To the editor: Leber’s congenital amaurosis, a common cause of blindness in infants and children,1 recently became the first human genetic retinal disease to show improved vision in response to treatment. Patients with mutations in the gene encoding retinal pigment epithelium–specific 65-kD protein (RPE65) had gains in vision within weeks after subretinal injection of a vector containing the gene in one eye.2–5 At 1-year follow-up after gene therapy, the three young adult patients in our trial4,5 remained without serious adverse events.
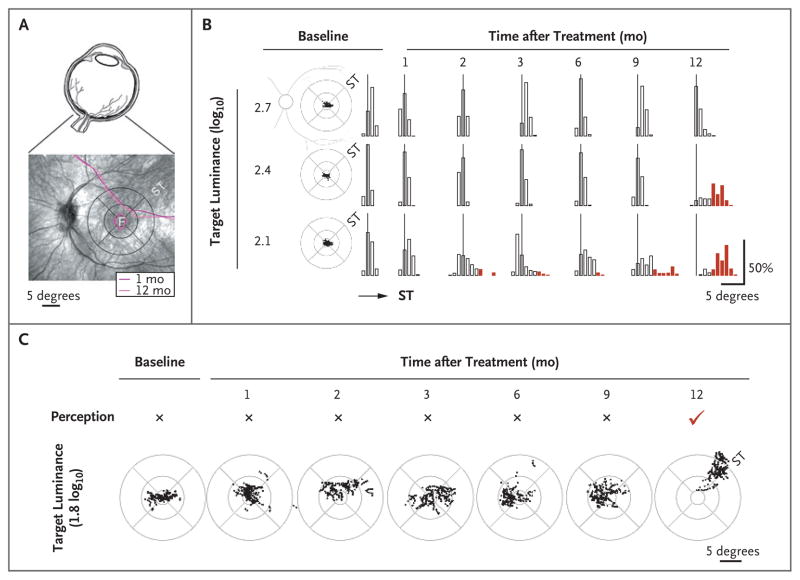
A noteworthy observation in one patient at 1 year after treatment prompted further studies. For the first time in her life, the patient reported that she could read the illuminated numerical clock display on the dashboard of the family vehicle while she was sitting in the front seat. The numerals subtended a visual angle equivalent to a visual acuity of 20/200, which is not different from her formally measured visual acuities at baseline or at 1 year after treatment. The simplest explanation of this development would be increased visual sensitivity either at the fovea or in the treated region of the superotemporal retina. However, visual sensitivity (measured by means of microperimetry) was unchanged at this visit as compared with earlier post-treatment visits (Fig. 1A).
Figure 1. Slow Emergence of a Pseudo-Fovea within the Treated Retinal Region and Perception of Previously Unseen Stimuli.
Panel A shows the eye (upper image) and the patient’s retina (lower image). Overlaid contours of constant sensitivity (measured by means of microperimetry) show no change in visual sensitivity between 1 and 12 months after treatment. F denotes the fovea, and ST the superotemporal retina that received treatment. The circular pattern is a standard grid centered on the fovea. Retinal distance calibration corresponding to 5 degrees of visual angle is shown. Panel B shows fixation clouds (scatter plots) in the study eye of the patient at baseline and the statistics of fixation dwell time (bar graphs) along the diagonal meridian as a function of the target luminance. All three luminances were perceived by the patient at all visits. At the 2.7- and 2.4-log10 luminances, fixation was within 3 degrees of the fovea more than 99% of the time at all visits except at the 12-month visit for 2.4-log10 luminance, when 68% of fixation time dwelled in an ST retinal region 4 to 9 degrees from the fovea. At 2.1-log10 luminance, fixations showed increasingly greater excursions into the ST retina between 2 and 9 months after treatment. At 12 months, 89% of fixation time dwelled in the ST region 4 to 9 degrees from the fovea. Thin vertical lines represent the foveal location. Red bars indicate significant (>3 degrees) excursions from the fovea. Panel C shows that a dimmer target (1.8 log10) was not perceived by the patient’s study eye during baseline though 9 months after treatment. At 12 months, this stimulus was perceived for the first time with a coincident shift of fixation into the ST retinal region.
We sought to determine the basis of this development by quantifying fixation of the patient’s gaze to dim targets over a range of luminances straddling her perception. At baseline, the patient had foveal fixation in both eyes over a range of target luminances from 2.1 to 2.7 log10 units higher than the normal foveal perceptual threshold (Fig. 1B), and the results were like those of other patients with Leber’s congenital amaurosis caused by RPE65 mutations and similar visual-acuity levels.5
Fixation dwell time, quantified along the diagonal meridian with a range of target luminances perceived by the patient, suggested a slow emergence of visual gain over many months causing progressively greater fixational use of the treated superotemporal retina (Fig. 1B). This gain was particularly evident at lower luminances. By 12 months after treatment, the patient reported perception of the lowest luminance target (1.8 log10) for the first time. This target was not seen during any previous visit. New perception was accompanied by a distinct shift in fixation into the treated superotemporal retina (Fig. 1C, and video in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Cone sensitivities in the control and study eyes of the patient were rendered as three-dimensional images on the view of the ocular fundus with a superimposed circular grid (Fig. 1 in the Supplementary Appendix). Foveal sensitivities in the two eyes were similar, but the superotemporal region of the treated eye, the “pseudo-fovea,” was remarkably different from the cone blindness in the comparable region of the control eye.
The change in fixation by the patient was driven by the treatment-created extrafoveal cone vision with better sensitivity and greater expanse than the untreated foveal region (Fig. 1 in the Supplementary Appendix).4,5 The unexpected late emergence of visual gain in the patient to spatially coded and sustained stimuli and a coincident change in preference for fixation from the fovea to the treated retinal region suggest a slow development of a pseudo-fovea and an underlying experience-dependent plasticity of the adult visual system. These results raise the possibility that this gene-based therapy may further improve visual function in an unexpected and useful way in previously untreatable congenital blindness.
Supplementary Material
Acknowledgments
Supported by a grant from the National Eye Institute of the National Institutes of Health, Department of Health and Human Services (U10 EY017280).
Drs. Byrne and Hauswirth report having a financial interest in the use of adeno-associated virus (AAV) therapies and owning equity in Applied Genetics Technologies, a company that might, in the future, commercialize some aspects of this work; Dr. Kaushal, serving as a principal investigator of a clinical trial of AAV-RPE65 to treat Leber’s congenital amaurosis sponsored by Applied Genetics Technologies; and Drs. Hauswirth and Jacobson, being coinventors on a patent (20070077228) held by the University of Pennsylvania, the University of Florida, and Cornell University on “a method for treating or retarding the development of blindness” (Dr. Jacobson has waived all claims to any financial benefit as a coinventor on the patent). No other potential conflict of interest relevant to this letter was reported.
Contributor Information
Artur V. Cideciyan, Email: cideciya@mail.med.upenn.edu, University of Pennsylvania, Philadelphia, PA
William W. Hauswirth, University of Florida, Gainesville, FL
Tomas S. Aleman, University of Pennsylvania, Philadelphia, PA
Shalesh Kaushal, University of Florida, Gainesville, FL
Sharon B. Schwartz, University of Pennsylvania, Philadelphia, PA
Sanford L. Boye, University of Florida, Gainesville, FL
Elizabeth A.M. Windsor, University of Pennsylvania, Philadelphia, PA
Thomas J. Conlon, University of Florida, Gainesville, FL
Alexander Sumaroka, University of Pennsylvania, Philadelphia, PA
Alejandro J. Roman, University of Pennsylvania, Philadelphia, PA
Barry J. Byrne, University of Florida, Gainesville, FL
Samuel G. Jacobson, University of Pennsylvania, Philadelphia, PA
References
- 1.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Bainbridge JWB, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 3.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–90. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–7. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.