Abstract
Linear ion-trap multiple-stage mass spectrometric approach (MSn) towards nearly complete structural elucidation of triacylglycerol (TAG) including (1) assignment the fatty acid substituents on the glycerol backbone and (2) location of the double bond(s) on the unsaturated fatty acyl groups is reported. The characterization is established by the findings that MS2 on the [M + Li]+ ions of TAG yields more abundant ions reflecting losses of the outer fatty acid substituents either as free acids (i.e., [M + Li − R1CO2H]+ and [M + Li − R3CO2H]+ ions) or as lithium salts (i.e., [M + Li − R1CO2Li]+ and [M + Li − R3CO2Li]+ ions) than the ions reflecting the similar losses of the inner fatty acid substituent (i.e., [M + Li − R2CO2Li]+ and [M + Li − R2CO2Li]+ ions). Further dissociation (MS3) of [M + Li − RnCO2H]+ (n=1, 2, or 3) gives rise to the ion series locating the double bonds along the fatty acid chain. These ions arise from charge-remote fragmentations involving β-cleavage with γ-H shift, analogous to those seen for the unsaturated long chain fatty acids characterized as dilithiated ions. Significant differences in abundances in the ion pairs reflecting the additional losses of the fatty acid moieties, respectively, were also seen in the MS3 spectra of the [M + Li − RnCO2H]+ and [M + Li − RnCO2Li]+ ions, leading to confirmation of the fatty acid substituents on the glycerol backbone. MSn on the [M + Na]+ and [M + NH4]+ adduct ions also affords location of fatty acid substituents on the glycerol backbone, but not the position of the double bond(s) along the fatty acid chain. Unique ions from internal losses of the glycerol residues were seen in the MS3 spectra of [M + Alk − RnCO2H]+ (n=1,2,3) and of [M + Alk − RnCO2Alk]+ (Alk= Li, Na, NH4; n=1,3). They are signature ions for glycerides and the pathways leading to their formation may involve rearrangements.
Keywords: Triacylglycerol, Ammonium adduct ions, Alkali metal adduct ions, Tandem mass spectrometry, Linear ion-trap mass spectrometry, ESI, Rearrangements
Introduction
High-energy CAD with a tandem sector instrument permits structural characterization of triacylglycerol (or triacylglyceride) (TAG) as sodiated adducts desorbed by FAB, leading to identify each fatty acid substituent and positions of fatty acid substituents on the glycerol backbone. However, the sensitivity is poor and the application in elucidation of the location of double bond(s) for the unsaturated fatty acid substituents has not been demonstrated [1]. High energy CAD product-ion spectra of the sodiated adducts of TAG obtained with tandem sector instrument coupled with ESI were unsatisfactory for structural characterization, but those from the [M + NH4]+ ions identified the fatty acid substituents, nevertheless, are not informative for assignment of the fatty acid substituents on the glycerol backbone [1]. Similarly, tandem quadrupole mass spectra of the [M + Na]+ ions of TAG did not contain structurally informative fragment ions, but those from [M + NH4]+ ions contained fragment ions that identify the fatty acid groups [2]. Neither the positions of the fatty acid substituents on the glycerol backbone nor the locations of double bonds in unsaturated fatty acid chains could be determined from these spectra [2]. Recently, Pittenauer and Allmaier demonstrated high-energy CAD using MALDI-TOF/RTOF-MS for characterization of TAGs as alkali adduct ions. The product ions from [M + Na]+ ions of TAG afford determination of the position of fatty acid substituents on the glycerol backbone and in some favorable cases the location of double bonds or hydroxy groups. However, the method is limited by the insufficient precursor ion gating after MS1 (gating window of 4 Da), and ion species differed by 2 Da cannot be separated [3].
By contrast, tandem quadrupole [4] and quadrupole ion-trap [5] product-ion spectra from the [M + Li]+ ions of TAG is readily applicable for identification of the fatty acid substituents and location their position on the glycerol backbone. Skimmer CAD on the lithiated adduct ion of TAG, followed by tandem quadrupole mass spectrometry on the dilithiated adduct ions of fatty acid substituents generated by skimmer CAD are applicable for location of double bonds. But the method for reliable location of the unsaturated sites for the fatty acid chain in TAG mixtures remains untested [4]. The employment of ozone electrospray ionization and ozone induced dissociation mass spectrometry to unveil the double bond position of trieicosatetraenoin and trioleoryglycerol standards was recently reported by Thomas et al, but assignment of the fatty acid substituents on the glycerol backbone has not been demonstrated [6-8]. The method also required a special set-up to generate harmful ozone gas to be emitted to an ion-trap instrument. Herein, We report a multiple-stage (MSn) (n=2,3,4) mass spectrometric approach with an LIT instrument for near-complete characterization of TAG as the [M + Li]+ ions, including identification of fatty acid substituents, the site of double bond of fatty acyl substituents and their positions on the glycerol backbone. The application of this LIT MSn method on the [M + Na]+ and [M + NH4]+ ions of TAG for structural characterization and the mechanism(s) underlying the fragmentation processes leading to ion formation are also discussed.
Experimental
Material and chemicals
Triacylglycerol standards of rac-glyceryl-1,3-dipalmitate-2-oleate [(16:0/Δ918:1/16:0)-TAG], rac-glyceryl-2,3-dipalmitate-1-oleate [(Δ918:1/16:0/16:0)-TAG], rac-glyceryl-1-palmitate-2-oleate-3-stearate [(16:0/Δ918:1/18:0)- TAG], rac-glyceryl-1-palmitate-2-stearate-3-oleate [(16:0/18:0/Δ918:1)-TAG], rac-glyceryl-1,3-distearate-2-oleate [(18:0/Δ918:1/18:0)-TAG], rac-glyceryl-2,3-distearate-1-oleate [(Δ918:1/18:0/18:0)-TAG], and tripalmitin [(16:0/16:0/16:0)-TAG] were purchased from Matreya (Pleasant Gap, PA). Synthetic triarachidonin [(Δ5,8,11,1420:4/Δ5,8,11,1420:4/ Δ5,8,11,1420:4)-TAG] and trilinolein [(Δ9,1218:2/Δ9,1218:2/Δ9,1218:2)-TAG] standards were purchased from NuChek Prep (Elysian, MN). 1,3(d5)-diheptadecanoyl-2-(10Z-heptadecenoyl)-glycerol [d5-(17:0/ Δ1017:1/17:0)-TAG] was purchased from Avanti Polar lipid (Alabaster, Al). All TAG standards are > 98% purity. Solvents and other chemicals were obtained from Fisher Chemical.
Mass spectrometry
Low-energy CAD tandem mass spectrometry experiments were conducted on a Finnigan (San Jose, CA) linear ion-trap (LIT) mass spectrometer (MS) with Xcalibur operating system. Triacylglycerols were dissolved in chloroform/methanol (1/4) at final concentration of 10 pmol/μL and lithium hydroxide was added to achieve a final [Li+] concentration of 1 mM before infusion (2 μL/min) to the ESI source, where the skimmer was set at ground potential, the electrospray needle was set at 4.5 kV, and temperature of the heated capillary was 350 °C. The automatic gain control of the ion trap was set to 5 × 104, with a maximum injection time of 200 ms. Helium was used as the buffer and collision gas at a pressure of 1×10−3 mbar (0.75 mTorr). The MSn experiments were carried out with an optimized relative collision energy ranging from 16-25% and with an activation q value at 0.25, and the activation time at 30-100 ms to leave a minimal residual abundance of precursor ion (around 20%). The isolation window for the precursor ions were set at 1 Da. Mass spectra were accumulated in the profile mode, typically for 3-10 min for MSn (n = 2, 3, and 4) spectra. The mass resolution of the instrument was tuned to 0.6 Da at half peak height.
Results and Discussion
TAG molecules that differ only with respect to the fatty acyl groups at sn-1 and sn-3, such as (A/B/C)-TAG vs. (C/B/A)-TAG (where A, B, and C are distinct fatty acid residues) are enantiomers and cannot be distinguished by the present mass spectrometric approach. In this premise for assignment of the fatty acid substituents on the glycerol backbone as described in the text, only the fatty acid substituent assigned to sn-2 is specific, and the fatty acid substituents assigned to sn-1 and at sn-3 is exchangeable (i.e., A/B/C)-TAG and (C/B/A)-TAG are not distinguishable). No effort was made to determine the chirality of TAGs in this study.
Structural determination of the TAG species that possesses different fatty acid substituents (A/B/C-TAG; A≠B≠C))
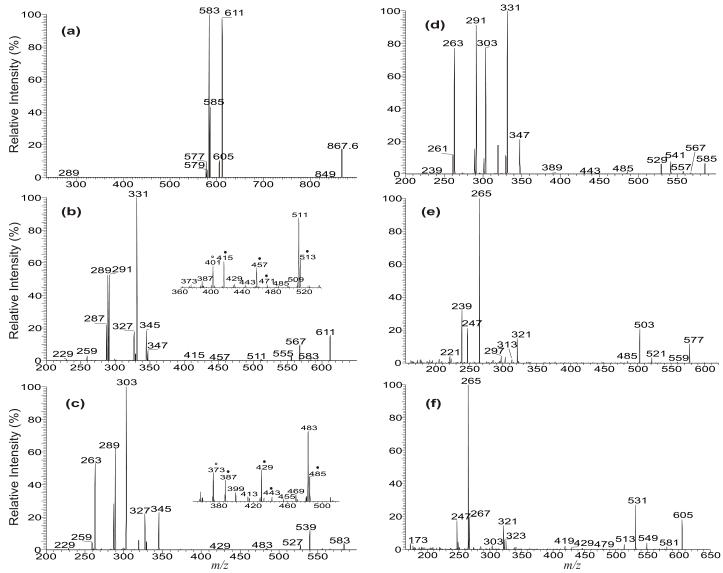
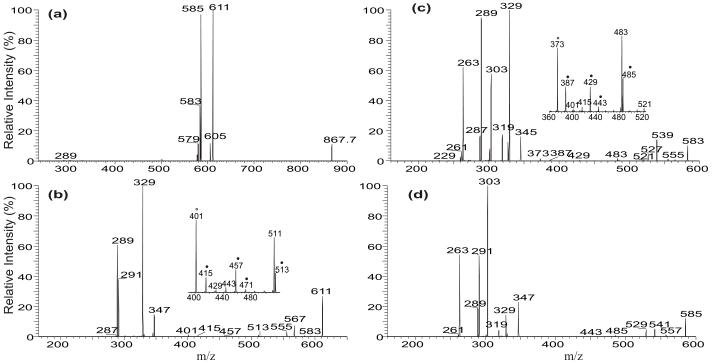
Upon resonance excitation in an ion-trap, the [M + Li]+ ions of TAG yielded abundant fragment ions corresponding to [M + Li − RnCO2H]+ and [M + Li − RnCO2Li] (where n denotes the glycerol carbon to which the fatty acid is esterified), which identify the fatty acid substituents. The differential formation of these ions also leads to the location of the fatty acid substituents on the glycerol backbone, similar to those previously observed with a tandem quadrupole instrument [4]. As shown in Figure 1a, the LIT MS2 spectrum of the [M + Li]+ ion of (16:0/18:1/18:0)-TAG at m/z 867 contain ions at m/z 611, 583, and 585, reflecting neutral losses of palmitic acid (16:0), stearic acid (18:0), and oleic acid (18:1), respectively. Ions at m/z 605, 577, and 579 reflect neutral losses of these fatty acids as the lithium salts.
Figure 1.
The LIT MS2 spectrum of the [M + Li]+ ion of the 16:0/18:1/18:0-TAG at m/z 867 (a), its MS3 spectra of the ions at m/z 611 (867 → 611) (b), at m/z 583 (867 → 583) (c), at m/z 585 (867 → 585) (d), at m/z 577 (867 → 577) (e), and at m/z 605 (867 → 605) (f). In the subsets (Panel b and c), the ions labeled with “●” identified the position of double bond(s).
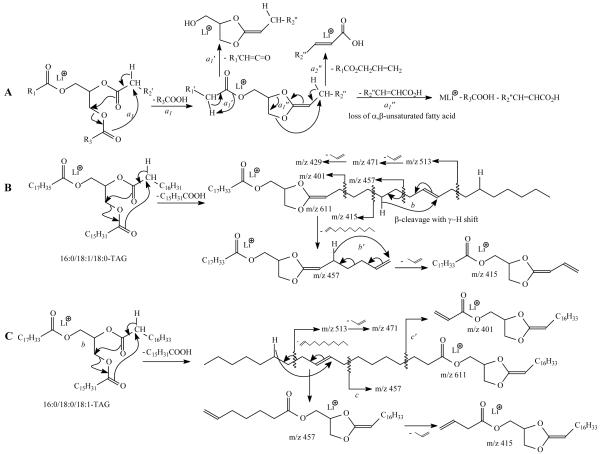
The ions at m/z 583 and 611 are of similar abundance, but are significantly more abundant than the ion at m/z 585; the ions at m/z 605 and 577 are also of similar abundance but are more abundant than the ion at m/z 579. The results indicate that neutral loss of the sn-2 fatty acid substituent either as a free fatty acid or as a lithium salt was less abundant than the corresponding losses of either the sn-1 or the sn-3 fatty acid substituent, resulting in assignment of the fatty acid substituents and their position on the glycerol backbone. These results are consistent with the notion that the α-hydrogen of the fatty acid substituent at sn-2 that participates in the formation of the ions at m/z 611 and 583 via elimination of the 16:0-fatty acid at sn-1 and of the 18:0-fatty acid at sn-3 (Scheme 1a) is more labile than that located at sn-1 or sn-3, which involves in the loss of the adjacent 18:1-fatty acid at sn-2 (i.e., the m/z 585 ion), leading to a greater abundance of the ions at m/z 583 and 611 than the m/z 585 ion [9, 10].
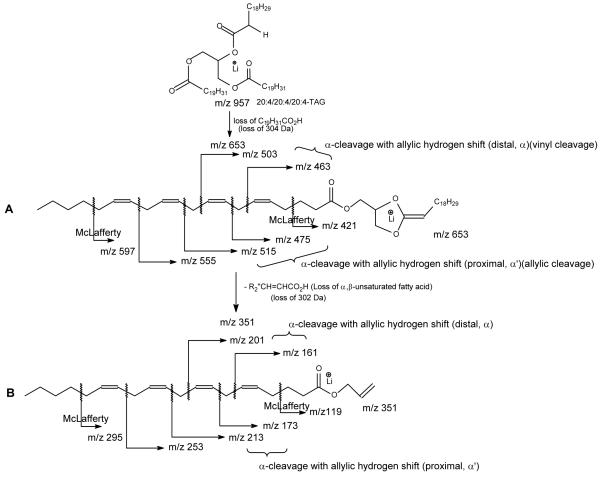
Scheme 1.
The mechanisms proposed for fragmentation of TAG (A), the β cleavage with γ-hydrogen shift mechanisms leading to location of double bond(s) for 16:0/18:1/18:0-TAG (B), and for 16:0/18:0/18:1-TAG (C). *All the schemes as shown do not imply that the fragmentation is a one-step process.
Further dissociation of the ion at m/z 611 (867 → 611, Figure 1b) gives rise to m/z 331, arising from loss of the 18:1-fatty acid at sn-2 as an α,β-unsaturated fatty acid (Scheme 1a, route a1”). This is in agreement with the presence of the ion at m/z 287, corresponding to a lithiated 18:2-fatty acid (Scheme 1a, route a2”). The results support the mechanism that the primary loss of the fatty acid substituents involves the participation of the α-hydrogen of the neighboring fatty acid substituent and the 5-member intermediate has been formed [4, 11]. The spectrum also contains the ions at m/z 345 and 327 arising from losses of the 18:0-fatty acid at sn-1 as a ketene and as an acid, respectively, along with the ions at m/z 289 and 291, corresponding to the lithiated ions of 18:1- and 18:0-fatty acids, respectively. This loss of the fatty acid substituent at sn-1 (or sn-3), followed by further elimination of the sn-2 fatty acid as an α,β-unsaturated fatty acid is further evidenced by the IT MS3 spectrum of the ion at m/z 583 (867 → 583, Figure 1c), in which the ion at m/z 303, arising from loss of the 18:1-fatty acid at sn-2 as an α,β-unsaturated fatty acid (loss as 18:2) is prominent, while the ions at m/z 345 and 327 represent further loss of the 16:0-fatty acid at sn-1 as a ketene and as a fatty acid, respectively. The observation of these ions is also consistent with the presence of the ions at m/z 289, 287 and 263 that respectively represent a lithiated 18:1-, 18:2, and a 16:0-fatty acid ions.
The ions at m/z 513 and 457 (Figure 1b, inset) may arise from charge-remote fragmentation (CRF) that involves β-cleavage with rearrangement of γ-H (Scheme 1b, route b); whereas the ions at m/z 415 (457 – propylene), 471 (513 – propylene), and 429 (471 – propylene) arise from consecutive β-cleavage with McLafferty rearrangement (Scheme 1b, route b’) [11]. These ions are analogous to the ions at m/z 197, 141, 99, 155, 113 seen in the MS2 spectrum of the dilithiated ion of Δ918:1 [12], suggesting that the double bond of the 18:1-fatty acid substituent at sn-2 is located at C(9). Similarly, the MS3 spectrum of m/z 583 (Figure 1c, inset) also contains the ions at m/z 485, 429, 387, 443, 401, which are 28 Da lighter than the analogous ions at m/z 513, 457, 415, 471, and 429 seen in Figure 1b. The results also show that the double bond of the 18:1-fatty acid moiety is located at C(9). The combined results from LIT MSn (n=2,3) on the [M + Li]+ ions of TAG readily afford near-complete structural characterization of the major regioisomer, including assignment of its fatty acid substituents on the glycerol backbone, and location the double bonds along the unsaturated fatty acid chain.
The MS3 spectrum of the ion at m/z 585 (867 → 585, Figure 1d) is dominated by the ion at m/z 331 and 303, arising from losses of the 16:0-fatty acid (loss as 16:1-FA) at sn-1 and 18:0-fatty acid (loss as 18:1-FA) at sn-3 as an α,β-unsaturated fatty acids, respectively. The ions at m/z 567 (585 – 18), 557 (585 – 28), 541 (585 – 44) may arise from losses of H2O, CO, CO2, respectively; while the m/z 529 (585 –56) ion may arise from cleavage of glycerol residue (loss of [glycerol – 2H2O]) involving rearrangements (Discussion later). The ion of m/z 529 gives rise to m/z 485 (529 – 44) by loss of CO2 as previously reported [11]. The analogous ions arising from the similar losses were also seen in Figure 1b and 1c.
Further dissociation of the ions at m/z 577 (867 → 577, Figure 1e) gives rise to ions at m/z 265 and 239, representing the 18:1- and 16:0-acylium ion, respectively, along with the ions at m/z 247 (265 – H2O) and 221 (239 – H2O). The ions at m/z 265 and 247 are more abundant than the ions at m/z 239 and 221, respectively, reflecting that the 18:1- and 16:0-fatty acid substituents are located at sn-2 and sn-1 of the glycerol backbone [13]. This is also consistent with findings that the ions at m/z 265 and 247 are respectively more prominent than the ions at m/z 267 (18:0-acylium ion) and 249 (267 – H2O) in the MS3 spectrum of the ion at m/z 605 (867 → 605, Figure 1f), signifying that the 18:1-fatty acid substituent is located at sn-2, while the 18:0-fatty acid substituent is located at sn-3, representing a outer fatty acid substituent.. This difference in the abundances of the acylium ions in the MS3 spectrum of [M + Li − RnCO2Li]+ ions is readily applicable for confirmation of the fatty acid substituents on the glycerol backbone.
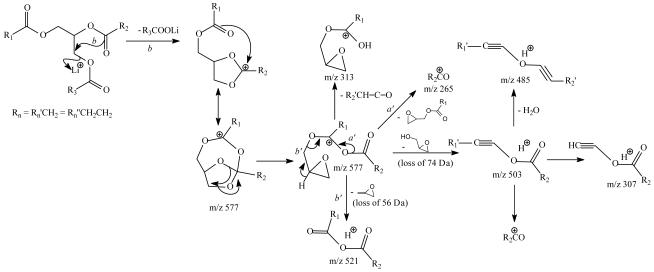
The ions at m/z 521 (577 – 56) and 503 (577 – 74) (Figure 1e) also arise from various cleavages of the glycerol backbone involving rearrangements [14, 15]. The proposed structure of the ion at m/z 503 is further supported by its MS4 spectrum (867 → 577 → 503; data not shown), which contains ions at m/z 485, 307 and 265 (Scheme 2). Similar losses of the glycerol residues were also seen for the ion at m/z 605 (Figure 1f), which arises from loss of the outer fatty acid; but the analogous losses were not seen in the MS3 spectrum of the ions of m/z 579 (data not shown), a [M + Li − R2CO2Li]+ arising from loss of inner fatty acid. These internal losses of glycerol residues are further confirmed by observation of the fragment ions that correspond to losses of 60 and 79 Da in the MS3 spectrum of the analogous [M + Li − RnCO2Li]+ ions arising from d5-17:0/17:1/17:0-TAG in which the glycerol hydrogen atoms are substituted by deuterium atoms (see Figure 5b and discussion therein). The ion at m/z 321 (577 – 256) from further loss of 16:0-fatty acid substituent at sn-1 is more abundant than the ion at m/z 313 (577 – 282), arising from further loss of 18:1-fatty acid substituent at sn-2. This differential loss of the fatty acid substituents dependent on the position of the fatty acid substituents on the glycerol backbone also applicable in the confirmation of the location of fatty acid substituents.
Scheme 2.
The fragmentation processes proposed for losses of various glycerol residues for the [M + Alk − RnCO2Alk]+ ions (Alk= Li, Na, NH4, n=1,3). The m/z values shown represent the ions seen for the [M + Li]+ ions of 16:0/18:1/18:0-TAG. The pathways were supported by the analogous spectrum of d5-17:0/17:1/17:0-TAG in which the hydrogen atoms (C-H) of the glycerol backbone were replaced by deterium atoms.
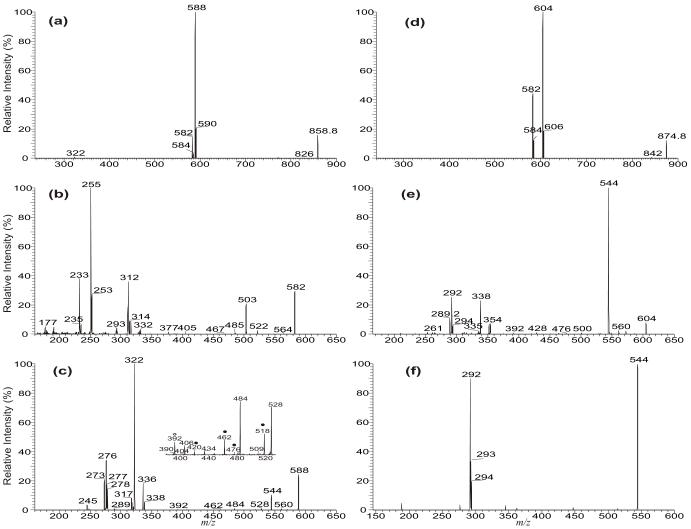
Figure 5.
The LIT MS2 spectrum of the [M + Li]+ ions of d5-17:0/17:1/17:0-TAG at m/z 858 (a), and its MS3 spectra of the ions at m/z 582 (858 → 582) (b), and at m/z 588 (858 → 588) (c). The LIT MS2 spectrum of the [M + Na]+ ions of d5-17:0/17:1/17:0-TAG at m/z 874 (d), its MS3 spectrum of the ions at m/z 604 (874 → 604) (e) and its MS4 spectrum of the ion at m/z 544 (874 → 604 → 544) (f). In the subset (Panel c), the ions labeled with “●” identified the position of double bond(s).
The IT MS2 spectrum of the [M + Li]+ ion of the 16:0/18:0/18:1-TAG regioisomer at m/z 867 (Figure 2a), again, is dominated by the ions at m/z 611, 585 and 583, arising from losses of the 16:0-, 18:1- and 18:0-fatty acid substituents at sn-1, sn-3, and sn-2, respectively. The former two ions are nearly of equal abundance and are more abundant than the ion at m/z 583, suggesting that the 18:0-FA is located at sn-2. The results are consistent with the finding that the ions at m/z 605 and 579, arising from losses of the 16:0-, 18:1-FA moieties at sn-1 and sn-3 as lithium salt are more abundant than the ion at m/z 577, arising from loss of the 18:0-FA acid substituent at sn-2 as a lithium salt.
Figure 2.
The LIT MS2 spectrum of the [M + Li]+ ion of the 16:0/18:0/18:1-TAG at m/z 867 (a), its MS3 spectra of the ions at m/z 611 (867 → 611) (b), at m/z 583 (867 → 583) (c), and at m/z 585 (867 → 585) (d). In the subsets (Panel b and c), the ions labeled with “●” identified the position of double bond(s).
The position of double bond of the 18:1-FA substituent at sn-3 is revealed by further dissociation of the ions at m/z 611 (867 → 611, Figure 2b) and at m/z 583 (867 → 583, Figure 2c). The former spectrum contains ions at m/z 513, 457, 471 and 415 (Figure 2b, inset), identical to those seen in Figure 1b, along with the ion at m/z 401 from β-cleavage (β to the carbonyl group) (Scheme 1c) indicating that the double bond is located at C(9). The latter spectrum contains ions at m/z 485, 429, 443, 387 and 373 (Figure 2c, inset), which are identical to those seen in Figure 1c, reflecting that the double bond is located at C(9). The IT MS3 spectrum of the ion at m/z 585 (867 → 585, Figure 2d) is dominated by the ion at m/z 303, corresponding to loss of an 18:1-fatty acid residue, in agreement with the earlier finding that the 18:0-fatty acyl group at sn-2 was further eliminated as α,β-unsaturated fatty acid.
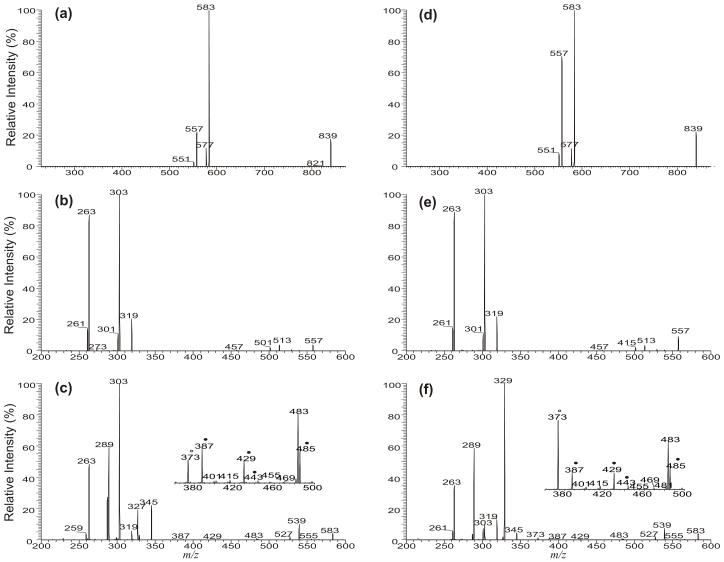
Structural characterization of A/B/A-TAG and A/A/B (or B/A/A)-TAG regioisomers
Both the LIT MS2 spectra of the lithiated ions of 16:0/18:1/16:0-TAG (Figure 3a) and 18:1/16:0/16:0-TAG (Figure 3d) at m/z 839 are dominated by the ion at m/z 583, arising from loss of 16:0-FA moiety. The ion at m/z 557 arising from loss of 18:1-FA is abundant in Figure 3d, but is of low abundance in Figure 3a. This is consistent with the fact that the 18:1/16:0/16:0-TAG contains a 18:1-FA at sn-1, while the 16:0/18:1/16:0-TAG (Figure 3a) contains a 18:1-FA at sn-2. The LIT MS3 spectrum of the ion at m/z 557 (839 →557, Figure 3b) originated from 16:0/18:1/16:0-TAG is identical to that originated from 18:1/16:0/16:0-TAG (Figure 3e). This consistent with the notion that the loss of the fatty acid substituents involve the participation of the α-hydrogen of the neighboring fatty acid substituent, resulting in an identical 5-member intermediate, which further eliminates the 16:0-FA as an α,β-unsaturated fatty acid (loss as 16:1-FA) to yield the prominent ion at m/z 303. The LIT MS3 spectrum of the ion at m/z 583 (839 →583, Figure 3c) contains the prominent ion at m/z 303 arising from elimination of the 18:1-FA at sn-2 as an α,β-unsaturated fatty acid (loss as 18:2-FA); while the LIT MS3 spectrum of the ion at m/z 557 (839 →557, Figure 3e) originated from 18:1/16:0/16:0-TAG isomer is dominated by the ion at m/z 303, arising from loss of the 16:0-fatty acid at sn-2 as an α,β-unsaturated fatty acid (loss as 16:1). The results confirm that the former molecule contains 18:1-fatty acid at sn-2; whereas the latter TAG consists of a 16:0-fatty acid substituent at sn-2. In Figure 3c (subset), the ions at m/z 485, 429, 443, 387, and 373 that identify the position of double bond of the 18:1-fatty acid residue are also present, leading to assignment of the double bond at C(9). The set of ions of m/z 485, 429, 443, 387 and 373 were also seen in Figure 3f (subset), revealing that the double bond of the 18:1-FA substituent is located at C(9).
Figure 3.
The LIT MS2 spectrum of the [M + Li]+ ions of 16:0/18:1/16:0-TAG at m/z 839 (a), its MS3 spectra of the ions at m/z 557 (839 → 557) (b), and at m/z 583 (839 → 583) (c), and the LIT MS2 spectrum of the [M + Li]+ ions of 18:1/16:0/16:0-TAG at m/z 839 (d), its MS3 spectra of the ions at m/z 557 (839 → 557) (e), and at m/z 583 (839 → 583) (f). In the subsets (Panel c and f), the ions labeled with “●” identified the position of double bond(s).
The profiles of the LIT MS2 spectrum of 18:1/18:0/18:0-TAG (see supplemental material, Figure S1a) and of the MS3 spectra of the ions at m/z 613 (Figure S1b) and at m/z 611 (Figure S1c) are identical to those seen in Figure 3d, 3e, and 3f, respectively, in agreement with the notion that both the 18:1/16:0/16:0-TAG and 18:1/18:0/18:0-TAG belong to the B/A/A (or A/A/B) class. The profiles of the MSn (n=2,3) spectra from 18:0/18:1/18:0-TAG (data not shown) are also identical to those seen for 16:0/18:1/16:0-TAG (Figure 3a-3c), indicating that they all belong to the A/B/A subclass. The distinction in the MSn spectra (Figure 3a vs. Figure 3d; Figure 3c vs. 3f) between the TAG isomers of A/B/A and A/A/B classes are substantial, leading to differentiation of isomers by mass spectrometry.
Characterization of TAG consisting of polyunsaturated fatty acid moieties
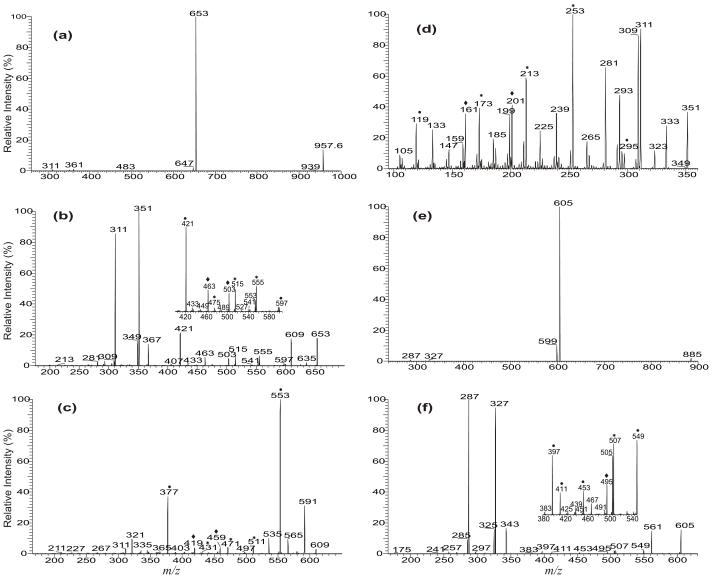
The MS2 spectrum of the [M + Li]+ ion of 20:4/20:4/20:4-TAG at m/z 957 (Figure 4a) is dominated by the ion at m/z 653, arising from loss of the 20:4-fatty acid substituent. By contrast, the ion at m/z 647 arising from loss of the 20:4-fatty acid as a lithium salt is of low abundance. Further dissociation of the ion at m/z 653 (957 → 653, Figure 4b) gives rise to the ion at m/z 351, by elimination of the 20:4-fatty acid moiety as an α,β-unsaturated fatty acid (loss as 20:5 fatty acid) as described earlier. The spectrum also contains two ion series at m/z 555, 515 and 475 and at m/z 503 and 463, indicating the presence of the homoconjugated double bonds at C(5), C(8), C(11), and C(14) (Scheme 3a). These ions are analogous to the ions at m/z 219, 179, 139, 167, and 127 that were previously observed for locating the double bonds for Δ5,8,11,14-20:4 fatty acid using LIT MS2 on the dilithiated adduct ions [12].
Figure 4.
The LIT MS2 spectrum of the [M + Li]+ ions of 20:4/20:4/20:4-TAG at m/z 957 (a), its MS3 spectrum of the ions at m/z 653 (957 → 653) (b), and its MS4 spectra of the ions at m/z 609 (957 → 653 → 609) (c), and at m/z 351 (957 → 653 → 351). The LIT MS2 spectrum of the [M + Li]+ ions of 18:2/18:2/18:2-TAG at m/z 885 (e), and its MS3 spectrum of the ions at m/z 605 (885 → 605) (f) are also shown. In Panels c and d, and in the subsets (Panel b and f), the ions labeled with “◆” (vinyl cleavage) “*” (allylic cleavage) and “●” (β-cleavage) are feature ions that identify the position of the double bonds of the 20:4-fatty acid moiety (see Scheme 3).
Scheme 3.
The fragmenation pathways proposed for location of the double bonds along the fatty acid chain of 20:4/20:4/20:4-TAG from MS3 on the [M + Li − R3CO2H] ions (A), and from MS4 on the [M + Li − R3CO2H − R2”CH=CHCO2H] ions.
The ion at m/z 609 (Figure 4b) may arise from loss of a CO2 residue. Further dissociation of the ion at m/z 609 (957 → 653 → 609, Figure 4c) also gives rise to the ion at m/z 553, arising from loss of a [glycerol – 2H2O] residue as described earlier. The spectrum also contains two sets of ions at m/z 511, 471, 431 and at m/z 459 and 419. These ions are analogous to those seen in Figure 4b, and are 44 Da lighter, consistent with the fact that the precursor ion at m/z 609 arises from loss of a 44 Da residue from m/z 653. The results further support the assignment of the position of double bonds of the 20:4-fatty acid substituents. The MS4 spectrum of the ion at m/z 553 (957 →653 →553, data not shown) contains two ion series at m/z 455, 415, 375 and at m/z 403, 363; and the MS4 spectrum of the ion at m/z 351 (957 →653 →351, Figure 4d) also contains two ion series at m/z 253, 213, and 173, and at m/z 201, 161 (Scheme 3b). The observation of these ions, again, is consistent with the assignment of the position of double bonds along the 20:4-fatty acyl chain (Scheme 3).
The LIT MS2 spectrum of the [M + Li]+ ion of 18:2/18:2/18:2-TAG at m/z 885 (Figure 4d) is dominated by the ion at m/z 605, arising from loss of the 18:2-fatty acid, along with the ion at m/z 599, arising from loss of 18:2-fatty acid as a lithium salt. The LIT MS3 spectrum of the ion at m/z 605 (885 → 605, Figure 4e) contains the prominent ion at m/z 327 (605 – 278), from further elimination of the 18:2-fatty acid as an α,β-unsaturated fatty acid (loss as 18:3 fatty acid). The position of the double bond of 18:2-fatty acid is seen by the ions at m/z 549 and 453 (Figure 4e, subset), arising from CRF cleavages of C(7)-C(8) and C(14)-C(15) bonds with McLafferty rearrangements, along with the ion at 453, arising from the similar fragmentation process (see supplemental material Scheme 1). These ions are analogous to those previously observed in the MS2 spectrum of the dilithiated ion of Δ9,12-18:2 [12], leading to locate the double bonds of the 18:2-fatty acyl groups at C(9) and C(12).
The fragmentation pathways supported by LIT MSn on 1,3(d5)-diheptadecanoyl-2-(10Z-heptadecenoyl)-glycerol [d5-(17:0/ Δ1017:1/17:0)-TAG]
The profile of the LIT MS2 spectrum of the [M + Li]+ ion of [d5-(17:0/ Δ1017:1/17:0)-TAG] at m/z 858 (Figure 5a) is similar to that shown in Figure 3a, arising from an A/B/A TAG subclass. The ions at m/z 588 (858 – C16H33CO2H) and 590 (858 – C16H31CO2H) arise from losses of the 17:0- and 17:1-fatty acid substituents, and the ions at m/z 582 and 584 arise from losses of the 17:0- and 17:1-fatty acid substituents as lithium salt, respectively. The observation of the losses of the C16H33CO2H and C16H31CO2H residues rather than losses of the C16H33CO2D and C16H31CO2D residues indicates that the elimination of the acid residues does not involve the participation of the hydrogen atom on the glycerol backbone. This is consistent with the proposed mechanisms in which the pathways leading to the fatty acid losses involve the participation of the α-hydrogen of the neighboring fatty acyl moiety (Scheme 1) [4]. The MS3 spectrum of the ion of m/z 582 (858 → 582, Figure 5b) contains the ions at m/z 522 (loss of C3D4O), 503 (loss of C3HD5O2), and 485 (loss of C3H3D5O3), of which the mass shifts match the proposed structures (Scheme 4) and support the earlier findings of the various internal losses of the glycerol residues.
Scheme 4.
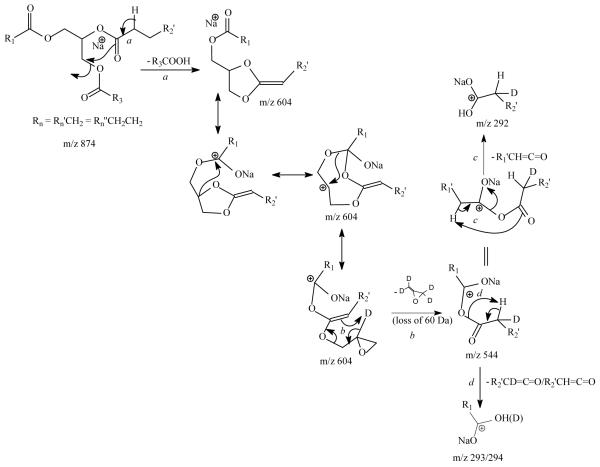
The proposed fragmenation processes leading to internal loss of [glycerol – 2H2O] (56 Da) produced by MSn on the [M + Na]+ ions of TAG. The m/z values shown in the scheme represent the observed mass seen for d5-17:0/17:1/17:0-TAG. The observation of loss of 60 Da (Figure 5e) supports the proposed internal loss of the glycerol residue. The internal loss was further supported by MS4 on the ion of m/z 544, which gives ions at m/z 292, 293 and 294 (see Figure 5f), deriving from the fragmentation processes as proposed..
Further dissociation of the ion of m/z 588 (858 → 588, Figure 5c) yielded ions at m/z 518 and 462 (figure 5c, inset), arising from β-cleavage with γ-H shift fragmentations. These ions together with ions at m/z 420, 406 and 392 demonstrate that the double bond of the 17:1-fatty acid substituent at sn-2 is located at C(10), consistent with the notion that the molecule is a d5-(17:0/ Δ1017:1/17:0)-TAG. The spectrum is dominated by the ion at m/z 322, arising from loss of the 17:1-fatty acyl group as an α,β-unsaturated fatty acid (loss as 17:2-fatty acid), along with ion at m/z 273, representing a lithiated 17:2-fatty acid cation. The observation of these ions along with the ions at m/z 336 arising from loss of 17:0-fatty acid as a ketene and at m/z 317 arising from loss of d1-17:0-fatty acid (loss as C16H33CO2D) supports the proposed mechanism depicted in Scheme 1a.
LIT MSn spectra of the [M+ Na]+ and [M + NH4]+ ions of TAG
The LIT MS2 spectrum of the [M+ Na]+ ions of TAG is similar to that arising from the [M+ Li]+ ions and is readily applicable for assignment of the fatty acyl substituents on the glycerol backbone. The product-ion spectrum of the sodiated (16:0/18:1/18:0)-TAG at m/z 883 (Figure 6a) contains the ions at m/z 627 and 599 arising from losses of the outer 16:0- and 18:0-fatty acids and the ion at m/z 601 arising from loss of the 18:1-fatty acid at sn-2. The ions at m/z 627 and 599 are more abundant than the ion at m/z 601. The ions at m/z 605 and 577 arising from losses of the outer 16:0- and 18:0-fatty acids as sodium salt (loss of RnCO2Na) are also more abundant than the ion at m/z 579, arising from the analogous loss of the 18:1 at sn-2. The results indicate that the 16:0- and 18:0-fatty acids are located at sn-1 and sn-3, respectively.
Figure 6.
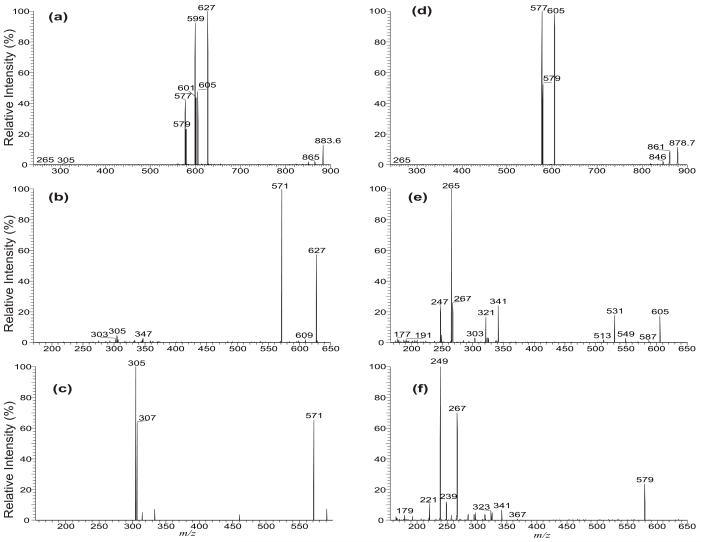
The LIT MS2 spectrum of the [M + Na]+ ions of 16:0/18:1/18:0-TAG at m/z 863.6 (a), its MS3 spectrum of the ions at m/z 627 (863 → 627) (b), and its MS4 spectrum at m/z 571 (863 → 627 → 571) (c). The LIT MS2 spectrum of the [M + NH4]+ ions of 16:0/18:1/18:0-TAG at m/z 878.7 (d), and its MS3 spectra of the ions at m/z 605 (878 → 605) (e), and at m/z 579 (878 → 579) (f).
The ions at m/z 605, 577, and 579 are more prominent than those seen in Figure 1a, indicating that the losses of fatty acids as sodium salts are more facile than the same losses as lithium salts. The ions corresponding to losses of the fatty acids as potassium salts become dominant in the MS2 spectrum of the corresponding [M+ K]+ ions of TAG (data not shown). The ions at m/z 605, 577 and 579 arising from losses of the fatty acid as ammonium salt become the exclusive ions in the MS2 spectrum of the [M + NH4]+ ions of (16:0/18:1/18:0)-TAG at m/z 878 (Figure 6d). The results indicate that the metal and NH4+ ions attached to TAG is in the order of Li+>Na+>K+>NH4+ [16]. The Li+ is localized on the [M + Li − RnCO2H]+ ions remote from the fragmentation sites, resulting in the formation of the ion series informative for location of the double bonds; while the Na+, K+, and NH4+ on the sodiated, potassiated, and ammoniated precursor ions were lost before the CRF processes take place. The loss of metal ions is evidenced by observation of metal ions in the tandem quadrupole product-ion spectra of the [M + Na]+ ion at m/z 881 (see supplemental material Figure 2, Panel a) and of the [M + K]+ ions at m/z 897 (Panel b) of 16:0/18:0/18:1-TAG, respectively. The former spectrum contained the ion at m/z 23, corresponding to the Na+ ion, while the latter spectrum is dominated by the K+ ion at m/z 39. The metal ion loss becomes even more prevalent, when MS2 on the [M + Cs]+ ions at m/z 991 with the same tandem quadrupole instrument was performed. The spectrum (see supplemental material Figure 2c) which was obtained with a much lower collision energy again, is dominated by the Cs+ ion at m/z 133. However, these metal ions are not detectable using a LIT because of the low-mass cut-off.nature of the instrument..
The MS3 spectrum of the ion at m/z 627 (883 → 627, Figure 6b) is dominated by the ion at m/z 571 (627 – 56), probably representing a sodiated ion of octadecanoyl octadecenoyl anhydride (18:0/18:1 acid anhydride) after removal of the glycerol residue from the ion of m/z 627 (Scheme 4). This speculation is supported by observation of the ions at m/z 307 and 305 representing the sodiated ions of 18:0- and 18:1-fatty acids, respectively, in the MS4 spectrum of the ions at m/z 571 (883 → 627→ 571, Figure 6c) (Scheme 4). The ion series reflecting the location of double bond of the 18:1-fatty acid substituent at sn-2 as seen in Figure 1b are absent in Figure 6b, attributable to the notion that the sodiated precursor ion is not stable and the internal energy of the precursor ion decomposed by CRF to yield the ions applicable for characterization is less than the energy required for releasing the Na+ ion [16]. The MS3 spectra of the ions at m/z 601, and at 599 (data not shown) are also dominated by the ions at m/z 545 and 543, respectively, arising from further loss of 56 Da (loss of [Glycerol – 2H2O]) and representing a 16:0/18:0 and a 16:0/18:1 fatty acid anhydride residues. This loss of 56 Da related to the glycerol backbone is supported by the MS3 spectrum of the analogous ion of m/z 604 (874 → 604) (Figure 5e) arising from the [M+ Na]+ ions of d5-(17:0/ Δ1017:1/17:0)-TAG (Figure 5d). The spectrum (Figure 5e) is dominated by the ion at m/z 544, representing a sodiated ion of d1-17:0/17:1 acid anhydride arising from the analogous loss of a d4-glycerol residue (Scheme 4), along with the ions at m/z 338 from loss of 17:1-fatty acid at sn-2 as a α,β-unsaturated acid (loss as 17:2-FA) and the ion at m/z 289, representing a sodiated 17:2-fatty acid ion. The observation of the ions at m/z 292, 293 and 294 arising from further losses of 17:0-, d1-17:1-, and 17:1-fatty acyl ketene from m/z 544, respectively, seen in the MS4 spectrum of the ion of m/z 544 (874 → 604 → 544) (Figure 5f) is consistent with the formation of the sodiated d1-17:0/17:1 acid anhydride ion by loss of the glycerol residue (loss of 60 Da) (Scheme 4). Similar losses of the glycerol residues were also observed in the MS3 spectrum of the ion of m/z 606 (874 → 606), which is dominated by the ion at m/z 506 (data not shown).
By contrast, the LIT MS2 spectrum of the [M + NH4]+ ion of (16:0/18:1/18:0)-TAG at m/z 878 (Figure 6d) contained ions at m/z 605, 577, and 579, arising from neutral losses of 16:0-, 18:0-, and 18:1-fatty acid substituents as ammonium salt (loss as RnCO2NH4), respectively (or the combined losses of the corresponding fatty acids and NH3 [17]), and the ions arising from losses of the fatty acid substituents were absent. The ions at m/z 605 and 577 are significantly more abundant than the ion at m/z 579. These preferential losses of the outer 16:0- and 18:0-fatty acid moieties as ammonium salt than the analogous loss of inner sn-2 18:1-fatty acid moiety are readily applicable for assignment of fatty acid substituents on the glycerol backbone.
The LIT MS3 spectrum of the ion at m/z 605 (878 → 605, Figure 6e) is nearly identical to that shown in Figure 1, consistent with the notion that the 18:1- and 18:0-fatty acyl groups are located at sn-2 and sn-3, respectively. However, the ion at m/z 341 from further loss of 18:1-fatty acyl ketene (designated as [RCO+ + 74] ion in references 1, 3, and 15) is more abundant than the similar ions seen in Figure 1, indicating that they may arise from different mechanisms [17]. The LIT MS3 spectrum of the ions at m/z 577 (data not shown) is similar to Figure 6e, consistent with the notion that both the ions of m/z 605 and 577 arise from loss of the outer fatty acid substituent. In contrast, the LIT MS3 spectrum of the ions at m/z 579 (Figure 6f), arising from loss of the fatty acid moiety at sn-2 is readily distinguishable from Figure 6e. The abundances of the ions at m/z 341 (loss of sn-1 fatty acyl ketene) and 323 (loss of sn-1 fatty acid) are close to those of the ions at m/z 313 (loss of sn-3 fatty acyl ketene) and 295 (loss of fatty acid at sn-3), respectively, in agree with the fact that they all retain the outer fatty acid moieties. The ions at m/z 523, 505, and 487 arising from various losses of the glycerol residues are absent in the spectrum (Figure 6f) supporting the notion that the ion at m/z 579 arises from loss of the fatty acid group at sn-2 as described earlier. These spectra features dependent on the origin of precursor ions deriving from elimination of the fatty acid substituents at different positions (on the glycerol backbone) are readily applicable for confirmation of the assignment of the fatty acyl groups on the glycerol backbone.
The [M + NH4 − RnCO2H]+ ions analogous to the [M + Li − RnCO2H]+ ions are absent in the MS2 spectra (Figure 6d), thus, ions from MS3 on [M + NH4 − RnCO2H]+ for location of double bonds for the unsaturated fatty acid moieties as seen for the [M + Li]+ ions (i.e., ions from MS3 on the [M + Li − RnCO2H]+) are not available.
Conclusions
The employment of LIT MSn on the [M + Li]+ ions of TAG desorbed by ESI affords near-complete structural characterization of TAG molecules, including the position of double bond(s) of the unsaturated fatty acyl moieties. The feature ions informative for location of the position of double bonds derived from further dissociation of the [M + Li − RnCO2H]+ ions in an ion-trap. However, these ions are not present in the MS3 spectra of [M + Na − RnCO2H]+ or [M + NH4 − RnCO2H]+ ions and characterization of TAG by LIT MSn on the [M + Na]+ and [M + NH4]+ ions is incomplete. Momchilova et al demonstrated the separation of isobaric triacylglycerol positional isomers by reversed-phase high-performance liquid chromatography (RP-HPLC) [18]. Thus, this LIT MSn with ESI approach coupled with RP-HPLC should have broad application in the characterization of TAGs, in particular, of biological origin which often consists of numerous isobaric species [17, 19] that are not separable by a mass spectrometer.
Supplementary Material
Figure 1. The LIT MS2 spectrum of the [M + Li]+ ions of 18:1/18:0/18:0-TAG at m/z 895 (a), its MS3 spectra of the ions at m/z 613 (895 → 613) (b), and at m/z 611 (895 → 611) (c).
Figure 2. The ESI product-ion spectra of (a) the [M + Na]+ ions at m/z 881 (collision energy 48 eV), (b) the [M + K]+ ions at m/z 897 (35 eV), and (c) of the [M + Cs]+ ions at m/z 991 (25 eV) of 16:0/18:0/18:1-TAG obtained with a Finnigan TSQ-7000 triple quadrupole instrument. The metal ions are dominant in the spectra (Panel b and c) even with a lower collision energy.
Scheme 1. The fragmentation pathways proposed for location of double bonds along the fatty acid chain of 18:2/18:2/18:2-TAG.
Acknowledgments
This research was supported by US Public Health Service Grants P41-RR-00954, R37-DK-34388, P60-DK-20579, P01-HL-57278 and P30-DK56341.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheng C, Gross ML, Pittenauer E. Complete Structural Elucidation of Triacylglycerols by Tandem Sector Mass Spectrometry. Anal. Chem. 1998;70:4417–4426. doi: 10.1021/ac9805192. [DOI] [PubMed] [Google Scholar]
- 2.Duffin KL, Henion JD, Shieh JJ. Electrospray and Tandem Mass Spectrometric Characterization of Acylglycerol Mixtures That Are Dissolved in Nonpolar Solvents. Anal. Chem. 1991;63:1781–1788. doi: 10.1021/ac00017a023. [DOI] [PubMed] [Google Scholar]
- 3.Pittenauer E, Allmaier G. The Renaissance of High-Energy Cid for Structural Elucidation of Complex Lipids: Maldi-Tof/Rtof-Ms of Alkali Cationized Triacylglycerols. J. Am. Soc. Mass Spectrom. 2009;20:1037–1047. doi: 10.1016/j.jasms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Hsu FF, Turk J. Structural Characterization of Triacylglycerols as Lithiated Adducts by Electrospray Ionization Mass Spectrometry Using Low-Energy Collisionally Activated Dissociation on a Triple Stage Quadrupole Instrument. J. Am. Soc. Mass Spectrom. 1999;10:587–599. doi: 10.1016/S1044-0305(99)00035-5. [DOI] [PubMed] [Google Scholar]
- 5.Lin J-T, Arcinas A. Regiospecific Analysis of Diricinoleoylglycerols in Castor (Ricinus Communis L.) Oil by Electrospray Ionization-Mass Spectrometry. J. Agric. Food Chem. 2007;55:2209–2216. doi: 10.1021/jf063105f. [DOI] [PubMed] [Google Scholar]
- 6.Thomas MC, Mitchell TW, Blanksby SJ. Ozonolysis of Phospholipid Double Bonds During Electrospray Ionization: A New Tool for Structure Determination. J. Am. Chem. Soc. 2006;128:58–59. doi: 10.1021/ja056797h. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MC, Mitchell TW, Harman DG, Deeley JM, Murphy RC, Blanksby SJ. Elucidation of Double Bond Position in Unsaturated Lipids by Ozone Electrospray Ionization Mass Spectrometry. Anal Chem. 2007;79:5013–5022. doi: 10.1021/ac0702185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas MC, Mitchell TW, Harman DG, Deeley JM, Nealon JR, Blanksby SJ. Ozone-Induced Dissociation: Elucidation of Double Bond Position within Mass-Selected Lipid Ions. Anal. Chem. 2008;80:303–311. doi: 10.1021/ac7017684. [DOI] [PubMed] [Google Scholar]
- 9.Hsu FF, Turk J. Electrospray Ionization/Tandem Quadrupole Mass Spectrometric Studies on Phosphatidylcholines: The Fragmentation Processes. J. Am. Soc. Mass Spectrom. 2003;14:352–363. doi: 10.1016/S1044-0305(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 10.Hsu F-F, Turk J. Electrospray Ionization with Low-Energy Collisionally Activated Dissociation Tandem Mass Spectrometry of Glycerophospholipids: Mechanisms of Fragmentation and Structural Characterization. J. Chromatogr. B. 2009;877:2673–2695. doi: 10.1016/j.jchromb.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu F-F, Turk J. Structural Characterization of Unsaturated Glycerophospholipids by Multiple-Stage Linear Ion-Trap Mass Spectrometry with Electrospray Ionization. J. Am. Soc. Mass Spectrom. 2008;19:1681–1691. doi: 10.1016/j.jasms.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu FF, Turk J. Elucidation of the Double-Bond Position of Long-Chain Unsaturated Fatty Acids by Multiple-Stage Linear Ion-Trap Mass Spectrometry with Electrospray Ionization. J. Am. Soc. Mass Spectrom. 2008;19:1673–1680. doi: 10.1016/j.jasms.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu FF, Turk J. Studies on Phosphatidylserine by Tandem Quadrupole and Multiple Stage Quadrupole Ion-Trap Mass Spectrometry with Electrospray Ionization: Structural Characterization and the Fragmentation Processes. J. Am. Soc. Mass Spectrom. 2005;16:1510–1522. doi: 10.1016/j.jasms.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Hsu FF, Turk J, Owens RM, Rhoades ER, Russell DG. Structural Characterization of Phosphatidyl-Myo-Inositol Mannosides from Mycobacterium Bovis Bacillus Calmette Guerin by Multiple-Stage Quadrupole Ion-Trap Mass Spectrometry with Electrospray Ionization. I. PIMs and Lyso-PIMs. J. Am. Soc. Mass Spectrom. 2007;18:466–478. doi: 10.1016/j.jasms.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu FF, Turk J, Owens RM, Rhoades ER, Russell DG. Structural Characterization of Phosphatidyl-Myo-Inositol Mannosides from Mycobacterium Bovis Bacillus Calmette Guerin by Multiple-Stage Quadrupole Ion-Trap Mass Spectrometry with Electrospray Ionization. II. Monoacyl- and Diacyl-PIMs. J. Am. Soc. Mass Spectrom. 2007;18:479–492. doi: 10.1016/j.jasms.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams J, Gross ML. Energy Requirement for Remote Charge Site Ion Decomposition and Structural Information from Collisional Activation of Alkali Metal Cationized Fatty Alcohols. J. Am. Chem. Soc. 1986;108:6915–6921. [Google Scholar]
- 17.McAnoy AM, Wu CC, Murphy RC. Direct Qualitative Analysis of Triacylglycerols by Electrospray Mass Spectrometry Using a Linear Ion Trap. J. Am. Soc. Mass Spectrom. 2005;16:1498–1509. doi: 10.1016/j.jasms.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Momchilova S, Tsuji K, Itabashi Y, Nikolova-Damyanova B, Kuksis A. Resolution of Triacylglycerol Positional Isomers by Reversed-Phase High-Performance Liquid Chromatography. J. Sep. Sci. 2004;27:1033–1036. doi: 10.1002/jssc.200401746. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda K, Oike Y, Shimizu T, Taguchi R. Global Analysis of Triacylglycerols Including Oxidized Molecular Species by Reverse-Phase High Resolution LC/ESI-QTOF MS/MS. J. Chromatogra. B. 2009;877:2639–2647. doi: 10.1016/j.jchromb.2009.03.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. The LIT MS2 spectrum of the [M + Li]+ ions of 18:1/18:0/18:0-TAG at m/z 895 (a), its MS3 spectra of the ions at m/z 613 (895 → 613) (b), and at m/z 611 (895 → 611) (c).
Figure 2. The ESI product-ion spectra of (a) the [M + Na]+ ions at m/z 881 (collision energy 48 eV), (b) the [M + K]+ ions at m/z 897 (35 eV), and (c) of the [M + Cs]+ ions at m/z 991 (25 eV) of 16:0/18:0/18:1-TAG obtained with a Finnigan TSQ-7000 triple quadrupole instrument. The metal ions are dominant in the spectra (Panel b and c) even with a lower collision energy.
Scheme 1. The fragmentation pathways proposed for location of double bonds along the fatty acid chain of 18:2/18:2/18:2-TAG.