Abstract
The adaptor protein Shc is phosphorylated downstream of many cell surface receptors, including antigen and cytokine receptors. However, the role of Shc in B cell development has not been addressed. Here, through conditional expression of a dominant negative Shc mutant and conditional loss of Shc protein expression, we tested a role for Shc during early B lymphopoiesis. We identified a requirement for Shc beginning at the transition from the pre-pro-B to pro-B stage, with a strong reduction in the number of pre-B cells. This developmental defect is due to increased cell death rather than impaired proliferation or commitment to the B lineage. Additional studies suggest a role for Shc in IL-7-dependent signaling in pro-B cells. Shc is phosphorylated in response to IL-7 stimulation in pro-B cells, and pro-B cells from mice with impaired Shc signaling display increased apoptosis. Together, these data demonstrate a critical role for Shc in early B lymphopoiesis with a requirement in early B cell survival. In addition, we also identify Shc as a required player in signaling downstream of the IL-7 receptor in early B cells.
Introduction
B cell development is a highly ordered process that requires cells to progress through multiple checkpoints during maturation. B cells originate from the hematopoietic stem cell (HSC) pool and pass through multiple stages of development before becoming committed to the B lineage (1-3). Prior to B lineage commitment, potential B cells exist as common lymphoid progenitor cells (CLPs). Although CLPs maintain the capacity to differentiate into other non-B lineages, they are considered to be B lineage specified and are actively undergoing DH-JH IgHC gene rearrangement. In addition, CLPs express the IL-7 receptor, which is required for progression along the B lineage. CLPs progress to the pre-pro-B cell stage where expression of B220 can first be detected. At this stage DH-JH IgHC gene rearrangement is nearly complete, and these cells are considered B lineage committed although lineage plasticity remains (4).
Transition from the pre-pro-B to pro-B stage is accompanied by surface expression of CD19. The IgHC locus then begins to undergo VH-DHJH rearrangement (4). If an in-frame coding region is produced from these recombination events, the IgHC protein is expressed on the cell surface along with components of the surrogate light chain (SLC), including the λ5 and VpreB proteins and the Igα/Igβ heterodimer. This forms the pre-B cell receptor (pre-BCR). Proper expression and intracellular signal transmission of the pre-BCR is crucial for progression from the pro-B to pre-B stage, and only a third of pro-B cells successfully complete this process (5). Disruption of components of the pre-BCR (such as in Rag1-/- mice) can lead to a complete block at the pre-BCR checkpoint. (6-8). A functional antigen receptor is also required for mature B cell maintenance in the periphery, since deletion of the B cell receptor in mature splenic B cells results in rapid cell death (9). Successful signaling through the pre-BCR leads to survival, proliferation, and developmental progression. Once cells advance to the late pre-B stage, the recombination machinery initiates recombination of the light chain locus (10). The light chain pairs with IgHC and the Igα/Igβ heterodimer is expressed on the BCR in immature B cells.
Functionally immature B cells leave the bone marrow via the bloodstream and travel to the periphery where they undergo further maturation (11). Immature B cells in the spleen are denoted transitional B cells and show a high rate of turnover in vivo with rapid cell loss (12). A significant amount of negative selection occurs at the T1-T2 transition when immature B cells from the bone marrow encounter new antigens (13). Once cells reach the T2 stage, they progress to the mature B pool after positive selection. Three distinct types of mature B cells exist: follicular (FO), marginal zone (MZ), and B-1 cells, and there are different requirements for the development of each cell type. One current hypothesis for the formation of different mature B cell populations is the signal strength hypothesis. This hypothesis suggests that strong signaling from the BCR skews development to the FO B developmental pathway whereas weaker signaling through the BCR results in MZ B cell formation (14).
Ras signaling to Raf is required for B development as well as signaling through the BCR. Overexpression of a dominant negative Ras (H-rasN17) in early B cells blocked B cell development at the pre-pro-B to early pro-B transition (15). This block was partially rescued by expression of Raf-CAAX, which mimics Ras-induced activation of Raf. Transgenic mice that express dominant inhibitory Ras (Asn-17 HA-Ras) in the late stages of pro-B development showed decreased survival of B lymphocytes although proliferation was not impaired (as assessed by BrdU incorporation in the bone marrow) (16). Interestingly, expression of an activated Ras (c-HA-rasV12) on a Rag deficient background generated B lineage cells in peripheral tissues, suggesting that activated Ras was able to induce pro-B cells to differentiate beyond the pre-BCR checkpoint in the absence of the μ heavy chain (17). Thus, Ras plays important roles in B cell development both prior to and during the pre-BCR checkpoint.
Cytokines and their cognate receptors have been shown to play critical roles in B cell development prior to the pre-B cell receptor checkpoint (18-20). Deletion of cytokine receptors can inhibit B cell development at the earliest stages by blocking proliferation, differentiation, and/or survival of developing B lymphocytes. IL-7 and its cognate receptor are particularly important at the pre-pro-B to pro-B transition. Inhibiting the IL-7 receptor by injection of blocking antibodies (21) or genetic knockout (22) severely disrupts B cell development at the pre-pro-B to pro-B stage. This block has been linked to loss of commitment to the B lineage through decreased expression of the transcription factor EBF (early B factor) (23). In addition to B lineage commitment, IL-7 also functions to promote survival (24) and proliferation (25, 26) of early B subsets.
Proper intracellular transmission of receptor signals through appropriate cytoplasmic signaling intermediates is also required for successful B lymphopoiesis. The adaptor protein Shc signals downstream of multiple receptors, including antigen and cytokine receptors (27). During receptor signal transduction the p52 isoform of ShcA (hereinafter Shc) is phosphorylated on three critical tyrosine residues (Y239, Y240, and Y317), allowing the binding/recruitment of Grb2/Sos proteins leading to the activation of Ras, and in turn the MAPK cascade (28, 29). Shc protein expression and Shc tyrosine phosphorylation have been shown to be critical for thymocyte development at the β selection checkpoint. Impaired Shc-mediated signaling blocks T cell development by inhibiting the proliferative burst that accompanies successful pre-TCR signal transduction (30, 31). However the role of Shc in B cell development remains unknown.
In this report, attempt to address the role of Shc during B cell development by conditionally expressing a dominant negative Shc protein with mutation of its three critical tyrosine residues (hereinafter ShcFFF), or conditional loss Shc protein expression, in early B lymphocytes. Mice conditionally expressing ShcFFF had severely reduced pre-B cell numbers. However, we also noticed a surprising defect in cellularity of the pro-B population. Upon further analysis, we identified Shc as an important player in signaling downstream of the IL-7 receptor and in providing survival signals to pro-B cells. In vivo and ex vivo studies indicate a critical role for Shc during early B cell development that is distinct from its role during T cell development.
Materials and Methods
Mice
The conditional ShcFFF transgenic mouse line and conditional Shc1 knockout have been described previously (31). The Mb1-Cre and Cd19-Cre mice have also been previously described (32-34). Rag-1-deficient mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Mice were bred and maintained under specific pathogen-free conditions at the University of Virginia animal facility according to approved IACUC protocols.
Flow cytometry
Single-cell suspensions were prepared from bone marrow and spleen of 8-12 week-old mice. Red blood cells were lysed, and cells were stained for FACS analysis in 0.5% BSA in PBS with 0.05% NaN3. Antibodies specific for the following epitopes were used: B220 (RA3-6B2), CD19 (1D3), CD93 (AA4.1), BP-1 (6C3), CD24 (30-F1), TER119, CD111b (M1/70), CD3ε (145-2C11), IgM (II/41), Ly-6G (RB6-8C5), CD43 (S7), CD127 (A7R34), CD117 (2B8), Sca1 (D7), and BrdU. Annexin V-FITC was used to detect surface exposure of phosphatidyl serine. Antibodies were conjugated to FITC, Alexa488, PE, PE-Texas Red, PE-Cy5.5, PE-Cy7, V450, APC, Alexa647, APC-Alexa750, or APC-eFluor780. Biotin-conjugated antibodies were detected using streptavidin-FITC, PE, APC, or Pacific Orange. Cell viability or DNA content was analyzed with 4′, 6-Diamidino-2-phenylindole dihydrochloride (DAPI), 7-Amino-actinomycin D (7-AAD), or propidium iodide (PI). Antibodies and other reagents were purchased from ebiosciences, BD PharMingen, or Sigma-Aldrich. FACS data was collected at the University of Virginia Flow Cytometry Core Facility on a CyAn ADP 9 color machine. FACS analysis was performed using FlowJo software and gating on “singlets” as determined by pulse width versus forward scatter.
In vivo BrdU incorporation assay
To observe cell cycle kinetics, we utilized the protocol outlined by Kincade, et al (1). For bone marrow analysis, mice received an initial 0.1mg BrdU (Sigma-Aldrich) in 200μL PBS injected intraperitoneally (IP) followed by continuous BrdU administration in water (0.8mg/mL; changed daily). A minimum of five control and five mutant mice were sacrificed at 24, 48, and 72 hours post-injection. Bone marrow from two femurs per mouse was harvested, RBC lysed, counted (by trypan exclusion), and stained for surface phenotype. BrdU incorporation was determined by flow cytometry using a BrdU incorporation kit (Becton Dickinson). The least squares analysis was performed to obtain the renewal and production rates.
Ex vivo proliferation assays
Bone marrow B cells were sorted at the University of Virginia Flow Cytometry Core Facility with a Becton Dickinson FACSVantage SE Turbo Sorter with DIVA Option. Sorted cells were collected into fetal bovine serum, rinsed in 1× PBS, and plated at 7,000 cells per well in triplicate in a 96-well plate. For mice on a Rag1-/- background, pro-B cells were collected using MACS anti-CD19 microbeads (Miltenyi) per the manufacturer's protocol and assessed to be >95% pure by FACS analysis of anti-B220 by anti-CD19. Pro-B cells were grown for three days in the indicated concentration of IL-7 (Pepro Tech) in 100μL RPMI 1640 with 5% FCS, 2mM L-glutamine, and 50μM β-mercaptoethanol (complete RPMI) while incubating at 37°C in a humidified chamber with a 5% CO2 atmosphere. On the third day, 50μL of fresh complete RPMI with the indicated concentration of IL-7 were added to feed the cells. During long-term cultures on OP-9 stromal cells, cultures were fed every three days. For [3H]-thymidine uptake assays, 1μCi of [3H]-thymidine was added to each well 6-8 hours before harvesting. Cells were collected on Filtermat A filters (PerkinElmer Life Sciences). For total cell counts, 100μL of cell suspension were added to a FACS tube along with 50μL of 5μm counting beads (SpheroTech) and PI for viability. Live cells were gated using PI versus FSC, and total live cell number was calculated per the manufacturer's protocol. Remaining cell suspension was used to phenotype B cell subsets by FACS analysis.
Cell activation, immunoprecipitation, and immunoblotting
Pro-B cells from Rag1-/- bone marrow were collected using anti-CD19 microbeads (Miltenyi) per the manufacturer's protocol. Cells were allowed to rest in plain RPMI in a 37°C humidified chamber with a 5% CO2 atmosphere for one hour before stimulation. Cells were then transferred to Eppendorf tubes in a 37°C water bath for stimulation. Cells received 100ng/mL IL-7, removed at the indicated times, and immediately boiled in 1× Laemmli buffer with 2% β-mercaptoethanol. “Unstimulated” cells received PBS alone and were incubated for 5 minutes. Lysates were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes. Phosphorylated Shc was detected with anti-phospho-Shc antibody (pY239/pY240, Santa-Cruz). Total Shc protein was detected with rabbit polyclonal antibody (BD Biosciences).
For detection of FLAG-tagged ShcFFF transgenic protein, B cells from bone marrow or spleen were positively selected using anti-CD19 microbeads (Miltenyi). Cells were rinsed with PBS and lysed. Total protein from cleared lysates was determined with Bradford Reagent. Equal protein levels between control (Cre-ShcFFF+) and mutant (Cre+ShcFFF+) were subjected to immunoprecipitation by anti-FLAG agarose (Sigma Chemical Co.). Immunoprecipitates were rinsed 4× with 500μL cold lysis buffer and immunoblotted as described above. For detection of endogenous Shc protein in the conditional Shc1 knockout mice, total bone marrow B cells were first enriched with anti-CD19 microbeads (Miltenyi) and then sorted as described above. Total cell lysates were probed for total Shc and Erk1/2 protein as a loading control.
Results
Targeted disruption of Shc during B cell development
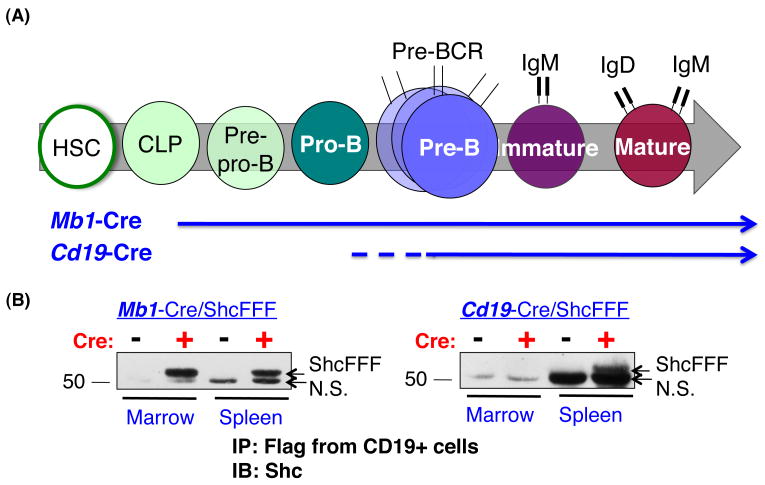
To determine the role of Shc during B cell development (Figure 1A), we took two genetic approaches to impair Shc-mediated signaling via the Cre/loxP approach: first, we targeted expression of a transgene encoding a dominant negative FLAG-tagged Shc protein (Shc Y239F/Y240F/Y317F, hereinafter ShcFFF); and second, we selectively deleted Shc1 at different stages of B cell development. To target the early B cell developmental stages, we used the Cre transgenic lines Mb1-Cre and Cd19-Cre. Mb1-Cre expression begins at the common lymphoid progenitor (CLP) stage and continues throughout B cell development, allowing strong expression of Cre recombinase during early B development (32, 33). In Mb1-Cre/ShcFFF mice, the transgenic ShcFFF protein was detected by anti-FLAG immunoprecipitation of CD19+ B cells from both the bone marrow and spleen, consistent with the expected Cre expression pattern (Figure 1B). Cd19-Cre expression begins weakly at the pro-B stage, but this Cre strain is largely beneficial for examining the role of Shc during later B cell development (Figure 1A) (34). Although Cd19-Cre expression s reported to weakly begin at the pro-B stage, we were only able to detect transgenic ShcFFF protein in CD19+ cells from the spleen of Cd19-Cre/ShcFFF mice (Figure 1B). Since these mice only expressed detectable levels of ShcFFF in splenic B cells, they were used as a model to study the requirement of Shc in later stages of B development
Figure 1. Reduction in bone marrow B cells in Mb1-Cre ShcFFF mice.
(A) Schematic of bone marrow B cell development showing early expression of Mb1-Cre in the common lymphoid progenitor (CLP) stage and later expression of Cd19-Cre within the pro-B stage. (B) Expression of ShcFFF in marrow and spleen. CD19+ cells were positively selected from bone marrow and spleen of the indicated mice, immunoprecipitated with anti-FLAG, and immunoblotted with anti-Shc antibody to detect ShcFFF transgenic protein. ShcFFF is visible in both bone marrow and spleen of Mb1-Cre/ShcFFF mice, but only in the spleen of Cd19-Cre/ShcFFF mice. Non-specific band, N.S.
Disruption of Shc results in a block at the pre-pro-B to pro-B transition
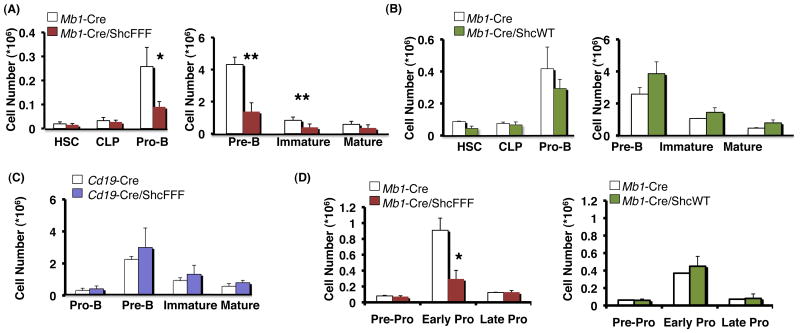
Conditional expression of ShcFFF or deletion of Shc1 with Mb1-Cre resulted in a severe block in B cell development that was first apparent in the pro-B cell compartment (Figure 2A and Supplemental Figure 2), suggesting a developmental block prior to the pro-B stage. Importantly, we saw no defect in the hematopoietic stem cell (HSC) compartment, which is prior to Mb1-Cre expression, suggesting that the fidelity of Cre (and in turn ShcFFF) expression is retained in Mb1-Cre/ShcFFF mice. There was also no detectable difference in the numbers of common lymphoid progenitors (CLPs), the stage when Mb1-Cre-mediated recombination begins (Figure 2A). This places the defect in Mb1-Cre/ShcFFF mice during early B cell development after the CLP stage but prior to the pro-B stage.
Figure 2. Shc is required during early B cell development prior to the pro-B transition.
(A) Absolute numbers of B lineage cells in bone marrow. Conditional expression of ShcFFF with Mb1-Cre shows an initial developmental block from the CLP to Pro-B stage. (B) Mb1-Cre/ShcWT mice do not show a defect in B cell numbers in the bone marrow. (C) Conditional expression of ShcFFF during later stages of development with Cd19-Cre does not affect lymphocyte numbers in the bone marrow. (D) Detailed analysis of early subsets from Mb1-Cre/ShcFFF mice reveals an initial defect in cell numbers at the pre-pro-B to pro-B transition that is not present in Mb1-Cre/ShcWT mice. *P<0.05, **P<0.01.
To control for expression of a transgenic protein, we crossed the Mb1-Cre mice to transgenic mice that conditionally express wild-type Shc (“ShcWT”), based on the same Cre/loxP approach as the ShcFFF mutant. Mb1-Cre/ShcWT mice had no detectable defect in B cell development (Figure 2B). Instead, these mice displayed a reproducible increase in B cell numbers beginning at the pre-B stage. Although this increase was not statistically significant, it could be explained by overexpression of the wild-type Shc protein, which has previously been shown to be beneficial to cell proliferation (35).
In contrast to Mb1-Cre/ShcFFF mice, conditional expression of ShcFFF under Cd19-Cre resulted in no detectable defect in marrow B cell subsets (Figure 2C). Although this result does not rule out a role for Shc in peripheral B cells, it suggests that Shc plays a non-redundant role during early stages of B cell development and may not have a similar requirement during later stages of development.
In addition to conditional expression of transgenic Shc proteins, we also examined the requirement for Shc during B cell development with conditional Shc1 knockout mice (Shcf/f) (Supplemental Figure 2). Although Shc protein was efficiently removed in sorted bone marrow B cells beginning at the pre-B stage, we noticed residual protein in the pro-B subset of Mb1-Cre/Shcf/f mice (Supplemental Figure 2A). In addition, there was not a reproducible, statistically significant defect in B cell subsets in the bone marrow or spleen (Supplemental Figure 2B). Since residual Shc protein was present in pro-B cells, we examined mice that were allowed to age to 9 months. Senescence can allow subtle defects in B cell development to become more apparent, as CLPs, pre-pro-B, and pro-B cell numbers are reduced in aged mice (36). Aged Mb1-Cre/Shcf/f mice showed developmental defects in the bone marrow beginning at the pro-B stage (Supplemental Figure 2C), consistent with Mb1-Cre/ShcFFF mice. This demonstrates that both Shc protein and its ability to be tyrosine phosphorylated on the three critical tyrosine residues are required for early B cell development.
We then sought to narrow the window at which the developmental defect occurred in Mb1-Cre/ShcFFF mice. Using flow cytometry, we first gated out non-B lineage and mature B cells (CD3ε-, Gr1-, Ter119-, Mac1-, IgM-), then gated on B220+, CD43+, AA4.1+ cells and displayed early B fractions as CD19-BP1- (pre-pro-B), CD19+BP1- (early pro-B), and CD19+BP1+ (late pro-B). This revealed a 67% decrease in the early pro-B stage of Mb1-Cre/ShcFFF mice compared to littermate controls. Importantly, this was not seen when the wild-type Shc transgene was expressed in Mb1-Cre/ShcWT mice (Figure 2D). Since a consensus in defining the pre-pro-B compartment is lacking, we used multiple gating strategies with flow cytometry but observed no defect in the pre-pro-B cell compartment of Mb1-Cre/ShcFFF mice (Supplemental Figure 3). This places the most proximal defect in B cell development due to impaired Shc-mediated signaling at the pre-pro-B to early pro-B transition.
Diminished B cell compartment in the spleen of Mb1-Cre/ShcFFF mice
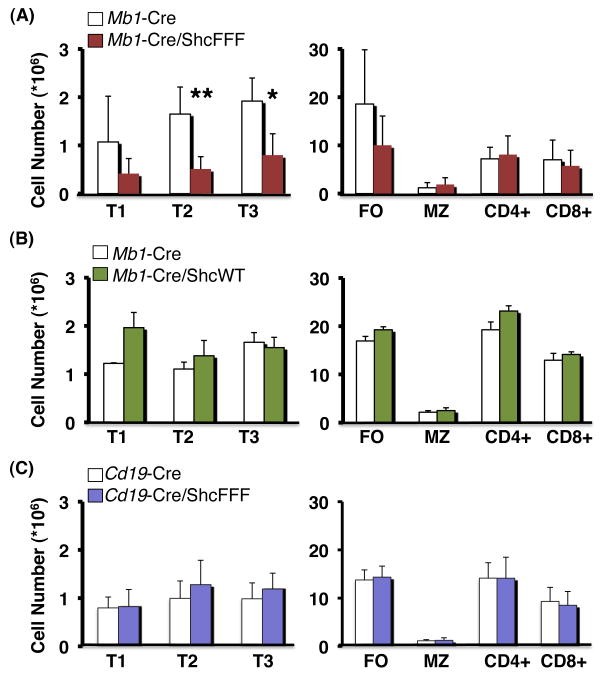
Immature B cells from the bone marrow populate the spleen as transitional B cells. Transitional B cells are nonproliferative and undergo measurable cell loss as they mature (11, 12, 37). Analysis of splenic B cell subsets from Mb1-Cre/ShcFFF mice revealed decreased immature, AA4.1+ transitional B cells (T1, T2, and T3 based on IgM versus CD23 expression) and AA4.1- mature follicular B cells, but no apparent defect in marginal zone B cells or T cells (Figure 3A).
Figure 3. Mb1-Cre/ShcFFF mice show diminished transitional and follicular but not marginal zone B cells in the spleen.
Absolute numbers of B lineage cells in spleen. (A) Mb1-Cre/ShcFFF mice are deficient in transitional (T1 to T3) and follicular (FO) but not marginal zone (MZ) B cells or T cells. (B) Mb1-Cre/ShcWT mice show no defect in peripheral lymphocyte numbers (C) Cre expression later in development in the Cd19-Cre/ShcFFF mice does not affect peripheral lymphocyte numbers. *P<0.01, **P<0.001.
Importantly, B cell development was not impaired in mice conditionally expressing the wild-type Shc transgene (Figure 3B). Moreover, when we assessed the spleen of mice expressing ShcFFF under Cd19-Cre, we saw no defect in splenic B cell subsets (Figure 3C). Taken together, these studies point to a critical role for Shc-mediated signaling specifically during early B cell development rather than later maturational events.
Pro-B cells from Mb1-Cre/ShcFFF mice fail to accumulate in response to IL-7
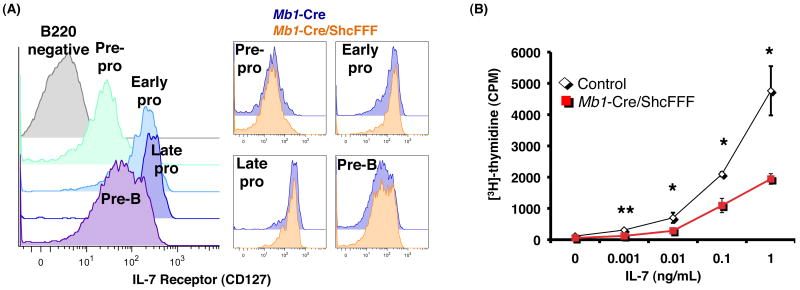
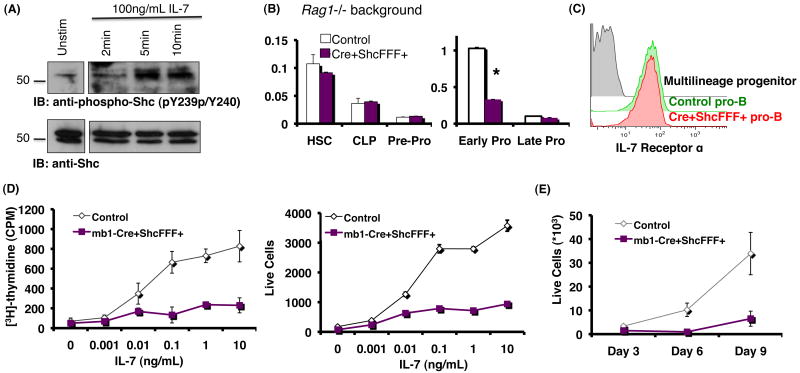
Both the cytokine IL-7 and its receptor (composed of the IL-7Rα and common gamma chain) have been shown to be critically required for development of early B cell subsets (18, 22, 38). Expression of IL-7Rα is increased from pre-pro-B to early pro-B, maintained through the late pro-B, and begins to decline by the pre-B stage (Figure 4A, left panel) (39). IL-7Rα expression in Mb1-Cre/ShcFFF mice is comparable to littermate controls during early B cell development (Figure 4A, right panel). To determine if Mb1-Cre/ShcFFF bone marrow B lymphocytes could respond to IL-7 stimulation, we measured [3H]-thymidine incorporation of pro-B cells after culture with varying concentrations of IL-7 (Figure 4B). Mb1-Cre/ShcFFF pro-B cells had significantly lower [3H]-thymidine incorporation compared to littermate controls. This suggests that although Mb1-Cre/ShcFFF mice express normal levels of the IL-7 receptor, bone marrow B cells are defective in their ability to respond to IL-7 when Shc-mediated signaling is impaired.
Figure 4. ShcFFF expression impairs response of pro-B cells to IL-7 although IL-7 receptor is present.
(A) (left) Normal expression pattern of the IL-7 receptor during early B cell development. (right) Comparable expression of IL-7 receptor in Mb1-Cre and Mb1-Cre/ShcFFF littermates in the early B cell subsets. (B) Sorted pro-B cells were cultured with the indicated concentration of IL-7. On the fourth day after plating, cells were pulsed with 1μCi of [3H] thymidine for eight hours, and thymidine incorporation was determined. *P<0.01, **P<0.001, ***P<0.0001.
It has been reported that IL-7 receptor signaling can be influenced by the pre-BCR (40, 41). Since we were specifically interested in early pro-B cells, which do not express a pre-BCR, and to rule out any contributions from the pre-BCR, we utilized Rag1-/- mice. To test the phosphorylation of Shc in response to IL-7 stimulation, pro-B cells from Rag1-/- bone marrow were stimulated with IL-7 for the indicated times (Figure 5A). Phosphorylated Shc was detected as early as 2 minutes after IL-7 stimulation, increased at 5 minutes, and began to decrease by 10 minutes. This demonstrated that Shc is phosphorylated in freshly isolated pro-B cells in response to IL-7 stimulation.
Figure 5. Pre-pro to pro-B block in Mb1-Cre ShcFFF mice and Shc tyrosine phosphorylation in response to IL-7 occur independently of the pre-BCR.
(A) CD19+ cells from Rag1-/- bone marrow were allowed to rest for 1 hour in plain RPMI before incubation with either no IL-7 or 100ng/mL IL-7 for the indicated times. Total lysates were probed with antibodies against phospho-Shc (pY239/pY240) and then total Shc. (B) Total bone marrow cell numbers. Block in pre-pro-B to early pro-B in Mb1-Cre ShcFFF mice on a Rag1-/- background. (C) Pro-B cells from Mb1-Cre ShcFFF Rag1-/- mice express IL-7 receptor at levels comparable to littermate controls. (D) CD19+ bone marrow pro-B cells from mice on a Rag1-/- background were grown in varying concentrations of IL-7, and proliferation was measured after four days. Cell growth was assessed with [3]H incorporation (left panel) or by total cell counts (right panel). (E) CD19+ pro-B cells from mice on a Rag1-/- background were grown on OP-9 stromal cells in the presence of 5ng/mL IL-7 for the indicated times. Total cell counts were quantified with a flow cytometry-based bead assay.
Pro-B cells cultured in IL-7 ex vivo can differentiate over time to yield a mixed population of early B cells. To avoid this caveat, along with any possible influence of impaired pre-BCR signaling in ShcFFF-expressing mice, we crossed the Mb1-Cre/ShcFFF mice onto a Rag1-/- background, blocking B cell development at the pro-B stage. Once again we observed a defect in development to the early pro-B cell stage in mice conditionally expressing ShcFFF (Figure 5B). These pro-B cells expressed the IL-7Rα chain at levels comparable to littermate controls (Figure 5C); yet the pro-B cells from Mb1-Cre/ShcFFF/Rag1-/- mice failed to respond to varying concentrations of IL-7 as shown by diminished [3H]-thymidine incorporation as well as decreased live cell numbers compared to littermate controls. (Figure 5D).
Although the previous experiments demonstrated that ShcFFF-expressing pro-B cells were defective in their ability to respond to IL-7, we wanted to determine whether placing cells under a more robust ex vivo culture system could overcome this defect. To this end, we plated pro-B cells from Mb1-Cre/ShcFFF/Rag1-/- mice on OP-9 stromal cells in the presence of 5ng/mL IL-7. Even in the presence of OP-9 stromal cells, ShcFFF-expressing pro-B cells were reduced in cellularity compared to littermate controls (Figure 5E). By placing Mb1-Cre/ShcFFF mice onto a Rag1-/- background, we were able to test the effects of impaired Shc signaling on IL-7 response specifically in pro-B cells. ShcFFF-expressing pro-B cells displayed an impaired response to IL-7 even in the presence of OP-9 stromal cells. Furthermore, this defect is independent of the pre-BCR.
In addition to IL-7, we tested the ability of ShcFFF-expressing bone marrow B cells to migrate to SDF-1α, which has been shown to be critical for B cell development by directing developing lymphocytes to distinct areas in the bone marrow niche (19, 20, 42). We did not observe any defect in ShcFFF-expressing B cells to migrate to SDF-1α over a range of concentrations (data not shown). Moreover, we did not observe an accumulation of immature B cells in the spleen, which is observed in Cxcr4 conditional knockout mice (43). Normal migration to SDF-1α in addition to defective response of pro-B cells to IL-7 when cultured on OP-9 stromal cells allowed us to rule out defective migration in the bone marrow niche as a possible cause for the defect in Mb1-Cre/ShcFFF mice.
Expression of ShcFFF does not affect renewal rates but impairs population production
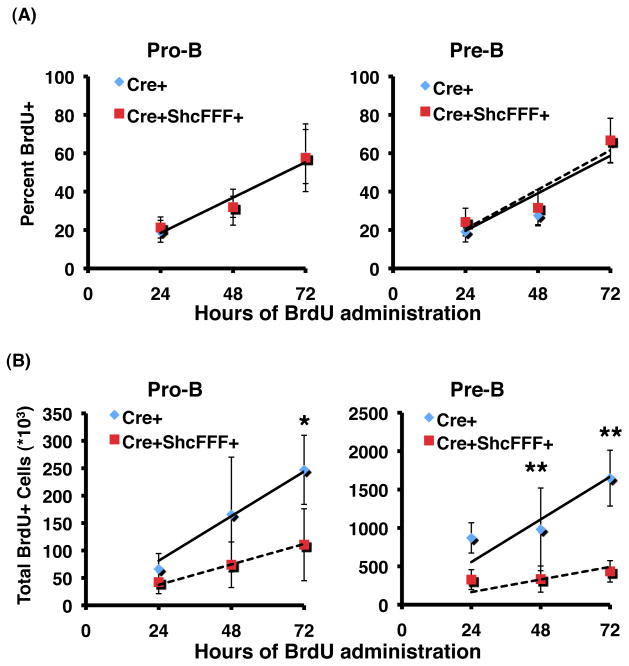
To determine how conditional expression of ShcFFF affected the population kinetics of developing B lymphocytes in the bone marrow, we analyzed in vivo BrdU incorporation rates during continuous BrdU administration (Figure 6). Both Mb1-Cre control and Mb1-Cre/ShcFFF pro-B subsets show equivalent turnover rates with 18% labeling per day (Table 1). However, the production rate of Mb1-Cre/ShcFFF pro-B cells was decreased by 54% compared to Mb1-Cre littermate controls (37×103 cells per day versus 81×103, respectively). Similarly, control and ShcFFF-expressing mice exhibited comparable labeling of the pre-B compartment, but pre-B cells from ShcFFF-expressing mice had a production rate of only 30% relative to littermate controls (164×103 cells per day versus 555×103 in controls). This suggests that ShcFFF affects the number of pro-B and pre-B cells generated under steady-state conditions, rather than the renewal rate of the respective pools.
Figure 6. Early B subsets expressing ShcFFF show normal turnover, but decreased production of lymphocyte populations.
Mice were given an initial intraperitoneal injection of BrdU and then continuous BrdU administration in water. Mice were then sacrificed at the indicated times. Bone marrow was collected, stained for surface antigens, then fixed and stained for BrdU incorporation. (A) Relative fraction of dividing BrdU+ labeling of the indicated subsets over time. The slope provides the renewal rate of the population. (B) Absolute numbers of BrdU+ ProB and pre-B cells over the time course of the experiment. Slope provides the renewal rate of the subsets. A minimum of five mice were used for each genotype at each time point. Solid and dashed lines are linear regressions for Mb1-Cre control and Mb1-Cre ShcFFF mice, respectively. *P<0.01, **P<0.001.
Table 1.
Renewal and Production Rates of Bone Marrow B Cell Subsets
| Mb1-Cre | Mb1-Cre ShcFFF | ||
|---|---|---|---|
| Pro | Renewal Rate (% of pool/day) |
18.5 | 18.4 |
| Production Rate (cells/day *103) |
81.3 | 37.3 | |
| Pre | Renewal Rate (% of pool/day) |
19.6 | 20.5 |
| Production Rate (cells/day *103) |
389.02 | 54.016 | |
Percentages of BrdU+ Pro-B and Pre-B cells for each mouse were determined by flow cytometry and multiplied by total bone marrow cell counts (trypan) from two femurs of each mouse to obtain total BrdU+ cell numbers. The regression coefficients of percent and absolute BrdU labeling versus time provide an estimate of renewal and production rates, respectively.
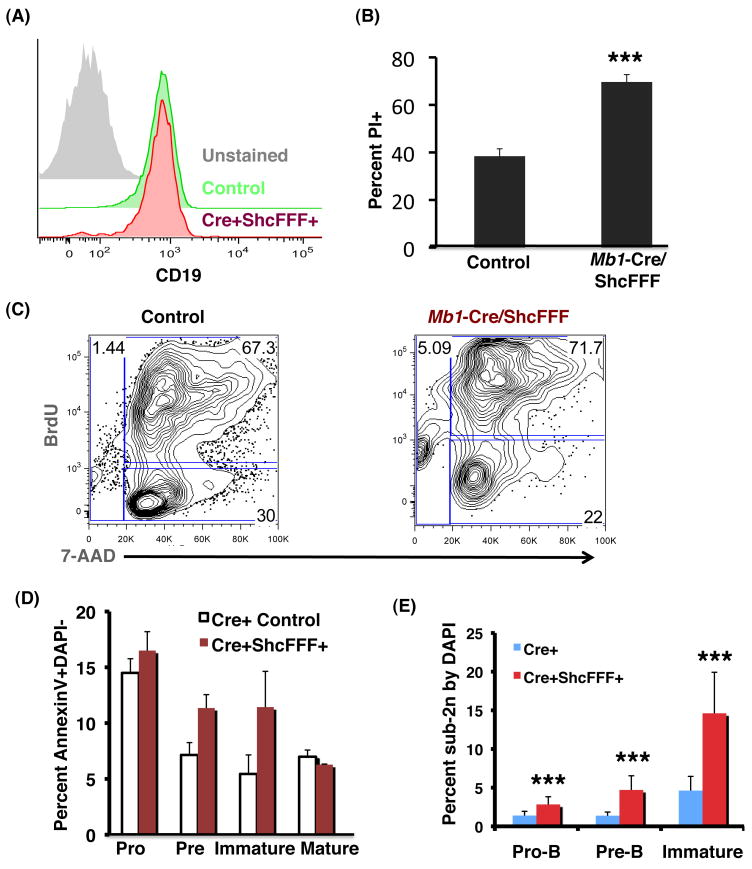
ShcFFF-expressing pro-B cells abnormally undergo apoptosis
Pro-B cell response to IL-7 is manifested as proliferation, survival, and commitment to the B lineage. Although pro-B cells from Mb1-Cre/ShcFFF/Rag1-/- mice maintained B lineage even after nine days ex vivo culture on OP-9 stromal cells (Figure 7A), they displayed a 65% increase in the fraction of propidium iodide positive cells (indicative of late-stage apoptosis) after three days (Figure 7B). We pulsed pro-B cells cultured ex vivo in IL-7-containing medium with BrdU to examine proliferation and observed a similar percentage of BrdU+ pro-B cells between Mb1-Cre/ShcFFF and littermate controls (Figure 7C), suggesting that proliferation in response to IL-7 was not impaired. However, in these experiments, we noticed an increased percentage of cells in the sub-2n gate with 7-AAD, suggesting that ShcFFF-expressing cells stimulated with IL-7 ex vivo were abnormally induced to undergo apoptosis.
Figure 7. Mb1-Cre/ShcFFF pro-B cells show increased apoptosis.
(A) Mb1-Cre/ShcFFF Rag1-/- pro-B cells grown on OP-9 stromal cells in 5ng/mL IL-7 for nine days retain B lineage as shown by expression of CD19. (B) Mb1-Cre/ShcFFF Rag1-/- pro-B cells grown on OP-9 stromal cells in 5ng/mL IL-7 for three days show an increase in percent PI+ cells. (C) FACS sorted pro-B cells from Mb1-Cre/ShcFFF mice or littermate controls were grown in IL-7-containing medium for three days. Cell cultures were pulsed with BrdU for 12 hours before assessing BrdU incorporation into pro-B cells. (D) Freshly isolated early B lymphocytes from Mb1-Cre/ShcFFF show an increase in Annexin V staining, as well as (E) an increase in the sub-2n gate (by DAPI).
We next asked whether the increase in cell death observed in the ex vivo culture of Mb1-Cre/ShcFFF could be observed in B cells freshly isolated from bone marrow. ShcFFF-expressing mice showed an increase in the percentage of cells that stained positive for Annexin V, beginning at the pro-B stage (Figure 7D). This Annexin V staining is likely underrepresented for multiple reasons: first, apoptotic cells are generally cleared quite rapidly in vivo; second, the cells isolated from the bone marrow were washed and stained for surface immunophenotyping (potentially losing the more fragile apoptotic cells in the washing/centrifugation steps); yet, the ShcFFF-expressing bone marrow B cells clearly demonstrated increased Annexin V staining compared to littermate controls. In addition, early bone marrow B cell subsets displayed an increased percentage of cells in the sub-2n gate beginning at the pro-B stage and continuing through the immature B stage (Figure 7E). Together with the data under ex vivo culture conditions in the presence of IL-7, these in vivo data provide evidence that ShcFFF-expressing pro-B cells undergo apoptosis in the bone marrow, and this likely contributes to the developmental defects seen in Mb1-Cre/ShcFFF mice.
Discussion
The adaptor protein Shc was initially shown to become phosphorylated in B cell lines in response to BCR and FcR ligation (44-46). However, due to conflicting results seen in B cell lines (41, 42), the role of Shc in primary B cells during development has remained unknown. By utilizing the Cre/loxP system we demonstrate a novel requirement for Shc during B cell development. Disruption of Shc by conditional deletion of Shc1 or transgenic expression of ShcFFF blocked B cell development prior to the pro-B stage (Supplemental Figure 2 and Figure 2). The developmental block was further defined to lie at the pre-pro-B to pro-B transition (Figure 2). Although critical roles for cell surface receptors have been demonstrated during this stage of development, the requirements for intracellular signaling components responsible for the pleiotropic effects of these receptors remains incomplete. Previous studies have demonstrated requirements of other adaptor proteins during multiple stages of B cell development, yet their functional significance is found to lie beyond to pro-B stage (47-52). To our knowledge, the data presented here are the first demonstrations of a requirement for an adaptor protein in the developmental transition from the pre-pro-B to pro-B stage.
We also present here evidence that Shc may function downstream of the IL-7 receptor. The IL-7 receptor promotes proliferation, differentiation, survival, and commitment to the B lineage. Deficiency of the cytokine IL-7 or the IL-7 receptor severely disrupts early B cell development (18, 22). Defective survival is associated with decreased Bcl-2 protein, although expression of Bcl-2 is not sufficient to rescue B lymphopoiesis in IL-7 receptor deficient mice (53). However, deletion of the pro-apoptotic Bcl-2 family member Bim was able to partially rescue B lymphopoiesis in the absence of IL-7 (54). It appears that the IL-7 receptor mediates a balance of pro-apoptotic and anti-apoptotic proteins to allow survival during early B lymphopoiesis, although the requirements for this balance remain elusive. In our initial tests, we did not observe a defect in Bcl-2 mRNA in Mb1-Cre/ShcFFF bone marrow subsets (pro, pre, immature, or mature recirculating) (data not shown). Nevertheless, the contribution of other Bcl-2 family members must be considered as possible causes for the observed increase in apoptotic cells.
Shc is linked to proliferation in response to cytokine and antigen receptor signaling (27, 30, 31). However, it also appears to play a role in cell survival. Expression of a phosphorylation-defective Shc mutant (Y239F/Y240F) in the IL-3-dependent Ba/F3 cell line caused these cells to become sensitive to apoptosis in response to IL-3 withdrawal and serum starvation (55). This was linked to reduced expression of c-Myc mRNA. Likewise, the Y239F/Y240F Shc mutant abolished c-Myc mRNA induction in response to TCR/CD3 crosslinking in Jurkat T cells (29). This was correlated with an increase in activation-induced cell death. Thus, Shc can affect multiple cellular responses. In developing thymocytes, conditional expression of ShcFFF blocked proliferation whereas in early B cells, conditional expression of ShcFFF appears to impair cell survival and lead to apoptosis.
To date, the role of Shc is best characterized as a signaling component of the Ras/MAP kinase pathway. Interestingly, expression of a dominant negative Ras mutant during early B cell development severely disrupted B lymphopoiesis at the pre-pro-B to pro-B transition. Pro-B and pre-B cells from the dominant negative Ras transgenic mice incorporated BrdU at a rate equivalent to littermate controls, suggesting no defect in the renewal rates of these subsets (15, 16). This is quite similar to the phenotype observed in B cells expressing ShcFFF. Disruption of Ras has also been shown to affect survival of pre-B cells, although upstream players have remained elusive (16). Given the known involvement of Shc in the Ras signaling pathway, it is intriguing to consider the possibility that ShcFFF may disrupt Ras signaling at the pre-pro-B to pro-B transition and induce cells to undergo apoptosis.
The intracellular signaling components of early B lymphocytes prior to the pre-BCR checkpoint remain largely unresolved, although these early B progenitors must pass multiple checkpoints before advancing along the developmental pathway. For the first time, we show here that Shc plays a key role during B cell development. Intriguingly, we identify a novel requirement for Shc in IL-7 receptor signaling that is independent of pre-BCR signaling. Expression of ShcFFF impairs B cell development at the pre-pro-B to pro-B transition with a reduction in the production rate of early B subsets, although the turnover rate is unaffected. We have shown that Shc is phosphorylated in response to IL-7 in pro-B cells. Furthermore, although pro-B cells from Mb1-Cre/ShcFFF mice proliferate normally in response to IL-7 when cultured ex vivo, as demonstrated with BrdU incorporation, they fail to accumulate and show a substantial increase in the percentage of cells undergoing apoptosis. This represents a novel role for Shc during B lymphopoiesis prior to expression of the pre-BCR that is linked to survival. These data also reveal a new player in the IL-7 receptor-mediated survival pathway during B lymphopoiesis.
Supplementary Material
Supplemental Figure 1. Diminished B cells in marrow of Mb1-Cre/ShcFFF mice. (A) FACS gating strategy to identify pro-B, pre-B, immature, and mature recirculating B cells in marrow of Mb1-Cre control (upper panel) or Mb1-Cre/ShcFFF mice (lower panel).
Supplemental Figure 2. Impaired B lymphopoiesis in Mb1-Cre Shcf/f marrow. (A) Total lysates from sorted lymphocytes from marrow of Mb1-Cre Shcf/f conditional knockout mouse littermates immunoblotted for Shc (upper panel), or Erk2 (lower panel) as a loading control. (B-C) Absolute numbers of bone marrow B lymphocytes (left panels) or splenic B lymphocytes (right panels) in eight-week old (B) or nine-month old Mb1-Cre Shcf/f mice versus littermate controls (C). *P<0.05.
Supplemental Figure 3. Normal pre-pro-B cell numbers in Mb1-Cre ShcFFF mice. (A-C) FACS gating strategies employed to examine pre-pro-B subsets in Mb1-Cre (upper panels) or Mb1-Cre/ShcFFF mice (lower panels). (A) Pre-pro-B cells gated as Ly6C-, NK1.1-, B220+, IgM-, CD43+, CD19-, CD24-. (B) Pre-pro-B cells gated as CD19-, CD11c-, NK1.1-, CD43+, B220+, Ly6C-. (C) Pre-pro-B cells gated as Lineage negative (CD3ε, Ly6G, Ly6C, NK1.1, Ter119) and IgM-, B220+, CD43+, AA4.1+, CD19-, BP1-. (D) Absolute numbers generated from total cell counts from the bone marrow, combined with the indicated FACS gating strategy.
Acknowledgments
We would like to thank the members of the Ravichandran and Bender laboratories for helpful input and suggestions on this manuscript. We would also like to thank the University of Virginia Flow Cytometry Core for technical assistance and help with cell collection.
This work was supported by a grant from the NIAID (2RO1AI43425) to K.S.R.
References
- 1.Pelayo R, Miyazaki K, Huang J, Garrrett KP, Osmond DG, Kincade PW. Cell cycle quiescence of early lymphoid progenitors in adult bone marrow. Stem Cells. 2006;24:2703–2713. doi: 10.1634/stemcells.2006-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy RR, Hayakawa K. B Cell Developmental Pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 3.Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- 4.Rumfelt LL, Zhou Y, Rowley BM, Shinton SA, Hardy RR. Lineage specification and plasticity in CD19- early B cell precursors. J Exp Med. 2008;203:675–687. doi: 10.1084/jem.20052444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alt FW, Blackwell TK, Yancopoulos GD. Development of the primary antibody repertoire. Science. 1987;238:1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- 6.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 7.Shinkai Y, Rathbun G, Lam KP, Oitz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura D, Kudo A, Schaal S, Müller W, Melchers F, Rajewski K. A critical role of lambda 5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 9.Lam KP, Kühn R, Rajewski K. In Vivo Ablation of Surface Immunoglobulin on Mature B Cells by Inducible Gene Targeting Results in Rapid Cell Death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 10.Grawunder U, Leu TMJ, Schatz DG, Werner A, Rolink AG, Melchers F, Winkler TH. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 11.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. TRENDS in Immunol. 2003;24:342–348. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 12.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of Three Nonproliferative Immature Splenic B Cell Subsets Reveals Multiple Selection Points During Peripheral B Cell Maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 13.Niiro H, Clark EA. Regulation of B-Cell Fate by Antigen-Receptor Signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 14.Wang LD, Clark MR. B-cell antigen-receptor signaling in lymphocyte development. Immunity. 2003;110:411–420. doi: 10.1111/j.1365-2567.2003.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iritani BM, Forbush KA, Farrar MA, Perimutter RM. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagaoka H, Takahashi Y, Hayashi R, Nakamura T, Ishii K, Matsuda J, Ogura A, Shirakata Y, Karasuyama H, Sudo T, Nishikawa SI, Tsubata T, Mizuochi T, Asano T, Sakano H, Takemori T. Ras mediates effector pathways responsible for pre-B cell survival, which is essential for the developmental progression to the late pre-B cell stage. J Exp Med. 2000;192:171–182. doi: 10.1084/jem.192.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw AC, Swat W, Ferrini R, Davidson L, Alt FW. Activated Ras Signals Developmental Progression of Recombinase-activating Gene (RAG)-deficient Pro-B Lymphocytes. J Exp Med. 1999;189:123–129. doi: 10.1084/jem.189.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JP, Izon D, DeMuth W, Gerstein R, Bhandoola A, Allman D. The earliest step in B lineage differentiation from common lymphoid progenitors is critically dependent upon interleukin 7. J Exp Med. 2002;196:705–711. doi: 10.1084/jem.20020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fuji N, Kishimoto T, Katsura Y, Nagasawa T. The earliest stages of B cell development require a chemokine stroma cell-derived factor/pre-B growth-stimulating factor. Immunity. 2001;15:323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 21.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, Nishikawa SI. Expression and Function of the Interleukin 7 Receptor in Murine Lymphocytes. Proc Natl Acad Sci. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peschon JJ, Morrissey PJ, Grabstein KH, R FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kikuchi K, Kasai H, Watanabe A, Lai AY, Kondo M. IL-7 Specifies B Cell Fate at the Common Lymphoid Progenitor to Pre-ProB Transition Stage by Maintaining Early B Cell Factor Expression. J Immunol. 2008;181:383–392. doi: 10.4049/jimmunol.181.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Chaudhury P, Osmond DG. Regulation of Cell Survival During B Lymphopoiesis: Apoptosis and Bcl-2/Bax Content of Precursor B Cell in Bone Marrow of Mice with Altered Expression of IL-7 and Recombinase-Activating Gene-2. J Immunol. 1999;162:1931–1940. [PubMed] [Google Scholar]
- 25.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmiere A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 26.Morrissey PJ, Conlon P, Charrier K, Braddy S, Alpert A, Williams D, Namen AE, Mochizuki D. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J Immunol. 1991;147:561–568. [PubMed] [Google Scholar]
- 27.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 28.Basu T, Warne PH, Downward J. Role of Shc in the activation of Ras in response to epidermal growth factor and nerve growth factor. Oncogene. 1994;9:3483–3491. [PubMed] [Google Scholar]
- 29.Patrussi L, Savino MT, Pelligrini M, Paccani SR, Migliaccio E, Plyte S, Lanfrancone L, Pelicci PG, Baldari CT. Cooperation and selectivity of two Grb2 binding sites of p52Shc in T-cell antigen receptor signaling to Ras family GTPases and Myc-dependent survival. Oncogene. 2005;24:2218–2228. doi: 10.1038/sj.onc.1208384. [DOI] [PubMed] [Google Scholar]
- 30.Pratt JC, v d Brink MRM, Igras VE, Walk SF, Ravichandran KS, Burakofff SJ. Requirement for Shc in TCR-Mediated Activation of a T Cell Hybridoma. J Immunol. 1999;163:2586–2591. [PubMed] [Google Scholar]
- 31.Zhang L, Camerini V, Bender TP, Ravichandran KS. A nonredundant role for the adapter protein Shc in thymic T cell development. Nature Immunology. 2002;3:749–755. doi: 10.1038/ni820. [DOI] [PubMed] [Google Scholar]
- 32.Pelanda R, Hobeika E, Kurokawa T, Zhang Y, Kuppig S, Reth M. Cre Recombinase-Controlled Expression of the mb-1 Allele. Genesis. 2002;32:154–157. doi: 10.1002/gene.10070. [DOI] [PubMed] [Google Scholar]
- 33.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing Gene Function Early in the B Cell Lineage in mb1-cre Mice. Proc Natl Acad Sci. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanfrancone L, Pelicci G, Brizzi MF, Aronica MG, Casciari C, Giuli S, Pegoraro L, Pawson T, Pelicci PG, Arouica MG. Overexpression of Shc proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SoS and Grb2/p140 complexes to the beta receptor subunit. Oncogene. 1995;10:907–917. [PubMed] [Google Scholar]
- 36.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J Immunol. 2006;176:1007–1012. doi: 10.4049/jimmunol.176.2.1007. [DOI] [PubMed] [Google Scholar]
- 37.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B Cell Development in the Spleen Takes Place in Discrete Steps and is Determined by the Quality of B Cell Receptor-derived Signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Seminars in Immunology. 2006;18:20–30. doi: 10.1016/j.smim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi K, Lai AY, Hsu CL, Motonari K. IL-7 Receptor Signaling is Necessary for Stage Transition in Adult B Cell Development Through Up-Regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 Dose-Response Threshold During Pro-B Cell Differentiation is Dependent on Pre-B Cell Receptor Expression. J Immunol. 1998;161:6038–6045. [PubMed] [Google Scholar]
- 41.Fleming HE, Paige CJ. Pre-B Cell Receptor Signaling Mediates Selective Response to IL-7 at the Pro-B to Pre-B Cell Transition via an ERK/MAP Kinase-Dependent Pathway. Immunity. 2001;15:521–531. doi: 10.1016/s1074-7613(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 42.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zhou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smit L, de Vries-Smits AM, Bos JS, Borst J. B cell antigen receptor stimulation induced formation of a Shc-Grb2 complex containing multiple tyrosine-phosphorylated proteins. J Biol Chem. 1994;269:20209–20212. [PubMed] [Google Scholar]
- 45.Lankester AC, van Schijndel GM, Rood PM, Verhoeven AJ, van Lier R. B cell antigen receptor cross-linking induces tyrosine phosphorylation and membrane translocation of a multimeric Shc complex that is augmented by CD19 co-ligation. Eur J Immunol. 1994;24:2818–2825. doi: 10.1002/eji.1830241136. [DOI] [PubMed] [Google Scholar]
- 46.Kumar G, Wang S, Gupta S, Nel A. The membrane immunoglobulin receptor utilizes a Shc/Grb2/hSos complex for activation of the MAPK cascade in a B cell line. Biochem J. 1995;307:215–223. doi: 10.1042/bj3070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi K, Nittono R, Okamoto N, Tsuji S, Hara Y, Goitsuka R, Kitamura D. The B Cell-Restricted Adaptor BASH is Required for Normal Development and Antigen Receptor-Mediated Activation of B cells. Proc Natl Acad Sci. 2000;97:2755–2760. doi: 10.1073/pnas.040575697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu S, Tan JEL, Wong EPY, Manickam A, Ponniah S, Lam KP. B Cell Development and Activation Defects Resulting in xid-like Immunodeficiency in BLNK/SLP-65-Deficient Mice. Int Immunol. 2000;12:397–404. doi: 10.1093/intimm/12.3.397. [DOI] [PubMed] [Google Scholar]
- 49.Herzog S, Hug E, Meixlsperger S, Paik JH, DePinho RA, Reth M, Jumaa H. SLP-65 Regulates Immunoglobulin Light Chain Gene Recombination through the PI(3)K-PKB-Foxo Pathway. Nat Immunol. 2008;9:623–631. doi: 10.1038/ni.1616. [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki T, Takeda K, Gotoh K, Takeshima H, Akira S, Kurosaki T. Essential Immunoregulatory Role for BCAP in B Cell Development and Function. J Exp Med. 2002:195. doi: 10.1084/jem.20011751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donahue AC, Hess KL, Ng KL, Fruman DA. Altered Splenic B Cell Subset Development in Mice Lacking Phosphoinositide 3-Kinase p85alpha. Int Immunol. 2004;16:1789–1798. doi: 10.1093/intimm/dxh180. [DOI] [PubMed] [Google Scholar]
- 52.de la Fuente MA, Kumar L, Lu B, Geha RS. 3BP2 Deficiency Impairs the Response of B Cell, but not T Cells, to Antigen Receptor Ligation. Mol Cell Biol. 2006;26:5214–5225. doi: 10.1128/MCB.00087-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maraskovsky E, Peschon JJ, McKenna H, Teepe M, Strasser A. Overexpression of Bcl-2 does not rescue impaired B lymphopoiesis in IL-7 receptor-deficient mice but can enhance suvival of mature B cells. Int Immunol. 1998;10:1367–1375. doi: 10.1093/intimm/10.9.1367. [DOI] [PubMed] [Google Scholar]
- 54.Huntington ND, Labi V, Cumano A, Vieira P, Strasser A, Villunger A, Di Santo JP, Alves NL. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim sustains B lymphopoiesis in the absence of IL-7. Int Immunol. 2009 doi: 10.1093/intimm/dxp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gotoh N, Tojo A, Shibuya M. A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J. 1996;15:6197–6204. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Diminished B cells in marrow of Mb1-Cre/ShcFFF mice. (A) FACS gating strategy to identify pro-B, pre-B, immature, and mature recirculating B cells in marrow of Mb1-Cre control (upper panel) or Mb1-Cre/ShcFFF mice (lower panel).
Supplemental Figure 2. Impaired B lymphopoiesis in Mb1-Cre Shcf/f marrow. (A) Total lysates from sorted lymphocytes from marrow of Mb1-Cre Shcf/f conditional knockout mouse littermates immunoblotted for Shc (upper panel), or Erk2 (lower panel) as a loading control. (B-C) Absolute numbers of bone marrow B lymphocytes (left panels) or splenic B lymphocytes (right panels) in eight-week old (B) or nine-month old Mb1-Cre Shcf/f mice versus littermate controls (C). *P<0.05.
Supplemental Figure 3. Normal pre-pro-B cell numbers in Mb1-Cre ShcFFF mice. (A-C) FACS gating strategies employed to examine pre-pro-B subsets in Mb1-Cre (upper panels) or Mb1-Cre/ShcFFF mice (lower panels). (A) Pre-pro-B cells gated as Ly6C-, NK1.1-, B220+, IgM-, CD43+, CD19-, CD24-. (B) Pre-pro-B cells gated as CD19-, CD11c-, NK1.1-, CD43+, B220+, Ly6C-. (C) Pre-pro-B cells gated as Lineage negative (CD3ε, Ly6G, Ly6C, NK1.1, Ter119) and IgM-, B220+, CD43+, AA4.1+, CD19-, BP1-. (D) Absolute numbers generated from total cell counts from the bone marrow, combined with the indicated FACS gating strategy.