Abstract
Methyl CpG binding protein-2 (MeCP2) is an essential epigenetic regulator in human brain development. Rett syndrome, the primary disorder caused by mutations in the X-linked MECP2 gene, is characterized by a period of cognitive decline and development of hand stereotypies and seizures following an apparently normal early infancy. In addition, MECP2 mutations and duplications are observed in a spectrum of neurodevelopmental disorders, including severe neonatal encephalopathy, X-linked mental retardation, and autism, implicating MeCP2 as an essential regulator of postnatal brain development. In this review, we compare the mutation types and inheritance patterns of the human disorders associated with MECP2. In addition, we summarize the current understanding of MeCP2 as a central epigenetic regulator of activity-dependent synaptic maturation. As MeCP2 occupies a central role in the pathogenesis of multiple neurodevelopmental disorders, continued investigation into MeCP2 function and regulatory pathways may show promise for developing broad-spectrum therapies.
Keywords: Rett syndrome, Angelman’s syndrome, Autism, Epigenetic, Neurodevelopmental
Introduction
The MECP2 gene encodes methyl CpG binding protein-2 (MeCP2), a transcriptional regulator required for proper neuronal development. Mutations in MECP2 are the major cause of the neurodevelopmental disorder Rett syndrome (RTT), an X-linked disorder that primarily affects females. MECP2 mutations and dysfunctions have also been associated with a broad array of other neurodevelopmental disorders in males and females, including X-linked mental retardation (XLMR), severe neonatal encephalopathy, Angelman’s syndrome (AS), and autism, demonstrating that disruption of MeCP2 can have wide-ranging effects on neurodevelopment and lead to a spectrum of disorders [1]. However, the extent of MECP2 mutations within these individual disorders is variable. Although MECP2 mutations can account for up to 96% of classic RTT [2], they are rare in other disorders (eg, autism), indicating that MECP2 is just one of many genes that could contribute to the broader spectrum of neurodevelopmental phenotypes. Additionally, a clear correlation does not always exist between specific MECP2 mutations and individual disorders or phenotypic severity, suggesting that genetic, epigenetic, and/or environmental factors interact with the MECP2 mutation to determine the final phenotypic outcome in terms of type and severity of the disorder.
MECP2 mutations can be grouped into three general categories: severe loss-of-function mutations, mild loss-of-function mutations, and a broad group of duplications and other noncoding mutations that affect MeCP2 expression levels. Each mutation type is associated with a subset of disorders (Fig. 1). The involvement of MeCP2 in multiple neurodevelopmental disorders demonstrates that it occupies a central role in the postnatal development of the human brain. Understanding the role that MeCP2 plays in brain development and maturation is important for understanding the pathogenesis of these disorders and identifying potential therapeutic targets. To achieve this will require a thorough understanding of the molecular mechanisms through which MeCP2 acts, as well as how MeCP2 interacts with signaling and epigenetic pathways in the developing brain.
Fig. 1.
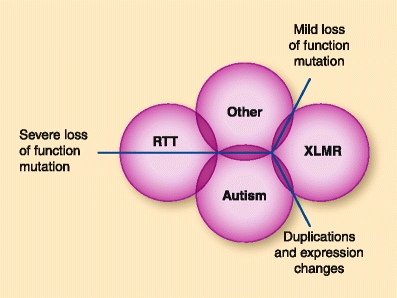
Association of neurodevelopmental disorders with specific classes of Methyl CpG binding protein-2 (MeCP2) mutation. The schematic diagram depicts the major MeCP2-based neurodevelopmental disorders and their association with each class of MeCP2 mutation. RTT—Rett syndrome; XLMR—X-linked mental retardation
MECP2 Inheritance and X Inactivation
MECP2 is located on the X chromosome. As such, it is subjected to an X-linked inheritance pattern. Females inherit two X chromosomes, one maternal and one paternal, whereas males inherit their single X chromosome maternally. Therefore, whereas females can inherit MECP2 mutations from either parent, male inheritance of MECP2 mutations is exclusively maternal. This has dramatic effects on the transmittance of MECP2 mutations as well as the stratification of MECP2-associated disorders by gender (Table 1). The MECP2 gene is also subject to X-chromosome inactivation (XCI) in females. XCI is the process by which one of the two X chromosomes carried by females is inactivated to achieve gene expression patterns similar to those found in males, who carry only one copy of the X chromosome. XCI occurs on a cell-by-cell basis and is a predominantly random process. However, extreme degrees of skewing can occur with preferential inactivation of the maternal or paternal X chromosome. Therefore, the phenotypic results of MeCP2 mutations in females are likely not only based on the type of mutation but also on the degree of inactivation between the mutant and wild-type alleles. With a single X chromosome, males are more severely affected by MECP2 mutations than females, who along with the mutant gene on one X chromosome carry a normal copy of MECP2 on the other.
Table 1.
Neurodevelopmental disorders associated with MeCP2 mutations
| Studies | Disorder | Mutation | Gender | Parent of origin |
|---|---|---|---|---|
| Amir et al. [5] | Rett syndrome | Severe loss of function | Female | Paternal |
| Schanen et al. [8], Villard et al. [9] | Severe neonatal encephalopathy | Severe loss of function | Male | Maternal |
| Carney et al. [21] | Autism | Severe loss of Function | Female | Paternal (?) |
| Couvert et al. [18] | X-linked mental retardation | Mild loss of function | Male | Maternal |
| Lugtenberg et al. [19•] | X-linked mental retardation | Duplication | Male | Maternal |
| Loat et al. [22], Nagarajan et al. [23], Shibayama et al. [24], Ramocki et al. [25••] | Autism | Duplication/noncoding variants | Male | Maternal |
| Adegbola et al. [27], Cohen et al. [28], Watson et al. [29] | Other | Severe/mild | Male/female | Either (?) |
MeCP2 methyl CpG binding protein-2
MECP2 mutations are mostly sporadic, occurring preferentially as C>T transitions of CpG dinucleotides and preferentially on the paternal X chromosome [3, 4]. It has been postulated that the low number of males with MECP2 mutations is the result of a lethal in utero effect of having no functional MECP2 from their single X chromosome. However, the predominantly paternal origin of MeCP2 mutations likely prevents them from being transmitted to male offspring in the first place.
MeCP2 and Neurodevelopmental Disorders
Rett Syndrome
MeCP2 dysfunction is associated with several neurodevelopmental disorders. The primary neurodevelopmental disorder associated with MECP2 mutations is RTT, which affects about 1 in 15,000 females [5]. Classic RTT is characterized by apparently normal early development for the first 6 to 18 months of life followed by a period of developmental regression and loss of acquired skills that results in intellectual disability (ID), loss of speech and purposeful hand movements, ataxia, seizures, and respiratory abnormalities. There are several recognized variants of RTT in addition to the classic form, including the congenital, early-onset seizure, and preserved speech variants, which have similar yet distinct clinical phenotypes from classic RTT. About 96% of classic RTT cases are associated with MECP2 mutations [2], whereas other forms are largely associated with other genetic mutations, such as CDKL5 in the early-onset seizure variant [6] and FOXG1 mutations in the congenital variant [7•]. Thus, whereas MECP2 mutations are clearly responsible for most classic RTT, they are not the only genetic lesion that can yield RTT phenotypes.
Significant phenotypic variability exists in classic RTT, even among individuals with the same MECP2 mutation. Skewed XCI has been used to explain the mild phenotype associated with rare maternal carriers of MECP2 mutations [8, 9]. In addition, three different Mecp2-deficient murine models showed variably skewed XCI favorably inactivating the mutant allele and correlating with phenotypic severity [10, 11]. However, most girls with classic RTT show random X chromosome inactivation patterns [12]. Furthermore, recent studies have questioned the exclusive ability of XCI status to explain discordance between individuals with identical MECP2 mutations but different severity, suggesting the influence of genetic modifiers [13•, 14•].
Severe Neonatal Encephalopathy
The classic RTT phenotype in males is exceedingly rare and associated with a 47,XXY karyotype or somatic mosaicism for an MECP2 mutation [15, 16]. Both of these situations recapitulate the female scenario of XCI and allow for expression of some wild-type MECP2. In karyotypically normal boys, RTT-associated MECP2 mutations present as severe neonatal encephalopathy, a disorder characterized by a static encephalopathy, severe developmental delays, hypotonia, seizures, and respiratory abnormalities that often leads to death at an early age [8, 9]. Most of these individuals inherited the MECP2 mutation from mildly or unaffected mothers with favorable XCI skewing [8, 9]. Because XCI of the mutant MECP2 allele is random in most females, favorably skewed maternal carriers of RTT-associated mutations are rare. Therefore, the prevalence of severe neonatal encephalopathy caused by MECP2 mutations is also thought to be rare. However, most boys with this disorder have been identified because they have female siblings with RTT. It is possible that more cases exist outside of known RTT families that have yet to be identified.
X-linked Mental Retardation
XLMR is a source of inherited ID affecting 1 in 600 to 1 in 1000 males, as well as a smaller—but still significant—number of females [17]. XLMR is a genetically diverse disorder arising from mutation or duplication of genes across the X chromosome, including MECP2. In early studies, MECP2 point mutations were identified in up to 2% of individuals with XLMR [18]. In males, these mutations result in severe ID, speech impairment, and motor abnormalities—including progressive spasticity—whereas females display mild ID or appear unaffected. To date, XLMR-associated MECP2 mutations have not been identified in RTT or severe neonatal encephalopathy; also, RTT mutations have not been found in cases of XLMR. Duplications in Xq28, a region of the X chromosome that includes MECP2, also have been implicated in 1% to 2% of XLMR cases [19•]. Analysis of these duplications in multiple XLMR families has identified a region containing MECP2 and its neighbor IRAK1 as the minimal genomic region associated with XLMR and Xq28 duplications [19•]. MECP2 duplication syndrome is characterized by severe ID, lack of speech, progressive spasticity, and susceptibility to respiratory infections in males and mildly affected or unaffected females. The molecular situation arising from MECP2 duplication is very different from that caused by MECP2 mutations in XLMR. Whereas mutations are thought to cause neurodevelopmental disorders through reduced levels of functional MeCP2, MeCP2 duplications actually result in elevated MeCP2 levels [20]. That both under- and overexpression of functional MeCP2 can lead to neurodevelopmental disorders serves to highlight the requirement for correct regulation of MeCP2 expression for proper neuronal development.
Female carriers of XLMR MECP2 mutations generally show balanced XCI, signifying that XLMR mutations are less severe compared with those that result in RTT. Alternatively, MECP2 duplication carrier females display extreme XCI skewing (85%–100%) favoring inactivation of the duplicated allele [20]. This suggests that the effects of MECP2 duplication are more severe than those of the XLMR mutations and that the mild phenotype in these females is due to preferential inactivation of the duplicated allele. Both the relatively mild nature of XLMR mutations in females and the preferential XCI skewing of duplications allow for maternal transmission of defective MECP2 alleles to male and female offspring [19•, 20], a situation that is not possible with the more severe RTT mutations. Because of this, MECP2 mutations associated with XLMR are much more common in males than those associated with RTT.
Autism
Autism and RTT have significant phenotypic overlap, and both are classified as pervasive developmental disorders. Although autism and RTT have a strong genetic component, the genetic basis of autism is unclear and likely involves multiple genes. Mutations in MECP2 identical to some classic RTT mutations have been identified in a small number of autistic females who do not meet the diagnostic criteria for RTT [21]. In addition to discrete MECP2 mutations, linkage analysis has identified the over- and undertransmittance of specific MECP2 variants in families with autistic children, suggesting a link between MECP2 and increased susceptibility to autism [22]. Reduced expression of MeCP2 protein frequently occurs in the frontal cortex of autistic individuals and is correlated with increased MECP2 promoter methylation [23]. Decreased MeCP2 expression may also be caused by variations in 3΄ regulatory elements of MECP2, which are found more commonly in autistic individuals than in controls [24]. Finally, a recent study found a high comorbidity of autism in males with MECP2 duplications inherited from maternal carriers with neuropsychiatric symptoms [25••]. Therefore, as with XLMR, over- and underexpression of MeCP2 can lead to an autistic phenotype in some individuals.
Autism is relatively rare in girls, making the association of MECP2 mutations in girls with an autistic phenotype particularly interesting. Because of the phenotypic overlap between RTT and autism, as well as the phenotypic variability between individuals in both disorders, it is not clear if an MECP2 mutation with an autism diagnosis is a different disorder than RTT, or if the two are simply different representations on a spectrum associated with MECP2 mutations. It was reported that an initial diagnosis of autism in girls with MECP2 mutations was not maintained as they grew older, and it was suggested that the autistic phenotype may actually represent an RTT variant [26]. Regardless of whether another autistic phenotype associated with MECP2 mutations is truly autism, it is likely that the phenotypic differences from RTT or XLMR arise from differences in genetic background and environmental exposures in each affected individual.
Other Disorders
MECP2 mutations also have been reported in other neurological cases. A father and daughter were identified with a mild neurobehavioral phenotype including cognitive and motor difficulties. Both carry a proline-to-alanine point mutation at amino acid 152 in MeCP2, and the daughter displays balanced XCI, suggesting that this mutation has a mild effect on MeCP2 function [27]. It is interesting to note that a different mutation at the same site (proline to arginine) is commonly found in classic RTT. The arginine mutation has a much more dramatic effect on the biochemical properties of MeCP2 than does the alanine mutation, which is likely related to the differences in phenotypic severity between the two mutations [27]. Another report described a boy with early-onset schizophrenia and developmental receptive language disorder with a mutation in MECP2 [28]. This same mutation is found in XLMR but presents with a vastly different phenotype in this case. Cases of AS with MECP2 mutations also have been reported [29]. AS is a neurodevelopmental disorder with some phenotypic similarities to RTT but with an earlier onset characterized by hypotonicity at birth. AS is primarily caused by deletions, mutations, or imprinting errors of the UBE3A gene on chromosome 15, but 15% of AS cases have no identified genetic abnormality at this site. Several different MECP2 mutations, most of which are also found in RTT, have been found in boys and girls diagnosed with AS. Some individuals transitioned to a more RTT-like phenotype with age, whereas others maintained an AS-like phenotype. The appearance of MECP2 mutations usually associated with other disorders in these cases is another demonstration of variable expressivity. In these cases, rare genetic variants and/or environmental insults may interact with the MECP2 mutations to produce the uncommon phenotypes described previously. Therefore, not only is there extensive variability of phenotypes associated with single mutations within specific disorders, but the capacity also exists for specific mutations to give rise to distinct disorders in different individuals.
MeCP2 Function
MeCP2 is a member of a family of proteins that specifically bind to methylated DNA sequences in the genome. DNA methylation is a covalent modification to CpG dinucleotides and an epigenetic mark used by cells to alter expression of genes without permanently changing the DNA sequence. In this way, DNA methylation represents a mitotically heritable yet reversible modification to the genome. By binding to methylated DNA, MeCP2 can read these epigenetic marks in the genome and translate them into functional effects of modified gene expression.
Historically, DNA methylation has been thought to be a repressive mark, primarily when found at CpG-rich gene promoters. Therefore, MeCP2 was predicted to be a global repressor of transcription of genes by binding to methylated gene promoters and recruiting transcriptional repressors to silence gene expression [30]. However, gene promoters are vastly de-enriched for DNA methylation, and global analyses of MeCP2-dependent expression have not yielded many clear target genes with densely methylated promoters that require MeCP2 for silencing, suggesting that the function of MeCP2 is broader than the proximal repression of methylated gene promoters. Recently, new models of MeCP2 function have been proposed in which MeCP2 acts as a transcriptional modulator capable of regulating increases and decreases in expression of transcriptionally active genes by long-range regulation of chromatin structure from distal methylation sites outside of gene promoters [31, 32•]. An additional study suggested an activating role for MeCP2 by its interaction with the transcription factor CREB at the promoters of active genes [33•].
Many gene targets of MeCP2 undergo rapid changes in expression following activity-dependent neuronal activation. In the case of the MeCP2 target gene BDNF, this is achieved through activity-dependent phosphorylation of MeCP2 [34]. By acting as an activity-dependent regulator of gene expression, MeCP2 is positioned to integrate signals from neuronal activation and epigenetic marks to control the expression of its target genes. It is likely that the heterogeneous nature of activity-dependent activation and epigenetic status of individual neurons or neuronal networks in the maturing brain have led to the inability to fully delineate the entire spectrum of MeCP2 target genes.
MeCP2 is ubiquitously expressed in human tissues, but particularly high protein levels are found in the brain [35]. Within the brain, neurons contain the highest levels of MeCP2, but expression in these cells is heterogeneous and based primarily on the maturational state of the individual neurons. The level of MeCP2 in neurons is initially low and increases over the course of postnatal neuronal development, reaching a maximum in a subpopulation of mature postmitotic neurons [36]. This pattern of expression suggests that MeCP2 is involved in the maturation of existing neurons rather than the development of new neurons from precursor cells. Imaging studies have shown that girls with MECP2 mutations and RTT have an overall reduction in brain volume compared with controls [37], but there have been no reports of major neuropathological abnormalities in girls with RTT. Instead, MECP2 mutations seem to result in subtle changes in neuronal morphology. Neuroanatomic studies demonstrate that specific populations of neurons from girls with RTT are smaller and have reduced dendritic complexity than those found in control samples, adding support to the idea that MeCP2 is involved in neuronal maturation as opposed to formation. Although MeCP2 expression is highest in neurons, glial cells have detectable levels of MeCP2 expression as well. Recent reports have suggested that MeCP2 expression in astrocytes may be critical for RTT pathogenesis by disrupting support for neuronal dendritic maturation [38•, 39•].
Murine models have been developed by targeted disruption of Mecp2 that recapitulates many of the neurodevelopmental phenotypes seen in humans [40–42]. Deficiency of MeCP2 specifically in postmitotic neurons is responsible for the neurodevelopmental deficits in these mice [40, 43]. Combined with the lack of major anatomic defects in neurons lacking functional MeCP2, this suggests that a lack of MeCP2 does not result in irreversible damage to neurons. Instead, MeCP2 mutations may cause a premature arrest in neuronal development with relative sparing of neuronal commitment. Accordingly, reintroduction of functional MeCP2, even in symptomatic mice, can rescue the developmental phenotypes associated with MeCP2 deficiency [44•, 45••]. However, it is important to note that overexpression of MeCP2 by even twofold can cause related severe neurological defects [43, 46], indicating that levels of MeCP2 are tightly regulated and that too little as well as too much functional MeCP2 can cause neurodevelopmental disorders. The sensitivity of neurons to aberrant levels of MeCP2 means that attempts to rescue MeCP2 dysfunction in humans through reintroduction of a functional MECP2 transgene will be challenging and require a deeper understanding of MeCP2’s regulation and mechanism(s) of action.
Molecular Mechanisms of Disease
Although much progress has been made in understanding the functions of MeCP2 in recent years, it is still unclear exactly how MECP2 mutations contribute to the pathogenesis of RTT and related syndromes. The major anatomic differences in brains of both girls with RTT and Mecp2-deficient mice are reduced dendritic arborization and dendritic spine formation, both of which are indicative of immature neurons and would be predicted to represent a deficit in synaptic formation and/or transmission. In support of this hypothesis, it has been shown that brain samples from Mecp2-deficient mice display reduced spontaneous synaptic transmission as well as reduced synaptic plasticity—molecular processes that underlie synaptic formation, learning, and memory [47]. Reduction of spontaneous transmission and synaptic plasticity in these mice is a result of reduced synaptic connectivity and a weakening of the remaining neuronal connections caused by loss of Mecp2 [48•]. Synaptic deficits also have been reported in transgenic mice with twofold Mecp2 overexpression [46], again demonstrating the strict requirements for regulation of MeCP2 levels in neuronal maturation. Synapse formation and maturation requires activity-dependent gene expression in neurons. Activation of an immature neuron causes the induction of a large number of genes that help strengthen and mature the developing synapse. MeCP2 controls the expression of several of these genes, including BDNF, ID1, EGR2, and JUNB. Activity–dependent phosphorylation of MeCP2 is required for the activity-dependent expression of BDNF [34] and proper dendritic branching and dendritic spine formation in neurons. Therefore, a loss of MeCP2-regulated, activity-dependent gene expression may lead to the widespread deficiencies in synaptic maturation observed in the brains of Mecp2-null mice and girls with RTT. This in turn is thought to be the underlying cause of RTT and other MeCP2-associated neurodevelopmental disorders. The late-infancy onset of RTT symptoms corresponds to the occurrence of elevated MeCP2 levels in maturing neurons and fits with the predicted role for MeCP2 in activity-dependent postnatal maturation of neuronal synapses.
A large number of MECP2 mutations have been reported, including point mutations that change single amino acids in MeCP2, truncations that cause premature termination that results in shortened MeCP2 protein, and small deletions that are missing stretches of amino acids from the interior of MeCP2. Mutations can be found throughout the protein but tend to cluster in or include regions that are important for MeCP2 functions. Through mutation of critical amino acids or deletion of important functional domains, MECP2 mutations result in a loss of MeCP2 function. Mutant proteins show a reduced affinity for methylated DNA and a reduced ability to regulate gene expression [49]. Because of this, MeCP2 mutants cannot properly regulate activity-dependent gene expression in response to neuronal stimulation. Alternatively, MECP2 duplication or other mutations in noncoding regions of the gene can lead to changes in MeCP2 expression levels and do not change the intrinsic functions of the protein. Instead, abnormal levels of MeCP2 will result in misregulation of MeCP2 target genes. Both situations lead to improper modulation of MeCP2-regulated pathways and result in deficient neuronal maturation.
MECP2 mutations are involved in many different disorders with substantial phenotypic overlap (Fig. 1). In some instances, there is a clear segregation of specific MECP2 mutations and particular disorders. MECP2 mutations found in RTT are rarely seen in XLMR and vice versa. This difference is due to the effects of different types of mutations on MeCP2 function. Mutations found in XLMR have a relatively mild effect on the biochemical functions of MeCP2 when compared with those found in RTT [50]. This is likely to result in different patterns of neuronal maturation and dysfunction, which will lead to different phenotypic outcomes. However, a clear segregation between mutation type and symptoms is not always apparent. Identical MECP2 mutations can be found in individuals with many different diagnoses, ranging from classic RTT to autism to AS. Even within RTT, a large phenotypic variability exists between individuals with the same MECP2 mutation. Some of this variability can be attributed to differences in mutation type and XCI, but these alone cannot explain all the variability seen in RTT. Much of this variability across disorders and within RTT is likely due to genetic modifiers—that is, variants of genes that are a part of MeCP2-regulated pathways. On their own, variations in these genes may not lead to visible phenotypes, but they can affect the phenotypic outcome of MECP2 mutations. It has been reported that a common polymorphism in the BDNF gene found in healthy and affected individuals correlates with increased severity and susceptibility to seizures in RTT [51•]. Additional genetic modifiers likely have yet to be identified, many of which may have no apparent effect on their own but when combined with mutations in MECP2 can lead to different neurodevelopmental phenotypes. Therefore, it is likely a complex interaction between mutation type, XCI skewing (in the case of females), and other genetic modifiers that leads to the final phenotype in MECP2 mutations.
Conclusions
As is apparent by the spectrum and severity of associated disorders, MeCP2 seems to be a critical regulator of neuronal activity–dependent synaptic maturation. To achieve this, MeCP2 integrates epigenetic marks and signals from neuronal activation to regulate the gene expression patterns required for neuronal maturation through long-range and local regulation of chromatin structure. As such, MeCP2 occupies a central role in the postnatal development of the human brain. However, MeCP2 does not work in isolation. It is influenced by and acts upon countless other genetic, epigenetic, and environmental factors, all of which work in concert to insure proper development of the human brain. Although each of these MECP2-associated disorders is distinct, they share significant phenotypic overlap, including core deficits in cognition and motor function. A more complete understanding of the molecular mechanisms surrounding MeCP2, as well as the pathways in which it resides will not only enhance our understanding of neuronal maturation but will help us to understand the pathogenesis of multiple neurodevelopmental diseases. The relative sparing of neuronal commitment in MECP2-related disorders provides hope that potential therapies to restore MECP2 function or reactivate MeCP2-dependent pathways may provide significant benefit to individuals suffering from these disorders.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Michael L. Gonzales, Email: migonzales@ucdavis.edu
Janine M. LaSalle, Email: jmlasalle@ucdavis.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Hammer S, Dorrani N, Dragich J, et al. The phenotypic consequences of MECP2 mutations extend beyond Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:94–98. doi: 10.1002/mrdd.10023. [DOI] [PubMed] [Google Scholar]
- 2.Zoghbi HY. MeCP2 dysfunction in humans and mice. J Child Neurol. 2005;20:736–740. doi: 10.1177/08830738050200090701. [DOI] [PubMed] [Google Scholar]
- 3.Girard M, Couvert P, Carrie A, et al. Parental origin of de novo MECP2 mutations in Rett syndrome. Eur J Hum Genet. 2001;9:231–236. doi: 10.1038/sj.ejhg.5200618. [DOI] [PubMed] [Google Scholar]
- 4.Trappe R, Laccone F, Cobilanschi J, et al. MECP2 mutations in sporadic cases of Rett syndrome are almost exclusively of paternal origin. Am J Hum Genet. 2001;68:1093–1101. doi: 10.1086/320109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amir RE, Van den Veyver IB, Wan M, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl- CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 6.Lin C, Franco B, Rosner MR. CDKL5/Stk9 kinase inactivation is associated with neuronal developmental disorders. Hum Mol Genet. 2005;14:3775–3786. doi: 10.1093/hmg/ddi391. [DOI] [PubMed] [Google Scholar]
- 7.Ariani F, Hayek G, Rondinella D, et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schanen NC, Kurczynski TW, Brunelle D, et al. Neonatal encephalopathy in two boys in families with recurrent Rett syndrome. J Child Neurol. 1998;13:229–231. doi: 10.1177/088307389801300507. [DOI] [PubMed] [Google Scholar]
- 9.Villard L, Kpebe A, Cardoso C, et al. Two affected boys in a Rett syndrome family: clinical and molecular findings. Neurology. 2000;55:1188–1193. doi: 10.1212/wnl.55.8.1188. [DOI] [PubMed] [Google Scholar]
- 10.Braunschweig D, Simcox T, Samaco RC, LaSalle JM. X-chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2−/+ mouse brain. Hum Mol Genet. 2004;13:1275–1286. doi: 10.1093/hmg/ddh142. [DOI] [PubMed] [Google Scholar]
- 11.Young JI, Zoghbi HY. X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of Rett syndrome. Am J Hum Genet. 2004;74:511–520. doi: 10.1086/382228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahbazian MD, Sun Y, Zoghbi HY. Balanced X chromosome inactivation patterns in the Rett syndrome brain. Am J Med Genet. 2002;111:164–168. doi: 10.1002/ajmg.10557. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S, Ohinata J, Makita Y, et al. Skewed X chromosome inactivation failed to explain the normal phenotype of a carrier female with MECP2 mutation resulting in Rett syndrome. Clin Genet. 2008;73:257–261. doi: 10.1111/j.1399-0004.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 14.Bao X, Jiang S, Song F, et al. X chromosome inactivation in Rett syndrome and its correlations with MECP2 mutations and phenotype. J Child Neurol. 2008;23:22–25. doi: 10.1177/0883073807307077. [DOI] [PubMed] [Google Scholar]
- 15.Leonard H, Silberstein J, Falk R, et al. Occurrence of Rett syndrome in boys. J Child Neurol. 2001;16:333–338. doi: 10.1177/088307380101600505. [DOI] [PubMed] [Google Scholar]
- 16.Topcu M, Akyerli C, Sayi A, et al. Somatic mosaicism for a MECP2 mutation associated with classic Rett syndrome in a boy. Eur J Hum Genet. 2002;10:77–81. doi: 10.1038/sj.ejhg.5200745. [DOI] [PubMed] [Google Scholar]
- 17.Gecz J, Shoubridge C, Corbett M. The genetic landscape of intellectual disability arising from chromosome X. Trends Genet. 2009;25:308–316. doi: 10.1016/j.tig.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Couvert P, Bienvenu T, Aquaviva C, et al. MECP2 is highly mutated in X-linked mental retardation. Hum Mol Genet. 2001;10:941–946. doi: 10.1093/hmg/10.9.941. [DOI] [PubMed] [Google Scholar]
- 19.Lugtenberg D, Kleefstra T, Oudakker AR, et al. Structural variation in Xq28: MECP2 duplications in 1% of patients with unexplained XLMR and in 2% of male patients with severe encephalopathy. Eur J Hum Genet. 2009;17:444–453. doi: 10.1038/ejhg.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Esch H, Bauters M, Ignatius J, et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet. 2005;77:442–453. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carney RM, Wolpert CM, Ravan SA, et al. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr Neurol. 2003;28:205–211. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 22.Loat CS, Curran S, Lewis CM, et al. Methyl-CpG-binding protein 2 polymorphisms and vulnerability to autism. Genes Brain Behav. 2008;7:754–760. doi: 10.1111/j.1601-183X.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagarajan RP, Hogart AR, Gwye Y, et al. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:e1–e11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibayama A, Cook EH, Jr, Feng J, et al. MECP2 structural and 3′-UTR variants in schizophrenia, autism and other psychiatric diseases: a possible association with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;128B:50–53. doi: 10.1002/ajmg.b.30016. [DOI] [PubMed] [Google Scholar]
- 25.Ramocki M, Sarika UP, Tavyev YJ, et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MECP2duplication syndrome. Ann Neurology. 2009;66:771–782. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zappella M, Meloni I, Longo I, et al. Study of MECP2 gene in Rett syndrome variants and autistic girls. Am J Med Genet B Neuropsychiatr Genet. 2003;119B:102–107. doi: 10.1002/ajmg.b.10070. [DOI] [PubMed] [Google Scholar]
- 27.Adegbola AA, Gonzales ML, Chess A, et al. A novel hypomorphic MECP2 point mutation is associated with a neuropsychiatric phenotype. Hum Genet. 2009;124:615–623. doi: 10.1007/s00439-008-0585-6. [DOI] [PubMed] [Google Scholar]
- 28.Cohen D, Lazar G, Couvert P, et al. MECP2 mutation in a boy with language disorder and schizophrenia. Am J Psychiatry. 2002;159:148–149. doi: 10.1176/appi.ajp.159.1.148-a. [DOI] [PubMed] [Google Scholar]
- 29.Watson P, Black G, Ramsden S, et al. Angelman syndrome phenotype associated with mutations in MECP2, a gene encoding a methyl CpG binding protein. J Med Genet. 2001;38:224–228. doi: 10.1136/jmg.38.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones PL, Veenstra GJ, Wade PA, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 31.Horike S, Cai S, Miyano M, et al. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat Genet. 2005;37:31–40. doi: 10.1038/ng1570. [DOI] [PubMed] [Google Scholar]
- 32.Yasui DH, Peddada S, Bieda MC, et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci U S A. 2007;104:19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chahrour M, Jung SY, Shaw C, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z, Hong EJ, Cohen S, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent BDNF transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet. 2002;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- 36.Balmer D, Goldstine J, Rao YM, LaSalle JM. Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J Mol Med. 2003;81:61–68. doi: 10.1007/s00109-002-0396-5. [DOI] [PubMed] [Google Scholar]
- 37.Carter JC, Lanham DC, Pham D, et al. Selective cerebral volume reduction in Rett syndrome: a multiple-approach MR imaging study. AJNR Am J Neuroradiol. 2008;29:436–441. doi: 10.3174/ajnr.A0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maezawa I, Swanberg S, Harvey D, et al. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 41.Guy J, Hendrich B, Holmes M, et al. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 42.Shahbazian M, Young J, Yuva-Paylor L, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/S0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 43.Luikenhuis S, Giacometti E, Beard CF, Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci U S A. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci U S A. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guy J, Gan J, Selfridge J, et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins AL, Levenson JM, Vilaythong AP, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- 47.Moretti P, Levenson JM, Battaglia F, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudo S, Nomura Y, Segawa M, et al. Heterogeneity in residual function of MeCP2 carrying missense mutations in the methyl CpG binding domain. J Med Genet. 2003;40:487–493. doi: 10.1136/jmg.40.7.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kudo S, Nomura Y, Segawa M, et al. Functional characterisation of MeCP2 mutations found in male patients with X linked mental retardation. J Med Genet. 2002;39:132–136. doi: 10.1136/jmg.39.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeev BB, Bebbington A, Ho G, et al. The common BDNF polymorphism may be a modifier of disease severity in Rett syndrome. Neurology. 2009;72:1242–1247. doi: 10.1212/01.wnl.0000345664.72220.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]


