Abstract
Cytokine-induced killer (CIK) cells are polyclonal T effector cells generated when cultured under cytokine stimulation. CIK cells exhibit potent, non-MHC-restricted cytolytic activities against susceptible tumor cells of both autologous and allogeneic origins. Over the past 20 years, CIK cells have evolved from experimental observations into early clinical studies with encouraging preliminary efficacy towards susceptible autologous and allogeneic tumor cells in both therapeutic and adjuvant settings. This paper is our attempt to summarize the available published literature related to CIK cells. Looking into the future, we anticipate that the continuous therapeutic application of CIK cells will likely be developed along two major directions: overcoming the challenge to organize large prospective randomized clinical trials to define the roles of CIK cells in cancer immunotherapy and expanding its spectrum of cytotoxicity towards resistant tumor cells through experimental manipulations.
1. Background
One of the first prototypes of cytokine-induced immuneeffector cells is the Lymphokine-Activated Killer (LAK) cells. Firstly described in the early 1980s, LAK cells are cytotoxic effector lymphocytes whose cytolytic activities are not restricted by major histocompatibility complex (MHC) and have the ability to kill fresh tumor cells and NK-resistant tumor cell lines [1]. LAK cells are generated following expansion in the presence of IL-2 for a relatively short culture period of approximately 5 days. After culture, the heterogeneous LAK cell population consists of CD3−Leu19+ NK cells, CD3+Leu19+ cells, and CD3+Leu19− T cells. Leu19 was subsequently redesignated as CD56 and these CD3+CD56+ cells are also termed non-MHC-restricted cytotoxic T cells. The two cell subsets the CD3+Leu19+ T cells and CD3−Leu19+NK cells contribute to the cytolytic property of LAK cells [2]. Over the years, various improvements in the methods to culture LAK cells have been developed. These included the addition of OKT3 at the initiation of culture, prolongation of culture duration, and the addition of various different types of cytokines at the end of culture. These improved methodologies to culture LAK cells resulted in better expansion over the originally described method [3]. LAK cells demonstrated potent in vitro cytotoxicity against susceptible tumor cells and led to the regression of established tumors in animal models [4, 5]. In clinical studies, LAK cells had demonstrated modest efficacy against metastatic cancer such as renal cell carcinoma and melanoma [6]. In a randomized controlled trial in the 1990s, adoptive immunotherapy using ex vivo activated T cells showed clinical efficacy in terms of prolongation of relapse-free survival for patients with hepatocellular carcinoma following resection of the primary tumor [7].
The discovery, generation, and therapeutic use of immune-active host effector cells that can kill cancer cells are continuously being developed. The pioneering work that accelerated the field of cellular immunotherapy with CIK cells was performed in Stanford. The authors described CIK cells as non-MHC-restricted T cells with marked ability to proliferate and demonstrated superiority over LAK cell in cytolytic activities against B cell lymphoma [8]. Furthermore, CIK cells exhibit potent in vivo cytolytic activities without the need for coadministration of IL-2. CIK cells are generated by the timed addition of IFN-γ 1000 u/ml on day 1 of culture, followed 24 hours later by the addition of anti-CD3 at 50 ng/ml and IL-2 at 300 IU/ml. Together with the periodic addition of IL-2, the culture medium is regularly replenished throughout the culture period of 21–28 days [8]. At the end of the culture, the CD3+CD56+ cells, derived from CD3+CD56− cells, could expand by up to 1000-fold and gave the greatest cytotoxicity against various tumor cell targets, including K562 and B cell lymphoma cell lines, as compared to CD3+CD56− cells [9]. The expression of CD56 on these non-MHC-restricted effector T cells was found to be the result of IFN-γ-priming that induced the production of IL-12 by monocytes and the upregulation of CD58 (LFA-3), both of which were demonstrated to be crucial for the expansion of CD56+ T cells [10]. Subsequently, this unique subset of non-MHC-restricted CD3+CD56+ T cells was referred to as NK-like T cells since, similar to the NK cells, they do not require prior specific sensitization to induce the recognition of target cells.
2. Functional, Phenotypic, and Genotypic Characterization of CIK Cells
Following bulk culture in vitro, the effector cell population obtained is heterogeneous and comprises of a small fraction (~2%) of CD3−CD56+ NK cells and >90% of CD3+ cells of which ~35% expresses CD56 while the remaining cells are CD3+CD56− [11]. The sorted NK cell fraction (CD3−CD56+) behaves like classical NK cells and the killing of autologous acute myeloid leukemia (AML) target cells mediated by these cells can be enhanced by blocking the HLA class I molecules on the target cells [12]. The CD3+CD56+ subset, termed non-MHC-restricted T cells, is able to kill both autologous and allogeneic susceptible tumor targets such as primary AML cells of disparate HLA types [11]. However, we have earlier shown that this subset of cells recognizes target cells through the T cell receptor (TCR) and requires the presence of MHC molecules on the target cells, a phenomenon similar to that observed for classical cytotoxic T lymphocytes. Furthermore, blockade of either TCR on the effector cells or the MHC class I molecules on the target cells abrogates the killing of target cells [12]. This discrepant observation of being not restricted by the MHC specificity in its cytolytic activities but with the effector function dependent on the presence of MHC is inconsistent with the current T cell paradigm, which dictates that cytotoxic effector T lymphocytes recognize target peptides in the context of self-MHC molecules. Besides our report, similar observations had also been independently made by others [13–15]. A hypothetical working explanation is that T cells in an “activated” state induced by cytokines could express elevated levels of adhesion molecules which result in the recognition of target cells more readily and efficiently, overriding the requirement for stringent matching of the MHC molecules. While these T cells are labeled as “NK-like”, we demonstrated that the well-characterized conventional NK receptors, including the KIR, NKG2C/E, NKG2D, and DNAM-1, are not involved in the recognition and killing of AML targets [12]. The exact molecules responsible for the recognition of AML targets await further molecular characterization. While CIK cells have been shown to kill the myeloma cell line U266 via NKG2D-mediated recognition of its cognate ligands MICA and MICB on the target cells [16], the same mechanism is unlikely to be operative for AML targets as these target cells do not express MICA and MICB [17].
Investigation into the possible explanations for the better cytolytic activities against tumor cells demonstrated for the CD3+CD56+ cells over its CD3+CD56− counterpart revealed that the CD3+CD56+ cells consist of a higher proportion of CD8+ cells compared to the CD3+CD56− cell subset. Furthermore, the CD3+CD56+ subset is a more terminally differentiated late effector T cell population bearing the CD27+CD28− or CD27−CD28− phenotypes. In contrast, the CD3+CD56− cells are early effector T cells expressing mainly the CD27+CD28+ and CD62L+ phenotypes. The granzyme content is also higher in the CD3+CD56+ T cells, consistent with the report that late effector T cells possess more potent cytotoxicity than early effector T cells [12]. Table 1 summarizes the characteristics of the three subsets of cells in the bulk CIK culture.
Table 1.
Comparison of the three subsets of cells in a CIK culture. NA = not applicable, ND = not done.
| Subset | Name | Cytotoxicity | Target class I | Immunophenotype | |||||
|---|---|---|---|---|---|---|---|---|---|
| versus | versus | versus | Blocked | Enhanced | %CD8 | Memory T cell subset | Granzyme | ||
| AML | ALL | K562 | content | ||||||
| CD3−D56+ | NK cells | + | 0 | ++++ | Killing ↑ | Killing ↓ | NA | NA | ND |
| CD3+CD56+ | NK-like T cells | +++ | 0 | 0 | Killing ↓ | Killing ↑ | higher | CD27+/−CD28−CD62L+ late T effector | higher |
| CD3+CD56− | T cells | + | 0 | 0 | Killing ↓ | Killing ↑ | lower | CD27+CD28+CD62L+early T effector | lower |
Our research focus is on the cellular immunotherapy of hematological malignancies and we have observed that CIK cells kill AML blasts efficiently but not acute lymphoblastic leukemia (ALL) blasts [11]. The resistance of ALL blasts to killing by immune effector cells has also been well reported by others [18, 19]. We therefore also studied the possible molecular event that might explain this discrepancy. We observed that the CD3+CD56+ fraction of CIK cells cultured from acute leukemic marrow at diagnosis expressed high levels of immune function genes including IFN-γ, TNFα, CXCR3, CCR1, granzyme B, CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), IL-7R, IL-12Rβ2, and caspase-1, consistent with a Th1 and Tc1 polarization [20]. Upon stimulation by the corresponding leukemic targets, that is, CIK cells derived from AML patients coincubating with autologous or allogeneic AML blasts and similarly, CIK cells derived from ALL patients coincubating with autologous or allogeneic ALL blasts, immune response-related genes like IFN-γ, GM-CSF, IL-4, IL-8, cytokine receptor genes IL-2Rβ, IL-2Rα, IL-4R, IL-12Rβ, IL-15Rα, chemokine and chemokine receptor genes, and genes belonging to the TNF and TNF receptor superfamily were upregulated. Many proapoptotic genes were downregulated whilst antiapoptotic genes were upregulated. One observation of great interest was that genes encoding the NK receptors NKG2C, NKG2E, and CD94 were exclusively upregulated in CIK cells stimulated with myeloid blasts but not lymphoid blasts. In contrast, TGFβ-1 gene transcript was upregulated in CIK stimulated with lymphoid blasts. The differential regulation of these immune-related genes corroborated with our observed findings of good CIK killing against myeloid but poor killing against lymphoid blasts [20]. However we did not detect surface expression of NKG2C and NKG2E molecules on the CIK cells both before and after stimulation with myeloid target cells [12]. Therefore, the molecular and cellular significance of the upregulation of these immune-related genes needs to be further investigated.
3. In Vitro Antitumor Activity of CIK Cells
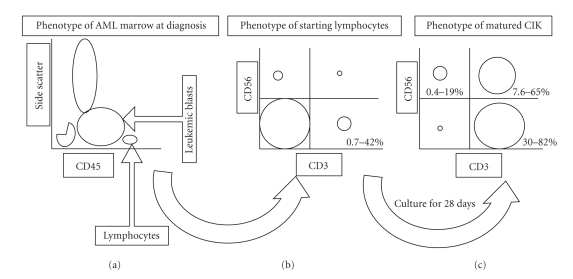
Over the years, CIK cells have been tested for its antitumor activity against a variety of tumor targets. It was first shown to be able to kill t(14;18)-positive lymphoma cell lines but not against normal human hemopoietic precursors [8]. Doxorubicin- and vinblastine-resistant tumor cell lines expressing high level of Pgp (P-glycoprotein) were also susceptible to CIK-mediated lysis [21]. Furthermore, chronic myeloid leukemia (CML) colony growth was reported to be suppressed by CIK cells and after 28 days of coincubation, the remaining colonies in culture were exclusively composed of Philadelphia- (Ph-) negative cells [22, 23]. Previously, we have shown that CIK cells could be generated from the marrow or peripheral blood samples from acute leukemia patients collected at diagnosis. Figure 1 shows the change in the composition of cells cultured under CIK condition from a starting cell population consisting of a majority of leukemic blasts with typically less than 10% T cells, to a majority of T cells at maturity. These cells were lytic against both autologous and allogeneic AML targets [11]. Additionally, CIK cells could also be generated from untreated chronic lymphocytic leukemia (CLL) patients, where cytotoxicity against autologous CLL targets could be induced by addition of anti-CD3 MoAb [24, 25].
Figure 1.
Marrow cells obtained from newly diagnosed AML samples analyzed by side scatter versus CD45 staining show a large proportion of leukemic blasts that are CD45dim and a much smaller fraction of normal lymphocytes that are CD45bright (a). Culture of the bulk marrow cells comprising of majority of leukemic blasts with a small fraction of lymphocytes under CIK condition (b) is able to generate an end product comprising of a majority of CD3+ cells with a variable CD3+CD56+ fraction (c).
Immunological manipulations aimed to potentiate the antitumor activity of CIK cells have been explored. Hence, dendritic cells (DC) were cultured and engineered to present tumor antigens to CIK cells hoping that this might enhance the recognition of tumor cells and its subsequent killing. This was shown to be feasible for multiple myeloma (MM) when the DC was pulsed with target-derived idiotype before coculture with the CIK cells [26]. CIK-resistant pancreatic carcinoma target cells also became susceptible to CIK cells that were cocultured with DC loaded with tumor-restricted RNA and the CA19-9 peptide [26, 27]. Furthermore, transfection of IL-2 genes into CIK cells to enhance its IL-2 production potentiated its cytotoxicity against a pancreatic cancer cell line following coculture with DC when compared to non-IL-2-transfected CIK cells [28].
4. Mice Studies
Earlier work had shown the antitumor activity of CIK cells in mice. Human B cell lymphoma cell lines harboring the t(14;18) chromosomal translocation were injected into SCID mice to evaluate the efficacy of CIK cells. When allogeneic CIK cells were injected 1 day after inoculation of tumor cells, the SCID mice receiving the CIK cells had prolonged survival compared to control mice and LAK cell-treated mice, with long-term survival of 30% and no molecular evidence of lymphoma [9]. In SCID mice engrafted with human CML, autologous CIK cells transfer 4 weeks after inoculation of tumor cells resulted in eradication of bcr-abl which remained detectable in control mice [22]. This study showed that CIK cells, which are Ph chromosome-negative, could be expanded from patients with CML and had potent in vitro and in vivo efficacy against autologous tumor cells. In the same study, an additional series of SCID mice was not engrafted with CML but they instead unexpectedly developed large human Epstein-Barr virus-associated lymphomas. Within this series, the CIK cells-treated group developed no or small tumor as compared to large tumor developed in the untreated group [22]. These experimental data served as convincing early evidence of in vivo efficacy of CIK cells.
With the availability of the bioluminescence imaging (BLI) technology [29], the in vivo functional activities of CIK cells could be visualized in a real-time fashion in mice inoculated with bioluminescent gene-transfected tumor cells and treated with CIK cells for cellular immunotherapy. To monitor tumor regression by BLI, mice were implanted intraperitoneal with HeLa-luc (a luciferase gene-transfected human cervical carcinoma cell line) and subsequently treated with CIK cells. Mice treated with CIK cells had significant tumor regression or complete eradication compared to saline-treated mice [30]. Similar tumor response was visualized for murine lymphoma cell lines [31]. Using the same strategy, CIK cells were transfected with the gfp/luc genes to visualize its trafficking by BLI [31]. Following injection, it was observed that luc+ CIK cells first reached the lungs within 30 minutes followed by a general distribution to other sites of the body. By the 7th hour, a population of the labeled CIK cells migrated to the tumor sites and remained detectable at these sites for an additional 9 days with resultant tumor regression [31]. Importantly, this antitumor effect of CIK cells occurred without the need for exogenous IL-2, a clinically relevant observation.
5. CIK Cells Across MHC Barrier
Donor lymphocyte infusion (DLI) is used to increase the graft-versus-tumor (GVT) effect after allogeneic hematopoietic cell transplant. The role of DLI in the management of malignancies remains restricted mainly due to the limited spectrum of activity and high risk of graft-versus-host disease (GVHD). The finding of new cell populations for adoptive immunotherapy with the ability to separate GVT from GVHD would be useful. Being non-MHC-restricted and active against autologous and allogeneic tumor cells with comparable efficacy [11], CIK cells have therefore been exploited in studies related to both its efficacy and possible toxicity due to GVHD across MHC barrier. In an allogeneic model where transplant with purified hemopoietic stem cell was done across major MHC barrier from H-2b donors into murine lymphoma-inoculated H-2d recipient mice, transplanted mice died from persistent lymphoma [32]. However, recipient mice infused with CIK cells expanded from donor mice had reduction of lymphoma and 50% survived long term, with no or minimal GVHD. In contrast, recipient mice that were treated with unmanipulated donor splenocytes died from acute GVHD [32]. This observation suggests that expanded CIK cells mediate significant graft-versus-tumor effect without significant GVHD even when transplanted across MHC barrier. The mechanism of reduced GVHD induction was postulated to be related to IFN-γ production [33]. In an MHC-mismatched model when mice bearing the A20 leukemia/lymphoma B cells were treated with allogeneic CIK cells, the donor CIK cells were observed to infiltrate GVHD target tissues to a lesser extent and for a shorter duration than unmanipulated splenocytes, resulting in milder histological changes in the gut and liver [34]. Instead, CIK cells were found to accumulate and persist at tumor sites resulting in eradication of established tumor [34]. These encouraging experimental data may be important information for application in human allogeneic transplantation where donor CIK cells may prove to be superior and hopefully safer than unmanipulated DLI in the prevention or treatment of relapse.
6. Novel Development
Exciting development that promised to broaden the application of CIK cell was reported over recent years. Primary ovarian carcinoma cells are resistant to CIK-mediated lysis. However, it was demonstrated recently that the addition of bispecific antibodies (BsAb) CD3xCA125 or CD3xHer2 (heteroconjugates of anti-CD3 with anti-CA125 or Her2, resp.) could efficiently target the CD3+ CIK cells to ovarian carcinoma bearing the specific ovarian tumor antigens and overcome the resistance. This strategy was shown to be effective against autologous primary ovarian tumor cells in vitro and in ovarian tumor-bearing mice. The survival of mice with ovarian carcinoma treated with CIK cells redirected by the BsAb was prolonged compared to control mice treated with CIK cells alone [35]. In an attempt to treat the Ewing's family tumors (EFTs), the low levels of cell surface Her2/neu expression were employed to redirect ex vivo activated CIK cells to tumor targets using the BsAb CD3xHer2/neu [36]. It was demonstrated that CD3xHer2/neu could be used to redirect CIK cells to mediate cytotoxicity against EFTs.
Besides using BsAb, other methods of harnessing antigen-antibody affinity to redirect CIK cells to target tumor cells involved the transfection of CIK cells to express tumor-specific receptors. CIK cells are known to be inactive against B cell ALL targets in vitro, but CIK cells engineered to express the chimeric receptor specific for the CD19 antigen could redirect CIK cells and become cytotoxic towards CD19-expressing B cell ALL targets [37].
Another innovative genetic modification of CIK cells was the transfection of CIK cells with oncolytic viruses. These are viruses with the ability to infect only transformed (tumor) cells. A modified double-deleted vaccinia virus (vvDD) was able to infect CIK cells without affecting its activity. CIK cells were employed as a carrier vehicle bringing the oncolytic virus to tumor cells through the NKG2D receptor which is highly expressed by CIK cells to its ligands MICA and MICB on the tumor cells. VvDD-carrying CIK cells migrated to the tumor sites where the oncolytic viruses were then released to specifically lyse tumor cells. This approach has been successfully shown to work using mouse models for human ovarian tumor and murine breast carcinoma, resulting in reduction of tumor burden and prolongation of survival [38].
7. Clinical Trials
CIK cells can be generated successfully from healthy donors as well as patients treated with chemotherapy for various malignancies and undergoing peripheral blood progenitor cell (PBSC) leukapheresis [39]. Feasibility of large-scale expansion was also reported for cord blood and even from washout of leftover mononuclear cells from cord blood unit bags [40]. Several phase I trials to test the clinical efficacy of CIK cells on a small number of patients have been reported. The first clinical trial using CIK cells was reported in 1999 by Schmidt-Wolf et al. in Germany using autologous CIK cells electroporated with IL-2 genes for infusion into 10 patients with metastatic renal carcinoma, colorectal cancer, and lymphoma. One patient with follicular lymphoma showed complete response. No major side effect was observed, except for 3 patients who developed fever that spontaneously resolved [41]. Negrin's group at Stanford reported giving 9 patients autologous CIK cells to treat relapsed Hodgkin disease (HD) and non-Hodgkin lymphoma (NHL) postautologous transplantation. Besides demonstrating the feasibility of large-scale expansion of CIK cells and the absence of adverse reaction, this trial achieved 2 partial responses and 2 stabilization of disease in the recipients [42]. In another phase I/II study, autologous CIK cells were given as an adjuvant immunotherapy postautologous transplantation for 9 high risk HD and 4 MM patients, again demonstrating safety as well as the maintenance of disease response in these high risk patients in the first year post-transplantation [43]. In the adjuvant therapy of acute leukemia, a study from China reported that 73.4% of the 19 patients who received 1–4 courses of autologous CIK cell infusions in combination with consolidation chemotherapy remained in continuous remission for a follow-up period of 4 years, compared to 27.3% in the chemotherapy-only group [44].
CIK cells have also been explored in clinical trials to treat solid tumors by investigators in China. In one report, patients with stage IV stomach cancer who were treated with chemotherapy and received additional CIK cell infusions had a higher overall short-term remission rate of 56.3% as compared to 48% in the chemotherapy-only group [45]. In another study, patients with hepatocellular carcinoma who achieved complete remission with transcatheter arterial chemoembolization and radiofrequency ablation were divided into two groups, where intrahepatic arterial CIK cell infusion was given to a study group of 45 patients. Relapse at 1.5 years occurred in 31.1% of patients in the CIK group as compared to 85% in the control group, with the difference mainly accounted for by the significantly lower relapses seen at local sites (15.56% versus 65%, resp.) [46]. The same group also reported their experience in 10 patients with renal cell carcinoma postnephrectomy who were given intradermal autologous dendritic cells pulsed with autologous tumor lysate and followed by autologous CIK cell infusions. In 4 patients with evaluable disease, one case of partial response in terms of resolution and reduction in the size of metastatic lung nodules was observed [47].
In the allogeneic setting, an early abstract from Stanford in 2006 reported 10 patients with various hematological malignancies relapsing after allogeneic transplant-received cytoreductive treatment followed by donor CIK cell infusions in escalating doses up to 1 × 108 CD3/kg. Grade I acute GVHD occurred in 1 patient and limited chronic GVHD in 2 patients [48]. Another report from Italy studied 11 patients in similar clinical settings and with variable doses administered for each patient. As often occurs in such settings where other salvage therapies are being used concurrently, it was difficult to assess the efficacy. Nevertheless, 3 patients (1 each with MDS, HD, and AML) achieved measurable response, in terms of improvement in donor chimerism or clearance of disease, that could be solely attributable to donor CIK cell infusions. Similarly, GVHD rate was not higher than that of unmanipulated DLI, with grade I and II acute GVHD in 4 patients, 2 of whom progressed to extensive chronic GVHD [49]. In our own experience using allogeneic donor CIK cells to treat patients in relapse who are refractory to chemotherapy and DLI, response attributable solely to CIK infusions was also seen in a few patients. GVHD involving mainly skin and liver was sometimes observed but was easily controlled (Linn and Hui, unpublished data).
8. Future Directions
Over the years, investigators have learnt to generate various types of cytotoxic effector cells and have used them for the cellular immunotherapy of human cancer. CIK cells, either in autologous or allogeneic context, have demonstrated encouraging results both in vitro and in clinical studies by various groups, as summarized in Table 2. Advances in genetic engineering technologies could further broaden the potential clinical applications of CIK cells if the challenge to conform these genetic engineering technologies to GMP compliance could be overcome. However, clinical studies with CIK cells are still in their infancy and only involved a relatively small number of patients in most of these studies. The relatively robust and simple cell culture procedures to expand CIK cells have enabled this approach of adoptive cellular immunotherapy to be increasingly studied across the world. As an immunotherapeutic modality, infusion of CIK cells is most likely to show efficacy in a relatively low tumor burden stage or in an adjuvant setting, rather than in high tumor burden diseases. With the amount of encouraging experimental and clinical evidence currently available, randomized clinical trials are justifiable and have to be done under stringent compliance with the CONSORT principles. This will likely involve a substantial number of patients in order to demonstrate statistical significance for a modest degree of outcome superiority. Such studies are urgently needed in order to provide unequivocal evidence of the clinical usefulness of CIK cells, so as to enable its integration into cancer treatment protocols to improve overall cure rate.
Table 2.
Activities of CIK cells from preclinical experiments to clinical studies.
| In vitro cytotoxicity | Ref | |
| Cell lines | B cell lymphoma SU-DHL | [8, 9] |
| Multiple myeloma OPM-2,U266 | [16, 26] | |
| T cell ALL CCRF-CEM-VBL (Vinblastine resistant) | [21] | |
| Erythroleukemia K562/Dox(Doxorubicin resistant) | [21] | |
| Primary tumor cells | Acute myeloid leukemia | [11] |
| Chronic myeloid leukemia | [22, 23] | |
| Chronic lymphocytic leukemia | [24, 25] | |
| Multiple myeloma | [26] | |
| Mice studies | ||
| Cell lines | Human B cell lymphoma Su-DHL | [8, 9] |
| Human cervical cancer HeLa | [30] | |
| Murine B cell lymphoma BCL1 | [31, 32] | |
| Primary tumor cells | Chronic myeloid leukemia | [22] |
| Chronic lymphocytic leukemia | [24] | |
| CIK cells administered with additional manipulation | ||
| With BsAb | CIK + BSAbxCA125 or BSAbxHER2 versus ovarian primary tumor and cell lines in vitro and in mice | [35] |
| CIK + CD3xHER2/neu bsAb versus Ewing's family tumor cell lines in vitro and in mice | [36] | |
| Gene transfection | IL-2 gene electroporated CIK + DC versus pancreatic tumor cell line Dan G | [28] |
| Anti-CD19 chimeric receptor-redirected CIK versus ALL cell line REH and primary ALL cells | [37] | |
| Oncolytic virus transfected CIK versus human ovarian cell lines UCI-101 and SK-OV-3 in vitro and in mice | [38] | |
| Clinical studies with demonstrated tumor response | ||
| Treatment: autologous | Follicular lymphoma (1 case) | [41] |
| Hodgkin's disease (2 cases) | [42] | |
| Renal cell carcinoma (1 case) | [47] | |
| Treatment: allogeneic | Myelodysplastic syndrome (1 case), Hodgkin's disease (1 case), Acute myeloid leukemia (1 case) | [49] |
| Clinical studies in adjuvant setting with demonstrated reduction in relapse | ||
| Autologous | Acute myelogenous and lymphoblastic leukemia | [44] |
| Hepatocellular carcinoma (intra-arterial) | [46] | |
References
- 1.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. Journal of Experimental Medicine. 1982;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips JH, Lanier LL. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. Journal of Experimental Medicine. 1986;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochoa AC, Gromo G, Alter BJ, Sondel PM, Bach FH. Long-term growth of lymphokine-activated killer (LAK) cells: role of anti-CD3, β-IL 1, interferon-γ and -β. Journal of Immunology. 1987;138(8):2728–2733. [PubMed] [Google Scholar]
- 4.Lafreniere R, Rosenberg SA. Successful immunotherapy of murine experimental hepatic metastases with lymphokine-activated killer cells and recombinant interleukin 2. Cancer Research. 1985;45(8):3735–3741. [PubMed] [Google Scholar]
- 5.Mazumder A, Rosenberg SA. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. Journal of Experimental Medicine. 1984;159(2):495–507. doi: 10.1084/jem.159.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Lotze MT, Muul LM. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. The New England Journal of Medicine. 1987;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 7.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. The Lancet. 2000;356(9232):802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Wolf IGH, Negrin RS, Kiem H-P, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. Journal of Experimental Medicine. 1991;174(1):139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu P-H, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. Journal of Immunology. 1994;153(4):1687–1696. [PubMed] [Google Scholar]
- 10.Lopez RD, Waller EK, Lu P-H, Negrin RS. CD58/LFA-3 and IL-12 provided by activated monocytes are critical in the in vitro expansion of CD56+ T cells. Cancer Immunology, Immunotherapy. 2000;49(11):629–640. doi: 10.1007/s002620000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linn YC, Lau LC, Hui KM. Generation of cytokine-induced killer cells from leukaemic samples with in vitro cytotoxicity against autologous and allogeneic leukaemic blasts. British Journal of Haematology. 2002;116(1):78–86. doi: 10.1046/j.1365-2141.2002.03247.x. [DOI] [PubMed] [Google Scholar]
- 12.Linn YC, Lau SKJ, Liu BH, Ng LH, Yong HX, Hui KM. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology. 2009;126(3):423–435. doi: 10.1111/j.1365-2567.2008.02910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alajez NM, Schmielau J, Alter MD, Cascio M, Finn OJ. Therapeutic potential of a tumor-specific, MHC-unrestricted T-cell receptor expressed on effector cells of the innate and the adaptive immune system through bone marrow transduction and immune reconstitution. Blood. 2005;105(12):4583–4589. doi: 10.1182/blood-2004-10-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(18):7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magarian-Blander J, Ciborowski P, Hsia S, Watkins SC, Finn OJ. Intercellular and intracellular events following the MHC-unrestricted TCR recognition of a tumor-specific peptide epitope on the epithelial antigen MUC1. Journal of Immunology. 1998;160(7):3111–3120. [PubMed] [Google Scholar]
- 16.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103(8):3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 17.Pende D, Spaggiari GM, Marcenaro S, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Polio virus receptor (CD 155) and Nectin-2 (CD 112) Blood. 2005;105(5):2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 18.Galandrini R, Albi N, Zarcone D, Grossi CE, Velardi A. Adhesion molecule-mediated signals regulate major histocompatibility complex-unrestricted and CD3/T cell receptor-triggered cytotoxicity. European Journal of Immunology. 1992;22(8):2047–2053. doi: 10.1002/eji.1830220814. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–339. [PubMed] [Google Scholar]
- 20.Linn YC, Wang SM, Hui KM. Comparative gene expression profiling of cytokine-induced killer cells in response to acute myloid leukemic and acute lymphoblastic leukemic stimulators using oligonucleotide arrays. Experimental Hematology. 2005;33(6):671–681. doi: 10.1016/j.exphem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Wolf IGH, Lefterova P, Johnston V, et al. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cellular Immunology. 1996;169(1):85–90. doi: 10.1006/cimm.1996.0094. [DOI] [PubMed] [Google Scholar]
- 22.Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3+CD56+ cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92(9):3318–3327. [PubMed] [Google Scholar]
- 23.Scheffold C, Brandt K, Johnston V, et al. Potential of autologous immunologic effector cells for bone marrow purging in patients with chronic myeloid leukemia. Bone Marrow Transplantation. 1995;15(1):33–39. [PubMed] [Google Scholar]
- 24.Kornacker M, Moldenhauer G, Herbst M, et al. Cytokine-induced killer cells against autologous CLL: direct cytotoxic effects and induction of immune accessory molecules by interferon-γ. International Journal of Cancer. 2006;119(6):1377–1382. doi: 10.1002/ijc.21994. [DOI] [PubMed] [Google Scholar]
- 25.Lefterova P, Schakowski F, Buttgereit P, Scheffold C, Huhn D, Schmidt-Wolf IGH. Expansion of CD3+CD56+ cytotoxic cells from patients with chronic lymphocytic leukemia: in vitro efficacy. Haematologica. 2000;85(10):1108–1109. [PubMed] [Google Scholar]
- 26.Märten A, Renoth S, von Lilienfeld-Toal M, et al. Enhanced lytic activity of cytokine-induced killer cells against multiple myeloma cells after co-culture with idiotype-pulsed dendritic cells. Haematologica. 2001;86(10):1029–1037. [PubMed] [Google Scholar]
- 27.Ziske C, Märten A, Schöttker B, et al. Resistance of pancreatic carcinoma cells is reversed by coculturing NK-like T cells with dendritic cells pulsed with tumor-derived RNA and CA 19-9. Molecular Therapy. 2001;3(1):54–60. doi: 10.1006/mthe.2000.0230. [DOI] [PubMed] [Google Scholar]
- 28.Nagaraj S, Ziske C, Schmidt-Wolf IGH. Human cytokine-induced killer cells have enhanced in vitro cytolytic activity via non-viral interleukin-2 gene transfer. Genetic Vaccines and Therapy. 2004;2(1, article 12) doi: 10.1186/1479-0556-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edinger M, Sweeney TJ, Tucker AA, Olomu AB, Negrin RS, Contag CH. Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. 1999;1(4):303–310. doi: 10.1038/sj.neo.7900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweeney TJ, Mailänder V, Tucker AA, et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(21):12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edinger M, Cao Y-A, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101(2):640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 32.Verneris MR, Ito M, Baker J, Arshi A, Negrin RS, Shizuru JA. Engineering hematopoietic grafts: purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biology of Blood and Marrow Transplantation. 2001;7(10):532–542. doi: 10.1016/s1083-8791(01)70014-6. [DOI] [PubMed] [Google Scholar]
- 33.Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon γ production. Blood. 2001;97(10):2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura R, Baker J, Beilhack A, et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112(6):2563–2574. doi: 10.1182/blood-2007-06-092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan JK, Hamilton CA, Cheung MK, et al. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study. Clinical Cancer Research. 2006;12(6):1859–1867. doi: 10.1158/1078-0432.CCR-05-2019. [DOI] [PubMed] [Google Scholar]
- 36.Verneris MR, Arshi A, Edinger M, et al. Low levels of Her2/neu expressed by Ewing’s family tumor cell lines can redirect cytokine-induced killer cells. Clinical Cancer Research. 2005;11(12):4561–4570. doi: 10.1158/1078-0432.CCR-05-0157. [DOI] [PubMed] [Google Scholar]
- 37.Marin V, Dander E, Biagi E, et al. Characterization of in vitro migratory properties of anti-CD19 chimeric receptor-redirected CIK cells for their potential use in B-ALL immunotherapy. Experimental hematology. 2006;34(9):1219–1229. doi: 10.1016/j.exphem.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311(5768):1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 39.Alvarnas JC, Linn Y-C, Hope EG, Negrin RS. Expansion of cytotoxic CD3+CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2001;7(4):216–222. doi: 10.1053/bbmt.2001.v7.pm11349808. [DOI] [PubMed] [Google Scholar]
- 40.Introna M, Franceschetti M, Ciocca A, et al. Rapid and massive expansion of cord blood-derived cytokine-induced killer cells: an innovative proposal for the treatment of leukemia relapse after cord blood transplantation. Bone Marrow Transplantation. 2006;38(9):621–627. doi: 10.1038/sj.bmt.1705503. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Wolf IGH, Finke S, Trojaneck B, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-1 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. British Journal of Cancer. 1999;81(6):1009–1016. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biology of Blood and Marrow Transplantation. 2005;11(3):181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Arai S, Sheehan K, Moore S, et al. Autologous cytokine-induced killer cells as post-transplant cellular immunotherapy. ASH Annual Meeting Abstracts. 2007;110(11):p. 580. [Google Scholar]
- 44.Jiang H, Liu KY, Tong CR, Jiang B, Lu DP. The efficacy of chemotherapy in combination with auto-cytokine-induced killer cells in acute leukemia. Zhonghua Nei Ke Za Zhi. 2005;44(3):198–201. [PubMed] [Google Scholar]
- 45.Jiang J, Xu N, Wu C, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Research. 2006;26(3B):2237–2242. [PubMed] [Google Scholar]
- 46.Weng D-S, Zhou J, Zhou Q-M, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. Journal of Immunotherapy. 2008;31(1):63–71. doi: 10.1097/CJI.0b013e31815a121b. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Zhou FJ, Wang QJ, et al. Efficacy of autologous renal tumor cell lysate-loaded dendritic cell vaccine in combination with cytokine-induced killer cells on advanced renal cell carcinoma—a report of ten cases. Ai Zheng. 2006;25(5):625–630. [PubMed] [Google Scholar]
- 48.Laport GG, Sheehan K, Lowsky R, et al. Cytokine induced killer (CIK) cells as post-transplant immunotherapy following allogeneic hematopoietic cell transplantation. ASH Annual Meeting Abstracts. 2006;108(11):p. 412. [Google Scholar]
- 49.Introna M, Borleri G, Conti E, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92(7):952–959. doi: 10.3324/haematol.11132. [DOI] [PubMed] [Google Scholar]