Abstract
Tight junctions (TJs) are essential for normal function of epithelia, restricting paracellular diffusion and contributing to the maintainance of cell surface polarity. Superficial cells of the urothelium develop TJs, the basis for the paracellular permeability barrier of the bladder against diffusion of urinary solutes. Focusing on the superficial cell layer of stratified cell cultures of an immortalized human ureteral cell line, TEU-2 cells, we have examined the presence of TJ and TJ-associated proteins. TEU-2 cells were treated with calcium chloride and fetal bovine serum culture conditions used to induce stratification that resembles the normal transitional epithelial phenotype. Cultures were examined for TJ and TJ-associated proteins by confocal immuno-fluorescence microscopy and evaluated for TJ mRNA by reverse transcriptase-polymerase chain reaction (RT- PCR). TEU-2 cultures exhibited immunoreactivity at intercellular margins for claudins 1, 4, 5, 7, 14 and 16 whereas claudins 2, 8 and 12 were intracellular. RT-PCR corroborated the presence of these claudins at the mRNA level. The TJ-associated proteins occludin, JAM-1, and zonula occludens (ZO-1, ZO-2 and ZO-3) were localized at cell margins. We have found that numerous TJs and TJ-associated proteins are expressed in stratified TEU-2 cultures. Further, we propose TEU-2s provide a useful ureteral model for future studies on the involvement of TJs proteins in the normal and pathological physiology of the human urinary system.
Keywords: urothelium; tight junctions; claudins, cell culture
Introduction
The urothelium consists of basal, intermediate, and superficial cells lining the urinary tract from the renal pelvis to the proximal urethra (review, Lewis 2000). Superficial cells develop tight junctions (TJs) that restrict urine movement across the urothelium. Claudins, along with occludin and junction adhesion molecules (JAM), are integral membrane proteins that comprise the barrier elements whereas TJ-associated proteins zonula occludens (ZOs) in the cell cortex link the transmembrane proteins to cytoskeletal elements. Twenty four claudin isoforms and numerous TJ and TJ-associated proteins have been reported in mammals (reviews, Turksen and Troy 2004, Aijaz et al. 2006).
Occludin, ZO-1, claudin 4, 8, and 12 proteins and claudin 2, 4, 8, 12, and 13 mRNA have been identified in the urothelium of animal models (Acharya et al. 2004). Claudins 1, 4, occludin and and ZO-1 in cultured human urothelial cells and a differentiation-associated profile of claudins 3, 4, 5, 7, ZO-1 and occludin for normal utereric urothelium in situ has been reported (Cross et al. 2005, Varley et al. 2006). These investigators employed human primary and subcoultured cells which have a finite lifespan.
TEU-2 cells are an immortalized cell line derived from normal human ureter (Klumpp et al. 2001) that form a stratified epithelium in vitro and thus provide a potential model for studies on the presence, distribution and junctional properties of TJs. Our observations characterize the presence and distribution of specific TJ elements in cultured urothelial cells and provide reference data for future studies on the involvement of TJs in pathological conditions of the urinary system.
Materials and Methods
Cell Culture
TEU-2 cells were generated by immortalization of normal human ureteral cells with human papillomavirus type 16E6E7 (Klumpp et al. 2001). Cultures were grown in EpiLife®. Medium with 60 µM calcium chloride and growth factor supplements (Cascade Biologics, Inc. Portland, OR), 20units/ml penicillin and 100 µg/ml streptomycin (Sigma Chemical Company, St. Louis, MO). Post confluence, cells were grown 3 days in the same medium with 10% fetal bovine serum (FBS) and 1.4 mM calcium chloride.
Adenocarcinoma colon (Caco-2, HTB-37), ovarian adenocarcinoma (SW 626, HTB-78) and Madin Darby canine kidney cells (MDCK, CCL-34) were obtained from American Type Culture Collection (Rockville, MD).
RT-PCR
Total RNA was isolated from TEU-2 cells using the Versagene RNA Cell Kit with on-column DNase treatment (Gentra Systems, Minneapolis, MN). First strand cDNA was synthesized using random hexamer primers with the ThermoScript RT-PCR System (Invitrogen, Carlsbad, CA). Claudins were PCR amplified using PCR with 8 touchdown cycles of 94°C 15s, 70 to 56 °C 30s, 72 °C 2 min, followed by 35 regular cycles: 94°C 15s, 54 °C 30s, 72 °C 2 min. Amplicons were separated on 1.5% agarose gels, ethidium bromide stained and visualized on a UV transilluminator.
Confocal Immuno-fluorescence Microscopy
Cultured TEU-2 cells were washed in phosphate buffered saline, 1 mM calcium chloride, 37° C, fixed in cold methanol, washed, and refrigerated until the time of staining. Cells were treated on ice with 0.5% Triton X-100, 10 mM piperazine ethane sulfonic acid, 50 mM NaCl, 300 mM sucrose and 3 mM MgCl2, pH 6.8 for 2 minutes, washed, blocked, and incubated with primary antibodies to occludin, JAM-1, ZOs, claudins (Invitrogen Corporation, Frederick, MD) or non-immune serum. Following treatment with Alexa Fluor secondary antibodies (Molecular Probes, Eugene, OR), samples were viewed by confocal microscopy [MRC 1024: Bio-Rad, Hercules, CA)], and processed [Confocal Assist (Todd Clark Brelje, University of Minnesota), Image J software and Image Pro Plus (MediaCybernetics, Silver Spring, MD).
Light and Transmission Electron Microscopy
Cultured TEU-2 cells were fixed with 3.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.25), 5%sucrose, 2 mM calcium chloride for 3 hours and then for 16 hours in fresh fixative at 4°C. Cells were washed in 0.1 M sodium cacodylate buffer, 5% sucrose at room temperature and postfixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer, 5% sucrose for 3 hours. Cells were washed in water, incubated 1 hour in 2% aqueous uranyl acetate, dehydrated through graded ethanols to 100%, rinsed in propylene oxide, and infiltrated with a 1:1 mixture of Polybed resin (Polysciences, Inc., Warrington, PA) and propylene oxide for 3 hours. Cells were incubated in Polybed resin for 3 hours, transferred to fresh resin, and polymerized overnight at 70°C. Small plastic blocks containing selected cell areas were cut, attached to blank plastic stubs, trimmed and sectioned using a Reichert Ultracut E ultramicrotome (Depew, NY). “Thick” (0.5 µm) sections were cut, heat attached to glass slides, stained with toluidine blue and examined with a light microscope. Ultrathin (0.05 µm) sections were cut with a diamond knife, collected on 200 mesh copper grids, poststained with uranyl acetate and lead citrate, and photographed with a JEOL (Peabody, MA) 100CX transmission electron microscope. Negatives were scanned with an Epson V700 scanner and processed using Microsoft Power Point software.
Scanning Electron Microscopy
Cells were processed as described above up to 100% ethanol dehydration. Membranes were critically point dried in a Tousimis Research Corporation Autosamdri-810 using CO2 as transitional solvent, mounted on aluminum stubs, and gold-palladium coated in a Polaron Instruments E5100 SEM. Samples were photographed with a JEOL (Peabody, MA) 5800 scanning electron microscope.
Results
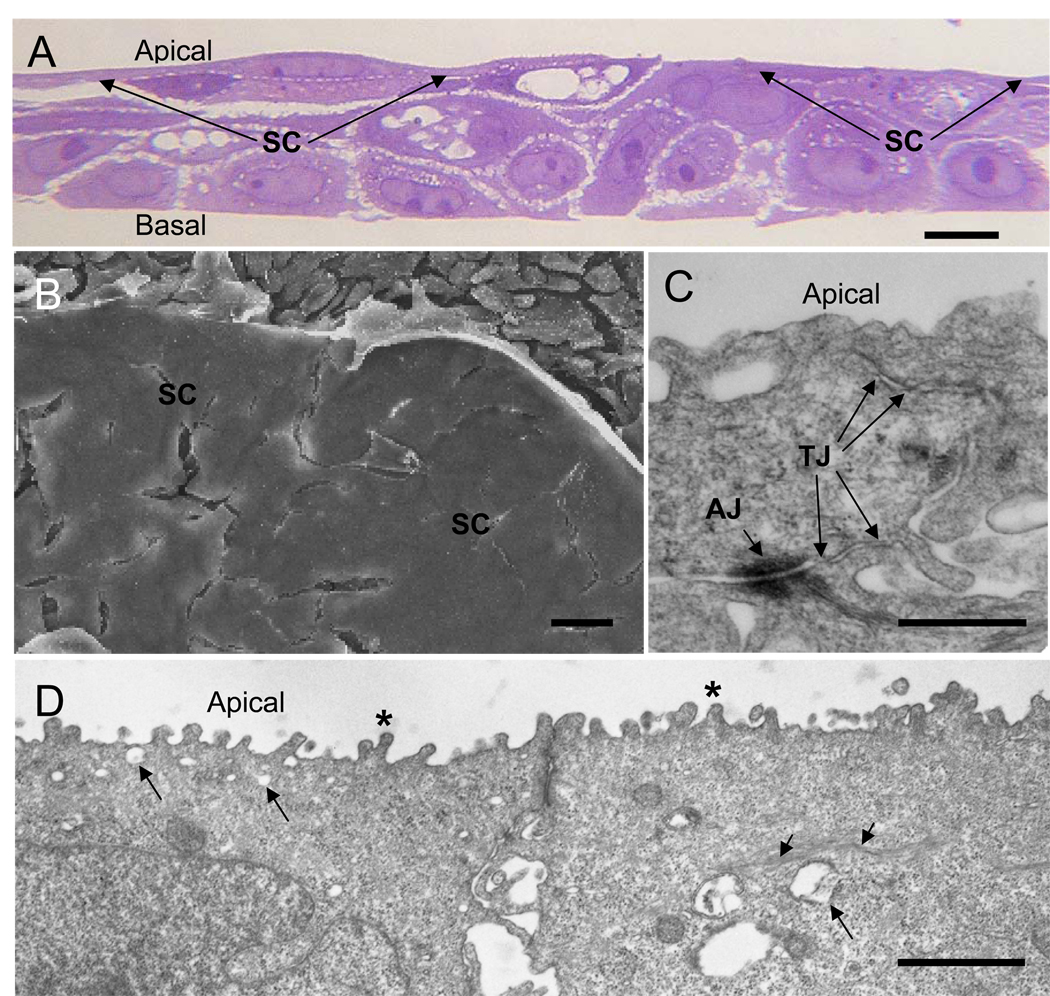
Increasing the calcium concentration in the culture medium of normal human urothelial cells induces stratification (Southgate et al. 1994). Under culture conditions with 1.4 mM calcium and 10% FBS, TEU-2 cells differentiate into a stratified epithelium consisting of thin, elongated and tightly apposed apical cells and more loosely connected underlying cells (Fig. 1a). The apical surface appears flat and in areas where the superficial cell layer has pulled away, the loosely connected underlying cells are apparent (Fig. 1b). The superficial cells form TJs and adherens junctions (Fig. 1c), and develop stubby apical microvilli, clear vesicles in the apical cytoplasm, cytoplasmic strands of tonofilaments and adherens junctions (Fig. 1d), all characteristic features of urothelial superficial cells in vivo.
Figure 1.
TEU-2 cells differentiate into a stratified epithelial culture consisting of thin, tightly apposed apical superficial cells and more loosely connected underlying cells. (a) Light micrograph of cultured TEU-2 cells stained with toluidine blue. Apical and basal surfaces are indicated and the superficial cell (SC) layer is indicated with arrows. (b) SEM image of the apical surface of TEU-2 cell culture. The apical superficial cell (SC) surface appears flat and featureless. The loosely connected underlying cells are apparent where the SC layer has separated from the underlying cells during processing. (c) TEM of apical tight junctions (TJ) and an adhering junction (AJ) between two SCs. (d) Lower magnification TEM of SCs illustrating the stubby microvilli (asterisks), the clear cytoplasmic vesicles (long arrows), the strands of tonofilament fibers (short arrows) and adhering junctions. Magnification bars in a, b, c and d are 10, 10, 0.5 and 1 µm, respectively.
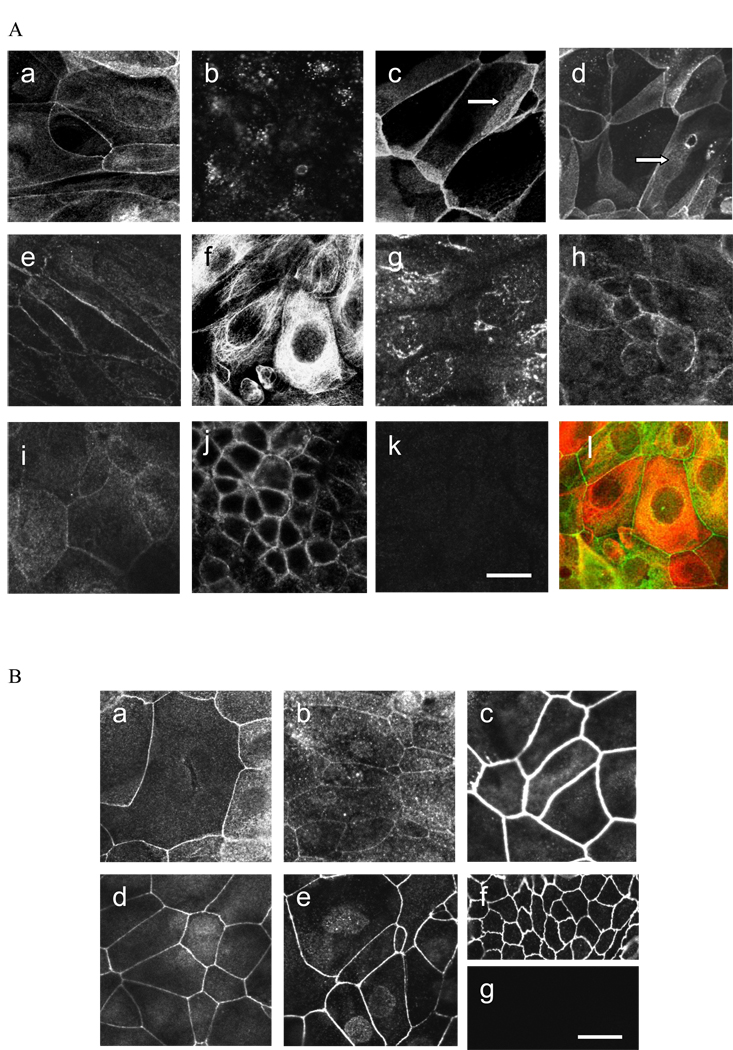
Because TJ are located in the superficial cells of the urothelium, analysis of TJ and TJ-associated proteins focused on these cells. Of the 9 expressed claudins, 1, 4, 5, 7, 14 and 16 were expressed at cell-cell contact points (Fig. 2A) where occludin and the ZO proteins were also observed (Fig. 2B) indicating these proteins were present in or closely associated with the TJs around the apical periphery. Although the most intense immunostaining for claudins 1, 4, 5 and 7 was at the apical cell-cell junctions, these claudins also were expressed along the lateral surfaces of overlapping cells (Fig. 2A). Claudin 14 was present in a discontinuous pattern along cell-cell margins and claudin16 displayed weak fluorescence at cell junctions.
Figure 2.
(A) Expression of claudins in TEU-2 cells: (a) claudin 1, (b) claudin 2, (c) claudin 4, (d) claudin 5, (e) claudin 7, (f) claudin 8, (g) claudin 12, (h) claudin 14, (i) claudin 16. Arrows in (c) and (d) indicate claudin immuno-staining along apical-lateral cell borders between overlapping cells. (j) Positive control staining for claudin 4 in Caco-2 cells. (k) Representative example of negative control. Normal rabbit IgG for claudin 4. (l) Merged double-labeled image of claudin 8 (red) and ZO-2 (green). (B) Expression of TJ-associated proteins in TEU-2 cells: (a) occludin, (b) JAM-1, (c) ZO-1, (d) ZO-2, (e) ZO-3. (f) Representative positive control for ZO-3 in MDCK cells. (g) Representative negative control of TEU-2 superficial cells stained with normal rabbit IgG. Magnification bar = 25 µm.
Claudins 8 and 12 had a cytoplasmic peri-nuclear distribution but were not observed at cellcell junctions (Fig. 2A). Cultures were double-labeled for the individual claudins and ZO-2. A merged image of red claudin 8 peri-nuclear staining and green ZO-2 junctional labeling in the same plane is shown. When available, antibody specificity was confirmed in positive control cell types; a representative example is shown for claudin 4 in Caco-2 cells.
Transmembrane proteins occludin and JAM-1, as well as TJ-associated proteins ZO-1, ZO-2, and ZO-3 were observed at junctions between cells (Fig.2B). MDCK or Caco-2 cells were used as positive controls and normal rabbit IgG as a negative control, for which representative examples are shown.
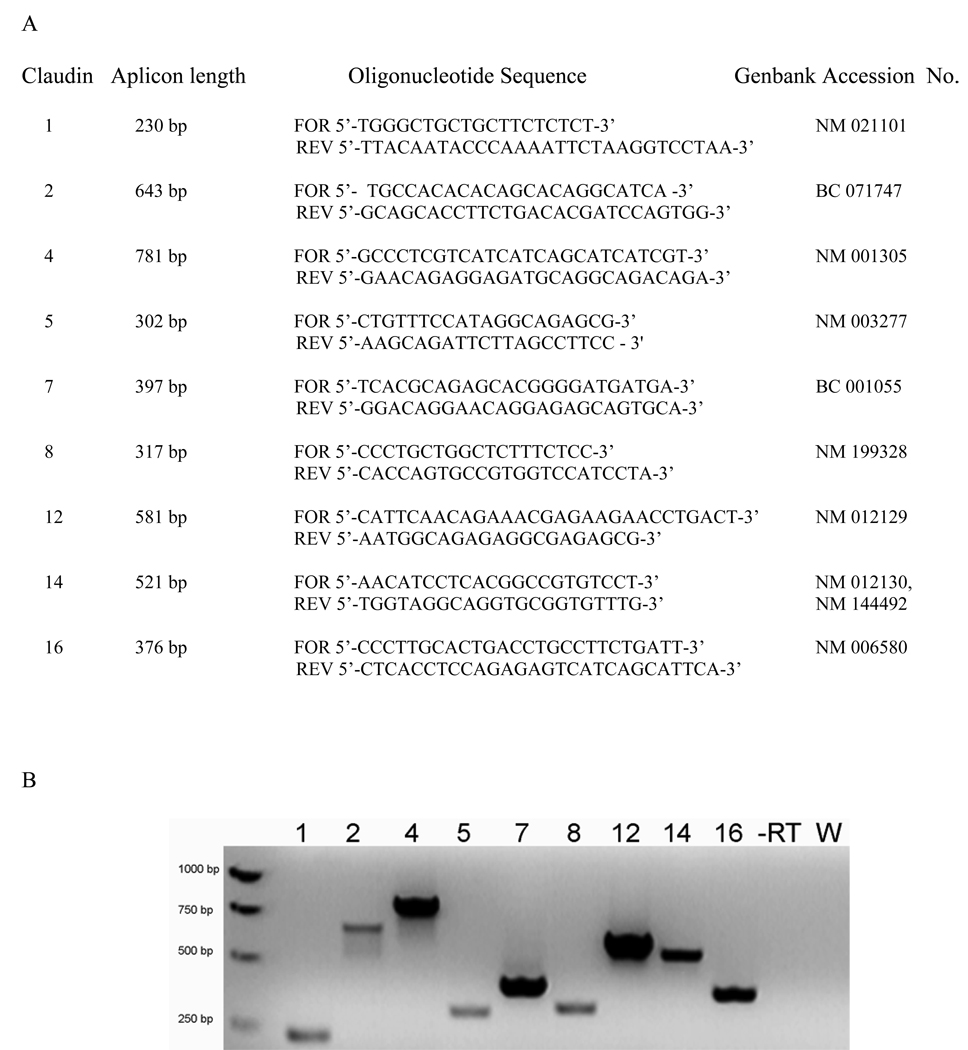
RT-PCR detected transcripts encoding claudins 1, 2, 4, 5, 7, 8, 12, 14, and 16. Results yielded the predicted sizes (Fig. 3). These mRNAs represent transcripts from the entire stratified culture. Therefore, stratified urothelial cultures express a broad repertoire of claudins.
Figure 3.
(A) Primers used for RT-PCR including the expected amplicon length in base pairs and Genebank accession numbers from which the primer sequences were derived. (B) Expression of claudin mRNA in TEU-2 cell cultures by RT-PCR. Amplicons were synthesized using the primer sets listed in Table 1 and electrophoresed on 1.5% agarose gels. Numbers above the gel refer to claudin isoforms, -RT—a representative control sample without reverse transcriptase, W—a representative water control (all controls were negative).
Discussion
TJs are essential for normal function of epithelia, restricting paracellular diffusion and contributing to the maintainance of cell surface polarity (review, Aijaz et al. 2006). The urothelial barrier prevents the leakage of urine into the body tissues and is characterized by a unique lumenal epithelium in which the apical cells are large, flattened, umbrella-shaped cells called superficial cells. Adjacent superficial cells develop TJs along their apical borders and form the paracellular barrier.
When grown in FBS and millimolar calcium in vitro, superficial TEU-2 cells develop TJs, adherens junctions with inserted tonofilaments, cytoplasmic vesicles and stubby cellular projections on the apical plasma membrane. Primary cultures of human urothelial cells have a limited lifespan in vitro and may undergo changes in differentiation state and morphology during the time that they remain viable, thus TEU-2 cultures represent a stable in vitro model to study specific TJ proteins in a stratified epithelial tissue derived from immortalized human ureteral urothelial cells. Although cells grown under these conditions form multiple layers, it should be noted that it is only a model and cannot adequately be classified as duplicate urothelium as we did not characterize it for other urothelial characteristics, such as cell cytokeratin profiles or presence of asymmetric unit membrane on cells of the apical layer.
We describe the transcription and protein localization of 9 claudin isoforms in the ureteral cell model. Six of the 9 are located at cell junctions (claudins 1, 4, 5, 7, 14 and 16) and 3 (claudins 2, 8, 12) are located in the cell cytoplasm. Additionally, we report that TJ and TJ associated proteins, occludin, JAM-1, ZO-1, ZO-2, and ZO-3 are expressed in areas of cell-cell contact between adjacent cells. This represents a surprising complexity of TJ and TJ-associated elements and extends the list of transcripts and proteins which have been reported for junctional proteins in cultured urothelial cells.
When overexpressed in MDCK II cells, claudins 1 and 4 increase transepithelial electrical resisistance (McCarthy et al. 2000, Van Itallie et al. 2001). Localization of claudins 4 at TJs of TEU-2 cells is consistent with its role as a component in barrier function. Only a punctate staining pattern for claudin 2 in the TEU-2 cell cytoplasm was observed. In contrast to claudin 4, claudin 2 is associated with “leaky” epithelia (Furuse et al. 2001). Further, claudin 2 transcripts, but not protein, have been previously observed in bladder tissue, the absence of protein expression being attributed to possibly low levels of synthesis or rapid turnover (Acharya et al. 2004). Relatively heavy labeling of both claudins 4 and 5 was observed at contact points of TEU-2 cells with immunofluorescence extending along the lateral surfaces of overlapping superficial cell processes. Over-expression of claudin 4 increases transepithelial resistance via a selective decrease in Na permeability (Van Itallie et al. 2001). Claudins 4 and 5 have been reported in superficial cells of human ureteric paraffin-embedded sections (Varley et al. 2006), thus suggesting TEU-2 cultures as a valuable model expressing these claudins.
In control Caco-2 cells, staining was observed for claudins 7 and 8 at areas of cell-cell contact. In TEU-2 cultures, a similar pattern for claudin 7 was observed whereas claudin 8 staining was consistently observed in the cytoplasm. Claudin 7 has been localized to the tight junctions of ureteric urothelial intermediate cells (Varley et al. 2006) as well as to the basolateral membranes of the distal nephron (Li et al. 2004) where it may function as a storage pool prior to recruitment to the junctional complex. Claudin 8 is expressed in MDCK II kidney cells, suggesting a role for modulation of permeability to ions involved in acid excretion (Angelow et al. 2006). Claudin 12 is associated with high resistance epithelia, such as in the bladder (Acharya et al. 2004). Expression of these claudins in the TEU-2 culture system provides a basis to further study their role in the urothelium.
To our knowledge, this is the first report of claudin 14 and 16 in urothelial cells in vitro. Mutations in claudin 14 are known to cause the non-syndromic autosomal recessive deafness condition, DFNB29 (Wilcox et al. 2001). Claudin 16 has been located in the TJs of the thick ascending limb of Henle, is required for Mg2+ resorption and is over-expressed in human ovarian cancer cells (Simon et al. 1999, Rangel et al. 2003). Specific roles of claudins 14 and 16 in functional properties of TJs in the urothelium remain to be elucidated.
Occludin is a tetraspan integral TJ protein (Furuse et al.1993). Expression results in increased trans-epithelial resistance while occludin nulls show no evidence of altered TJ morphology or barrier function indicating that occludin is sufficient but not necessary for TJ formation or barrier function (McCarthy et al. 1996, Saitou et al. 2000). siRNA suppression of occludin in MDCKs results in increased expression of claudins 3 and 4, and decreased expression of other claudins suggesting a compensatory mechanism (Yu et al. 2005).
Zonula occludens (ZOs), the “undercoat” scaffolding proteins in the cortical cytoplasm interact with cytoplasmic actin filaments and claudin and occludin proteins (review, Aijaz et al. 2006). ZO-1 has previously been reported in mammalian bladder and cultured human urothelial cells (Acharya et al. 2004, Cross et al. 2005). We have confirmed the presence of ZO-1 in TEU-2 cultures and further shown the presence of ZO-2 and ZO-3. Thus, the TEU-2 model system provides a stable system to study these important scaffolding proteins.
We have demonstrated the presence of several TJ and TJ-associated proteins in human TEU-2 cultures. Because TEU-2 cultures are of ureteral origin, further studies are needed to characterize immortalized urothelial cells of bladder, renal pelvis, and proximal uretheral origin as they may show different tight junction protein and pattern expression results.
Conclusions
We have demonstrated the presence of several TJ and TJ-associated proteins in human TEU-2 cultures. This system employs immortalized ureteral urothelial cells, thus providing a stable model to study the identified tight junction and associated proteins.
Acknowledgements
Supported by National Institute of Diabetes and Digestive and Kidney Diseases Award DK66119
References
- Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin−4, −8, and −12 in bladder epithelium. Am J Physiol Renal Physiol. 2004;287:F305–F318. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- Aijaz S, Balda MS, Matter K. Tight Junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- Angelow S, Kim K-J, Yu ASL. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J Physiol. 2006;571:15–26. doi: 10.1113/jphysiol.2005.099135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross WR, Eardley I, Leese HJ, Southgate J. A biomimetic tissue from cultured normal human urothelial cells: analysis of physiological function. Am J Physiol Renal Physiol. 2005;289:F459–F468. doi: 10.1152/ajprenal.00040.2005. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp DJ, Wieser AC, Sengupta S, Forrestal SG, Batler RA, Schaeffer AJ. Uropathogenic E. coli potentiates type I pilus-induced apopotosis by suppressing NF-κB. Infect Immun. 2001;69:6689–6695. doi: 10.1128/IAI.69.11.6689-6695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- Li WY, Huey CL, Yu ASL. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol. 2004;286:F1063–F1071. doi: 10.1152/ajprenal.00384.2003. [DOI] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Skare IB, Lynch RD, Schneeberger EE. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J Cell Sci. 2000;113:3387–3398. doi: 10.1242/jcs.113.19.3387. [DOI] [PubMed] [Google Scholar]
- Rangel LBA, Sherman-Baust CA, Wernyj RP, Schwartz DR, Cho KR, Morin PJ. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene. 2003;22:7225–7232. doi: 10.1038/sj.onc.1207008. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke J-D, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriquez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg (2+) resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- Southgate J, Hutton KAR, Thomas DFM, Trejdosiewicz LK. Normal human urothelial cells in vitro: proliferation and induction of stratification. Lab Invest. 1994;71:583–594. [PubMed] [Google Scholar]
- Turksen K, Troy T-C. Barriers built on claudins. J Cell Sci. 2004;117:2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley CL, Garthwaite MAE, Cross W, Hinley J, Trejdosiewicz LK, Southgate J. PPARγ-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol. 2006;208:407–417. doi: 10.1002/jcp.20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ASL, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, Kachar B, Wu DK, Griffith AJ, Riazuddin S, Friedman TB. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]