Abstract
Background
White matter hyperintensities (WMH) and MRI-defined brain infarcts (BI) have individually been related to stroke, dementia, and mortality in population-based studies, mainly in older people. Their significance in middle-aged community-dwelling persons and the relative importance of these associations remain unclear. We simultaneously assessed the relation of WMH and BI with incident stroke, mild cognitive impairment (MCI), dementia and mortality in a middle-aged community-based cohort.
Methods
2,229 Framingham Offspring Study participants aged 62±9yrs underwent volumetric brain MRI and neuropsychological testing (1999–2005). Incident stroke, dementia and mortality were prospectively ascertained, and, for 1,694 participants in whom a second neuropsychological assessment was performed (2005–2007), incident MCI was evaluated. All outcomes were related to WMH volume (WMHV), age-specific extensive WMHV (EXT-WMHV) and BI, adjusting for age and sex.
Results
EXT-WMHV and BI were associated with an increased risk of stroke (HR=2.28[95%CI: 1.02–5.13], HR=2.84[1.32–6.10]). WMHV, EXT-WMHV and BI were associated with an increased risk of dementia (HR=2.22[1.32–3.72], HR=3.97[1.10–14.30], HR=6.12[1.82–20.54]), independently of vascular risk factors, and interim stroke. WMHV and EXT-WMHV were associated with incident amnestic MCI in participants aged ≥ 60 only (OR=2.47[1.31–4.66] and OR=1.49[1.14–1.97]). WMHV and EXT-WMHV were associated with an increased risk of death (HR=1.38[1.13–1.69], HR=2.27[1.41–3.65]) independent of vascular risk factors, and of interim stroke and dementia.
Conclusion
In a large community-based sample of middle-aged adults, BI predicted an increased risk of stroke and dementia, independent of vascular risk factors. WMH portended an increased risk of stroke, amnestic MCI, dementia, and death, independent of vascular risk factors and interim vascular events.
Keywords: Epidemiology, Cerebrovascular disease/stroke, Computerized tomography and Magnetic Resonance Imaging
Introduction
Several longitudinal population-based studies have suggested that white matter hyperintensities (WMH) and MRI-defined brain infarcts (BI) are associated with an increased risk of stroke,1–6 dementia,7 and death.8, 9 Some studies observed an association only for certain subtypes of outcomes such as vascular dementia,10 or certain types of WMH such as periventricular hyperintensities.4, 11
The majority of these studies were performed in older individuals over age 65.2, 3, 7–11 Furthermore all studies, except two,6, 9 have measured WMH volume (WMHV) using visual semi-quantitative rating scales. Finally, only few studies have assessed the relative contribution of WMH and BI to the risk of stroke, dementia and mortality, in elderly people.9 Disentangling the relative effects of WMH and BI on these outcomes in middle-aged adults could improve our understanding of the underlying mechanisms and help refine risk prediction.
Our aim was to assess simultaneously the relation of both a quantitative measure of WMHV and presence of BI with the risk of incident stroke, mild cognitive impairment (MCI), dementia and mortality in a large community-based sample of middle-aged adults.
Methods
Population
The Framingham Heart Study is a community-based, prospective cohort study that was initiated in 1948 and now comprises three generations of participants. The present study includes participants from the intermediate generation, the Framingham Offspring Cohort, comprising 5,124 persons examined approximately once every 4 years since enrollment (1971).12
Offspring participants who survived to the seventh examination (1998–2001) and had attended at least one among the fifth, sixth or seventh examinations, or had moved away from Framingham but continued to be followed up offsite, were invited to take a neuropsychological test battery (NP) and undergo volumetric brain MRI (1999–2005). Among the participants who were invited (n=3623), 2,229 participants were available for this study (see flow diagram, Supplemental Figure 1).
Since 2005, all participants included in the ancillary study have been invited to undergo a second NP and MRI. Exams performed between 2005 and 2007 (n=1,694) could be used for the analysis of incident MCI (data from 2008–2009 are still being processed at this time).
MRI scans
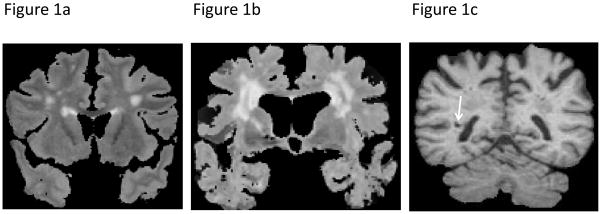
MRI techniques used in the Framingham Offspring Study have been described previously.13, 14 Briefly, participants were evaluated with a 1 or 1.5-tesla Siemens Magnetom. 3D T1 and double echo proton density (PD) and T2 coronal images acquired in 4-mm contiguous slices were performed. All images were transferred to a centralized reading center (University of California Davis Medical Center) and analyses were performed on QUANTA 6.2, a custom-designed image analysis package operating on a Sun Microsystems Ultra 5 workstation. Semiautomated analysis of pixel distributions based on mathematical modeling of MRI pixel intensity histograms for cerebrospinal fluid (CSF) and brain matter (white matter and gray matter) were used to determine the optimal threshold of pixel intensity to best distinguish CSF from brain matter based on methods published previously.15 For segmentation of WMH from other brain tissues, the first and second echo images from T2 sequences were summed and a log-normal distribution was fitted to the summed data. A segmentation threshold for WMH was determined as 3.5 standard deviations (SD) in pixel intensity greater than the mean of the fitted distribution of brain parenchyma.14 White matter hyperintensity volume (WMHV) was computed as the ratio of total white matter hyperintensity volume to totalintracranial volume using a previously validated method.14 WMHV was not normally distributed and was log-transformed for all analyses. In addition we created (five-year) age-group specific z-scores, zWMHV, and participants with zWMHV>1 were designated as having extensive WMHV (EXT-WMHV) (Figure 1a and 1b and see Supplemental Table 1 for age-specific thresholds). BI were defined as an area of abnormal signal intensity in a vascular distribution, at least 3mm in size, with a cerebrospinal fluid density on the subtraction image and, for lesions in the basal ganglia area, distinct separation from the circle of Willis vessels (Figure 1c).13, 16 We used size, location, shape and tissue contrast to distinguish BI from dilated perivascular spaces. Indeed, our analysis method allows for the superimposition of T1, PD and T2 images and using this method, vessels can often be seen within perivascular spaces, particularly on T2 images.
Figure 1. Illustration of MRI-markers of vascular brain injury.
a: MRI-scan (coronal T2-weighted sequence) of an 65-year old male participant with extensive white matter hyperintensities (EXT-WMH); b: MRI-scan (coronal T2-weighted sequence) of an 84-year old female participant with EXT-WMH; c: MRI-scan (coronal T1-weighted sequence) of an 84-year old male participant with an MRI-defined brain infarct in the right centrum semiovale (arrow)
Outcomes
Participants have been monitored since 1974, using previously described surveillance techniques, for the development of stroke or dementia.17, 18 Stroke was defined as an acute onset focal neurological deficit of presumed vascular etiology, lasting ≥ 24 hours. Ischemic stroke was diagnosed if a focal neurological deficit was documented and either the imaging showed no hemorrhage, imaging showed an infarct that correlated with the clinical deficit, or an infarct was documented at autopsy. Dementia was diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition,19 and Alzheimer’s disease (AD) based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association for definite, probable, or possible AD.20
The diagnosis of MCI was based on the results of the NP. We defined five domain-specific composite cognitive factors (Supplemental Table 2). Participants were defined as having MCI if they were non-demented and scored ≥ 1.5 SD below the gender-specific mean of the distribution for ≥ 1 factor. Amnestic MCI was defined by a score ≥ 1.5 SD below the mean for factor 1 (verbal memory), factor 2 (visuospatial memory) or both. Incident MCI was defined as new onset MCI on the second NP in participants without MCI at baseline. Incident amnestic MCI corresponded to new onset amnestic MCI in participants without amnestic MCI at baseline (this included participants with non-amnestic MCI at baseline who developed a memory deficit during follow-up).
Vascular death was established upon review of all available records if the cause of death was deemed most likely to be coronary heart disease (including sudden cardiac death), congestive heart failure, stroke or any other vascular event, and no other cause could be ascribed.21 Cardiovascular death was defined similarly, but excluded stroke death.
Definition of covariates
Vascular risk factors (systolic blood pressure, smoking, diabetes mellitus and history of cardiovascular disease) were defined as in the Framingham Stroke Risk Profile (FSRP).18 Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or use of antihypertensive medication.22 Educational achievement was studied as a four-class variable (no high-school degree; high-school degree, no college; some college; college degree). Participants were categorized according to presence or absence of ≥ 1 Apolipoprotein E ε 4 (ApoEε 4) allele.
Statistics
Chi-square tests for binary variables and t-tests for continuous variables were used to compare baseline characteristics between persons with and without EXT-WMHV or BI.
We used multivariable Cox regression adjusted for sex and age at MRI to examine the associations of WMHV, EXT-WMHV and BI with incident stroke, dementia and mortality, and logistic regression adjusted for sex, age at MRI, education and interval between first and second NP for the association with incident MCI. When analyzing associations with incident stroke, participants with prevalent stroke were excluded. Similarly, participants with prevalent dementia were excluded when testing associations with incident dementia and participants with prevalent MCI or dementia were excluded when looking at the association with incident MCI.
In secondary analyses we adjusted for vascular risk factors, for ApoEε 4-status in the analyses of cognitive outcomes, and we conducted analyses stratified on gender, age (<60 versus ≥ 60 years) and hypertension, as well as ApoEε 4 carrier status for incident MCI (given the small number of events we did not perform any stratified analysis for incident dementia). To assess whether associations of WMHV or BI with dementia and MCI were mediated by their associations with stroke, we ran regression models excluding prevalent stroke and adjusting for interim stroke as a time-varying covariate. Similarly we ran a regression model excluding prevalent stroke and dementia and adjusting for interim stroke and dementia for the association with mortality. Finally, we tested for the presence of an interaction between WMHV and BI, and EXT-WMHV and BI. In the absence of statistical interaction (p<0.05) we ran a multivariable regression model including both WMHV and BI (or EXT-WMHV and BI) as independent variables.
Analyses were performed using Statistical Analyses System software version 9® (SAS Institute, Cary, NC).
Results
The baseline characteristics of the study population are presented in Table 1. Participants with EXT-WMHV were significantly older, and more often had hypertension, prevalent stroke and MCI. Participants with BI were significantly older, and more often had hypertension, diabetes and stroke. The mean duration of follow-up was 5.6±1.4 years for stroke, 5.9±1.4 years for dementia, and 5.2±1.5 years for mortality, and 6.2±1.2 years between the first and last NP evaluation for MCI. During follow-up, 32 participants sustained a stroke (26 ischemic, 5 hemorrhagic and 1 of unspecified type), 11 participants developed dementia (7 AD, 3 vascular dementia, 1 other), and 97 participants died (21 vascular deaths, of which 3 were stroke deaths). Between the first and second NP, 93 of the 1134 participants without cognitive deficit at baseline developed new-onset MCI, and 93 of the 1344 participants without memory deficit at baseline developed new-onset amnestic MCI. BI was correlated with WMHV (ρ=0.20, p<0.001) and EXT-WMHV (ρ=0.14, p<0.001).
Table 1.
Baseline characteristics
| All | EXT-WMHIV = 0 | EXT-WMHIV = 1 | p | BI = 0 | BI = 1 | p | |
|---|---|---|---|---|---|---|---|
| N | 2229 | 1912 | 313 | 1975 | 253 | ||
| Age (mean±SD) | 62±9 | 62±9 | 63±9 | 0.030 | 62±9 | 65±9 | <0.001 |
| Women, n (%) | 1179 (53) | 911 (48) | 139 (44) | 0.288 | 923 (47) | 126 (50) | 0.357 |
| High-school graduate | 2149 (97) | 1842 (96) | 303 (97) | 0.745 | 1907 (97) | 241 (95) | 0.255 |
| Hypertension | 916 (42) | 779 (41) | 136 (47) | 0.048 | 770 (40) | 146 (58) | <0.001 |
| Active smokers | 267 (12) | 226 (12) | 41 (14) | 0.261 | 234 (12) | 33 (13) | 0.661 |
| Diabetes | 255 (127) | 214 (11) | 41 (14) | 0.151 | 215 (11) | 40 (16) | 0.028 |
| Prevalent stroke | 38 (2) | 25 (1) | 13 (4) | <0.001 | 17 (0.9) | 21 (8) | <0.001 |
| Prevalent dementia | 6 (0.3) | 4 (0.2) | 2 (0.6) | 0.202 | 5 (0.3) | 1 (0.4) | 0.515 |
| Prevalent MCI | 534 (24) | 431 (23) | 103 (33) | <0.001 | 469 (24) | 66 (27) | 0.381 |
| Prevalent amnestic MCI | 283 (13) | 234 (12) | 49 (16) | 0.091 | 252 (13) | 31 (12) | 0.813 |
| log (WMHIV) | − 3.0±1.0 | − 3.2±0.9 | − 1.4±0.9 | <0.001 | − 31±1.0 | − 2.5±1.2 | <0.001 |
| EXT-WMHIV | 313 (14) | - | - | - | 242 (12) | 70 (28) | <0.001 |
| BI | 253 (11) | 183 (10) | 70 (22) | <0.001 | - | - | - |
MCI: Mild Cognitive Impairment; WMHV: White Matter Hyperintensity Volume; EXT-WMHV: Extensive WMHV; BI: MRI-defined Brain Infarcts; 0: Absent; 1: Present
Association of WMHV, EXT-WMHV and BI with incident stroke
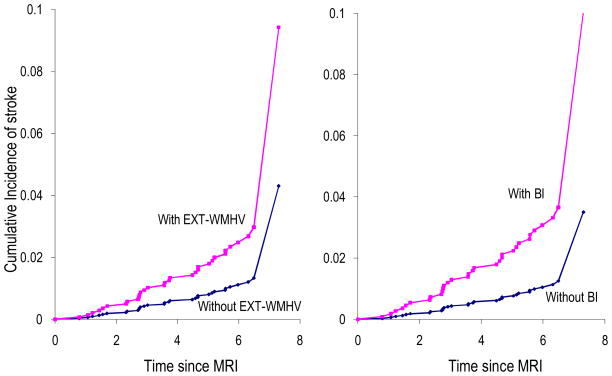
BI and EXT-WMHV, but not WMHV, were associated with an increased risk of incident stroke (Table 2, Figure 2), especially ischemic stroke (HR=3.49[95%CI: 1.54–7.91] for BI and HR=2.97[1.28–6.85] for EXT-WMHV). These associations were independent of vascular risk factors (Table 2) and were unchanged when stratifying on age, sex, and hypertension (data not shown). The associations were stronger for BI compared to EXT-WMHV (Table 2).
Table 2.
Association of WMHV, EXT-WMHV and BI with incident stroke, dementia and mortality
| Hazard ratio (95% confidence interval) for incident | |||||||
|---|---|---|---|---|---|---|---|
| Stroke | p | Dementia | p | Death | p | ||
| N events / sample size | 32 / 2177 | 11 / 2013 | 97 / 2208 | ||||
| WMHV | Model 1 | 1.33 (0.94–1.88) | 0.113 | 2.18 (1.33–3.58) | 0.002 | 1.43 (1.18–1.73) | 0.001 |
| Model 2 | 1.33 (0.93–1.90) | 0.120 | 2.22 (1.32–3.72) | 0.003 | 1.38 (1.13–1.69) | 0.002 | |
| Model 3 | - | - | 2.24 (1.31–3.77) | 0.002 | 1.27 (1.03–1.57) | 0.025 | |
| Model 4* | 1.23 (0.86–1.75) | 0.257 | 1.97 (1.16–3.35) | 0.012 | 1.38 (1.13–1.68) | 0.002 | |
| EXT-WMHV | Model 1 | 2.33 (1.04–5.21) | 0.040 | 4.02 (1.13–14.32) | 0.032 | 2.31 (1.45–3.67) | 0.001 |
| Model 2 | 2.28 (1.02–5.13) | 0.046 | 3.97 (1.10–14.30) | 0.035 | 2.27 (1.41–3.65) | <0.001 | |
| Model 3 | - | - | 2.07 (1.35–19.00) | 0.016 | 1.77 (1.05–2.98) | 0.031 | |
| Model 4* | 1.96 (0.86–4.46) | 0.108 | 2.75 (0.74–10.19) | 0.130 | 2.13 (1.33–3.43) | 0.002 | |
| BI | Model 1 | 3.06 (1.44–6.51) | 0.004 | 6.31 (1.88–21.18) | 0.003 | 1.73 (1.08–2.78) | 0.024 |
| Model 2 | 2.84 (1.32–6.10) | 0.008 | 6.12 (1.82–20.54) | 0.003 | 1.53 (0.94–2.48) | 0.087 | |
| Model 3 | - | - | 8.97 (2.39–33.72) | 0.001 | 1.54 (0.91–2.60) | 0.104 | |
| Model 4† | 2.80 (1.29–6.06) | 0.009 | 4.55 (1.33–15.56) | 0.016 | 1.40 (0.85–2.30) | 0.183 | |
WMHV: natural log from ratio of WMHV over total intracranial volume; EXT-WMHV: extensive WMHV; BI: MRI-defined Brain Infarcts; Model 1: Cox regression adjusted for age and sex; Model 2: Model 1 additionally adjusted for systolic blood pressure, current smoking, diabetes, history of cerebrovascular disease; Model 3: Model 1 additionally adjusted for interim stroke and excluding prevalent stroke (for dementia) or for interim stroke and dementia and excluding prevalent stroke and dementia (for death); Model 4: Model 1 additionally adjusted for BI* or WMHV†.
Figure 2. Cumulative incidence of stroke based on age- and sex-adjusted Cox models.
Association of WMHV, EXT-WMHV and BI with incident dementia and MCI
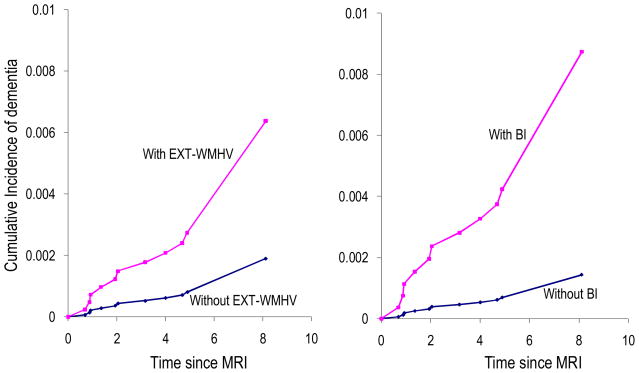
WMHV, EXT-WMHV and BI were all associated with an increased risk of incident dementia, independently of vascular risk factors (Table 2, Figure 3), and of ApoEε 4 (data not shown). The results were similar after excluding prevalent stroke and adjusting for interim stroke (Table 2). None of the MRI-markers was associated with incident all-MCI or amnestic MCI overall (Table 3). There was a significant interaction of EXT-WMHV and WMHV with age (p<0.05), EXT-WMHV and WMHV being associated with an increased risk of amnestic MCI only among participants aged ≥ 60 (OR=2.47[1.31–4.66] for EXT-WMHV and OR=1.49[1.14–1.97] for WMHV). There was also a significant interaction of WMHV with gender (p<0.05), WMHV being associated with an increased risk of amnestic MCI only among women (OR=1.43[1.06–1.93]).
Figure 3. Cumulative incidence of dementia based on age- and sex-adjusted Cox models.
Table 3.
Association of WMHV, EXT-WMHV and BI with incident MCI
| Odds ratio (95% confidence interval) for incident | |||||
|---|---|---|---|---|---|
| MCI | p | amnestic MCI | P | ||
| N events / sample size | 93 / 1134 | 93 / 1344 | |||
| WMHV | Model 1 | 1.06 (0.83–1.36) | 0.617 | 1.24 (0.98–1.58) | 0.071 |
| Model 2 | 1.06 (0.83–1.36) | 0.635 | 1.24 (0.98–1.57) | 0.071 | |
| Model 3 | 1.04 (0.81–1.33) | 0.750 | 1.22 (0.96–1.55) | 0.102 | |
| Model 4 | 1.09 (0.85–1.39) | 0.516 | 1.22 (0.96–1.56) | 0.100 | |
| EXT-WMHV | Model 1 | 1.27 (0.68–2.40) | 0.452 | 1.70 (0.97–2.97) | 0.062 |
| Model 2 | 1.26 (0.67–2.39) | 0.471 | 1.67 (0.96–2.93) | 0.072 | |
| Model 3 | 1.13 (0.59–2.17) | 0.719 | 1.65 (0.94–2.92) | 0.084 | |
| Model 4 | 1.35 (0.71–2.56) | 0.363 | 1.64 (0.93–2.89) | 0.087 | |
| BI | Model 1 | 0.76 (0.37–1.57) | 0.456 | 1.39 (0.77–2.52) | 0.277 |
| Model 2 | 0.77 (0.37–1.59) | 0.478 | 1.39 (0.77–2.52) | 0.278 | |
| Model 3 | 0.68 (0.31–1.45) | 0.314 | 1.24 (0.65–2.38) | 0.511 | |
| Model 4 | 0.73 (0.35–1.52) | 0.400 | 1.26 (0.69–2.31) | 0.452 | |
WMHV: natural log from ratio of WMHV volume over total intracranial volume; EXT-WMHV: extensive WMHV; BI: MRI-defined brain infarcts; MCI: mild cognitive impairment; Model 1: logistic regression adjusted for age, sex, education, interval between exams; Model 2: Model 1 additionally adjusted for systolic blood pressure, current smoking, diabetes, history of cerebrovascular disease; Model 3: Model 1 additionally adjusted for interim stroke, after excluding participants with prevalent stroke at baseline; Model 4: Model 1 additionally adjusted for BI* or WMHV†
Association of WMHV, EXT-WMHV and BI with mortality
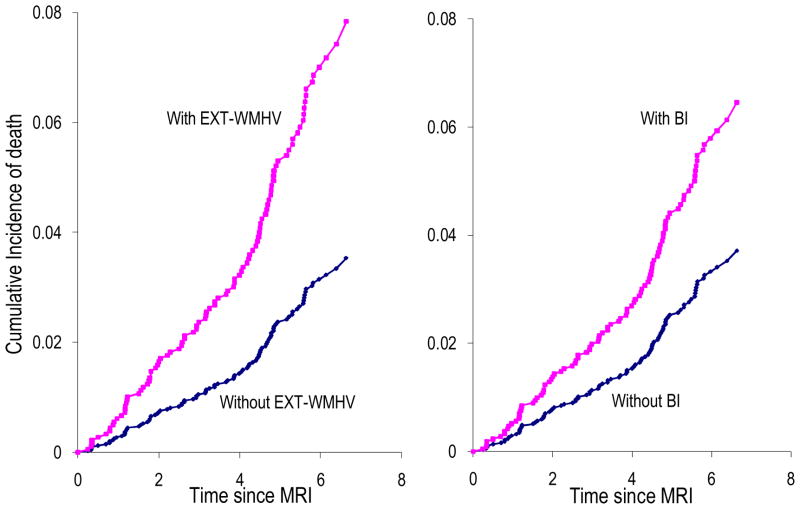
Both WMHV and EXT-WMHV were associated with an increased risk of death (Table 2, Figure 4), especially vascular death (HR=1.96[1.31–2.92] for WMHV and HR=4.18[1.72–10.15] for EXT-WMHV) or cardiovascular death (HR=1.86[1.20–2.89] for WMHV and HR=3.49[1.30–9.37] for EXT-WMHV). These associations were not attenuated by adjustment for vascular risk factors, exclusion of prevalent stroke and dementia, and adjustment for interim stroke and dementia (Table 2). BI predicted an increased risk of death (Table 2), especially vascular death (HR=3.42[1.40–8.34]) or cardiovascular death (HR=2.76[1.02–7.44]), but these associations became non significant after adjustment for vascular risk factors (Table 2 and data not shown). The associations of WMHV and EXT-WMHV with mortality were unchanged when stratifying on age and hypertension (data not shown).
Figure 4. Cumulative incidence of death based on age- and sex-adjusted Cox models.
Discussion
In 2,229 middle-aged community-dwelling participants from the Framingham Offspring Study, BI and the presence of excessive white matter hyperintensity for age (EXT-WMHV) were each associated with an increased risk of incident stroke, especially ischemic stroke, independently of vascular risk factors. WMHV as a continuous measure, EXT-WMHV and BI were associated with an increased risk of incident dementia, independent of vascular risk factors, prevalent and interim stroke. WMHV and EXT-WMHV were associated with an increased risk of incident amnestic MCI among participants aged 60 years or older only. Finally, WMHV, EXT-WMHV and BI were associated with an increased risk of death, particularly vascular death. The associations of WMHV and EXT-WMHV with mortality were also independent of concomitant vascular risk factors, and they were still significant but weakened after accounting for prevalent and interim stroke and dementia.
The strengths of this study are the prospective population-based setting with careful surveillance of clinical events and the quantitative measurement of WMHV. Original features are the younger age of the study sample compared to previous publications, and the simultaneous assessment of the impact of WMH and BI on correlated outcomes, allowing an estimation of the relative contribution of each risk marker. Our study also has limitations. Despite the substantial sample size, there were a limited number of events, especially for dementia. Even though the acceptance rate was high, persons included in this study are not perfectly representative of the general population. Finally, we did not look at differential associations according to location, number and size of BI or location of WMH.
The association of BI and EXT-WMHV with incident stroke is in line with previous findings in other population-based studies,1–6 both in middle-aged,1, 4 and elderly individuals.2, 3, 5, 6 In addition, we found that the continuous quantitative measure of WMHV was not associated with an increased risk of stroke, which could suggest a threshold effect, with WMH being associated with an increased stroke risk only beyond a certain volume.
Only a few community-based studies have assessed the relation of WMH with incident dementia,7, 10, 11 and these were in older adults (≥ 65 years). One study found an association of extensive WMH with an increased risk of all dementia, AD and vascular dementia.7 A second study found an association of WMH volume with vascular dementia only.10 A third study observed that increasing periventricular hyperintensities were associated with all dementia and AD.11 To our knowledge, our study is the first to test the association of BI and WMHV with incident dementia in middle-aged community-dwelling persons. It is also the first population-based study to test the association of these MRI-markers with incident MCI. Small-vessel disease is commonly considered to be the main determinant of WMH.23, 24 However, the fact that the associations between WMH and dementia persisted after adjusting for vascular risk factors, interim stroke, and BI, could suggest that mechanisms other than arteriolosclerosis may be involved in the relation between WMH and cognition. Other potential mechanisms have been suggested such as amyloid angiopathy,25 or wallerian degeneration due to cortical atrophy or AD-related pathological changes.26, 27 Interestingly, WMHV and EXT-WMHV were associated with an increased risk of amnestic MCI in older participants only (age≥ 60). This could either suggest that increasing WMHV matters only above a certain absolute threshold, reached at an older age, or that other neurodegenerative lesions, which are more frequent in older persons, need to be present concomitantly in order to affect cognition.
The association of WMHV and EXT-WMHV with mortality in our middle-aged sample extends previous observations in elderly individuals.8, 9 While these associations were unchanged after adjusting for vascular risk factors, they became weaker after adjusting for interim dementia and stroke, suggesting that these events explain a substantial part of the increased mortality. Furthermore, the stronger associations with death due to vascular disease than with overall mortality, even after exclusion of stroke death, may imply that increasing amounts of WMH are correlated with vascular disease not only in the cerebral vasculature, but also in other territories such as coronary arteries. In line with these findings, a study conducted on patients aged 62 years on average with proven atherosclerotic disease had identified a significant association of periventricular white matter hyperintensities (but not deep white matter hyperintensities) with an increased risk of incident myocardial infarction.28 Interestingly however, another more recent study did not observe any association of WMH volume with the occurrence of major vascular events other than stroke (including myocardial infarction and non stroke vascular death) in non-institutionalized community-dwelling participants aged ≥ 65.6 A potential explanation for this discrepancy could be a selection bias, as participants with an increased risk of non stroke vascular events, which generally occur earlier in life than stroke, may have been less likely to be included in the latter study due to death or institutionalization before the age of 65. In the present study the association of BI with mortality disappeared when adjusting for vascular risk factors, suggesting that the association may be driven mainly by the presence of an increased burden of conventional vascular risk factors, while the association of WMH with mortality may be more complex, involving other factors, such as an increased risk of death from dementia.
Overall, our findings show that, in middle-aged community-dwelling subjects, BI and WMH are important predictors of incident stroke, amnestic MCI and dementia, and also portend an increased risk of death. Interestingly, the associations of WMH with cognition and mortality were at least partly independent of concomitant vascular disease. Further epidemiological studies in middle-aged individuals are warranted to confirm or refute our findings, as well as studies looking at the association of WMHV progression and incident BI with the risk of stroke, MCI and dementia, as these MRI markers could potentially serve as intermediate endpoints in prevention trials.
Supplementary Material
Acknowledgments
This work was supported by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195) and by grants from the National Institute of Neurological Disorders and Stroke (R01 NS17950) and from the National Institute on Aging (R01 AG16495; AG08122; AG033193; AG031287). Dr. Debette is supported by a Fulbright grant and received an award from the Bettencourt-Schueller Foundation.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, Hubbard LD, Mosley TH. Cerebral white matter lesions, retinopathy, and incident clinical stroke. Jama. 2002;288:67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 3.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 4.Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, Oguro H, Takahashi K. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15:57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, Price T. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 6.Buyck JF, Dufouil C, Mazoyer B, Maillard P, Ducimetiere P, Alperovitch A, Bousser MG, Kurth T, Tzourio C. Cerebral white matter lesions are associated with the risk of stroke but not with other vascular events: the 3-City Dijon Study. Stroke. 2009;40:2327–2331. doi: 10.1161/STROKEAHA.109.548222. [DOI] [PubMed] [Google Scholar]
- 7.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 8.Kuller LH, Arnold AM, Longstreth WT, Jr, Manolio TA, O’Leary DH, Burke GL, Fried LP, Newman AB. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging. 2009;30:450–456. doi: 10.1016/j.neurobiolaging.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Meguro K, Ishii H, Kasuya M, Akanuma K, Meguro M, Kasai M, Lee E, Hashimoto R, Yamaguchi S, Asada T. Incidence of dementia and associated risk factors in Japan: The Osaki-Tajiri Project. J Neurol Sci. 2007;260:175–182. doi: 10.1016/j.jns.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 12.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 13.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 15.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16:274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O’Donnell CJ, Yoshita M, D’Agostino RB, Sr, DeCarli C, Wolf PA. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D’Agostino RB. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 18.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 19.American_Psychiatric_Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Cupples LA, D’Agostino RB, Kiely D. The Framingham Heart Study, Section 35 An Epidemiological Investigation of Cardiovascular Disease Survival Following Cardiovascular Events: 30 Year Follow-up. Bethesda, MD: National Heart, Lung and Blood Institute; 1988. [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 24.van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114 (Pt 2):761–774. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- 26.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Gerdes VE, Kwa VI, ten Cate H, Brandjes DP, Buller HR, Stam J. Cerebral white matter lesions predict both ischemic strokes and myocardial infarctions in patients with established atherosclerotic disease. Atherosclerosis. 2006;186:166–172. doi: 10.1016/j.atherosclerosis.2005.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.