Abstract
Pesticide exposure has been implicated in the etiopathogenesis of Parkinson's disease (PD); in particular, the organochlorine insecticide dieldrin is believed to be associated with PD. Emerging evidence indicates that histone modifications play a critical role in cell death. In this study, we examined the effects of dieldrin treatment on histone acetylation and its role in dieldrin-induced apoptotic cell death in dopaminergic neuronal cells. In mesencephalic dopaminergic neuronal cells, dieldrin induced a time-dependent increase in the acetylation of core histones H3 and H4. Histone acetylation occurred within 10 min of dieldrin exposure indicating that acetylation is an early event in dieldrin neurotoxicity. The hyperacetylation was attributed to dieldrin-induced proteasomal dysfunction, resulting in accumulation of a key histone acetyltransferase (HAT), cAMP response element-binding protein. The novel HAT inhibitor anacardic acid significantly attenuated dieldrin-induced histone acetylation, Protein kinase C δ proteolytic activation and DNA fragmentation in dopaminergic cells protected against dopaminergic neuronal degeneration in primary mesencephalic neuronal cultures. Furthermore, 30-day exposure of dieldrin in mouse models induced histone hyperacetylation in the striatum and substantia nigra. For the first time, our results collectively demonstrate that exposure to the neurotoxic pesticide dieldrin induces acetylation of core histones because of proteasomal dysfunction and that hyperacetylation plays a key role in dopaminergic neuronal degeneration after exposure of dieldrin.
Parkinson's disease (PD) is a neurodegenerative disorder associated with progressive degeneration of nigral dopaminergic neurons in the mesencephalic region of the brain, resulting in irreversible motor dysfunction. This neurological disease affects approximately 1 million people in the United States, and an estimated 50,000 new cases are reported each year. Although the disorder has been studied for many years, the etiopathogenesis remains unclear because of PD's very complex causal relationship with both genetic and environmental factors (Le Couteur et al., 2002). The available data indicate that environmental exposure to certain chemicals, such as metals and pesticides, may cause the majority of idiopathic PD cases, whereas genetic defects (i.e., mutations in α-synuclein, Parkin, PINK-1, LRRK1, and DJ), are responsible for less than 10% of PD cases. Epidemiological data overwhelmingly support the possible involvement of pesticide exposure in the development of PD (Fleming et al., 1994; Corrigan et al., 2000; Priyadarshi et al., 2000).
The organochlorine pesticide dieldrin was widely used throughout the United States until the late 1980s, and the pesticide still persists heavily in the environment, particularly in soil sediments (Jorgenson, 2001). Thus, human exposure to dieldrin continues via contaminated food (Corrigan et al., 1996). Studies indicate that exposure to dieldrin is closely associated with Parkinson's disease (Fleming et al., 1994; Corrigan et al., 2000). Dopaminergic neurons are more sensitive to dieldrin-induced neurotoxicity as observed in cell culture models of PD (Fleming et al., 1994; Kitazawa et al., 2001; Kanthasamy et al., 2005). Recent reports also indicate that dieldrin damages the nigrostriatal system through oxidative stress and can significantly decrease dopamine metabolites, including 3,4-dihydroxyphenylacetic acid and homovanillic acid, in mouse models (Hatcher et al., 2007). Despite the established link, the mechanism of pesticide-induced dopaminergic degeneration has been understudied. Thus, clarification of the cellular mechanism of dieldrin-induced neurotoxicity in dopaminergic cells would elucidate the molecular mechanism underlying dopaminergic neurodegeneration after environmental chemical exposure.
We previously reported that the caspase-3-dependent proteolytic activation of protein kinase C δ (PKCδ) plays a critical role in the dieldrin-induced apoptotic cascade (Kitazawa et al., 2003; Kanthasamy et al., 2005, 2008). We also demonstrated that dieldrin can impair proteasomal activity, resulting in an accumulation of proteins degraded by the ubiquitin-proteasome pathway (Sun et al., 2005). Still, the effect of dieldrin at the nuclear level has never been studied.
Epigenetic changes induced by chemical exposure are believed to be involved in the pathogenesis of chronic neurodegenerative diseases, including PD and Alzheimer's disease (Mattson, 2003; Migliore and Coppedè, 2009). However, little is known about the exact epigenetic mechanisms underlying neurotoxic pesticide exposure in nigral dopaminergic systems and the relevance of these epigenetic changes to the pathogenesis of PD. Histone modification, including methylation, phosphorylation, acetylation, and ubiquitination, has been linked to many human diseases in recent years (Somech et al., 2004). Acetylation of the histone tail by histone acetyl transferase (HAT) neutralizes the positive charges of histone, resulting in binding of transcription factors to DNA, which eventually recruits the RNA-polymerase and enables transcription (Hasan and Hottiger, 2002). In addition to facilitation of transcription, HATs also are involved in many other cellular functions, such as DNA repair, cell cycle progression, and cell death (Chen et al., 2001). It is now known that histone deacetylase (HDAC) inhibitors, which cause the hyperacetylation of histones, can induce growth arrest, differentiation, or apoptosis of cancer cells (including neuroblastoma) in vitro and in vivo (Somech et al., 2004). The balance of histone acetyltransferase (HAT)/HDAC has recently been suggested to contribute to neurodegenerative conditions (Rouaux et al., 2003; Saha and Pahan, 2006). In the present study, we examined whether histone acetylation plays a role in dieldrin-induced apoptotic cell death and, if so, whether inhibition of histone acetylation by a HAT inhibitor protects against the neurotoxic effect of dieldrin in cell culture models of PD.
Materials and Methods
Chemicals.
Dieldrin (purity 90%) was purchased from Sigma Chemical Co. (St. Louis, MO). Anacardic acid was purchased from Alexis Co. (Lausen, Switzerland). The caspase-3 substrate Ac-DEVD-AFC was obtained from Bachem Biosciences (King of Prussia, PA). The Cell Death Detection enzyme-linked immunosorbent assay (ELISA) Plus Assay Kit was purchased from Roche Molecular Biochemicals (Indianapolis, IN). RPMI 1640 medium, fetal bovine serum, l-glutamine, penicillin/streptomycin, and Sytox green dye were obtained from Invitrogen (Carlsbad, CA). The Bradford protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA). The primary antibodies used in this study were PKCδ, caspase-3, CREB-binding protein (CBP; rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA), β-actin (mouse monoclonal; Sigma), acetyl-lysine (rabbit polyclonal), and histone H3 (mouse monoclonal; Millipore, Billerica, MA). [γ-32P] ATP was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). The Bradford protein assay kit was purchased from Bio-Rad Laboratories. IRDye 800-conjugated anti-rabbit (Rockland Immunochemicals, Gilbertsville, PA) and Alexa Fluor 680 conjugate anti-mouse (Invitrogen) were used.
Cell Culture and Treatment Paradigm.
Immortalized rat mesencephalic/dopaminergic cells (N27 cells) were grown in RPMI 1640 medium containing 10% fetal bovine serum, 2 mM l-glutamine, 50 units of penicillin, and 50 μg/ml streptomycin and maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells (2–3 days old) were used for experiments. Two different treatment paradigms were used in the study. The short-term exposure to high-dose (100 μM) dieldrin was used to evoke immediate neurotoxic responses in N27 cells, whereas long-term exposures to lower dose exposures (10–30 μM dieldrin for 24 h) are environmentally relevant. In the anacardic acid studies, cells with 60% confluence were pretreated with anacardic acid for 1 h and then exposed to 100 μM dieldrin under a serum-free condition. Primary mesencephalic neuronal cultures were prepared from timed-pregnant C57/bl mice (embryonic day 14) as described previously (Zhang et al., 2007). In brief, mesencephalic tissues were dissected and maintained in ice-cold Ca2+-free HBSS, and then HBSS solution containing trypsin-EDTA (0.25%) was used to dissociate the fetal brain tissues for 30 min at 37°C. The dissociated cells were then seeded at equal density (106 cells) in 30-mm diameter tissue culture wells, which were precoated with poly-d-lysine (1 mg/ml) and 10 μg/ml laminin. Cultures were maintained in a chemically defined medium consisting of neurobasal medium fortified with B-27 supplements, l-glutamine (500 μM), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (Invitrogen). The cells were then maintained in a humidified CO2 incubator (5% CO2, 37°C) for 6 to 7 days. Half of the culture medium was replaced every 2 days.
Animal Studies.
Dieldrin (5.0 mg/kg) was administered to male C57BL/6J mice every other day for 30 days, and control mice were injected intraperitoneally with vehicle (dimethyl sulfoxide). All animal use and related protocol procedures employed in this study were approved and supervised by the Committee on Animal Care at Iowa State University (Ames, IA). After treatment, striatum and substantia nigra tissues were dissected for histone extraction. Histone acetylation was examined by Western blot with the use of anti-acetyl-Lys antibody.
Histone Extraction.
After treatment, cells were collected by scraping and were washed thrice with ice-cold PBS. The whole histones were extracted with a NE-PER kit (Thermo Fisher Scientific, Waltham, MA) and were eventually dissolved into 0.2 N HCl. In brief, cell pellets were incubated with cytosolic extraction reagent I (supplied in NE-PER kit) plus 0.5% Triton X-100 for 10 min. Nuclei were collected by centrifugation at 2000g for 5 min. Then the pellet was resuspended in 0.2 N HCl and incubated on a rotator for 3 h at 4°C. After centrifuging for 10 min at maximum speed in a microcentrifuge, supernatant was collected for further analysis.
Proteolytic Activation of Caspase-3 and PKCδ.
After dieldrin exposure, cells were washed with PBS, pH 7.4, and resuspended in caspase lysis buffer at 37°C for 20 min. Lysates were centrifuged at 14,000 rpm and the cell-free supernatants were incubated with 50 μM Ac-DEVD-AFC at 37°C for 1 h. Formation of 7-amino-4-methylcoumarin (AFC), resulting from caspase-3 activity, was measured at an excitation wavelength of 400 nm and an emission wavelength of 505 nm with the use of a fluorescence plate reader. The caspase-3 cleavage and PKCδ cleavage were checked by Western blot (Kitazawa et al., 2003). In brief, cell lysates containing equal amounts of protein were loaded in each lane and separated on a 10-to-12% SDS-polyacrylamide gel. After separation, proteins were transferred to nitrocellulose membrane, and nonspecific binding sites were blocked by treating with LI-COR blocking buffer. The membranes were then incubated with primary antibodies directed against PKCδ (rabbit polyclonal; 1:2000 dilution) or caspase-3 (rabbit polyclonal; 1:1000). The primary antibody treatments were followed by treatment with secondary IR dye-800 conjugated anti-rabbit dye or Alexa Fluor 680 conjugated anti-mouse IgG for 1 h at room temperature. To confirm equal protein loading, blots were reprobed with β-actin antibody (1:5000 dilution). Western blot images were captured with the Odyssey IR Imaging system (LI-COR) and data were analyzed using Odyssey 2.0 software.
Proteasomal Enzymatic Activity Assay.
The proteasomal peptidase assay was performed as described previously (Sun et al., 2005). In brief, cells after treatment were harvested and lysed with hypotonic buffer (10 mM HEPES, 5 mM MgCl2, 10 mM KCl, 1% sucrose, and 0.1% CHAPS). Lysates were then incubated with fluorogenic substrate succinyl-LLVY-AFC (75 μM) in the assay buffer (50 mM Tris-HCl, 20 mM KCl, 5 mM magnesium acetate, and 10 mM dithiothreitol, pH 7.6) at 37°C for 30 min. Cleaved fluorescent products were then examined at an excitation wavelength of 400 nm and an emission wavelength of 505 nm by a fluorescence plate reader (Gemini Plate Reader; Molecular Devices, Sunnyvale, CA). Enzymatic activities were normalized by protein concentration, which was measured by Bradford method.
Assay of Protein Kinase Cδ Activity.
PKCδ kinase activity was examined by immunoprecipitation as described previously (Kitazawa et al., 2003). N27 cells were exposed to 100 μM dieldrin for 20 min, with or without the HAT inhibitor anacardic acid, and cell lysates were extracted. After immunoprecipitation with anti-PKCδ antibody, samples bound to Sepharose A beads were incubated with reaction buffer containing 0.4 mg of histone H1 and 5 μCi of [γ-32P]ATP (4500 Ci/mM) for 10 min at 30°C. The reaction was terminated by the addition of 2× SDS gel loading buffer and boiled for 5 min. The samples were separated on 15% SDS-PAGE and phosphorylated histone was detected by filmless autoradiographic analysis (Personal Molecular Imager FX; Bio-Rad) and quantified using Quantity One 4.2.0 Software (Bio-Rad).
DNA Fragmentation.
DNA fragmentation assays were performed using a Cell Death Detection ELISA Plus Assay Kit (Roche Diagnostics) as we have described previously (Sun et al., 2005). This highly sensitive assay was used for analysis of DNA fragmentation by quantification of histone-associated low-molecular-weight DNA in the cytoplasm of cells. In brief, after dieldrin exposure with or without anacardic acid pretreatment, cells were centrifuged and washed once with PBS and then incubated with cell lysis buffer (supplied with the kit) for 30 min at room temperature. After centrifuge, the supernatants were then dispensed into streptavidin-coated 96-well microtiter plates containing 80 μl of horseradish peroxidase-conjugated antibody cocktail. After 2-h incubation at room temperature, the absorbance of the ELISA reaction was measured at 490 and 405 nm using a microplate reader (SpectraMAX 190; Molecular Devices). The difference of absorbance between A405 and A490 nm was used to measure the actual DNA fragmentation level.
Sytox Cell Death Assay and Morphometric Studies.
Cell death was determined by the cell-impermeable dye Sytox green (Invitrogen) after exposing the cells to dieldrin with or without anacardic acid pretreatment. Sytox green enters only dead cells and binds with DNA to produce green fluorescence (Sherer et al., 2002). In brief, cells grown in 24-well plates were incubated with 1 μM Sytox green for 20 min and then exposed by 100 μM dieldrin with or without 8.5 μM anacardic acid pretreatment under serum-free conditions. In the Sytox assay, dead cells can be viewed directly under the fluorescence microscope as well as quantitatively measured using the fluorescence microplate with excitation at a wavelength of 485 nm and emission at a wavelength of 538 nm with the use of a fluorescent reader (SpectraMax Gemini XS Model, Molecular Devices).
ROS Generation Assay.
Flow cytometry analysis was performed on a FACScan flow cytometer (BD Biosciences, San Jose, CA). Hydroethidine, a sodium borohydride-reduced derivative of ethidium bromide, was used to detect reactive oxygen species (ROS) produced specifically inside the cell. When hydroethidine is loaded in the cells, it binds to cellular macromolecules. Once O2− is generated, it converts hydroethidine to ethidium bromide and increases red fluorescence (620 nm). A 15-mW air-cooled argon-ion laser was used as an excitation source for hydroethidine at 488 nm, and the optical filter was 585/42 nm bandpass. Cells were detected and distinguished from the background by forward-angle and orthogonal light scattering characteristics. All flow cytometric data were analyzed by CellQuest data analysis software to determine the significant increase or decrease in fluorescence intensity. N27 cells were resuspended with HBSS with 2 mM calcium at a density of 106 cells/ml. Cells were then incubated with 10 μM hydroethidine for 15 min at 37°C in the dark to allow loading of dye into the cells. After addition of anacardic acid (5, 10, 30, and 50 μM), ROS generation was measured at 60 min after the exposure.
siRNA Transfection.
Small interfering RNA (siRNA) specific to rat CBP mRNA or nonspecific siRNA were obtained from Integrated DNA Technologies (Coralville, IA). N27 cells were transiently transfected with specific and nonspecific siRNA duplexes by the Amaxa Nucleofector Kit (Amaxa Biosystems, Gaithersburg, MD). In brief, N27 cells were resuspended with transfection buffer provided with the kit to a final concentration of 4 to 5 × 106 neurons/100 μl and mixed with siRNA duplex. The final concentration of siRNA is 5 nM. Electroporation was executed with an Amaxa Nucleofector instrument according to the manufacturer's protocol. The transfected neurons were then transferred to a 75-ml flask for 24 h before further treatments.
TH+ Neuron Morphology Examination and Neurite Measurement.
Primary mesencephalic dopaminergic neurons labeled with tyrosine hydroxylase (TH) antibody were examined and analyzed. Immunocytochemical staining in cell cultures was conducted as described previously (Zhang et al., 2007). In brief, primary neuronal cells were grown on poly-d-lysine–coated glass coverslips. After treatment, the cells were fixed with 4% paraformaldehyde, and nonspecific sites were blocked by 5% normal goat serum containing 0.4% bovine serum albumin and 0.2% Triton X-100 in PBS for 1 h. Primary neurons were then incubated with antibodies against TH (rabbit polyclonal; 1:1000 dilution), overnight at 4°C followed by incubation with Cy3-conjugated (red; 1:1000) secondary antibody for 2 h at room temperature. For nucleus staining, Hoechst 33342 (10 μg/ml) was incubated with the cells for 10 min after secondary antibody treatments. Then the coverslips containing stained cells were washed with PBS, mounted on a slide, and viewed under a Nikon inverted fluorescence microscope (model TE-2000U); images were captured with a SPOT digital camera (Diagnostic Instruments). From those pictures, neurite length was measured by Nikon Metamorph software and analyzed by Prism 4.0 software (GraphPad Software, San Diego, CA).
Uptake of [3H]Dopamine.
The effects of dieldrin and anacardic acid on uptake of dopamine were assessed in fetal mouse mesencephalic cultures using [3H]dopamine (DA), as we described previously (Afeseh et al., 2009). In brief, after incubation for 36 h with 10 μM dieldrin, with or without anacardic acid, medium with the treatment was removed and cells were then washed once by assay incubation (Krebs-Ringer) buffer (5.6 mM glucose, 1.3 mM EDTA, 1.2 mM magnesium sulfate, 1.8 mM calcium chloride, 4.7 mM potassium chloride, 120 mM sodium chloride, and 16 mM sodium phosphate). Cells were incubated with 10 μM [3H]DA (30 Ci/mol) for 20 min at 37°C. Positive controls were obtained by incubating the cells with 10 μM [3H]DA together with 1 nM mazindol (potent dopamine reuptake inhibitor). The uptake was stopped by removing the reaction mixture and followed by three washes with fresh Krebs-Ringer buffer. Cells were then collected with the use of 1 N NaOH, and the radioactivity was measured by liquid scintillation counting after addition 5 of ml scintillation cocktail to each vial.
Data Analysis.
Data analysis was performed using Prism 4.0 software (GraphPad Software, San Diego, CA). Data were first analyzed using one-way ANOVA and then Bonferroni's post test was performed to compare all treatment groups. Differences with p < 0.05 were considered significant.
Results
Dieldrin Induces Acetylation of Core Histones H3 and H4 in a Time-Dependent Manner in Dopaminergic Neuronal N27 Cells.
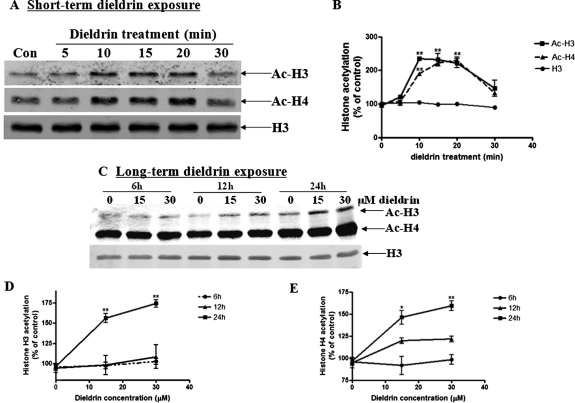
Emerging evidence indicates that acetylation and deacetylation of histones can profoundly influence various functions of neurons, including cell death in response to stress (Mattson, 2003; Rouaux et al., 2003; Soriano et al., 2009). First, we examined whether exposure to the neurotoxic pesticide dieldrin induces acetylation of histones in dopaminergic neuronal cell models. Dieldrin is a highly lipophilic compound that accumulates significantly in the central nervous system (Fleming et al., 1994; Corrigan et al., 2000). Based on human exposure reports (Campoy et al., 2001), the calculated cumulative lifetime exposure to dieldrin is approximately 30 μM. We previously demonstrated that short-term exposure to 100 μM dieldrin for 1 h induces dopaminergic cell death (Kitazawa et al., 2003). We used two different treatment paradigms to examine histone acetylation in N27 cells. The short-term exposure to high-dose (100 μM) dieldrin was used to evoke immediate neurotoxic responses in cells, whereas long-term exposures to lower doses (10–30 μM dieldrin for 24 h) are environmentally relevant. To understand the early changes, we studied the short-term effect of 100 μM dieldrin on histone acetylation. N27 cells were treated with 100 μM dieldrin for 5, 10, 15, 20, and 30 min, and then the nuclear fraction was isolated. Histone acetylation was examined in the nuclear extract by Western blot analysis using anti-acetyl-lysine antibody. As shown in Fig. 1, a time-dependent acetylation of H3- and H4-specific histones was observed in dieldrin-treated cells compared with control cells treated only with 0.1% DMSO solvent. The acetylation occurred as early as 10 min after dieldrin exposure. The H3 band showed equal loading of the protein levels. The acetylation increase on histone H3 and H4 was observed after exposure to either 15 or 30 μM dieldrin after 6 to 24 h as shown in Fig. 1C. Together, these results demonstrate that dieldrin exposure can alter histone acetylation in dopaminergic neuronal cells.
Fig. 1.
Dieldrin induces acetylation of core histones H3 and H4 in a time-dependent manner in dopaminergic neuronal cells. A, N27 dopaminergic neuronal cells were exposed to 100 μM dieldrin, and then acetylation of histones H3 and H4 was monitored at various time points. Native H3 was used as an internal control. B, densitometric quantification of acetylated H3 band and H4 band in A. Statistical significance between the control group and each treatment group was determined by ANOVA, p < 0.01. C, N27 dopaminergic neuronal cells were exposed to dieldrin at the physical concentration (30 μM), and then acetylation of histones H3 and H4 was also monitored at various time points. Native H3 was used as an internal control. Densitometric quantification of acetylated H3 band (D) and acetylated H4 band (E) of C. Statistical significance between the control group and each treatment group was determined by ANOVA, *, p < 0.05; **, p < 0.01.
Dieldrin Increases CBP level in Dopaminergic Cells via Inhibition of Ubiquitin Proteasome Function.
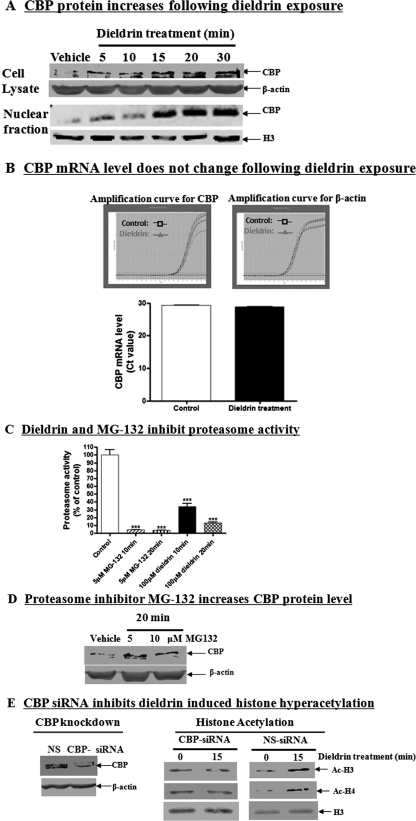
The HAT domain of CBP has been reported to play an important role in acetylation reactions in the neuronal system (Rouaux et al., 2003). Therefore, we examined whether the histone acetylation observed during dieldrin exposure was due to an increase in the cellular CBP levels. As shown in Fig. 2A, dieldrin treatment increased CBP protein levels in a time-dependent manner, and the increase was evident in both the whole-cell lysate and nuclear fraction. However, quantitative real-time-polymerase chain reaction analysis of the CBP mRNA levels showed no significant changes between control and dieldrin-treated cells (Fig. 2B), indicating that the increase in the CBP protein level was not due to an increase in transcription.
Fig. 2.
Dieldrin increases HAT CBP levels in dopaminergic cells via inhibition of ubiquitin proteasome function. CBP protein level increases during dieldrin treatment. A, N27 cells were treated with 100 μM dieldrin for 5, 10, 15, 20, and 30 min and then cell lysates and nuclear fractions were prepared as described under Materials and Methods. The CBP levels in lysates and nuclear fractions were measured by Western blot using anti-CBP antibody. Native H3 and β-actin were used as the loading control. B, CBP mRNA level does not change after dieldrin exposure. N27 cells were treated with 100 μM dieldrin for 30 min, and then mRNA was extracted. CBP mRNA level was performed by relative quantitative real-time-polymerase chain reaction with CBP specific primers. C, Dieldrin inhibits proteasome activity in dopaminergic N27 cells. Cells were treated with 100 μM dieldrin and then chymotrypsin-like proteasome activity was measured at 10 and 20 min after exposure using fluorogenic substrate. The proteasome inhibitor MG-132 was used as a positive control. All the data represent the mean ± S.E.M. for three samples in each group. Asterisks (***, p < 0.001, Student's t test) indicate statistically significant differences compared with vehicle-treated N27 cells. D, proteasome inhibitor MG-132 increases CBP protein level. N27 cells were treated with 5 or 10 μM MG-132 for 20 min, and the level of CBP was examined by Western blot using anti-CBP antibody. The membrane was reprobed with anti-β-actin antibody to show equal loading. E, CBP-specific siRNA inhibits dieldrin-induced histone hyperacetylation. N27 cells were transfected with CBP-specific siRNA or nonspecific (NS) siRNA for 24 h. After transfection, cells were treated with 100 μM dieldrin for 10 and 20 min. Histones were then extracted and acetylation level was examined by Western blot with anti-acetyl-lysine antibody.
Because cellular CBP levels are regulated mainly by the ubiquitin-proteasome protein degradative pathway (Yan et al., 2003), we hypothesized that dieldrin increases CBP protein levels as a result of the inhibition of proteasome function. Our recent studies indicate that dieldrin inhibits the ubiquitin-proteasome pathway to promote apoptosis in dopaminergic cells (Sun et al., 2005, 2007). Through measurement of proteasomal peptidase activity, we determined that dieldrin treatment inhibited proteasome activity in cells within 10 min of exposure (Fig. 2C). Treatment of N27 cells with the proteasome inhibitor MG-132 also increased CBP accumulation (Fig. 2D) similar to dieldrin, supporting our hypothesis that proteasome inhibition increases dieldrin-induced CBP protein levels.
To verify that CBP contributes to dieldrin-induced histone acetylation, we examined cellular acetylation after CBP knockdown by CBP-siRNA. As shown in Fig. 2E, N27 cells transfected with CBP-specific siRNA had significantly reduced expressions of CBP protein levels, whereas nonspecific siRNA transfection did not alter the CBP protein levels. It is noteworthy that CBP knockdown significantly blocked dieldrin-induced histone acetylation compared with that observed in the nonspecific siRNA-treated groups, demonstrating that CBP plays a role in the dieldrin-induced histone acetylation in dopaminergic cells.
HAT Inhibitor Anacardic Acid Attenuates Dieldrin-Induced H3 and H4 Acetylation.
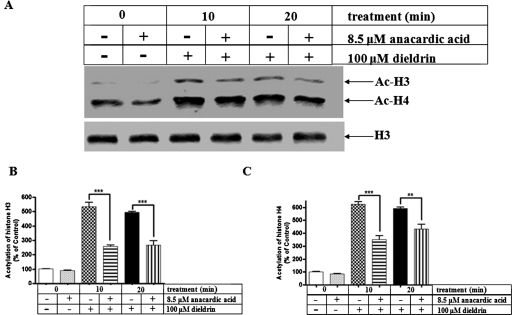
To further examine the role of hyperacetylation in the neurotoxicity induced by dieldrin in dopaminergic cells, we used anacardic acid, which has been shown to effectively inhibit HAT with an IC50 value of 8.5 μM (Balasubramanyam et al., 2003; Sun et al., 2006). N27 cells were pretreated with 8.5 μM anacardic acid for 1 h and then exposed to 100 μM dieldrin. As shown in Fig. 3A, histone (specifically on H3 and H4) acetylation dramatically increased as soon as 10 min after exposure to dieldrin alone. However, the anacardic acid treatment significantly attenuated the dieldrin-induced hyperacetylation of both H3 and H4 histones (Fig. 3, B and C), indicating that anacardic acid is an effective inhibitor of histone acetylation in dopaminergic neuronal cells.
Fig. 3.
HAT inhibitor anacardic acid attenuates dieldrin-induced H3 and H4 acetylation. A, N27 dopaminergic cells were pretreated with 8.5 μM anacardic acid for 1 h and then exposed to 100 μM dieldrin. H3 and H4 acetylation was measured from the nuclear histone extract by Western blot. Native H3 was used as an internal control. Densitometric quantification of acetylated H3 band (B) and acetylated H4 band (C). Statistical significance between the dieldrin exposed groups, with or without anacardic acid pretreatment, was determined by ANOVA, **, p < 0.01; ***, p < 0.001.
Hyperacetylation of Histones Promotes Dieldrin-Induced Caspase-3 Activation and PKCδ Proteolytic Activation in Dopaminergic Cells.
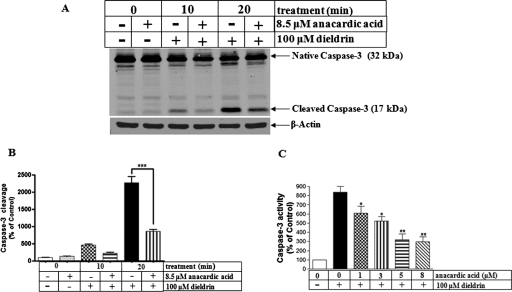
We have demonstrated previously that dieldrin induces apoptotic cell death by activating the mitochondrial-dependent pathway involving caspase-3 dependent proteolytic activation of PKCδ (Kitazawa et al., 2003). We routinely use PKCδ proteolytic activation as an apoptotic marker. To demonstrate the role of HAT, we examined whether anacardic acid would attenuate dieldrin-induced activation of PKCδ. To do this, we first reduced histone acetylation using the HAT inhibitor anacardic acid and then measured key markers of apoptosis including caspase-3 activation and PKCδ proteolytic activation during dieldrin treatment. As shown in Fig. 4A, 8.5 μM anacardic acid significantly attenuated dieldrin-induced caspase-3 proteolytic cleavage, as measured by Western blot analysis of cleaved caspase-3 product. Quantitative analysis revealed a time-dependent inhibition of caspase-3 proteolytic cleavage by anacardic acid (Fig. 4B). Furthermore, measurement of caspase-3 enzyme activity by using the fluorogenic caspase-3 substrate Ac-DEVD-AFC, revealed that anacardic acid dose-dependently attenuated dieldrin-induced caspase-3 activity (Fig. 4C), indicating that inhibition of histone acetylation can protect dopaminergic cells against neurotoxic pesticide dieldrin-induced apoptosis.
Fig. 4.
Anacardic acid attenuates dieldrin-induced caspase-3 proteolytic activation. A, N27 cells were pretreated with 8.5 μM anacardic acid for 1 h and then exposed to 100 μM dieldrin. Caspase-3 activation was measured by cleaved caspase-3 Western blot and densitometric quantification of cleaved caspase-3 band intensity (B). Statistical significance between the dieldrin exposure groups with or without anacardic acid pretreatment was determined by ANOVA, p < 0.001. C, measurement of caspase-3 enzyme activity by fluorogenic caspase-3 substrate Ac-DEVD-AFC. Asterisks (**, P < 0.01) indicate significant differences between anacardic acid pretreated and dieldrin-alone treated cells.
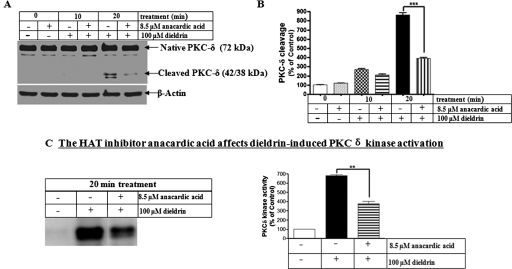
Next, we examined whether anacardic acid also can reduce dieldrin-induced proteolytic activation of the proapoptotic kinase PKCδ. Anacardic acid effectively blocked dieldrin-induced PKCδ proteolytic cleavage (Fig. 5A). The protective effect of anacardic acid was time-dependent (Fig. 5B). To determine the kinase activity resulting from PKCδ proteolytic cleavage, we measured PKCδ kinase activity in the absence of a lipid activator, as we described elsewhere (Kaul et al., 2005; Sun et al., 2008). PKCδ immunoprecipitation kinase assay was performed with the use of [32P]ATP and histone H1 substrate. A 20-min exposure to 100 μM dieldrin resulted in a 6-fold increase in PKCδ activity, compared with control cells, and pretreatment with 8.5 μM anacardic acid significantly suppressed the dieldrin-induced PKCδ kinase activity (Fig. 5C). These results indicate that anacardic acid can inhibit the activation of both caspase-3 and PKCδ induced by dieldrin exposure. Collectively, these results suggest that hyperacetylation may play a proapoptotic role in dopaminergic neuronal cells after treatment with the neurotoxic pesticide dieldrin.
Fig. 5.
The HAT inhibitor anacardic acid attenuates dieldrin-induced PKCδ proteolytic cleavage and kinase activation. A, N27 cells were pretreated with 8.5 μM anacardic acid for 1 h and then exposed to 100 μM dieldrin. PKCδ cleavage was measured in the cell lysate by immunoblotting. Equal loading of protein was demonstrated by using β-actin. B, densitometric quantification of cleaved PKCδ band statistical significance between the dieldrin-exposed groups, with or without anacardic acid pretreatment, was determined by ANOVA, p < 0.01. C, after treatment, cell lysates were collected and subjected to immunoprecipitation kinase assays as described under Materials and Methods. Phosphorylated histone bands were quantified by filmless autoradiographic analysis after scanning the dried gel, and the data are expressed as percentage of control. The values represent mean ± S.E. from two separate experiments performed in triplicate. Asterisks (**, P < 0.01) indicate significant difference compared with dieldrin-alone treated cells and anacardic acid together with dieldrin-treated N27 cells.
Inhibition of Hyperacetylation Protects Dopaminergic Cells from Dieldrin-Induced Apoptosis and Neurotoxicity.
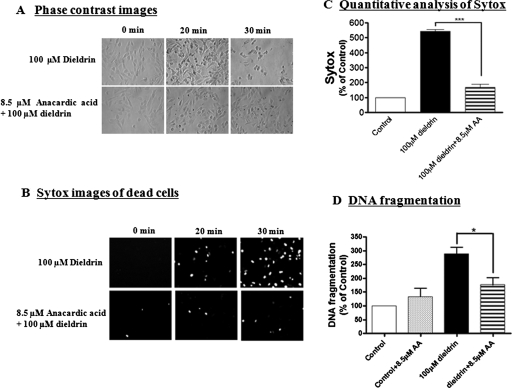
Next, we determined the effect of anacardic acid on dieldrin-induced cell death using the Sytox green cytotoxicity assay. As shown in Fig. 6, A and B, pretreatment with 8.5 μM anacardic acid for 30 min attenuated dieldrin-induced morphological changes measured by phase-contrast microscopy and Sytox green fluorescence microscopy. Quantification of Sytox fluorescence (Fig. 6C) revealed almost total protection of dieldrin-induced neurotoxicity by anacardic acid.
Fig. 6.
Anacardic acid protects against dieldrin-induced cytotoxicity and DNA fragmentation. N27 cells were pretreated with 8.5 μM anacardic acid for 1 h and then exposed to 100 μM dieldrin. Phase contrast images of N27 cells treated with dieldrin in the presence and absence of anacardic acid, B, Sytox fluorescence staining in N27 cells treated with dieldrin in the presence and absence of anacardic acid. C, Sytox green fluorescence in cells treated with dieldrin was also quantified using a microplate reader. The data represent n = 6. Asterisks (***, p < 0.001) represent significant differences between the dieldrin-treated group and the cells treated with dieldrin plus anacardic acid. D, apoptotic cell death was determined by measuring DNA fragmentation in an ELISA sandwich assay as described under Materials and Methods. Asterisks (*p < 0.05; *** p < 0.001) indicate significant difference compared with dieldrin-treated cells and dieldrin plus anacardic acid-treated cells.
DNA fragmentation has been regarded as a key marker of apoptosis; therefore, we examined whether inhibition of hyperacetylation by anacardic acid protects N27 dopaminergic neuronal cells from dieldrin-induced DNA fragmentation and cytotoxicity. N27 cells were treated with 100 μM dieldrin for 20 min in the presence or absence of 8.5 μM anacardic acid. Apoptotic cell death was determined by measuring DNA fragmentation in an ELISA sandwich assay, as we have described previously (Kaul et al., 2005; Sun et al., 2008). As shown in Fig. 6D, anacardic acid pretreatment led to a marked reduction in DNA fragmentation induced by dieldrin, showing the protective effect of the HAT inhibitor during neurotoxic response to the pesticide.
Anacardic Acid Does Not Show Antioxidant Effect.
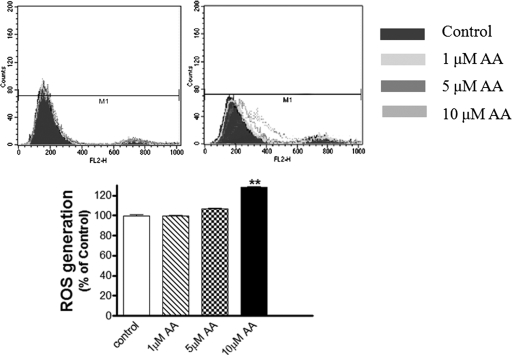
Because anacardic acid at higher concentrations has been shown to inhibit superoxide generation by acting as an antioxidant (Trevisan et al., 2006), we evaluated whether the protective function of anacardic acid was due to antioxidant properties or to HAT inhibition. Intracellular ROS generation after treatment with anacardic acid was then quantified by flow cytometry. Anacardic acid treatment did not reduce ROS generation at the concentration used in our study (Fig. 7). A slight increase in ROS generation was detected among N27 cells treated with higher doses of anacardic acid. The results from this study show that anacardic acid does not exhibit an antioxidant function; at higher concentrations, it increases ROS generation. Thus, anacardic acid reduces dieldrin-induced apoptotic cell death by inhibiting HAT and not by acting as an antioxidant.
Fig. 7.
Effect of anacardic acid on ROS generation. N27 dopaminergic cells were pretreated with 1 to 10 μM anacardic acid for 60 min, and then ROS was measured using hydroethidine in a flow cytometer. Top, representative flow cytometric histogram. Bottom, quantitative data. **, p < 0.01; ***, p < 0.001 compared with the control group (n = 3). **, p < 0.01; ***, p < 0.001 compared with the control group (n = 3).
Anacardic Acid Shows Neuroprotective Effect against Dieldrin-Induced Dopaminergic Degeneration in Primary Mesencephalic Neuronal Cultures.
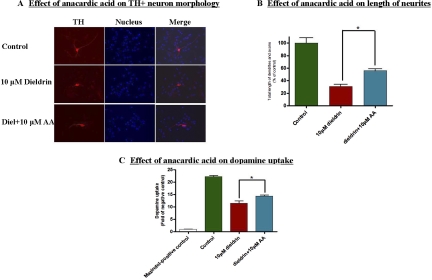
We further assessed the protective effect of anacardic acid on dopaminergic neurons using primary mesencephalic neuronal cultures. N27 cells were exposed to dieldrin for 24 h in the presence or absence of 8.5 μM anacardic acid. However, because the primary cultures will be exposed to dieldrin for 36 h, we increased the anacardic acid concentration to 10 μM. Mouse primary mesencephalic cultures were cotreated with 10 μM anacardic acid and 10 μM dieldrin, dopaminergic neuronal morphology was measured by TH immunochemistry, and the neuronal function was assessed by dopamine uptake assay. As shown in Fig. 8A, anacardic acid treatment significantly rescued the TH+ neuronal degeneration caused by dieldrin treatment. Measurement of the neurite lengths of the TH+ neurons indicated significant protection against dieldrin-induced neurotoxicity (Fig. 8B). The protective effect of anacardic acid against dieldrin toxicity was further confirmed by examination of dopamine uptake as a marker for loss of dopaminergic neurons. A [3H]DA uptake assay was performed in dieldrin and dieldrin-plus anacardic acid-treated cultures. As shown in Fig. 8C, a greater than 50% decrease in dopaminergic neuronal viability was observed after a 36-h treatment with 10 μM dieldrin, but anacardic acid effectively ameliorated dieldrin-induced loss of dopaminergic neurons. Together, these results demonstrate that the HAT inhibitor anacardic acid protects against the neurotoxic effect of dieldrin in nigral dopaminergic neurons.
Fig. 8.
Neuroprotective role of anacardic acid against dieldrin-induced dopaminergic neuronal degeneration in primary mesencephalic neuronal cultures. Mesencephalic cultures were cotreated with 10 μM dieldrin and 10 μM anacardic acid for 36 h. Immunocytochemical staining by anti-TH antibody reveals the morphology of the dopaminergic neurons, B, measurement of neurites length of TH+ neuron by Metamorph software (Molecular Devices). The data represent the mean ± S.E.M. for three samples in each group. Asterisks (*, p < 0.05, Student's t test) indicate statistically significant differences compared between dieldrin treated group and the treatment group exposed by dieldrin together with anacardic acid. C, assessment of viability of dopaminergic neurons using [3H]DA uptake assay. (*, p < 0.05).
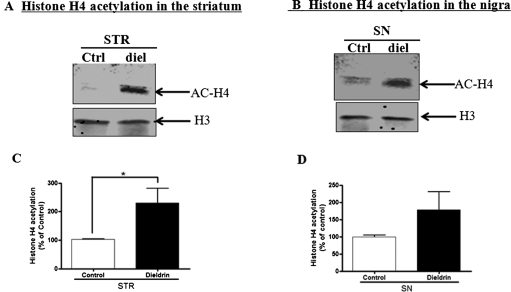
Dieldrin Exposure Induces Hyperacetylation of Histones in an Animal Model.
To validate our results obtained in cell culture models, we examined the effect of dieldrin on histone acetylation in animal models. C57BL/6J mice were treated for an intermediate term with 5.0 mg/kg via oral gavage every other day for 30 days, and then histone acetylation in the striatum and substantia nigra was measured. Intermediate-term exposure of dieldrin has been shown to produce neurochemical changes and oxidative damage in the nigrostriatal dopaminergic system (Hatcher et al., 2007). We also observed acute tremor and locomotor deficits in dieldrin-exposed animals. As shown in Fig. 9, A and B, an increased acetylation of histone H4 in both striatum and substantia nigra of dieldrin-treated animals was observed compared with the vehicle-treated group. The H4 acetylation was more pronounced in the striatum than substantia nigra (Fig. 9, C and D). This result validates our in vitro findings and suggests that environmental exposure to dieldrin can induce hyperacetylation of histones in the nigrostriatal dopaminergic system.
Fig. 9.
Hyperacetylation of histones in the substantia nigra and striatum in animal model of dieldrin neurotoxicity. C57 black mice were treated with 5 mg/kg dieldrin via oral gavage every other day for 30 days. B, acetylation of histones H3 and H4 was examined in the striatum and substantia nigra by Western blot with anti-acetyl-Lys antibody. Native H3 was used as an internal control. Densitometric quantification of acetylated H4 band in the striatum (C) and substantia nigra (D) are presented. Asterisks (*, p < 0.05, Student's t test) indicate statistically significant differences between dieldrin-treated group and the vehicle control group.
Discussion
In the present study, we demonstrate that dieldrin increases histones acetylation in dopaminergic neuronal cells, an event known to modulate cellular function. Major findings from our present study are as follows: 1) dieldrin rapidly can induce the hyperacetylation of histones, specifically histone H3 and H4, as early as 10 min after the start of dieldrin exposure in dopaminergic neuronal cells; 2) long-term dieldrin exposure in a mouse model also induces histone hyperacetylation in the striatum and substantia nigra; 3) dieldrin-induced histone acetylation is attributed to accumulation of a major histone acetyl transferase CBP, resulting from inhibition of proteasomal function; 4) HAT-inhibitor anacardic acid effectively attenuates dieldrin-induced hyperacetylation as well as the apoptotic cascade that includes caspase-3 activation, PKCδ proteolytic activation and DNA-fragmentation; 5) anacardic acid also shows a neuroprotective effect against dieldrin-induced nigral dopaminergic neuronal degeneration in primary mesencephalic culture models; and 6) the neuroprotective effect of anacardic acid is independent of its antioxidant effect. Collectively, these findings indicate that histone hyperacetylation is an early signaling event in the execution of apoptosis after neurotoxic exposure to the environmental toxicant dieldrin. To our knowledge, this is the first report of histone modification during the apoptotic cell death of nigral dopaminergic neurons after exposure to an environmental neurotoxicant.
A number of observations show that histone acetylation plays an important role in gene transcription, chromatin remodeling, development and oncogenesis (Yin et al., 2007; Gupta et al., 2008). Most strikingly, recent studies using disease models show that the balance in histone acetylation/deacetylation can be a determining factor in mediating cell survival and cell death (Marchion and Münster, 2007; Soriano et al., 2009). For example, an HDAC inhibitor, which induces histone hyperacetylation, could cause death in cancer cells (Aron et al., 2003; Condorelli et al., 2008). Histone acetylation and deacetylation also play very important roles in neurodegenerative models (Saha and Pahan, 2006; Selvi and Kundu, 2009). Studies have shown that HDAC inhibitors might slow or prevent neurodegeneration in the Huntington's disease model (Steffan et al., 2001). On the contrary, overexpression of HDAC-4 prolongs neuronal survival in a mouse model of retinal degeneration (Chen and Cepko, 2009). These findings further emphasize that any significant alterations in the critical balance of histone acetylation/deacetylation may contribute to degenerative processes. In the present study, we provide evidence that dieldrin can cause rapid impairment in homeostasis of histone acetylation in dopaminergic neuronal cells. Our data also show that the small-molecule HAT inhibitor anacardic acid can effectively suppress dieldrin-induced hyperacetylation of histones, suggesting that the increase in HAT during dieldrin treatment is responsible for the acetylation of histones. In addition, dieldrin-induced cytotoxicity is greatly blocked by anacardic acid, demonstrating that hyperacetylation plays a role in the cell death processes of neuronal cells during neurotoxic insult. We previously showed that dieldrin induces generation of ROS during dopaminergic neuronal cell death (Kitazawa et al., 2001) and that dieldrin induces apoptosis in dopaminergic neuronal cells through a caspase-3 and PKCδ-dependent manner (Kitazawa et al., 2003). The results of the present study suggest that the increase in histone acetylation contributes to apoptotic cell death, which may be an early step before the downstream cellular apoptotic process, including caspase-3 and PKCδ activation.
HATs and HDACs are the enzymes that influence histone acetylation levels in cells. Maintenance of the precise balance of HATs and HDACs is critical to cell survival, and aberrant changes in the homeostasis of HATs and HDACs can induce neuronal cell death (Saha and Pahan, 2006). Rouaux et al. (2003) found a critical loss of CBP/p300 histone acetylase activity in different models of neuronal cell death including K+-deprived cerebellar granule neuron apoptosis, amyloid precursor protein activation-induced neuronal apoptosis, and motor neuron degeneration. They further demonstrated that CBP is specifically targeted by caspases during the onset of neuronal apoptosis (Rouaux et al., 2003). In contrast, other studies have shown that treatment with the HDAC inhibitor trichostatin A or overexpression of CBP can induce hyperacetylation of histones and neuronal apoptosis (Salminen et al., 1998; Boutillier et al., 2003).
To better understand how dieldrin changes the level of histone acetylation, we investigated the possibility that HAT also participates in the process. Our results show that the protein level of CBP, a well known HAT, increases in a time-dependent manner during dieldrin treatment. It is noteworthy that the time-course increase in the CBP level mirrored the time course of hyperacetylation, revealing the interrelationship between dieldrin-induced CBP and hyperacetylation. CBP levels change in various disease models, including neurodegenerative diseases (Rouaux et al., 2003); like other short-lived regulatory proteins, CBP is degraded through the ubiquitin-proteasome pathway (Yan et al., 2003). The ubiquitin proteasomal system is present not only in the cytoplasm but also in the nucleus and may be directly involved in gene expression (Bader et al., 2007; Scharf et al., 2007). We have shown previously that dieldrin very rapidly impairs the proteasomal function and causes the accumulation of ubiquitin-conjugated protein within dopaminergic cells (Sun et al., 2005). We also showed that mutation of various polyubiquitination sites can differentially regulate fragmentation of nuclear DNA during dieldrin treatment (Sun et al., 2009). Based on these observations, it is likely that increased CBP protein in both cytoplasmic and nuclear fractions, observed in the present study, may be due to inhibition of cytosomal and nuclear ubiquitin proteasomal systems by dieldrin. The accumulated CBP eventually contributes to increased acetylation of histones in treated cells. Consistent with the hypothesis, we show that in addition to dieldrin, MG-132, a well known proteasome inhibitor, can also increase CBP protein levels. We also show that both dieldrin and MG-132 inhibited 70 to 90% of proteasome activity within 10 min, which is the time point in which significant hyperacetylation started to occur during dieldrin treatment. Furthermore, cells transfected by siRNA specific for CBP completely suppressed histone hyperacetylation induced by dieldrin, establishing the role for CBP in histone acetylation during dieldrin treatment. We cannot completely rule out the possibility that decreases in HDAC activity may contribute to hyperacetylation because we did not examine the effect of dieldrin on HDAC in the present study. Future efforts may be undertaken to examine HDAC activity; however, based on these results, we propose that the dramatic increase in histone acetylation may be due in part to an increase in the CBP level resulting from inhibition of proteasomal function during dieldrin treatment.
Anacardic acid, extracted from cashew nut shells, is reported to be a potent inhibitor of the histone acetyltransferase CBP/p300/PCAF or Tip60. The IC50 of anacardic acid was determined to be 8.5 μM in cell-free assays (Balasubramanyam et al., 2003). Recent reports have shown that anacardic acid has significant potential for development of anticancer therapeutics (Sun et al., 2006; Eliseeva et al., 2007). Besides being a CBP/p300/PCAF inhibitor, anacardic acid has been reported to have antioxidant activity (Trevisan et al., 2006). In our study, measurement of ROS during anacardic acid treatment revealed no antioxidant effect. In fact, we observed a slight increase in ROS after treatment with higher doses of anacardic acid. However, anacardic acid was effective in attenuating the increase in histone acetylation and the activation of caspase-3 and proapoptotic kinase PKCδ. Anacardic acid also attenuated dopaminergic neuronal cell death in primary mesencephalic cultures, suggesting that anacardic acid and its analogs may serve as a neuroprotective agent against dopaminergic degeneration. We attribute the neuroprotective effect of anacardic acid on dopaminergic neuronal cells to its HAT-inhibitory properties.
Although our study is mainly focused on neuronal cells, both astrocytes and microglia also might influence the overall neurotoxic effect in in vivo conditions. Valproate, an anticonvulsant with significant inhibitory properties on HDAC, has been shown to protect midbrain dopaminergic neurons by stimulating neurotrophic factor release from adjacent astrocytes (Chen et al., 2006). The role of microglia in neurotoxicant-induced dopaminergic degeneration has been increasingly realized. A recent study demonstrated that dieldrin induced a dose-dependent generation of ROS in microglia, resulting in enhanced neurotoxicity (Mao et al., 2007). It is possible that dieldrin neurotoxicity may result from a complex interaction between astrocytes, microglia, and neurons. The function of HAT/HDAC in neurotoxicity is beginning to emerge, but a detailed understanding of the role of histone acetylation in glial cells during neurotoxic insults may shed more light on the molecular mechanisms of dopaminergic degeneration.
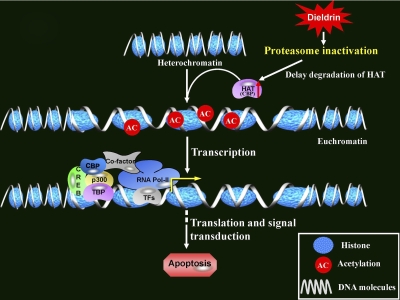
In summary, we present a novel finding that the environmental neurotoxicant dieldrin induces hyperacetylation of histones as an early event after neurotoxic insult, mainly because of increases in CBP resulting from proteasomal dysfunction (Fig. 10). We also show that the HAT inhibitor anacardic acid has a neuroprotective effect against dieldrin-induced neurotoxicity, indicating the translational potential of HAT inhibitors in the neurodegenerative process of dopaminergic neurons. These results advance the understanding of environmental neurotoxicant exposure and epigenetic mechanisms in the pathogenesis of Parkinson's disease and may facilitate the development of promising therapeutic treatments of this devastating disease.
Fig. 10.
Schematic representation of mechanisms underlying dieldrin-induced hyperacetylation. Exposure to neurotoxic insult dieldrin inhibits proteasome dysfunction, resulting in accumulation of a major HAT CBP. Increased CBP results in greater acetylation of nuclear histones in the chromatin, which ultimately results in alterations of gene expression associated with the neurodegenerative process, including oxidative damage and apoptosis in dopaminergic neurons. The symbols used in the scheme were taken from the SABiosciences Corporation web site.
Acknowledgments
We thank Mary Ann deVries for assistance in the preparation of the manuscript.
This work was supported by National Institutes of Health National Institute of Environmental Health Sciences [Grant ES10586]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants NS38644, NS45133]; and The W. Eugene and Linda Lloyd Endowed Chair (to A.G.K.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.062174.
- PD
- Parkinson's disease
- PKCδ
- protein kinase C δ
- HDAC
- histone deacetylase
- HAT
- histone acetyltransferase
- AFC
- 7-amino-4-methylcoumarin
- Ac-DEVD-AFC
- acetyl-Asp-Glu-Val-Asp-AFC
- ELISA
- enzyme-linked immunosorbent assay
- CBP
- CREB-binding protein
- CREB
- cAMP response element-binding protein
- HBSS
- Hanks' balanced salt solution
- PBS
- phosphate-buffered saline
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate
- ROS
- reactive oxygen species
- DA
- dopamine
- siRNA
- small interfering RNA
- TH
- tyrosine hydroxylase
- PCAF
- P300/CBP-associated factor
- ANOVA
- analysis of variance
- MG-132
- N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal.
References
- Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. (2009) Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson's disease. Toxicol Appl Pharmacol 240:273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron JL, Parthun MR, Marcucci G, Kitada S, Mone AP, Davis ME, Shen T, Murphy T, Wickham J, Kanakry C, et al. (2003) Depsipeptide (FR901228) induces histone acetylation and inhibition of histone deacetylase in chronic lymphocytic leukemia cells concurrent with activation of caspase 8-mediated apoptosis and down-regulation of c-FLIP protein. Blood 102:652–658 [DOI] [PubMed] [Google Scholar]
- Bader N, Jung T, Grune T. (2007) The proteasome and its role in nuclear protein maintenance. Exp Gerontol 42:864–870 [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. (2003) Small molecule modulators of histone acetyltransferase p300. J Biol Chem 278:19134–40 [DOI] [PubMed] [Google Scholar]
- Boutillier AL, Trinh E, Loeffler JP. (2003) Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J Neurochem 84:814–828 [DOI] [PubMed] [Google Scholar]
- Campoy C, Jiménez M, Olea-Serrano MF, Moreno-Frías M, Cañabate F, Olea N, Bayés R, Molina-Font JA. (2001) Analysis of organochlorine pesticides in human milk: preliminary results. Early Hum Dev 65 (Suppl):S183–S190 [DOI] [PubMed] [Google Scholar]
- Chen B, Cepko CL. (2009) HDAC4 regulates neuronal survival in normal and diseased retinas. Science 323:256–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Tini M, Evans RM. (2001) HATs on and beyond chromatin. Curr Opin Cell Biol 13:218–224 [DOI] [PubMed] [Google Scholar]
- Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, et al. (2006) Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry 11:1116–1125 [DOI] [PubMed] [Google Scholar]
- Condorelli F, Gnemmi I, Vallario A, Genazzani AA, Canonico PL. (2008) Inhibitors of histone deacetylase (HDAC) restore the p53 pathway in neuroblastoma cells. Br J Pharmacol 153:657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan FM, French M, Murray L. (1996) Organochlorine compounds in human brain. Hum Exp Toxicol 15:262–264 [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. (2000) Organochlorine insecticides in substantia nigra in Parkinson's disease. J Toxicol Environ Health A 59:229–234 [DOI] [PubMed] [Google Scholar]
- Eliseeva ED, Valkov V, Jung M, Jung MO. (2007) Characterization of novel inhibitors of histone acetyltransferases. Mol Cancer Ther 6:2391–2398 [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. (1994) Parkinson's disease and brain levels of organochlorine pesticides. Ann Neurol 36:100–103 [DOI] [PubMed] [Google Scholar]
- Gupta A, Guerin-Peyrou TG, Sharma GG, Park C, Agarwal M, Ganju RK, Pandita S, Choi K, Sukumar S, Pandita RK, et al. (2008) The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol Cell Biol 28:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S, Hottiger MO. (2002) Histone acetyl transferases: a role in DNA repair and DNA replication. J Mol Med 80:463–474 [DOI] [PubMed] [Google Scholar]
- Hatcher JM, Richardson JR, Guillot TS, McCormack AL, Di Monte DA, Jones DP, Pennell KD, Miller GW. (2007) Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol 204:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson JL. (2001) Aldrin and dieldrin: a review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ Health Perspect 109 (Suppl 1):113–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. (2005) Dieldrin-induced neurotoxicity: relevance to Parkinson's disease pathogenesis. Neurotoxicology 26:701–719 [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Yang Y, Anantharam V, Kanthasamy A. (2008) Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): Implications for neurodegeneration in Parkinson's disease. Mol Brain 1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Kanthasamy A, Kanthasamy AG. (2005) Wild-type alpha-synuclein interacts with pro-apoptotic proteins PKCdelta and BAD to protect dopaminergic neuronal cells against MPP+-induced apoptotic cell death. Brain Res Mol Brain Res 139:137–152 [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. (2001) Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med 31:1473–1485 [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. (2003) Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience 119:945–964 [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Muller M, Yang MC, Mellick GD, McLean AJ. (2002) Age-environment and gene-environment interactions in the pathogenesis of Parkinson's disease. Rev Environ Health 17:51–64 [DOI] [PubMed] [Google Scholar]
- Mao H, Fang X, Floyd KM, Polcz JE, Zhang P, Liu B. (2007) Induction of microglial reactive oxygen species production by the organochlorinated pesticide dieldrin. Brain Res 1186:267–274 [DOI] [PubMed] [Google Scholar]
- Marchion D, Münster P. (2007) Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther 7:583–598 [DOI] [PubMed] [Google Scholar]
- Mattson MP. (2003) Methylation and acetylation in nervous system development and neurodegenerative disorders. Ageing Res Rev 2:329–342 [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppedè F. (2009) Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res 674:73–84 [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. (2000) A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology 21:435–440 [PubMed] [Google Scholar]
- Rouaux C, Jokic N, Mbebi C, Boutillier S, Loeffler JP, Boutillier AL. (2003) Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J 22:6537–6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. (2006) HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ 13:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Tapiola T, Korhonen P, Suuronen T. (1998) Neuronal apoptosis induced by histone deacetylase inhibitors. Brain Res Mol Brain Res 61:203–206 [DOI] [PubMed] [Google Scholar]
- Scharf A, Rockel TD, von Mikecz A. (2007) Localization of proteasomes and proteasomal proteolysis in the mammalian interphase cell nucleus by systematic application of immunocytochemistry. Histochem Cell Biol 127:591–601 [DOI] [PubMed] [Google Scholar]
- Selvi RB, Kundu TK. (2009) Reversible acetylation of chromatin: implication in regulation of gene expression, disease and therapeutics. Biotechnol J 4:375–390 [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. (2002) An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci 22:7006–7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somech R, Izraeli S, Simon AJ. (2004) Histone deacetylase inhibitors—a new tool to treat cancer. Cancer Treat Rev 30:461–472 [DOI] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Bell KF, Hardingham GE. (2009) Role of histone acetylation in the activity-dependent regulation of sulfiredoxin and sestrin 2. Epigenetics 4:152–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Zhu YZ, Greenwald M, et al. (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413:739–743 [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. (2005) Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther 315:69–79 [DOI] [PubMed] [Google Scholar]
- Sun F, Kanthasamy A, Anantharam V, Kanthasamy AG. (2007) Environmental neurotoxic chemicals-induced ubiquitin proteasome system dysfunction in the pathogenesis and progression of Parkinson's disease. Pharmacol Ther 114:327–344 [DOI] [PubMed] [Google Scholar]
- Sun F, Kanthasamy A, Anantharam V, Kanthasamy AG. (2009) Mitochondrial accumulation of polyubiquitinated proteins and differential regulation of apoptosis by polyubiquitination sites Lys-48 and -63. J Cell Mol Med 13:1632–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Kanthasamy A, Song C, Yang Y, Anantharam V, Kanthasamy AG. (2008) Proteasome inhibitor-induced apoptosis is mediated by positive feedback amplification of PKCdelta proteolytic activation and mitochondrial translocation. J Cell Mol Med 12:2467–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jiang X, Chen S, Price BD. (2006) Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett 580:4353–4356 [DOI] [PubMed] [Google Scholar]
- Trevisan MT, Pfundstein B, Haubner R, Würtele G, Spiegelhalder B, Bartsch H, Owen RW. (2006) Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem Toxicol 44:188–197 [DOI] [PubMed] [Google Scholar]
- Yan F, Gao X, Lonard DM, Nawaz Z. (2003) Specific ubiquitin-conjugating enzymes promote degradation of specific nuclear receptor coactivators. Mol Endocrinol 17:1315–1331 [DOI] [PubMed] [Google Scholar]
- Yin W, Barkess G, Fang X, Xiang P, Cao H, Stamatoyannopoulos G, Li Q. (2007) Histone acetylation at the human beta-globin locus changes with developmental age. Blood 110:4101–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Yang Y, Anantharam V, Kanthasamy A. (2007) Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci 27:5349–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]