SUMMARY
Plasma FGF21 levels and hepatic FGF21 gene expression increase dramatically after birth in mice. This induction is initiated by suckling, requires lipid intake, is impaired in PPARα null neonates, and is mimicked by treatment with the PPARα activator, Wy14,643. Neonates exhibit reduced FGF21 expression in response to fasting, in contrast to the upregulation occurring in adults. Changes in FGF21 expression due to suckling or nutritional manipulations were associated with circulating free fatty acid and ketone body levels. We mimicked the FGF21 postnatal rise by injecting FGF21 into fasting neonates, and found that this enhanced the expression of genes involved in thermogenesis within brown fat, and increased body temperature. Brown adipocytes treated with FGF21 exhibited increased expression of thermogenic genes, higher total and uncoupled respiration, and enhanced glucose oxidation. We propose that the induction of FGF21 production by the liver mediates direct activation of brown fat thermogenesis during the fetal-to-neonatal transition.
INTRODUCTION
Fibroblast growth factor 21 (FGF21), a member of the FGF super-family, has recently emerged as a novel regulator of metabolism (Kharitonenkov et al., 2005). FGF21 is mainly produced by the liver and is induced after fasting in both rodents and humans (Inagaki et al., 2007; Badman et al., 2007; Gälman et al., 2008). Consistent with its proposed role as a regulator of the metabolic adaptations to fasting (Reitman, 2007), FGF21 causes paracrine effects, such as induction of hepatic ketogenesis, and endocrine actions, such as the promotion of lipolysis in white adipose tissue (Inagaki et al., 2007; Badman et al., 2007), although this last effect is controversial (Arner et al., 2008). Moreover, in rodent models, FGF21 corrects metabolic disorders of obese and diabetic mice, inhibiting hepatic lipogenesis and glucose production (Berglund et al., 2009; Coskun et al., 2008; Xu et al., 2009). FGF21 has also been reported to promote glucose uptake by white adipocytes (Kharitonenkov et al., 2005).
PPARα has a major role in the control of FGF21 expression and release by the liver (Badman et al., 2007; Inagaki et al., 2007); accordingly, treatment with fibrates increases FGF21 expression (Gälman et al., 2008). Free fatty acids appear to be major regulators of FGF21 expression, acting via PPARα-dependent activation of the FGF21 gene (Mai et al., 2009). This effect of fatty acids may explain not only the induction of FGF21 gene expression after fasting but also the paradoxically high levels of FGF21 reported recently in patients with type II diabetes and obesity (Chavez et al., 2009; Zhang et al., 2008).
Metabolic adaptations have key roles in development. Major metabolic changes take place rapidly in the transition from fetal life, characterized by the predominant utilization of glucose as a metabolic fuel, to postnatal life, when the use of lipids from milk predominates. With birth comes a sudden need to sustain physical muscle activity and thermogenesis. The first appearance of these physiological processes in the neonate is associated with a marked increase in energy metabolic activity, and, for instance, postnatal brown fat nonshivering thermogenesis requires a high metabolic fuel oxidation. These metabolic adaptations are accomplished by the coordinate regulation of expression of genes encoding components of the metabolic fuel oxidation machinery. PPARα, in particular, promotes the concerted expression of genes encoding lipid oxidative pathway components that mediate fatty acid catabolism and ketogesis, which are required for neonates to cope with the sudden availability of large amounts of fatty acids from milk (Yubero et al., 2004). Alterations in metabolism that occur during the perinatal period have long-term consequences in adulthood and influence the risks for development of obesity and type II diabetes (Levin, 2006). Thus, identifying the molecular actors that control these processes is not only essential for understanding neonatal metabolic pathophysiology but can also be expected to provide key insights into permanent metabolic alterations in adults. An analysis of the developmental regulation of FGF21 and the potential involvement of FGF21 in neonatal metabolic adaptations are the focus of the present study.
RESULTS
FGF21 Expression Is Induced after Birth and Is Regulated by Diet Composition at Weaning
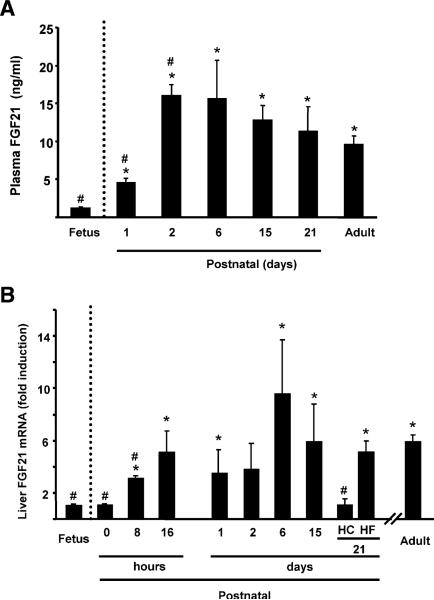
FGF21 levels in fetal plasma were very low (Figure 1A). After birth, plasma FGF21 levels increase rapidly, surpassing those seen in adult mice within 2 days of birth. Plasma FGF21 levels remained high in 6-day-old mice and declined thereafter. At 3 weeks, the time at which weaning has been completed, FGF21 levels were similar to those in adults.
Figure 1. Developmental Regulation of FGF21 Gene Expression: FGF21 Gene Expression in the Liver Is Induced and Plasma FGF21 Levels Are Increased at Birth.
Plasma FGF21 levels (A) and FGF21 mRNA levels in liver (B) at the indicated times during neonatal development. HC and HF denote mice spontaneously weaned onto a high-carbohydrate (HC) or high-fat (HF) diet between days 15 and 21. Bars are means ± SEM of three to six individual samples from separate litters. Statistically significant differences (p < 0.05) with respect to fetuses are denoted by *, and those with respect to adults by #.
In fetal liver and in newborn mice at birth (before initiation of suckling), FGF21 mRNA levels were very low (Figure 1B). FGF21 mRNA levels were induced soon thereafter, reaching levels similar to those in the adult liver within 16 hr. FGF21 mRNA levels during postnatal development were maximal in 6-day-old pups and were markedly reduced in the livers of 21-day-old mice compared with those from 15-day-old pups. Spontaneous weaning occurs in mice between neonatal days 15 and 21, when there is a progressive shift from a lipid-based diet (milk) to a mainly carbohydrate-based diet (regular chow). In mice provided access to a high-fat diet instead of the regular chow diet from day 15 onward, liver FGF21 mRNA levels were not reduced between day 15 and day 21 and were much higher than in 21-day-old mice weaned on the regular, high-carbohydrate diet.
Food Intake Is Required for the Induction of FGF21 Gene Expression at Birth, an Effect Mediated by Lipid Intake
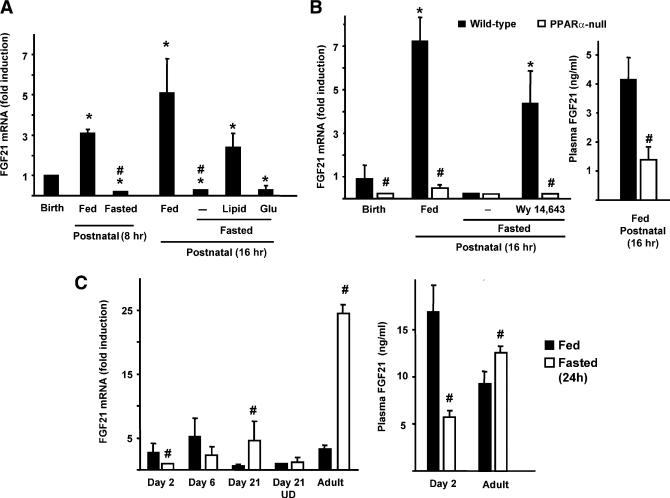
To investigate the mechanisms that elicit the increase in FGF21 gene expression at birth, we determined the effects of blunting the initiation of suckling. Figure 2A shows that postnatal feeding is an absolute requirement for FGF21 gene induction; pups that were not allowed to suckle showed no induction of FGF21 mRNA expression, indicating that milk intake induced the expression of the FGF21 gene. The components of food intake that stimulated FGF21 gene expression were assessed by feeding nonsuckling pups a lipid emulsion or a glucose solution by intragastric gavage. Lipid intake mimicked the action of milk on FGF21 expression, whereas glucose intake had no effect (Figure 2A). Injection of β-hydroxybutyrate to fasted pups did not modify significantly hepatic FGF21 mRNA expression (86% ± 18% respect to control levels).
Figure 2. Effects of Postnatal Starvation, Lipid or Glucose Intake, and PPARα Abrogation on the Neonatal Induction of FGF21 Gene Expression in the Liver.
(A) FGF21 mRNA levels in the livers of mice at birth (prior to suckling), of pups at 8 or 16 hr of life that were allowed to suckle (fed) or not allowed to suckle (fasted), and of fasted pups who received a lipid emulsion or glucose after birth.
(B) FGF21 levels in plasma and FGF21 mRNA levels in the livers of wild-type and PPARα null neonates. Pups were analyzed at birth, 16 hr after birth (fed and fasted), and after injection with Wy14,643 after birth.
(C) Effects of a 24 hr fast on FGF21 mRNA expression and plasma FGF21 levels in mice at distinct stages of development. UD, undernourished 21-day-old pups belonging to oversized litters (18 pups/litter). Bars are the means ± SEM of FGF21 mRNA levels, which are expressed as relative to the values in pups at birth (A and B, left) or relative to fasted 2-day-old pups (C, left). The bars in (B) (right) and (C) (right) are the means ± SEM of plasma FGF21 levels. Data are the means of four to six individual littermates obtained from four to six litters. Statistically significant differences (p < 0.05), with respect to values at birth, are denoted by * (A and B). Differences between fed and fasted mice are denoted by # (A and C), and those between PPARα null and wild-type littermates are denoted by # (B).
PPARα Is Required for FGF21 Gene Induction at Birth
To assess whether activation of PPARα could mediate the action of milk lipids on the FGF21 gene, we studied PPARα null pups (Figure 2B). Expression of FGF21 mRNA at birth in the liver of PPARα null mice was lower than in wild-type littermates. Moreover, postnatal induction of the FGF21 gene mediated by milk intake was completely suppressed in PPARα null pups. In wild-type pups starved after birth, injection of the PPARα activator Wy14,643 induced a significant increase in liver FGF21 mRNA levels, whereas there was no such response in PPARα null pups. The lack of PPARα caused a significant reduction in plasma FGF21 levels in neonates (Figure 2B).
FGF21 mRNA Levels in Liver and FGF21 Levels in Plasma Are Not Increased by Fasting during Early Stages of Development
Fasting caused a marked increase in FGF21 mRNA levels in the livers of 21-day-old pups and adult mice (Figure 2C). In contrast, FGF21 mRNA abundance decreased markedly in the livers of fasted 2-day-old pups and more modestly in fasted 6-day-old pups. When 21-day-old pups had been undernourished (oversized litters), white adipose tissue development was delayed (i.e., 62% ± 15% reduction in the weight of gonadal fat with respect to 21-day-old control pups, p < 0.05), and the induction of hepatic FGF21 mRNA expression by fasting was blunted. Plasma FGF21 levels were significantly increased in fasted adults, although to a much lesser extent than the increase in hepatic FGF21 mRNA levels. In contrast, fasting of 2-day-old pups results in the opposite response: a dramatic reduction in FGF21 plasma levels (Figure 2C, right).
Free Fatty Acid and β-Hydroxybutyrate Levels in the Plasma of Fetal, Newborn, and Adult Mice under Different Nutritional Regimens
A parallel assessment of free fatty acids levels in plasma was performed (see Table S1 available online). Fetal and newborn mice at birth showed very low levels of free fatty acids in plasma. There was a marked increase in circulating free fatty acids 16 hr after birth in suckling pups, but not in pups not allowed to suckle. When nonsuckling pups were administered a lipid emulsion, free fatty acid levels were increased to levels similar to those found in fed pups. There were no significant alterations in free fatty acid levels in PPARα null neonates. Once pups had begun suckling, starvation had differential effects on free fatty acids that depended on the stage of development. In 21-day-old and adult mice, fasting caused an increase in plasma free fatty acids but had no effect in 6-day-old mice, and it even caused a decrease in 2-day-old pups. However, fasting of undernourished 21-day-old pups did not increase plasma free fatty acids. In 21-day-old mice weaned on a high-fat diet, free fatty acid levels were greater than in mice weaned on the regular, high-carbohydrate diet.
Plasma β-hydroxybutyrate levels were very low in fetuses (Table S2) but increased suddenly after initiation of suckling. In contrast, plasma β-hydroxybutyrate levels were significantly lower in PPARα null neonates. Although fasting caused a marked induction of plasma b-hydroxybutyrate levels in wild-type adults, the induction was much more moderate in young (6-day-old) pups.
FGF21 Causes Thermogenic Activation of Brown Adipose Tissue in Neonatal Mice and Cultured Brown Adipocytes
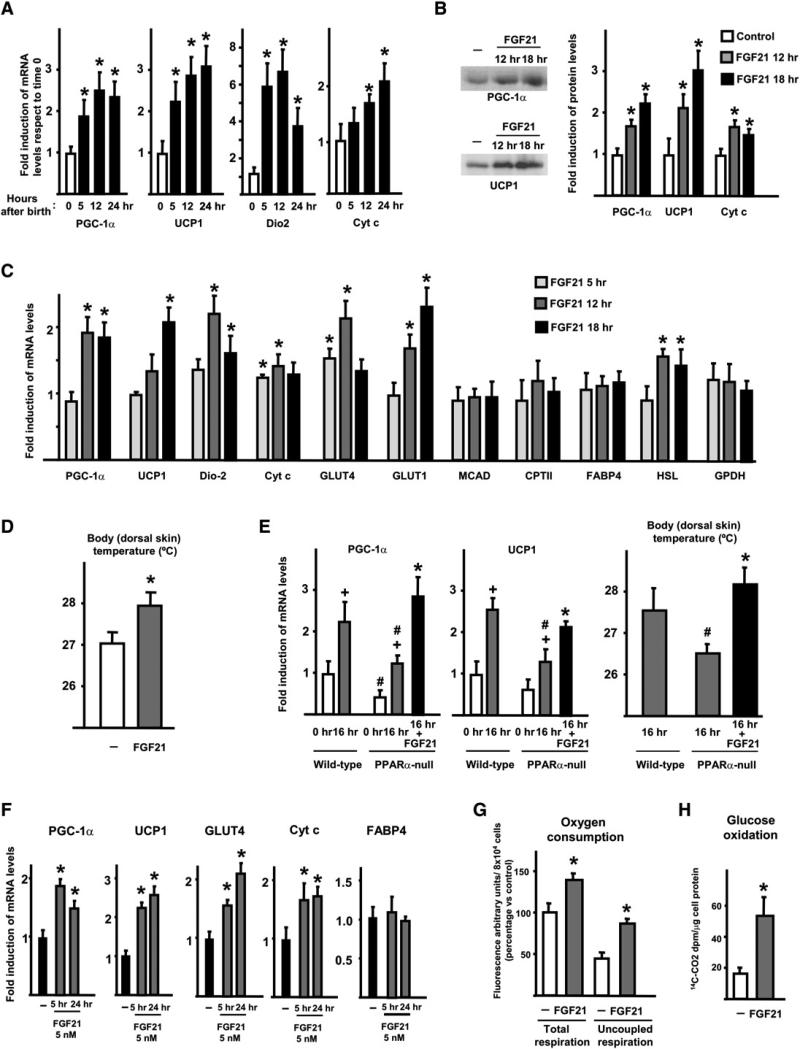
The expression of genes related to thermogenic activation is increased in neonatal brown adipose tissue (BAT) after birth (Figure 3A), in parallel with the rise in plasma levels of FGF21. To assess the potential effects of the postnatal burst in circulating FGF21 in response to the initiation of suckling, neonatal mice were treated 4 hr after birth with a single dose of recombinant mouse FGF21. The mice were not allowed to suckle further and were analyzed 5, 12, and 18 hr later. Treatment with FGF21 (12 hr) caused a mild, but statistically significant, reduction in blood glucose (25 ± 4.4 mM in FGF21-treated mice versus 39 ± 6 mM in controls, p < 0.05) and did not significantly modify the levels of free fatty acids (0.26 ± 0.05 mM in FGF21-treated mice versus 0.27 ± 0.03 mM in controls) or β-hydroxybutyrate (0.54 ± 0.14 mM in FGF21-treated versus 0.64 ± 0.06 mM in controls). FGF21 significantly induced the mRNA expression of genes encoding proteins involved in mitochondrial oxidation and BAT thermogenic activation (e.g., PGC-1α, UCP1, 5′-deiodinase-2 [Dio2], cytochrome c [Cyt c], and the hormone-sensitive lipase [HSL]), with specific time courses of induction for the distinct genes (Figure 3C). We also noted a significant increase in the expression of the mRNA for the glucose transporters GLUT1 and GLUT4. Other genes related to fatty acid oxidation (e.g., medium-chain acyl-CoA dehydrogenase [MCAD] and carnitine palmitoyltransferase-II [CPTII]) or overall adipogenic differentiation (e.g., fatty acid binding protein-4 [FABP4] and glycerol-3-phosphate dehydrogenase [GPDH]) were not altered. PGC-1α, UCP1, and Cyt c protein levels in BAT were significantly induced by FGF21 (Figure 3B). Body temperature of pups, recorded as skin temperature in the dorsal area, was significantly higher after FGF21 treatment (Figure 3D). Moreover, PPARα null pups exhibited a reduced postnatal induction of thermogenic genes after birth, which was rescued when PPARα null pups were treated with FGF21 just after birth (Figure 3E, left). Parallel results were obtained when body temperature was recorded in PPARα null pups treated with FGF21 (Figure 3E, right).
Figure 3. Gene Expression in Neonatal Mouse BAT, Effects of FGF21 Injection on Neonatal BAT, and Effects of FGF21 on Brown Adipocytes in Primary Culture.
(A) Transcript levels of thermogenic genes in BAT from pups at birth (0 hr) and at the indicated time after birth.
(B–D) Pups were injected 4 hr after birth with FGF21, and BAT was studied at the indicated times after injection. Representative immunoblots for PGC-1α and UCP1 are shown in (B), left. Levels of PGC-1α, UCP1, and Cyt c proteins are shown in (B), right. (D) Neonatal body temperature 12 hr after FGF21 injection.
(E) Transcript levels of PGC-1α and UCP1 (left) and body temperature (right) in PPARα null pups and wild-type littermates.
(F) Transcript levels of the indicated genes in differentiated brown adipocytes in culture treated with FGF21.
(G and H) Differentiated brown adipocytes in culture were treated with 5 nM FGF21 or saline for 24 hr; (G), total respiration and uncoupled respiration; (H), 14C-CO2 dpm levels after 3 hr incubation with 14C-glucose of control and FGF21-treated brown adipocytes. Bars are means ± SEM of five to seven pups or independent cell cultures. Statistically significant differences (p < 0.05) between controls and FGF21-treated conditions are denoted by *. In (E), statistically significant differences (p < 0.05) between PPARα null pups and wild-type littermates for the same experimental condition are shown as #, and those between 0 and 16 hr are shown as +.
In the liver, the mRNA expression of Cyt c and PGC-1α was significantly induced 12 hr after FGF21 injection (see Figure S1A). We noted no significant changes in the expression of phosphoenolpyruvate carboxykinase (PEPCK), which is indicative of gluconeogenesis; hydroxymethyl glutaryl-CoA synthase-2 (HMGCS), a key enzyme of ketogenesis; and MCAD, CPTII, and GLUT1, at any time of FGF21 treatment. Skeletal muscle and heart did not exhibit significant changes in the expression of any of the genes analyzed in response to FGF21 (see Figure S1B).
To assess whether FGF21 may act directly on BAT, mouse brown adipocytes were differentiated in culture and were then exposed to FGF21. Our results indicated that 5 nM FGF21 significantly induced the expression of the genes encoding proteins involved in thermogenic activation (PGC-1α, UCP1, GLUT4, Cyt c), whereas a higher concentration of FGF21 (50 nM) did not further enhance these effects (data not shown). The expression of FABP4 did not change. To determine directly the action of FGF21 on thermogenic function in brown adipocytes, total and uncoupled cell respiration was studied. FGF21 caused a 41% increase in brown adipocyte oxygen consumption. Moreover, the uncoupled (oligomycin-resistant) respiration was induced by FGF21 treatment (64% induction) (Figure 3G). FGF21 caused also a significant induction (around 3-fold) of glucose oxidation in brown adipocytes (Figure 3H).
DISCUSSION
The present findings provide the first characterization of the developmental regulation of FGF21 expression in mice, showing that circulating levels of FGF21 are markedly induced in the postnatal period, increasing from very low levels in the fetus to maximum levels just a few days after birth. A similar pattern was observed for hepatic FGF21 gene expression, consistent with a major role for the liver in the systemic production of FGF21. Previous studies have shown that physiological situations that lead to large changes in liver FGF21 gene expression, such as starvation or a ketogenic diet in adults, are usually associated with moderate changes in circulating FGF21 levels (Badman et al., 2007; and present results). The present results indicate that initiation of suckling not only profoundly modifies FGF21 gene expression in the liver but also results in a massive increase in plasma FGF21 levels.
The findings that initiation of food intake at birth is the trigger that activates FGF21 gene transcription and that FGF21 induction is blocked by postnatal food deprivation indicate that the response of neonates to starvation is opposite to that of adults, where FGF21 gene expression is activated by starvation. In neonates, high levels of free fatty acids appear following the initiation of suckling due to the high lipid content of milk. If suckling is prevented, blood free fatty acid levels remain low in neonates rather than increasing as they do in adults (Brun et al., 1999; and present data); this reflects the scarcity of white fat deposits at this stage of development that would otherwise lead to an increase in fatty acids through lipolysis of stored fat. Taken together with recent reports on a direct effect of fatty acids on FGF21 gene expression in adults (Mai et al., 2009), these findings support the notion that fatty acids are the key regulators of the FGF21 gene. This is also consistent with the fact that PPARα, which is known to be activated by fatty acid-related metabolites, was absolutely required for the induction of FGF21 gene expression in response to the initiation of suckling. Moreover, the shift in the response to starvation, from FGF21 gene repression to FGF21 gene activation, between postnatal days 2 and 21 is also consistent with this scenario. By day 21 after birth, substantial amounts of white adipose tissue have developed; at this point, fasting can therefore effectively increase circulating free fatty acid levels through the activation of lipolysis. When white fat development was delayed due to postnatal undernutrition, the induction of FGF21 gene expression was blunted. The maintenance of high levels of FGF21 gene expression in mice that are weaned onto a high-fat diet (fat content similar to milk) instead of onto a high-carbohydrate diet indicates that fatty acids from diet can also increase liver FGF21 gene expression beyond the immediate postnatal period. Collectively, these results indicate that hepatic FGF21 gene expression during development is under the control of fatty acids that reach the liver. This is true regardless of the origin of fatty acids, be it food or endogenous lipolysis in white fat. In this sense, the extent of white adipose tissue development appears to be a major determinant of the regulation of FGF21 gene expression in the liver.
The changes in FGF21 gene expression studied here not only parallel changes in plasma free fatty acids but also ketone body levels. For example, ketogenesis is activated spontaneously in newborns in response to milk intake, but not to starvation (Yubero et al., 2004). The data presented here support the proposition that FGF21 induction is associated with ketogenic states—in this case, that associated with initiation of lipid intake after birth.
The increase in FGF21 gene expression at birth suggests that FGF21 plays an important role in the metabolic adaptations that occur during the shift from fetal to neonatal metabolism. When we mimicked the postnatal burst in FGF21 levels that occur in fed pups by injecting FGF21 into fasted pups, we observed a coordinate induction of genes related to thermogenic activation and glucose utilization in BAT as well an increase in body temperature. Increased glucose utilization may contribute to the ability of FGF21 treatment to lower blood glucose levels in neonates. Although postnatal thermal stress is the main determinant of BAT thermogenic activation after birth, food intake is also required (R.I., unpublished data). Our present data strongly support the notion that FGF21 may be a food intake-dependent signal that favors neonatal BAT thermogenic activation. The impaired expression of thermogenic genes in BAT from pups lacking PPARα—and, therefore, exhibiting low plasma FGF21 levels—and the restoration of thermogenic gene expression and body temperature by exogenous FGF21 further support the role of the FGF21 released by liver in the control of neonatal BAT thermogenic activity.
Although we cannot exclude the possibility that FGF21 exerts indirect effects on thermogenic activation within BAT (i.e., by influencing sympathetic activation), our results in cultured brown adipocytes strongly support the notion that FGF21 acts directly on BAT. At birth, BAT expresses β-Klotho, an essential cofactor required for FGF21 action (Ito et al., 2000). The present findings raise questions as to whether FGF21 also acts as an activator of BAT thermogenesis in adults. Changes in the microscopy morphology of BAT in adult transgenic mice that overexpress FGF21 are consistent with enhanced activation (Kharitonenkov et al., 2005), and chronic treatment of mice with FGF21 increases the expression of UCP1 and Dio2 (Coskun et al., 2008) and glucose uptake (Xu et al., 2009) in BAT. However, in certain situations (e.g., when FGF21 levels increase during adult starvation), the positive effects of FGF21 on BAT thermogenic activation would hardly be compatible with the known reduction in thermogenic BAT activity. Research on these issues is currently underway.
Paracrine actions of the induction of FGF21 expression in postnatal liver may also take place. FGF21-induced PGC-1α gene expression in neonatal liver is consistent with recent data in FGF21-treated adult mice (Potthoff et al., 2009); however, gluconeogenesis does not appear to be enhanced in light of the observed effect of FGF21 reducing blood glucose in neonates and lack of effect on the PEPCK gene. With regards to ketogenesis, the injection of FGF21 did not modify either circulating ketone bodies or the expression of key genes of ketogenesis in neonates. The starved condition of FGF21-treated pups may not allow for the enhancement of ketogenesis, a process that is completely associated with milk consumption in the neonatal period (Yubero et al., 2004).
In summary, hepatic FGF21 gene expression and FGF21 levels in blood are developmentally regulated by nutritional determinants associated with fat availability in the liver, including the initiation of milk intake and a fat-enriched diet versus a carbohydrate-rich diet (Figure 4). The relationship between FGF21 expression, fatty acid availability, and ketogenesis during post-natal development, a physiological situation that is completely distinct from starvation in adults, suggests that FGF21 has a conserved functional role in the mediation of paracrine and endocrine metabolic adaptations to high fatty acid availability in the liver. Moreover, our data indicate that FGF21 specifically targets BAT thermogenic activation in neonates and indicate that FGF21 released by the liver may be a novel key signal contributing to neonatal activation of BAT thermogenesis in response to the initiation of milk intake.
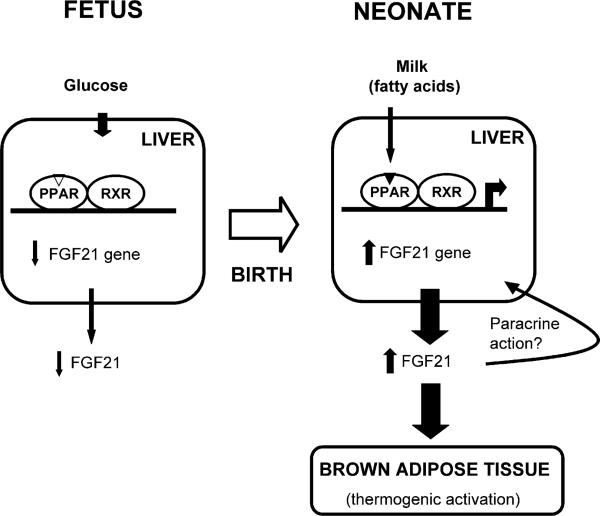
Figure 4. Schematic Representation of the Regulation of FGF21 Gene Expression and FGF21 Effects during the Transition from Fetal to Neonatal Metabolism.
Transcription of the FGF21 gene in the liver and release of FGF21 into the circulation are induced immediately after birth in response to the high fatty acid availability associated with the initiation of suckling. PPARα mediates the milk ingestion-dependent induction of FGF21 expression at birth. The burst in FGF21 levels after birth exerts endocrine actions that are relevant to metabolic adaptations in the neonatal period, mainly the induction of thermogenesis in BAT.
EXPERIMENTAL PROCEDURES
Mice were used in accordance with Directive 86/609/EEC. For studies in fetuses, cesarean sections were performed on Swiss mice on day 19 of gesta tion. Pups were studied starting at the time at which all pups had been born but had not yet started suckling (0 hr) and at the indicated times after birth. Adult mice were 3 months old. For studies on postnatal starvation, pups were separated from mothers prior to initiation of suckling, and placed in a humidified thermostatically controlled chamber (30° C). Pups were administered 100 μl of a triacylglycerol emulsion (Intralipid, Pharmacia) or 100 μl of a 0.3 g/ml glucose solution, by intragastric gavage. When indicated, pups were injected intraperitoneally 2 hr after birth with 50 μg Wy14,643 (Sigma)/g body weight, or β-hydroxybutyrate (0.1 mg/g body weight) and were studied 14 hr later. Heterozygote mice (129S4/SvJae-Pparatm1Gonz/J) were mated and experiments were performed on PPARα null pups and wild-type littermates. For studies on starvation at distinct stages of development, mice were fasted for 24 hr. The high-fat diet used was from Harlan Teklad (percent of total gross energy is as follows: 36% carbohydrate, 42% fat, 22% protein). For postnatal undernutrition, pups were adjusted to oversized litters (18 pups) from birth to day 21. To study the effects of FGF21, pups were intraperitoneally injected with saline (vehicle) or 2.5 μg mouse FGF21 (Phoenix Pharmaceuticals)/g of body weight 4 hr after birth. Pups were not allowed to suckle thereafter and were examined 5, 12, and 18 hr after the injection. A KM-1420 temperature recorder (Kane-May Measuring Instruments, UK) was attached to the dorsal skin area of pups for assessment of body temperature. Mice were killed by decapitation. Liver, interscapular BAT, leg skeletal muscle, and heart were dissected. Brown adipocytes were differentiated in primary culture as previously reported (Carmona et al., 2005). FGF21 (5 or 50 nM) was added to differentiated brown adipocytes (day 8 of culture), and cells were harvested 5 or 24 hr later. RNA was extracted (RNeasy, QIAGEN) and transcript levels were determined by quantitative RT-PCR using TaqMan Assay-on-demand probes (see the Supplemental Experimental Procedures) and the ABI/Prism 7700 Detector System (Applied Biosystems). The mean value of sample duplicates was normalized to that of the 18S rRNA gene using the comparative (2–ΔCT) method. Oxygen consumption of brown adipocytes was recorded using the Oxygen Biosensor System (BD) (see the Supplemental Experimental Procedures) in the absence or presence (uncoupled respiration) of 10 μg/ml oligomycin. Glucose oxidation was determined by the assessment of 14CO2 production from 14C-glucose added to cell culture medium 24 hr after 5 nM FGF21 treatment (see the Supplemental Experimental Procedures). FGF21 levels in plasma were determined by ELISA (Phoenix Secretomics). Immunoblot of PGC-1α and UCP1 in BAT extracts was performed as reported (Carmona et al., 2005; Yubero et al., 2004), whereas Cyt c was determined by ELISA (MitoSciences). Blood glucose levels were determined using Accutrend (Roche), and plasma nonesterified fatty acids and β-hydroxybutyrate using spectrophotometric methods (Wako). Statistical comparisons were performed using Student's t test.
ACKNOWLEDGMENTS
This work was supported by grants from MCIN (SAF2008-01896) and Instituto de Salud Carlos III (PI081715), Spain.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes two tables, one figure, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at doi:10.1016/j.cmet.2010.02.001.
REFERENCES
- Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. FGF21 attenuates lipolysis in human adipocytes—a possible link to improved insulin sensitivity. FEBS Lett. 2008;582:1725–1730. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun S, Carmona MC, Mampel T, Viñas O, Giralt M, Iglesias R, Villarroya F. Activators of peroxisome proliferator-activated receptor-α induce the expression of the uncoupling protein-3 gene in skeletal muscle: a potential mechanism for the lipid intake-dependent activation of uncoupling protein-3 gene expression at birth. Diabetes. 1999;48:1217–1222. doi: 10.2337/diabetes.48.6.1217. [DOI] [PubMed] [Google Scholar]
- Carmona MC, Hondares E, Rodríguez de la Concepción ML, Rodríguez-Sureda V, Peinado-Onsurbe J, Poli V, Iglesias R, Villarroya F, Giralt M. Defective thermoregulation, impaired lipid metabolism, but preserved adrenergic induction of gene expression in brown fat of mice lacking C/EBPbeta. Biochem. J. 2005;389:47–56. doi: 10.1042/BJ20050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 (FGF-21) is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32:1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI. Molecular cloning and expression analyses of mouse β-klotho, which encodes a novel Klotho family protein. Mech. Dev. 2000;98:115–119. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1107–1121. doi: 10.1098/rstb.2006.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai K, Andres J, Biedasek K, Weicht J, Bobbert T, Sabath M, Meinus S, Reinecke F, Möhlig M, Weickert MO, et al. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes. 2009;58:1532–1538. doi: 10.2337/db08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman ML. FGF21: a missing link in the biology of fasting. Cell Metab. 2007;5:405–407. doi: 10.1016/j.cmet.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yubero P, Hondares E, Carmona MC, Rossell M, Gonzalez FJ, Iglesias R, Giralt M, Villarroya F. The developmental regulation of peroxisome proliferator-activated receptor-gamma coactivator-1α expression in the liver is partially dissociated from the control of gluconeogenesis and lipid catabolism. Endocrinology. 2004;145:4268–4277. doi: 10.1210/en.2004-0099. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]