Short abstract
An inhibitor of microRNA-122 reduces viral load in chimpanzees that are chronically infected with hepatitis C virus, suggesting that such an approach might have therapeutic potential in humans.
Abstract
An inhibitor of microRNA-122 reduces viral load in chimpanzees that are chronically infected with hepatitis C virus, suggesting that such an approach might have therapeutic potential in humans.
Hepatitis C virus and microRNAs
MicroRNAs (miRNAs) are gaining an increasingly prominent role as regulators of numerous cellular processes, including virus-host interactions. They are short (21-23 nucleotide) non-coding regulatory RNAs that influence gene expression at a post-transcriptional level [1]. miRNAs are encoded as part of long nuclear transcripts, which are processed in the nucleus by Drosha, then exported to the cytoplasm and further processed by Dicer. The resulting mature miRNA strand is loaded into the RNA-induced silencing complex (RISC), which acts as the effector of miRNA activity [1]. In animals, target specificity is usually determined by a 6-8mer 'seed' at the 5' end of the miRNA. Typically, miRNAs bind sites in the 3' untranslated regions (UTRs) of mRNAs that have perfect complementarity to the seed but imperfect complementarity to the remainder of the miRNA. The precise mechanism of miRNA-mediated repression is not fully defined; both translational repression and degradation of miRNA-RISC-bound mRNAs have been observed in different studies [1].
Several viruses interact with the miRNA pathway. Certain viruses produce their own miRNAs, which regulate viral or cellular targets, whereas some viruses are regulated directly or indirectly by cellular miRNAs [2]. One important virus that has a requirement for a specific miRNA is hepatitis C virus (HCV). HCV infects the liver and is a major global health concern, with an estimated 170 million people infected worldwide [3]. In the majority of cases acute infection with HCV progresses to chronic infection, although infection can be cleared spontaneously in a minority of cases. Chronically infected individuals may then develop cirrhosis of the liver and may ultimately progress to hepatocellular carcinoma. HCV is predominantly spread through direct blood contact, although there is some evidence to suggest a possible (minor) route of sexual transmission [3]. A report recently published in Science [4] shows that inhibiting a specific miRNA in chimpanzees chronically infected with HCV reduces viral load.
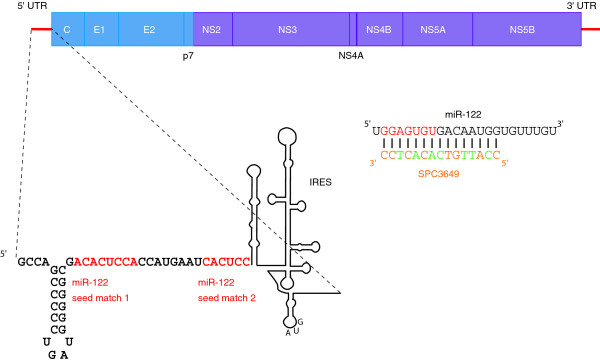
HCV has a single-stranded positive-sense RNA genome that encodes a single polyprotein that is processed to ten viral proteins (Figure 1). The single open reading frame is flanked by two structured UTRs that are required for replication [5]. The 5' UTR of HCV contains an internal ribosome entry site (IRES) that drives translation of the open reading frame [5]. Within the first 45 nucleotides of the 5' UTR are two seed matches for miR-122 (Figure 1), a highly expressed liver-specific miRNA accounting for about 70% of the total liver miRNA population (about 66,000 copies per cell) [6]. These sites bind to miR-122 and are conserved across all six HCV genotypes. This interaction is required for viral replication in cultured cells [7-9]. The mechanism by which miR-122 regulates HCV remains uncertain, with reports of enhancement at the level of either translation or replication [7,10]. It is possible that there is a complex regulatory mechanism that affects both processes.
Figure 1.
miR-122 targeting HCV. The HCV RNA genome is shown with coding regions as rectangles and the 5' and 3' UTRs as lines. Structural genes are in blue and non-structural genes in purple. The two seed matches bound by miR-122 are highlighted in red in an expanded view of the 5' UTR. The sequence of miR-122 is shown in black, with the seed (nucleotides 2-8) in red. The SPC3649 molecule that targets it is shown with LNA indicated in orange (C in orange indicates LNA methylcytosine) and DNA in green. The backbone is phosphorothioate.
It is possible to perturb miRNA activity by using complementary oligonucleotides directed against specific miRNAs. Following introduction into cells, the oligonucleotide is bound by the appropriate miRNA in complex with RISC. This prevents the miRNA from interacting with its targets. Various chemical modifications improve binding affinity and stability of these inhibitors. miR-122 has been targeted effectively in mice using 2'-O-methylated or 2'-O-methoxyethylated antisense oligonucleotides [11,12]. Researchers at Santaris Pharma took a similar approach to silence miR-122 in mice, using antisense oligomers containing locked nucleic acid (LNA), a bicyclic nucleic acid analog that provides superior target specificity and stability and low toxicity [13]. This strategy was extended to target miR-122 in primates using a molecule with an optimized combination of LNA and DNA bases and a phosphorothioate backbone (SPC3649; Figure 1) [14]. Effective, long-lasting knockdown of miR-122 levels was observed, coupled with derepression of endogenous targets and absence of significant associated toxicity [14].
miR-122 knockdown reduces HCV load in infected chimpanzees
The conserved and essential nature of the miR-122-HCV interaction, and the effective non-toxic in vivo suppression of miR-122 in primates by SPC3649, offers an exciting strategy to target HCV. In a new study [4], Lanford et al. have begun to assess the therapeutic potential of SPC3649 in chimpanzees chronically infected with HCV.
Four chimpanzees chronically infected with genotype 1 HCV isolates were used in this study. Two were treated with a high-dose regime (5 mg/kg SPC3649) and the remaining two were given a low-dose regime (1 mg/kg SPC3649). Baseline samples were taken for 4 weeks before treatment with SPC3649, and the two samples taken immediately before treatment were accompanied by administration of an intravenous saline placebo. SPC3649 was administered by weekly intravenous injection for 12 weeks followed by a 17-week treatment-free follow-up period [4].
This study [4] demonstrates that SPC3649 has a strong potential as a therapeutic agent. Treatment with the drug led to the de-repression of endogenous target mRNAs, in keeping with previous studies. Furthermore, SPC3649 therapy resulted in a reduction of viral load by up to 2.6 orders of magnitude for HCV genome equivalents in serum and up to 2.3 orders of magnitude in tissue in high-dose animals. One of the low-dose animals showed a similar but reduced response, whereas the other did not respond. HCV RNA fluctuated in the non-responding animal, and endogenous miR-122 targets were also unaffected, suggesting that miR-122 was not effectively inhibited [4].
Importantly, no escape mutants were detected by deep sequencing of HCV genome samples, implying that the interaction of miR-122 with HCV genomes is critical in vivo and suggesting that resistance to SPC3649 therapy might not be generated by mutation in miR-122 binding sites. Rebound of viral load did not occur during therapy, and took at least 15 weeks to return to pretreatment levels after withdrawal of the drug. Encouragingly, the half life in vivo of SPC3649 is in the order of 20 days, presenting the possibility of longer periods between administrations without sacrificing effectiveness once miRNA suppression is achieved. An improvement in liver histology also occurred in response to SPC3649 therapy, suggesting that damage induced by HCV infection might be reparable [4].
Implications for human HCV therapies
The results of this study are very exciting. Previous work demonstrating a role for miR-122 in the HCV life cycle was carried out in cell culture, so the discovery that this miRNA has similar effects in infected animals is highly significant. The good safety profile and stability of SPC3649 give it considerable promise for human therapy.
Clinical trials of SPC3649 in human patients will be very important, as results obtained in chimpanzees will not necessarily extrapolate to humans, and trials across larger populations may reveal different responses. The chimpanzee is a very useful model for HCV infection, but there are significant differences between the pathogenesis of viral infection in chimpanzees and humans [15]. Chimpanzees experience milder symptoms than humans, and cirrhosis has not been detected in infected animals. Chronically infected chimpanzees show no reduction in viral load in response to interferon therapy, and may therefore be more valid as a model for human non-responders [15]. The reduction in viral load following treatment with SPC3649 was accompanied by normalization of the endogenous interferon pathway, which is maximally induced in chronically infected chimpanzees [4]. SPC3649 might thus be able to convert human non-responders to responders and to allow effective interferon therapy.
Analysis of liver biopsies from HCV-infected humans showed no positive correlation between hepatic miR-122 expression and viral load [16]. Patients who were unresponsive to interferon therapy had significantly lower miR-122 levels prior to treatment than responders [16]. However, miR-122 expression is very high in the liver, so even reduced levels could be sufficient to support HCV replication. Interestingly, chimpanzees receiving a low dose of the drug in the Lanford et al. study [4] did not respond as well as high-dose animals, despite miR-122 being undetectable by Northern blot, lending support to the hypothesis that low levels of miR-122 can support viral replication [4]. It is also possible that the subpopulation of hepatocytes infected with HCV may show a different correlation between miR-122 and HCV levels to that observed in the liver as a whole.
The most encouraging aspects of this study [4] are the lack of liver toxicity in treated animals and the observation that escape mutations in the miR-122 binding sites did not emerge over the course of therapy. This is in contrast to the rapid acquisition of adaptive mutations in response to drugs that target viral proteins, and emphasizes the benefits of targeting a host factor. There are potential problems in targeting an endogenous miRNA as expression of endogenous targets will change; however, the overall effect of de-repression of miR-122 targets was a beneficial change in cholesterol levels. Many different measures of liver toxicity were examined over the course of the study without any apparent therapy-induced toxicity [4]. However, problems might arise over longer treatment courses, or some time after treatment. Follow-up of the treated chimpanzees will be important.
The current therapy for HCV uses interferon-α, covalently attached to a polyethylene glycol molecule to improve pharmacokinetics and stability, in combination with ribavirin, a guanosine analog. The mechanisms by which these drugs act are not well understood, and direct inhibition of HCV replication and modulation of the immune response may both be involved. Although this treatment is a great improvement on interferon monotherapy, it is ineffective in many cases, highly toxic, and poorly tolerated [17]. An effective alternative with few side effects is therefore highly desirable. Several clinical trials are underway to test compounds directed against viral or cellular targets. The results obtained with two HCV protease inhibitors in combination with existing therapy are especially promising and are now in phase III trials [18]. However, resistance to these new drugs has been detected, and the inclusion of interferon means that poor tolerance remains a problem [18]. An interferon-free treatment regime may require a combined small-molecule approach similar to that used in HIV treatment, combining protease inhibitors with other emerging anti-HCV drugs, such as polymerase inhibitors. If the anti-miR-122 drug proves to be effective and safe in humans, it could form part of such a therapy. An enhanced reduction in HCV replication in cell culture when miR-122 sequestration was accompanied by treatment with lovastatin, an inhibitor of isoprenoid biosynthesis, supports the possibility that SPC3649 could be effective in a combined therapy [19].
This research is likely to pave the way for future miRNA-based therapeutics. Altered expression of specific miRNAs is associated with many human diseases, particularly cancers. miR-122 is relatively easy to target because antisense oligonucleotides can be delivered to the liver by intravenous injection. miRNAs in other organs may be more difficult to target and thus require specialized delivery methods. For some miRNAs it may be necessary to improve delivery of antisense oligonucleotides by methods such as conjugation to cell penetrating peptides [20]. There is also potential for plasmid or viral-based delivery of inhibitors using miRNA 'sponges', in which multiple targets for the miRNA of interest compete with the endogenous target [21]. Overexpression of miRNAs in whole animals could also be achievable using techniques under development for RNA interference.
In conclusion, the results of this study show considerable promise for the development of an effective, well-tolerated therapy against HCV.
Acknowledgements
We thank Martin Bushell for critical reading of the manuscript. Research in the authors' laboratory is funded by a BBSRC David Phillips Fellowship to CLJ.
References
- Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach JL, Cullen BR. The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes Dev. 2009;23:1151–1164. doi: 10.1101/gad.1793309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson BJ. Hepatitis C virus: the growing challenge. Br Med Bull. 2009;89:153–167. doi: 10.1093/bmb/ldp003. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R, Zaret KS, Taylor JM. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Jopling CL, Schutz S, Sarnow P. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe. 2008;4:77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, Chien M, Weir DB, Russo JJ, Ju J, Brownstein MJ, Sheridan R, Sander C, Zavolan M, Tuschl T, Rice CM. Cellular cofactors affecting hepatitis C virus infection and replication. Proc Natl Acad Sci USA. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JI, Goergen D, Zheng J, Song Y, Schuttler CG, Fehr C, Junemann C, Niepmann M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Elmen J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjarn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- Boonstra A, Laan LJ van der, Vanwolleghem T, Janssen HL. Experimental models for hepatitis C viral infection. Hepatology. 2009;50:1646–1655. doi: 10.1002/hep.23138. [DOI] [PubMed] [Google Scholar]
- Sarasin-Filipowicz M, Krol J, Markiewicz I, Heim MH, Filipowicz W. Decreased levels of microRNA miR-122 in individuals with hepatitis C responding poorly to interferon therapy. Nat Med. 2009;15:31–33. doi: 10.1038/nm.1902. [DOI] [PubMed] [Google Scholar]
- Pawlotsky J-M. Mechanisms of antiviral treatment efficacy and failure in chronic hepatitis C. Antiviral Res. 2003;59:1–11. doi: 10.1016/S0166-3542(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Nelson DR. Hepatitis C drug development at a crossroads. Hepatology. 2009;50:997–999. doi: 10.1002/hep.23208. [DOI] [PubMed] [Google Scholar]
- Norman KL, Sarnow P. Modulation of hepatitis C virus RNA abundance and the isoprenoid biosynthesis pathway by microRNA miR-122 involves distinct mechanisms. J Virol. pp. 666–670. [DOI] [PMC free article] [PubMed]
- Fabani MM, Gait MJ. miR-122 targeting with LNA/2'-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. Rna. 2008;14:336–346. doi: 10.1261/rna.844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]