Abstract
Globozoospermia is an infrequent pathology in which spermatozoa lack acrosomes. Patients are considered sterile without IVF augmented with intracytoplasmic sperm injection (ICSI), as fertilization is impaired due to absence of oocyte activation. As far as is known, this is the first study to report results of a comprehensive approach to the treatment of the semen parameters, sperm DNA fragmentation, aneuploidy, transmission electron microscopy, Western blotting and immunofluorescence for detection of phospholipase C zeta (PLCζ), as well as ICSI outcome, of an affected patient. Morphological evaluation and transmission electron microscopy revealed complete globozoospermia with significant duplicate heads and tails. Analysis for DNA damage revealed fragmentation rates of approximately 80% in semen and 15–23% in swim-up fractions. PLCζ was not detected by immunofluorescence or Western blotting. Aneuploidy rates were within normal ranges. ICSI followed by oocyte activation with calcium ionophore resulted in high rates of fertilization, and an ongoing pregnancy was established after transfer of cryopreserved–thawed embryos.
Keywords: globozoospermia, ICSI, oocyte activation, phospholipase C zeta, pregnancy
Introduction
Globozoospermia is a term used to describe spermatozoa with an absence of acrosomes. Globozoospermia was first documented in 1965 (Meyhöfer, 1965). Later, Schirren and colleagues (1971) provided ultrastructural details of globozospermic spermatozoa with the important finding that these abnormal spermatozoa lacked acrosomes.
During spermiogenesis, the acrosome is formed by the Golgi apparatus of the developing spermatocyte. After positioning of the prospective acrosomal cap, the sperm head is remodelled cytoskeletally to a species-specific shape. In patients with globozoospermia, this process does not occur, leaving spermatozoa with a characteristic round-head appearance and functionally with limited capacity to fertilize (Dam et al., 2007). Incidence has been estimated to be less than 0.1% of infertility patients (Holstein et al., 1973) and considerable evidence indicates that complete globozoospermia can be of genetic origin (Dam et al., 2007). Prior to 1995, patients with complete globozoospermia were left with no options other than the use of donor spermatozoa or adoption.
With the advent of intracytoplasmic sperm injection (ICSI), globozoospermia became one of the many severe male factor conditions that could be effectively treated. In 1995, an initial report was published where globozoospermic spermatozoa were used for IVF augmented with ICSI (Liu et al., 1995). Later reports indicated that induction of oocyte activation using a calcium ionophore was necessary after ICSI in some patients with globozoospermia (Tejera et al., 2008), strongly pointing to an absence of a putative oocyte-activating factor. However, at least two reports in the literature demonstrated that calcium ionophore treatment was not necessary (Dirican et al., 2008). Sperm phospholipase C zeta (PLCζ) has recently been implicated in oocyte activation (Yoon et al., 2008). This enzyme has been documented as a ‘sperm factor’ or an oocyte-activating factor, necessary to induce Ca2+ oscillations after fertilization (Yoon et al., 2008).
This case study reports a comprehensive clinical approach to complete globozoospermia with analyses of ultrastructure, DNA fragmentation levels, aneuploidy rate, detection of PLCζ and ICSI outcome (with and without the use of a calcium ionophore in sibling oocytes), resulting in a pregnancy.
Materials and methods
A patient with known globozoospermia, age 28 years and in otherwise good health, was enrolled for ICSI. Physical examination was unremarkable and the karyotype was 46XY, negative for Y chromosome microdeletions. His family history was negative and a brother had children that were conceived naturally. Genetic counselling was given to the patient regarding potential heritable globozoospermia in male offspring and treatment was approved by the institutional review board at Eastern Virginia Medical School.
The patient collected four semen samples. The initial sample was used for semen analysis and TdT (terminal deoxynucleotidyl transferase)-mediated dUDP nick-end-labelling (TUNEL). A second sample was used for transmission electron microscopy. A third sample was split for semen analysis, TUNEL and immunoblotting and immunofluorescence for PLCζ. A fourth semen collection was evaluated for aneuploidy by fluorescence in-situ hybridization (FISH). A semen sample from a fertile, normozoospermic individual was processed as a control.
Semen analysis
Semen was collected by masturbation into a sterile specimen container. A portion of the initial semen sample was cultured for Ureaplasma urealyticum using 10B broth (Remel, Lenexa, KS, USA) with simultaneous culture on A8 agar (Hardy Diagnostics, Santa Maria, CA, USA). The semen was analysed according to WHO standards (WHO, 1999). The presence of leukocytospermia was evaluated by peroxidase staining. Strict criteria (Menveld, et al., 1990) were used for sperm morphology. Concentration and the percentage of motile spermatozoa were determined manually using Makler counting chambers in duplicate. Other motion parameters were analysed using CASA (Hamilton-Thorne Research, Beverly, MA) and manually monitored as appropriate (Oehninger et al., 1990). Morphology smears were stained using Stat III Andrology Stain (Mid-Atlantic Diagnostics, Mt Laurel, NJ, USA), a cytology staining system equivalent to Diff-Quik for strict criteria. Morphology slides were scanned at ×400 magnification to verify the absence of acrosomes, with final evaluation at ×1000 magnification.
The liquefied semen was washed twice with human tubal fluid supplemented with 0.5% human serum albumin (HSA) (Irvine Scientific, Irvine, CA, USA). A portion of the washed spermatozoa was processed by swim-up to obtain a highly motile fraction. Aliquots were centrifuged to a pellet and after removing the supernatant, the pellet was overlaid with 100 μl. A swim-up separation of motile spermatozoa progressed for 60 min in an incubator with a 5% CO2 in humidified air at 37°C. Upon completion of incubation, the overlay was removed with care not to disturb the pellet, guaranteeing a highly motile fraction. Aliquots of both washed and swim-up spermatozoa were evaluated for DNA fragmentation using TUNEL and immunoblotting and immunofluorescence for PLCζ.
TUNEL
TUNEL was performed as previously published (Weng, et al., 2002). Briefly, washed and swim-up processed spermatozoa were applied in 25 μl volumes to poly-L-lysine-coated slides and fixed in 2% paraformaldehyde. After each step, spermatozoa were washed using 1% HSA in phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO, USA). Membranes were permeabilized using 10% Triton X-100 in PBS. Positive controls were aliquots treated with DNase I (Sigma-Aldrich). Negative controls were treated with label only, according to the manufacturer’s recommendation. Staining was accomplished using the In situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics, Indianapolis, IN, USA). After staining, aliquots were mounted with an anti-fade compound prior to examination using fluorescent microscopy. Spermatozoa were categorized as either TUNEL positive or negative. For each preparation, a total of 200 spermatozoa were evaluated.
Immunoblotting and immunofluorescence for PLCζ
Immunoblot samples, frozen at −80°C and refrigerated for immunofluorescence (2–5°C), were shipped on cold packs overnight to Dr R Fissore at University of Massachusetts for analysis. Aliquots were prepared for Western blots as published (Yoon et al., 2008). Briefly, aliquots were thawed, boiled and resolved on 7.5% SDS-PAGE chromatograms. Proteins were transferred to polyvinylidene fluoride membranes using a Mini trans-Blot Cell (Bio-Rad, USA) and blocked in reconstituted 6% non-fat dry milk in PBS with 0.1% Tween 20 followed by overnight incubation with anti-human-PLCζ antibody (1:1000) and incubated for 1 h with horse-radish peroxidase-labelled secondary antibody (goat anti-rabbit, 1:3000; Bio-Rad) and detected using chemiluminescence with a Kodak Image Station 440CF according to the manufacturer’s instructions.
Aliquots were prepared for immunofluorescence as in Yoon et al., 2008. Briefly, aliquots were fixed in 3.7% paraformaldehyde (Sigma-Aldrich) in Dulbecco’s PBS (DPBS), washed three times in DPBS, and then stored at 4°C until assayed. Sperm suspensions were assayed on poly-L-lysine slides and permeabilized with 0.1% Triton X-100 in DPBS. After blocking with 5% normal goat serum, the slides were incubated overnight at 4°C with anti-PLCζ (1:100) in 5% normal goat serum. At completion, slides were washed in 0.1% Tween 20 in DPBS and labelled for 1 h with an Alexa Fluor 555-labelled goat anti-rabbit secondary antibody at a 1:200 concentration. Slides were counterstained with Hoechst 33342 and evaluated using a fluorescent microscope.
Electron microscopy
Transmission electron microscopy was performed using a standard methodology at the Cleveland Clinic (Cleveland, OH), following previously published methods (Carbone et al., 1998).
Aneuploidy analysis
Sperm aneuploidy analysis was evaluated by a commercial clinical laboratory (The Centre for Preimplantation Genetics, Rockville, MD, USA). Aneuploidy was evaluated by FISH, as published by Pfeffer et al., (1999). A total of 400 spermatozoa were analysed. Probes for chromosomes 15, 16, 17, X and Y were used.
ICSI
The female partner, age 27 years, nulligravida and with no health or gynaecological problems, underwent an IVF cycle. Ovarian stimulation utilized recombinant gonadotrophins with gonadotrophin-releasing hormone agonist adjuvant therapy (long protocol) following standard protocols (Arslan et al., 2005). A total of eight metaphase-II oocytes were recovered. Four of six oocytes treated with calcium ionophore (10 μmol/l for 10 min) 30 min after ICSI fertilized normally (two pronuclei). The remaining two oocytes were not treated with calcium ionophore. Two day-3 embryos were transferred to the uterus (8- and 7-cell-stage embryos, respectively, with good morphology) under transabdominal ultrasound guidance. Two remaining embryos were cryopreserved (slow freeze) (Riggs et al., 2008). Transfer of thawed embryos was performed in an oestrogen and progesterone-supplemented cycle (Riggs et al., 2008). The frozen–thawed embryos were at the 7- and 6-cell stages with good morphology at the time of transfer.
Results
Analysis of the semen and sperm parameters revealed normal and unremarkable characteristics with the exception of a near-complete abnormality in sperm morphology. Ureaplasma urealyticum culture was negative and no leukocytes were detected. Sperm viability, concentration, motion parameters and morphology are summarized in Table 1. Stained morphology smears, evaluated extensively at ×400 magnification revealed complete globozoospermia, with significant duplicate heads and/or tails. All spermatozoa lacked acrosomes.
Table 1.
Semen analysis data from two semen samples provided 6 months apart.
| Parameter | Sample 1 | Sample 3 |
|---|---|---|
| Volume (ml) | 4.2 | 4.8 |
| Concentration (×106/ml) | 43 | 22 |
| Motility | 78 | 53 |
| Progressive motility | 46 | 31 |
| Morphology (strict criteria) | ||
| Globozoospermic | 100 | 100% |
| Coiled tails | 1.5 | 5 |
| Duplicates (heads and/or tails) | 11.5 | 15 |
| TUNEL positive | ||
| Washed semen | 79.5 | 80.5 |
| Swim-up | 23 | 15 |
Values are% unless otherwise stated.
Sample 3 provided within 3 days of ICSI.
Transmission electron microscopy confirmed that the spermatozoa from this patient were globozoospermic and devoid of normal acrosomes. Additionally, abnormal, immature patterns of chromatin condensation were observed. Occasional cytoplasmic droplets enveloping the nucleus and the initial segment of the flagellum were noted. Cross-sections of tails demonstrated standard 9+2 patterns of microtubules with both inner and outer dynein arms. Isolated tail defects were noted in a few spermatozoa, including duplicate tails, wrapping of the flagellum around the nucleus and loss of microtubular doublets.
Aneuploidy analysis revealed 6% of 400 spermatozoa evaluated were aneuploid. This percentage falls within the normal range (3.9–7.7%) established by the examining laboratory. In total, nine spermatozoa (2.3%) had an extra copy of chromosome 15, five (1.3%) had an extra copy of chromosome 16 and five (1.3%) had an extra copy of chromosome 17. Lastly, two spermatozoa (0.5%) had extra copies of the X chromosome and two (0.5%) had extra copies of the Y chromosome.
To examine for the presence of DNA fragmentation, the TUNEL assay was used. In unprocessed samples, approximately 80% of the spermatozoa were TUNEL positive (Table 1). In swim-up fractions with >96% progressive motility, TUNEL-positive cells ranged from 15% to 23%.
Immunoblotting was utilized to detect PLCζ. As positive controls, a non-contemporaneous sperm sample from an individual with normal sperm parameters from the same clinic (control 1) and another sample from an individual previously shown to have normal expression of PLCζ (control 2) were used. The Western blots indicated that the swim-up sample from control 1 displayed immunoreactivity at about 70 kDa, representing PLCζ. Moreover, immunoreactivity consistent with PLCζ was also observed for control 2. However, this band was not detected in the patient’s swim-up sperm sample (Figure 1). The additional lower band detected in the patient’s sample and control 1 most likely reflects non-specific binding of the antibody to some component of the washing media, as it was observed neither in control 2 nor in previous studies utilizing this antibody (Yoon et al., 2008).
Figure 1.
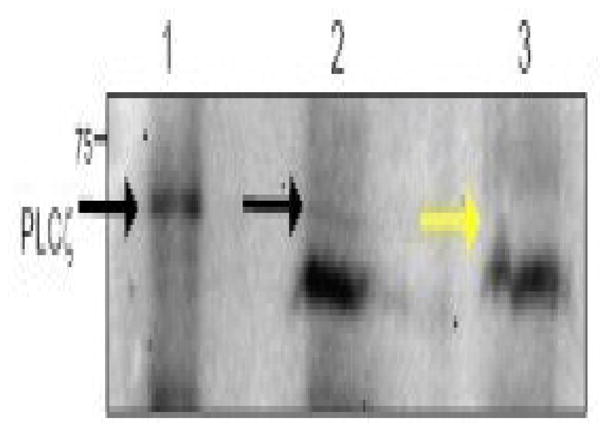
Immunoblotting of control and patient swim-up processed spermatozoa, illustrating PLCζ reactivity in the control spermatozoa but not in the patient’s sample: (1) unprocessed control 2; (2) swim-up processed control 1; (3) swim-up processed patient spermatozoa.
PLCζ expression was probed by immunofluorescence in individual spermatozoa. Control spermatozoa showed strong fluorescence in the equatorial region of the nucleus (Figure 2), whereas comparable patient samples lacked the characteristic band on the sperm head (Figure 2).
Figure 2.
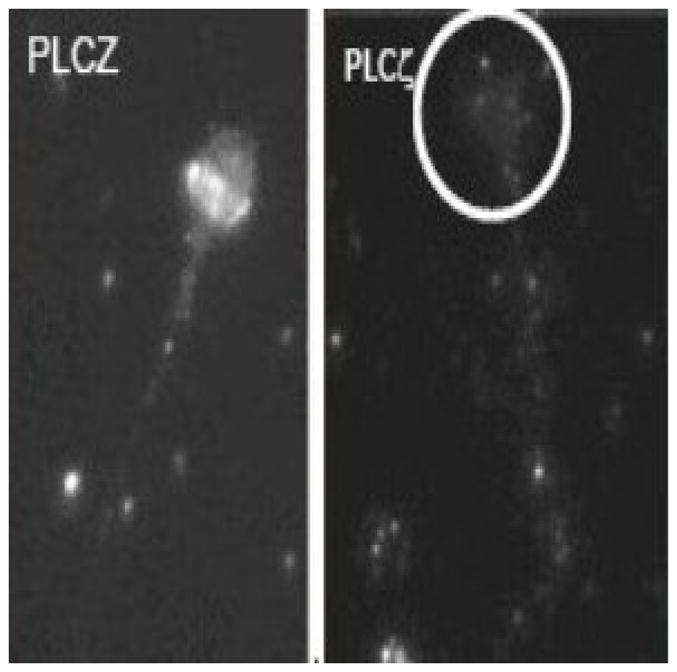
Representative PLCζ-labelled micrographs of spermatozoa. Left-hand panel: swim-up processed control 1; right-hand panel: swim-up processed patient (circled area indicates position of sperm head). PLCζ was prominent in the equatorial region of the sperm head of control 1 spermatozoa.
Following the initial ICSI attempt, two embryos were transferred. No pregnancy resulted from this attempt. A subsequent transfer of two cryopreserved–thawed embryos resulted in an ongoing pregnancy (as confirmed by transvaginal ultrasound visualization of a single intrauterine gestational sac with a crown–rump length consistent with 7.4 weeks and concordant with embryo transfer dates, as well as a positive fetal heart beat, at the time of submission).
Discussion
Although the rare incidence of globozoospermia has limited the amount of data collected, clinical effects of this abnormality have become clear over the years and can now be successfully treated through assisted reproduction technologies. The cause of this disorder is assumed to be genetic due to multiple reports of familial globozoospermia (Dirican et al., 2008). The actual genes involved in humans have not been unequivocally identified. Dam et al. (2007) identified a homozygous mutation in SPATA16 that resulted in familial globozoospermia with three affected brothers. The search for genetic causes is complicated by the fact that the gene abnormalities that cause globozoospermia do not seem to cause any identifiable clinical syndrome.
In addition to the lack of acrosomes, there is sufficient evidence in the literature to indicate that round-head spermatozoa are also subject to increased DNA damage (Vicari et al. 2002). Sutovsky et al. (2002) reported that round-head spermatozoa demonstrated a high level of ubiquinization, a known marker for DNA damage. These same authors found a correlation between ubiquinated spermatozoa and TUNEL. Using the sperm chromatin structure assay (SCSA), Larson et al. (2001) was unable to identify significant differences between globozoospermic and fertile individuals. DNA fragmentation in washed semen samples from this patient was highly elevated as measured by TUNEL. Swim-up samples were much improved over washed, unprocessed spermatozoa at an average of 23.4 ± 10.2% but still significantly elevated compared with a population of fertile sperm donors (27 ± 13% and 4 ± 3% in washed and swim-up samples, respectively; n = 30 ejaculates from 15 donors; SL Taylor et al., unpublished data). Therefore, the possibility exists that embryo development initiated by spermatozoa of these patients could be halted by a high degree of DNA fragmentation.
Both immunoblot and immunofluorescent results indicated that this subject’s spermatozoa do not contain PLCζ or if they do express it, they do so at concentrations below the sensitivity of assay (Figures 1 and 2). Research indicates that PLCζ is most likely the sperm factor that results in Ca2+ oscillations associated with oocyte activation at fertilization (Yoon et al., 2008). Consistent with this, in Yoon et al. (2008), which involved categorization of 17 patients into normal and low fertilization groups, two patients that demonstrated varying degrees of globozoospermia and lacked PLCζ were also unable to induce Ca2+ oscillations when injected into mouse oocytes. Interestingly, a third patient that had apparently normal morphology (strict criteria; Menkveld et al., 1990), although had vacuolated sperm heads, lacked equatorial PLCζ immunofluorescence in the majority of spermatozoa. Nonetheless, a few of these spermatozoa showed weak equatorial staining consistent with the presence of PLCζ, which coincided with a similar proportion of spermatozoa capable of initiating low frequency Ca2+ oscillations following injection into mouse oocytes. The 14 remaining patients, four of which were in the low fertilization category, demonstrated ability to induce Ca2+ oscillations after ICSI into mouse eggs, and 13 produced pregnancies after subsequent ICSI attempts.
A recent report indicates that calcium ionophore treatment is a necessary adjunct in at least some cases of globozoospermia (Tejera et al., 2008). The data from ICSI in this patient and the absolute requirement of a calcium ionophore for fertilization would tend to support the hypothesis that this protein is absent in this patient and necessary for fertilization to ensue, confirming its role as the oocyte-activating factor. However, at least one other report indicated that calcium ionophore stimulation of oocytes was not necessary (Dirican et al., 2008). This disparity may indicate that there may be a variable degree of absence of the enzyme in populations of globozoospermic patients. Importantly, more invasive injection techniques during ICSI might also overcome some of the activation failures observed in a subgroup of these patients. Therefore, future studies should examine the percentage of spermatozoa from fertile and infertile patients that show equatorial immunolocalization of PLCζ.
Spermatozoa from globozoospermic men have a number of deficiencies. They lack acrosomes and oocyte-activating factor, necessitating the use of ICSI and exogenous oocyte activation with a calcium ionophore. This study demonstrated that spermatozoa from this globozoospermic patient completely lacked, or expressed greatly reduced, PLCζ. In addition, it has to be kept in mind that round-headed sperm, although not showing abnormal degrees of aneuploidy, may exhibit significantly higher disturbances in chromatin condensation and DNA fragmentation. All of these factors must be considered when planning treatment and giving prognosis to these challenging patients. Future studies should investigate the role of recombinant human PLCζ as an activation agent for these patients.
Acknowledgments
This work was supported in part from grants by USDA 2007–35203 and NIH HDO51872 to RAF. The authors are grateful for the generous gift of anti-human-PLCζ antibody from Dr J Cibelli, Michigan State University, East Lansing, MI, USA.
Biography

Steven Taylor has been associated with the Andrology Laboratory of the Jones Institute for Reproductive Medicine for the past 10 years. He earned his PhD in biomedical sciences from Old Dominion University in Norfolk, Virginia in 2002. He has served as an ad hoc reviewer for several scientific publications and has either authored or co-authored eighteen manuscripts in peer-reviewed journals. Current interests include medical laboratory science and most aspects of reproductive sciences including male reproductive biology, sperm biology, sperm DNA fragmentation and its effects on subsequent embryo development and reproductive endocrinology.
Footnotes
Declaration: The authors report no financial or commercial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arslan M, Bocca S, Mirkin S, Barroso G, Stadtmauer L, Oehninger S. Controlled ovarian hyperstimulation protocols for in vitro fertilization: two decades of experience after the birth of Elizabeth Carr. Fertility and Sterility. 2005;84:555–569. doi: 10.1016/j.fertnstert.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Koehler JK, Klein NA, Tucker MJ. Failure of oocyte activation after intracytoplasmic sperm injection using round-headed sperm. Fertility and Sterility. 1997;68:118–122. doi: 10.1016/s0015-0282(97)81486-0. [DOI] [PubMed] [Google Scholar]
- Carbone DJ, Jr, McMahon JT, Levin HS, Thomas AJ, Jr, Agarwal A. Role of electron microscopy of sperm in the evaluation of male infertility during the era of assisted reproduction. Urology. 1998;52:301–305. doi: 10.1016/s0090-4295(98)00155-1. [DOI] [PubMed] [Google Scholar]
- Dam AHDM, Koscinski I, Kremer JAM, Moutou C, Jaeger A, Oudakker AR, Tournaye H, Charlet N, Lagier-Tourenne C, van Bokhoven H, Viville S. Homozygous mutation in SPATA 16 is associated with male infertility in human globozoospermia. American Journal of Human Genetics. 2007;81:813–820. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirican EK, Isik A, Vicdan K, Sozen E, Suludere Z. Clinical pregnancies and livebirths achieved by intracytoplasmic injection of round headed acrosomeless spermatozoa with and without oocyte activation in familial globozoospermia: case report. Asian Journal of Andrology. 2008;10:332–336. doi: 10.1111/j.1745-7262.2008.00248.x. [DOI] [PubMed] [Google Scholar]
- Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, de Angelis P, Claussen OP. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Human Reproduction. 1999;14:1039–1049. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- Holstein AF, Schirren CG, Schirren C, Mauss J. Round headed spermatozoa: a cause of male infertility. Deutsche Medizinische Wochenschrift. 1973;98:61–62. doi: 10.1055/s-0028-1106747. [DOI] [PubMed] [Google Scholar]
- Larson KL, Brannian JD, Singh NP, Burbach JA, Jost LK, Hansen KP, Kreger DO, Evenson DP. Chromatin structure in globozoospermia: a case report. Journal of Andrology. 2001;22:424–431. [PubMed] [Google Scholar]
- Liu J, Nagy Z, Joris H, Tournaye H, Devroey P, Van Steirteghem A. Successful fertilization and establishment of pregnancies after intracytoplasmic sperm injection in patients with globozoospermia. Human Reproduction. 1995;10:626–629. doi: 10.1093/oxfordjournals.humrep.a136000. [DOI] [PubMed] [Google Scholar]
- Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. Journal of Cellular Physiology. 2006;206:565–573. doi: 10.1002/jcp.20471. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Human Reproduction. 1990;5:586–592. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- Meyhöfer W. Beitrag zur cytophotometrischen beutreilung pathologisch verländerter samenzellen unter besonderer berücksichtiging der reunspermatozoen nach Feulgen- end fast greenfarbung. [Contribution to the cytophotometric evaluation of pathologically changed spermatozoa with special reference to round-shaped spermatozoa following Feuglen and fast green staining] Zeitschrift für Haut- und Geschlechtskrankheiten. 1965;39:174–182. [PubMed] [Google Scholar]
- Nardo LG, Sinatra F, Bartoloni G, Zafarana S, Nardo F. Ultrastructural features and ICSI treatment of severe teratozoospermia: report of two human cases of globozoospermia. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2002;104:40–42. doi: 10.1016/s0301-2115(01)00602-9. [DOI] [PubMed] [Google Scholar]
- Oehninger S, Acosta R, Morshedi M, Philput C, Swanson RJ, Acosta AA. Relationship between morphology and motion characteristics of human spermatozoa in semen and in the swim-up sperm fractions. Journal of Andrology. 1990;11:446–452. [PubMed] [Google Scholar]
- Pfeffer J, Pang MG, Hoegerman SF, Osgood CJ, Stacey MW, Mayer J, Oehninger S, Kearns WG. Aneuploidy frequencies in semen fractions from ten oligoasthenoteratozoospermic patients donating sperm for intracytoplasmic sperm injection. Fertility and Sterility. 1999;72:472–478. doi: 10.1016/s0015-0282(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Riggs R, Mayer J, Dowling-Lacey D, Chi TF, Jones E, Oehninger S. Does storage time influence postthaw survival and pregnancy outcome? An analysis of 11,768 cryopreserved human embryos. Fertility and Sterility. 2008 Nov 20; doi: 10.1016/j.fertnstert.2008.09.084. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schirren CG, Holstein AF, Schirren C. Uber die Morphologenese Rundkopfiger Spermatozoen des Menschen. Andrologie. 1971;3:117–125. [Google Scholar]
- Sutovsky P, Terada Y, Schatten G. Ubiquitin-based sperm assay for the diagnosis of male factor infertility. Human Reproduction. 2001;16:250–258. doi: 10.1093/humrep/16.2.250. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Neuber E, Schatten G. Ubiquitin-dependent sperm quality control mechanism recognizes spermatozoa with DNA defects as revealed by dual ubiquitin-TUNEL assay. Molecular Reproduction and Development. 2002;61:406–413. doi: 10.1002/mrd.10101. [DOI] [PubMed] [Google Scholar]
- Tejera A, Mollá M, Muriel L, Remohí J, Pellicer A, De Pablo JL. Successful pregnancy and childbirth after intracytoplasmic sperm injection with calcium ionophore oocyte activation in a globozoospermic patient. Fertility and Sterility. 2008;90:1202. doi: 10.1016/j.fertnstert.2007.11.056. [DOI] [PubMed] [Google Scholar]
- Vicari E, Perdichizzi A, De Palma A, Burrello N, D’Agata R, Calogero AE. Globozoospermia is associated with chromatin structure abnormalities: case report. Human Reproduction. 2002;17:2128–2133. doi: 10.1093/humrep/17.8.2128. [DOI] [PubMed] [Google Scholar]
- Weng SL, Taylor SL, Morshedi M, Schuffner A, Duran EH, Beebe S, Oehninger S. Caspase activity and apoptotic markers in ejaculated human sperm. Molecular Human Reproduction. 2002;8:984–991. doi: 10.1093/molehr/8.11.984. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO manual for the examination of semen and sperm/cervical mucus interaction. Cambridge University Press; Cambridge, UK: 1999. [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, Mager J, Fissore RA. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. Journal of Clinical Investigations. 2008;118:3671–3681. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]


