet QG, Hackeng TM. Design and synthesis of a bimodal target-specific contrast agent for angiogenesis. Organic Letters. 2004;6(26):4857–4860. doi: 10.1021/ol048084u. [DOI] [PubMed] [Google Scholar]
- 49.Buehler A, van Zandvoort MAMJ, Stelt BJ, Hackeng TM, Schrans-Stassen BHGJ, Bennaghmouch A, Hofstra L, Cleutjens JPM, Duijvestijn A, Smeets MB, de Kleijn DPV, Post MJ, de Muinck ED. cNGR: A novel homing sequence for CD13/APN targeted molecular imaging of murine cardiac angiogenesis in vivo. Arteriosclerosis Thrombosis and Vascular Biology. 2006;26(12):2681–2687. doi: 10.1161/01.ATV.0000245807.65714.0b. [DOI] [PubMed] [Google Scholar]
- 50.Puri A, Kramer-Marek G, Campbell-Massa R, Yavlovich A, Tele SC, Lee SB, Clogston JD, Patri AK, Blumenthal R, Capala J. HER2-Specific Affibody-Conjugated Thermosensitive Liposomes (Affisomes) for Improved Delivery of Anticancer Agents. Journal of Liposome Research. 2008;18(4):293–307. doi: 10.1080/08982100802457377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie JW, Libutti SK, Li KCP, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clinical Cancer Research. 2007;13(9):2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ponce A, Viglianti B, Yu D, Yarmolenko P, Michelich C, Woo J, Bally M, Dewhirst M. Magnetic resonance imaging of temperature-sensitive liposome release: Drug dose painting and antitumor effects. JOURNAL OF THE NATIONAL CANCER INSTITUTE. 2007;99(1):53–63. doi: 10.1093/jnci/djk005. [DOI] [PubMed] [Google Scholar]
- 53.Viglianti B, Abraham S, Michelich C, Yarmolenko P, MacFall J, Bally M, Dewhirst M. In vivo monitoring of tissue pharmacokinetics of liposome/drug using MRI: Illustration of targeted delivery. MAGNETIC RESONANCE IN MEDICINE. 2004;51(6):1153–1162. doi: 10.1002/mrm.20074. [DOI] [PubMed] [Google Scholar]
- 54.Langereis S, Keupp J, van Velthoven JLJ, de Roos IHC, Burdinski D, Pikkemaat JA, Grull H. A Temperature-Sensitive Liposomal H-1 CEST and F-19 Contrast Agent for MR Image-Guided Drug Delivery. Journal of the American Chemical Society. 2009;131(4):1380. doi: 10.1021/ja8087532. [DOI] [PubMed] [Google Scholar]
- 55.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 56.Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, Marks JD, Papahadjopoulos D, Benz CC. Tumor targeting using anti-her2 immunoliposomes. Journal of Controlled Release. 2001;74(1–3):95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]