Abstract
A new and efficient chlorination protocol is presented for the preparation of chlorosilanes from hydrosilanes. A variety of chlorinating agents in combination with palladium(II) chloride as the catalyst are examined. Among them, hexachloroethane is found to be the best choice, furnishing the desired product in good to quantitative yields under mild conditions. Various hydrosilanes are used as starting materials to explore the scope of this reaction.
Keywords: Hexachloroethane, Hydrosilanes, Chlorosilanes, Palladium(II) chloride, Chlorinating agents
Chlorosilanes play an important role in organic synthesis for the protection of highly reactive hydroxy and amino functional groups, as reagents for Mukaiyama aldol condensations,1–4 or as precursors of organochlorosilanes.5,6 In general, chlorosilanes are prepared by treatment of hydrosilanes with alkyl or allyl chlorides in the presence of CuCl2,7 NiCl2, PdCl28,9 or Pd/C10 as catalysts. However, these methods have certain limitations arising from the need for high temperatures, long reaction times or the use of expensive and toxic chlorinating agents.5,11 Recently, efficient systems were introduced for the conversion of alcohols into alkyl chlorides using the combination of PPh3 and chlorinating agents such as Cl3CCOCCl3, Cl3CCN, Cl3CCOOEt and Cl3CCONH2.12,13 Herein, we report a new and efficient procedure for the preparation of chlorosilanes from hydrosilanes using chlorinating agents in the presence of a catalytic amount of PdCl2.
Several chlorinating agents were explored for the conversion of triisopropylhydrosilane (TIPS-H) into triisopropylsilyl chloride (TIPS-Cl) (Table 1). The yields of TIPS-Cl were quantified by 1H NMR spectroscopy using toluene as an internal standard.
Table 1.
The effect of chlorinating agents on the conversion of TIPS-H into TIPS-Cl
 | ||||
|---|---|---|---|---|
| Entry | Chlorinating agent | Equivalents (mmol) | % Yielda Si–Cl | % Recovery Si–H |
| 1 | None | — | 2 | 98 |
| 2 | CH3COCl | 0.75 | 69 | 31 |
| 1.50 | Quant | 0 | ||
| 3 | Cl3CCOCCl3 | 0.50 | 17 (59)b (92)c | 83 (41)b (8)c |
| 4 | Cl3CCOOEt | 0.50 | 15 (39)b (35)c | 85 (61)b (65)c |
| 5 | Cl3CCOOH | 0.50 | 18 | 82 |
| 6 | Cl2CHCOOEt | 0.50 | 83 | 17 |
| 7 | ClCH2COOEt | 0.50 | 10 | 90 |
| 1.50 | 24 | 76 | ||
| 8 | Cl3CCN | 0.50 | 11 | 89 |
| 9d | Cl3CCONH2 | 0.50 | 15 | 85 |
| 10 | ClCH2CH2Cl | 0.50 | 16 | 84 |
| 11 | Cl2CHCHCl2 | 0.50 | 32 | 68 |
| 12 | Cl3CCCl3 | 0.50 | Quant (99)e,f | 0 |
| 13 | CCl4 | 0.50 | 18 | 82 |
| 14 | CHCl3 | 0.50 | 65 | 35 |
| 15 | CH2Cl2 | 0.50 | 14 | 86 |
| 16 | Cl3CCH3 | 0.50 | 54 | 46 |
% yield was determined by 1H NMR using toluene as an internal standard.
The reaction was carried out at 55 °C for 3 h.
The reaction was carried out at 55 °C for 6 h.
Not completely soluble.
20 mmol scale reaction was performed with 0.5 mol % PdCl2.
Isolated by distillation (bp 98 °C, 20 mmHg).
As shown in Table 1 (entry 1), PdCl2 can transfer its chlorine atoms quantitatively to TIPS-H. However, the use of hexachloroacetone, trichloroacetonitrile and trichloroacetamide in combination with this catalyst produced TIPS-Cl in poor yields only (entries 3, 8 and 9, respectively). Of the three ethyl chloroacetates (entries 4, 6 and 7), Cl2CHCOOEt provided the highest yield of TIPS-Cl, while Cl3CCOOEt, which has more chlorine atoms, gave a lower silyl chlorination yield. Comparison of the chloromethanes (entries 13–15) showed that CHCl3 furnished a higher yield of chlorination product compared to CCl4 or CH2Cl2. The product yield can be increased by carrying out these reactions at 55 °C for 3 h and 6 h, respectively (entries 3 and 4). The more reactive acetyl chloride afforded TIPS-Cl in quantitative yield (entry 2), however, due to its toxicity and acidity, it cannot be used in certain cases. On the other hand, comparison of different chloroethanes (entries 10–12) indicated that hexachloroethane afforded the highest yield of TIPS-Cl. In fact, 0.5 M equiv of this reagent led to quantitative conversion of TIPS-H into TIPS-Cl after only 1 h at 25 °C. When this reaction was repeated on a large scale, we were able to obtain a 99% yield of TIPS-Cl after vacuum distillation (98 °C, 20 mmHg).14
Table 2 reveals the effect of the amount and type of catalyst (PdCl2 or Pd/C) on this reaction. As expected, no reaction occurred when the Pd catalyst was not present demonstrating that the catalyst is essential (entry 1). Moreover, the product yield was reduced to 81% when 0.5 mol % PdCl2 was employed (entry 3).
Table 2.
The effect of the amount and type of catalyst on the conversion of TIPS-H into TIPS-Cl
 | |||
|---|---|---|---|
| Entry | Catalyst (% mmol) | % Yield Si–Cl | % Recovery Si–H |
| 1 | None | — | 100 |
| 2 | PdCl2 (1.0%) | Quant | 0 |
| 3 | PdCl2 (0.5%) | 81 | 19 |
| 4 | Pd/C (1.0%) | Quant | 0 |
The results of the optimization studies on the amount of Cl3CCCl3 required for this chlorination procedure are shown in Table 3. Use of 0.17 M equiv of this reagent (corresponding to 1 equiv of chlorine atoms) under standard reaction conditions produced only a 69% yield of TIPS-Cl (entry 2). The yield of TIPS-Cl was increased to 95% by using Cl3CCCl3 (0.30 mmol) (1.8 equiv of chlorine atoms). The use of 0.5 mmol of hexachloroethane (3 equiv of chlorine atoms) resulted in a quantitative yield of TIPS-Cl after only 30 min at room temperature (entry 5).
Table 3.
The effect of the amount of Cl3CCCl3 on the conversion of TIPS-H into TIPS-Cl
 | |||
|---|---|---|---|
| Entry | Cl3CCCl3 (mmol) | % Yield Si–Cl | % Recovery Si–H |
| 1 | 0.125 | 48 | 52 |
| 2 | 0.17 | 69 | 31 |
| 3 | 0.25 | 90 | 10 |
| 4 | 0.30 | 93 | 7 |
| 5a | 0.50 | Quant | 0 |
| Quant | 0 | ||
| 6 | 0.75 | Quant | 0 |
Reaction time: 30 min.
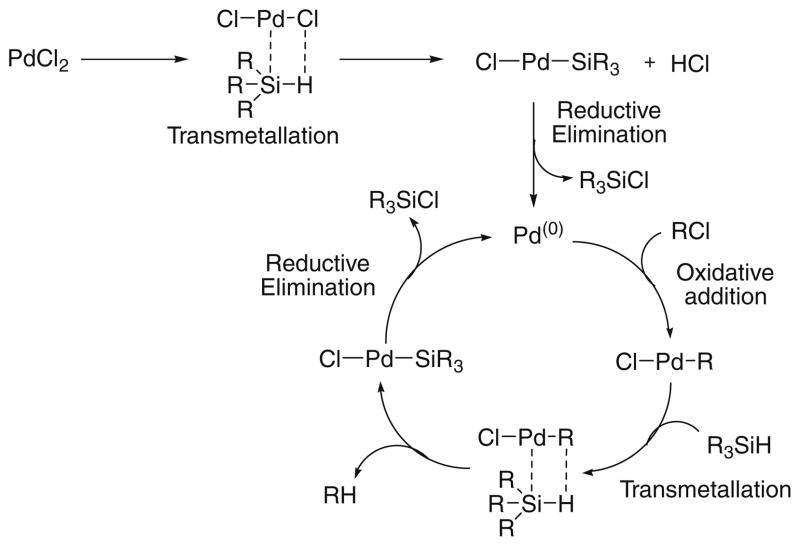
A general mechanism can be proposed (Scheme 1) which proceeds by the interaction of the chlorinating agent (RCl) with Pd(0) by oxidative addition, followed by transmetallation and reductive elimination to regenerate Pd(0).15
Scheme 1.
A general mechanistic pathway for the conversion of hydrosilanes into chlorosilanes.
This method is applicable to the synthesis of various chlorosilanes (Table 4). In the case of liquid hydrosilanes the chlorination proceeded best under neat conditions, but with solid hydrosilanes, for example, Ph3SiH, a solvent was required. Among the various solvents examined, THF was found to produce consistently higher chlorination yields. As shown in Table 4, this reaction can be applied to various hydrosilanes including monochloro diphenyl hydrosilane (entries 15–18). The reaction was performed under the optimized conditions: Cl3CCCl3 (0.25 mmol) and 1% PdCl2 at room temperature for one hour without any solvent unless otherwise stated. All the selected hydrosilanes were transformed successfully into the corresponding chlorosilanes with experimental modifications in some cases.
Table 4.
The conversion of monohydrosilanes into chlorosilanes
 | ||||
|---|---|---|---|---|
| Entry | Hydrosilane | Solvent (0.25 mL) | % Yield Si–Cl | % Recovery Si–H |
| 1 | Et3SiH | — | 78 | 22 |
| 2a | — | Quant (99.8)f,g | 0 | |
| 3 | (Me3Si)3SiH | — | Quant | 0 |
| 4 | THF | Quant | 0 | |
| 5 | Ph3SiH | THF | Quant | 0 |
| 6 | Ph(Me)2SiH | THF | 30 | 70 |
| 7a | THF | 66 | 34 | |
| 8a,c | THF | 55 | 45 | |
| 9d | THF | 61 | 39 | |
| 10e | THF | 60 | 40 | |
| 11b | t-Bu(Me)2SiH | — | 67 | 33 |
| 12a | THF | 86 | 14 | |
| 13a,c | THF | 89 | 11 | |
| 14 | THF | 80 | 20 | |
| 15 | Ph2SiClH | — | 89 | 11 |
| 16 | THF | 77 | 23 | |
| 17a | THF | 83 | 17 | |
| 18a,c | THF | 78 | 22 | |
Using 0.50 mmol of Cl3CCCl3.
The reaction was carried out in an ice bath.
The reaction was run for 2 h.
Using 0.75 mmol of Cl3CCCl3.
Using 1.00 mmol of Cl3CCCl3.
20 mmol scale reaction was performed with 0.5 mol % PdCl2.
Isolated by vacuum distillation (bp 45–47 °C, 20 mmHg).
In conclusion, hexachloroethane was found to be an efficient chlorinating agent for the conversion of hydrosilanes into chlorosilanes in the presence of PdCl2 as the catalyst. This chlorination reaction proceeds cleanly and rapidly under mild conditions and, in certain cases, in the absence of any solvent. Sub-equivalent amounts of hexachloroethane are needed for quantitative chlorination (typically between 0.25 and 0.5 equiv), which together with the fact that this compound is commercially available and inexpensive, renders this reaction attractive as a general method for the synthesis of chlorosilanes.
Acknowledgments
We gratefully thank the Natural Products Research Unit, Department of Chemistry, Faculty of Science, Chulalongkorn University, for the provision of chemicals and laboratory facilities. Financial support from TRF Master Research Grants, the Graduate school, Chulalongkorn University and the National Institutes of Health (Grant GM081484) is also acknowledged.
References and notes
- 1.Birkofer L, Stuhl O, Patai S, Rappoport Z. The Chemistry of Organic Silicon Compounds. Chapter 10. Wiley; Chichester: 1989. pp. 655–761. [Google Scholar]
- 2.Larson GL, Patai S, Rappoport Z. The Chemistry of Organic Silicon Compounds. Chapter 11. Wiley; Chichester: 1989. pp. 763–888. [Google Scholar]
- 3.Cunico RF, Bedell L. J Org Chem. 1980;45:4797–4798. [Google Scholar]
- 4.Khalafi-Nezhad A, Alamdari RF, Zekri N. Tetrahedron. 2000;56:7503–7506. [Google Scholar]
- 5.Masaoka S, Banno T, Ishikawa M. J Organomet Chem. 2006;691:174–181. [Google Scholar]
- 6.Kobayashi T, Pannell KH. Organometallics. 1991;10:1960–1964. [Google Scholar]
- 7.Kunai A, Sakurai T, Toyoda E, Ishikawa M. J Organomet Chem. 1996;15:2478–2482. [Google Scholar]
- 8.Kunai A, Ohshita J. Organometallic. 2003;686:3–15. [Google Scholar]
- 9.(a) Ferreri C, Costantino C, Chatgilialoglu C, Boukherroub R, Manuel G. J Organomet Chem. 1998;554:135–137. [Google Scholar]; (b) Ferreri C, Costantino C, Romeo R, Chatgilialoglu C. Tetrahedron Lett. 1999;40:1197–1200. [Google Scholar]
- 10.(a) Sommer LH, Citron JD. J Org Chem. 1967;32:2470–2472. [Google Scholar]; (b) Citron JD, Lyons JE, Sommer LH. J Org Chem. 1968;34:638–640. [Google Scholar]
- 11.Citron JD. J Org Chem. 1969;34:1977–1979. [Google Scholar]
- 12.Pluempanupat W, Chavasiri W. Tetrahedron Lett. 2006;47:6821–6823. [Google Scholar]
- 13.(a) Magid RM, Fruchey OS, Johnson WL, Allen TG. J Org Chem. 1979;44:359–363. [Google Scholar]; (b) Jang DO, Park DJ, Kim J. Tetrahedron Lett. 1999;40:5323–5326. [Google Scholar]
- 14.Typical experimental for the conversion of hydrosilanes into chlorosilanes: To a stirred mixture of hexachloroethane (2.37 g, 10 mmol, 0.5 equiv) and Pd(II)Cl2 (18.5 mg, 0.1 mmol, 0.5 mol %) at room temperature under an N2 atmosphere was added triisopropylsilane (3.16 g, 20 mmol, 1 equiv) over a period of 2 min. Note: the reaction is exothermic and large-scale reactions should be cooled using an ice bath. The reaction mixture became homogeneous after 5 min and was allowed to stir for 1 h. The crude residue was then distilled under vacuum (bp 98 °C, 20 mmHg) to yield triisopropylsilyl chloride (3.78 g, 19.7 mmol, 99%). In a similar manner triethylsilane (2.33 g, 20 mmol) was converted into triethylsilyl chloride (3.0 g, 19.96 mmol, quant. after distillation at 45–47 °C, 20 mmHg).
- 15.Cho YS, Han JS, Yoo BR, Kang SO, Jung IN. Organometallics. 1998;17:570–573. [Google Scholar]