Abstract
Aging is associated with an earlier timing of circadian rhythms and a shorter phase angle between wake time and the timing of melatonin secretion or the core body temperature nadir. Light has a phase-dependent effect on the circadian pacemaker, and modifications of habitual light exposure in older people could contribute to a change in the timing of circadian rhythms or in the phase angle of entrainment. In this study, we compare natural light exposure of community-dwelling older and young subjects studied at the same time of year, focusing on the pattern of light exposure across the waking day.
We recorded light exposure data for 3–8 days from 22 older (aged 66.01± 5.83 (SD)) and 22 young subjects (aged 23.41 ± 4.57 (SD)), living at home on self-selected sleep-wake schedules, and matched for time of year. All subjects were from New England (latitude 42.3° N to 43° N). We compared the percentage of the waking day spent by older and young subjects at four different light levels (from very dim to very bright). We compared hourly-averaged light exposure data in each group according to clock time and with respect to each subject’s daily sleep-wake times.
Although both age groups spent more than half of their waking hours in dim or moderate room light intensity (<100 lux), we found that the older subjects spent a significantly greater percentage of their waking day in the brighter light levels (>1,000 lux); their hourly-averaged light exposure levels were also significantly greater, whether we examined the data with respect to absolute clock time, to wake time, or to bedtime, and this was true across all seasons.
We found that healthy older people were exposed to significantly higher levels of light throughout their waking day than young people. Differences in natural light exposure may contribute to the age-related phase advance of the circadian pacemaker and its later timing relative to the sleep-wake cycle. This hypothesis should be explored further in carefully designed prospective studies.
Keywords: aging, circadian, sleep, entrainment, human, light, light exposure
INTRODUCTION
There are well-documented age-related changes in sleep timing, quality, and quantity with older people having earlier wake times and bedtimes, experiencing lighter and more fragmented sleep, and often waking earlier than desired (Bliwise, 1993; Buysse et al., 1992; Dijk et al., 2000). These changes have been hypothesized to be due to in part to underlying age-related changes in the circadian system (Cajochen et al., 2006; Dijk et al., 2000; Niggemyer et al., 2004).
Data from several laboratory studies have demonstrated that the absolute and relative timing of the circadian system is modified in older people compared to young adults (Carrier et al., 2002; Czeisler et al., 1992; Duffy et al., 1998; Duffy et al., 2002; Lewy et al., 2000; Yoon et al., 2003). Markers of circadian phase occur at an earlier clock time in older people compared to young adults (Duffy et al., 1998; Duffy et al., 2002; Yoon et al., 2003). In addition, it has been reported that the average wake time of older adults may also occur at an earlier phase of the circadian rhythm compared to young adults (Duffy et al., 1998; Duffy et al., 2002; Youngstedt et al., 2001), indicating that older adults are entrained at a different phase angle than young adults.
There are several potential causes of these age-related differences in the absolute and relative timing of the circadian system. Because light is the primary signal from the environment that entrains the circadian system in humans, a modification in the habitual pattern of light exposure with age could underlie the observed age-related changes in the timing of circadian phase. Studies of the response of the human circadian system to light have shown that the greatest sensitivity to light occurs during the habitual nighttime, when there is typically little light exposure (Honma and Honma, 1988; Khalsa et al., 2003; Minors et al., 1991).
It is therefore possible that the observed age-related modification of sleep and circadian rhythm timing in humans could be due to differences in self-selected light exposure. While several prior studies have examined light exposure levels in older and/or young adults, they typically have reported on average levels of light exposure across the 24h day, rather than examining the pattern of light exposure across the day (Guillemette et al., 1998; Jean-Louis et al., 2000; Savides et al., 1986). In that respect, by looking at differences in self-selected light exposure between young and older adults while controlling for photoperiod, our current report attempts to extend the results from previous studies (Kawinska et al., 2005).
The present study was conducted to examine self-selected light exposure levels and patterns in community-dwelling older and young adults who were studied in the same week, in order to determine whether there are differences that might contribute to the reported age-related differences in the timing of the circadian system. We focused our analyses on indoor as well as outdoor levels of light, and examined the patterns of light exposure across the waking day.
MATERIALS AND METHODS
Population
Data from two groups of healthy subjects, 22 older adults and 22 young adults, were selected for analysis. Subjects had participated in screening for an inpatient circadian rhythm or sleep study which included pre-study actigraphy and light recordings collected while still living at home in order to document their sleep-wake schedules.
Subjects were recruited for the inpatient studies using flyers, newspaper, and radio advertisements. All subjects were in good physical and psychological health, were not taking any medications, and reported no significant sleep complaints.
Each subject gave written, informed consent before participation in study procedures. The Human Research Committee at the Partners HealthCare System approved the protocols, which were conducted in accordance with the Declaration of Helsinki.
Ambulatory light monitoring
Light levels were recorded at 1- or 2-minute intervals using the Actiwatch-L (Respironics, Murrysville, PA) while the subjects lived at home on self-selected sleep-wake schedules (see below). For subjects to be included in our analysis, at least three consecutive days (but up to eight) of analyzable data from the Actiwatch-L had to be available, and the activity data had to be consistent with the schedule recorded by the subject in a sleep/wake diary. A total of 35 different light monitors were used to collect the data reported here; 17 in the 22 young subjects and 19 in the 22 older subjects, with one device used both in the young and in the older group.
To avoid including artifact data due to a sleeve covering the sensor, we first examined Actiwatch light exposure data recorded in the laboratory in verified sleeve-free recordings from very dim light conditions (less than 15 lux at 6 feet above the floor facing the ceiling, and less than 4 lux at approximately 4 feet above the floor facing the walls). In these lighting conditions, recorded levels from the Actiwatches did not drop below 1 lux. We therefore decided that light data below 1 lux during self-reported waking hours should be considered as artifact data (indicating the light sensor had been accidentally covered with a sleeve), and removed such data from analysis.
Bedtime and wake time recording
Subjects had to report a habitual sleep duration between 7 and 9 hours per night to take part in the inpatient study they were being screened for. Two to three weeks before the inpatient part of their study, they were asked to choose a target bedtime and wake time, close to their natural schedule, with bedtime and wake time 8 hours apart. They were asked to adhere to those times each day (+/− 30mn), including on week-ends and holidays and to record their wake and bed times in a sleep/wake diary. To supplement the diary, subjects also phoned in to a time-stamped voicemail system at each wake and bed time. During all or part of this 2–3 week period, subjects were given the Actiwatch-L to further document their adherence with a regular sleep-wake schedule. Subjects were excluded from the study if they were unable to maintain a regular sleep-wake schedule, and were excluded from our analysis if the diary and the time-stamped voicemail differed by more than 10 minutes.
Selection of subject pairs
We evaluated for inclusion in the present analysis actigraphy data from all the older subjects who had participated in four different inpatient sleep or circadian rhythm studies, totaling 37 individuals. In addition, we included three older subjects who had completed screening but who did not take part in an inpatient study. All 40 older subjects met the criteria outlined above for adherence to a regular sleep-wake schedule. However, data from five of the older subjects were eliminated from analysis because they had long periods of sleeve artifact (described above) on all days of recording.
Next, in order to minimize differences in light exposure due to weather or season, each older subject was paired with a young subject studied during the same days in the same calendar year. To do this, we reviewed actigraphy data from young subjects who participated in studies with similar medical, psychological, and sleep-wake inclusion/exclusion criteria described above (a total of nine different studies). An additional thirteen older subjects were not included in our final data set because we could not find a suitable young subject studied in the same week. For one of the older-young subject pairs, the subjects were studied on the same days one year apart, so that season, but not weather, was controlled. By this process of matching older with young subjects, we obtained our final 22 pairs of older and young subjects.
Because this is a retrospective analysis, we do not have information from the data collection period about what activities the subjects had during the days of recording. However, we assumed that their schedule might differ between week days and week-ends, and so to avoid this potentially confounding our comparison, we matched the older-young pairs so that they had the same number of week-end days.
Data analysis and statistical methods
The t-test (for normally distributed variables) or a non-parametric Wilcoxon sum of ranks test (for non-normally distributed variables) were used to compare the two groups’ characteristics such as age, bedtimes, wake times, average number of week-end days included in the analysis and average number of total study days included in the analysis. Comparison of sex distribution between the two groups and across seasons was done using a Chi-Square test.
Light exposure levels were analyzed from only the self-reported waking hours, as indicated from the time-stamped bedtime and wake time voicemail messages. Because of our instructions regarding the duration of sleep episodes, all subjects had wake episodes of about 16 hours per day.
Levels of light exposure across the waking day
We assigned each 1 or 2-minute epoch of light recording to one of four different light threshold categories: < 10 lux, corresponding to very dim indoor light; 10 to <100 lux, corresponding to dim-moderate levels of indoor light; 100 to <1,000 lux, corresponding to moderate-bright indoor light or dim outdoor light; and ≥1,000 lux, corresponding to outdoor levels of light. We calculated the average percentage of time spent at each light exposure level each day per subject and then compared whether both age groups spent comparable percentages of their waking day at these different light levels. We used an ordinal logistic regression with correlated responses (using SAS procedure GENMOD, specifying a multinomial distribution, an independent covariance structure, and link function cumlogit) to examine the association between percentage of the waking day spent in the brighter categories of light (100 to <1,000 and ≥1,000 lux) and age group. We ran this analysis for the first 8 hours of the waking day and then for the final eight hours before bedtime.
Light exposure pattern analysis
We first analyzed the waking light exposure data with respect to absolute clock hour for those hours each subject reported being awake. We averaged each subject’s three to eight days of light exposure data on an hourly basis to create a per-subject average with respect to clock hour, including only those hour bins containing at least 30mn of observations. Because of the large range of wake times (from 5am to 10:30 am) and of bedtimes (from 9pm to 2:30am), this resulted in twenty-two hourly bins ranging from the 5am-6am bin to the 2am-3am bin.
We next averaged light exposure levels hourly with respect to each subject’s sleep-wake timing. Because of the slightly variable wake durations (16h ± 30mn), each waking day was divided into two segments: the first eight hours following wake time, and the eight hours preceding bedtime. We averaged each subject’s three to eight days of light exposure data per hour for the eight hours following wake time (+1, +2, etc.). A similar procedure was followed for each hour in the eight hours before bedtime, beginning at bedtime and working back (−1, −2, etc.).
Comparisons between the two age groups were made on the following measures:
hourly light exposure with respect to absolute clock hour (twenty-two absolute clock hour bins).
hourly light exposure relative to wake time (for the first eight hours after wake time, resulting in eight 1-hour bins)
hourly light exposure preceding bed time (for the last eight hours preceding bedtime, resulting in eight 1-hour bins)
For each of these analyses, the hourly light exposure averages at each measurement occasion were right skewed, so we log-transformed them for statistical analysis. Log-transformation of the averaged raw data was successful in establishing a symmetrical distribution at each measurement occasion, meeting the criteria for a satisfactory multivariate normal distribution (Fitzmaurice et al., 2004). Due to reports that even brief episodes of exposure to bright light may have phase shifting effects (Gronfier et al., 2004; Nelson and Takahashi, 1991; van den Pol et al., 1998), we averaged the raw data prior to log transformation so that brief episodes of very bright light would be accounted for. Had we instead log-transformed the raw data first and then performed hourly averaging, this would have diluted the contribution of such brief episodes of very bright light exposure.
We analyzed the data using a mixed model analysis with repeated measures approach (proc MIXED in SAS). We included in our model the factors age group (older or young), time (hourly bin), gender, and season. The variable season was determined by photoperiod, rather than by calendar, with the winter season centered around December 21st (rather than beginning on December 21st), summer centered around June 21st, and spring and fall centered around the equinoxes.
Analyses were performed using the SAS system (SAS Institute, Cary, NC, version 9.1.2). Because we ran four different statistical analyses on the same data, we chose a conservative level of significance for our single comparison analyses (p = 0.01), and adjusted with a Bonferroni correction when there were multiple comparisons run simultaneously.
RESULTS
Population
The two age groups were comparable with respect to gender, the average number of days of light recording analyzed, and the proportion of week-end days studied (see Table 1). The older subjects woke up and went to bed significantly earlier than their younger counterparts (see Table 1). We had occupational information for 20 of the 22 older subjects and 20 of the 22 young subjects. Sixteen of the older subjects were retired and four were employed. In contrast, nine of the young subjects were employed, two were full-time students and also worked part-time, six were full-time students with no part-time employment, and three were unemployed.
Table 1.
Population characteristics (mean ± SD).
| Older (n=22) | Young (n=22) | P value | |
|---|---|---|---|
| Age | 66.01 ± 5.83 | 23.41 ± 4.57 | <0.0001 |
| Gender | 10F 12M | 9F 13M | NS |
| Study days | 5.11 ± 1.64 | 5.11 ± 1.59 | NS |
| Number of week end days | 1.77 ± 0.75 | 1.77 ± 0.75 | NS |
| Wake time | 6:57 ± 1:02 | 7:38 ± 1:03 | 0.03 |
| Bed time | 22:50 ± 1:06 | 23:38 ± 1:01 | 0.01 |
Time of study across the calendar year
Of the 22 pairs of subjects, four were studied in the winter, three were studied during the spring, five were studied during the summer and ten during the fall. There was no significant difference in the gender distribution across seasons (Chi square test: p=0.54).
Levels of light exposure across the waking day
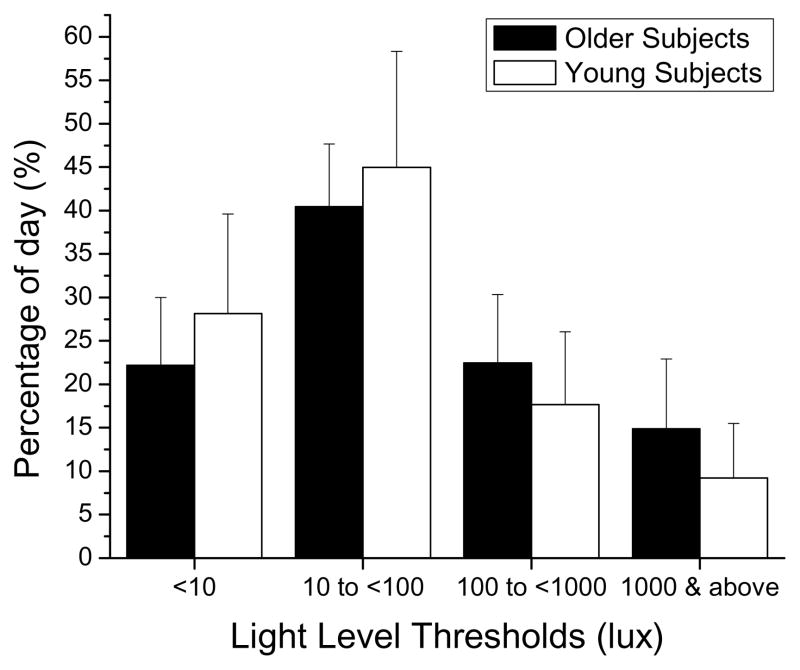
Older subjects spent on average 37.7% of their waking day in light levels above 100 lux and 14.7% in light levels above 1,000 lux (likely to be outdoor levels of light) whereas young subjects spent only 27% of their waking day in light exceeding 100 lux and only 9% in light levels above 1,000 lux (see Figure 1), across all seasons. These differences between the 2 groups were lowest in spring, with the older subjects spending 34% and the young spending 31% of their waking day light levels above 100 lux; differences were highest during the winter (30% and 13% only, respectively)..
Figure 1.
Average ± SD distribution of light threshold levels (<10 lux, 10 to <100 lux, 100 to <1,000 lux, ≥1,000 lux) in older (black bars) and young (white bars) subjects across the waking day.
Results from the ordinal logistic regression analysis (conducted to examine the association between the cumulative exposure to light and age group), showed older subjects had significantly greater odds of spending a larger percentage of their waking day in levels of light greater than 100 lux and greater than 1,000 lux, both in the first 8 hours (OR= 1.77; 95% CI=[1.17; 2.69]) and in the final eight hours of their waking day (OR= 1.52; 95% CI=[1.03; 2.24]).
Light exposure with respect to absolute clock hour
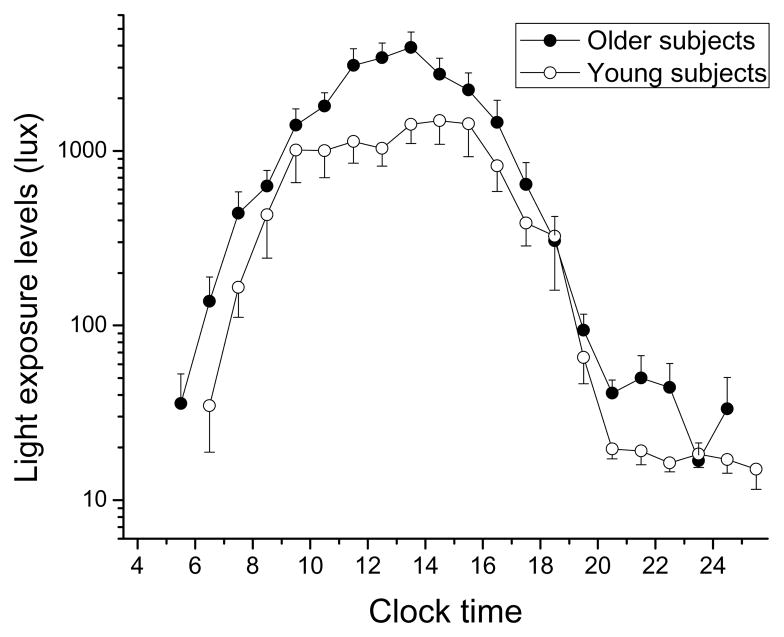
When examining how light exposure data varied by clock hour, we found a significant main effect of ‘absolute clock hour’ (F(21,566)=52.43; p<0.0001), with subjects exposed to increasing light levels beginning in the morning and continuing until midday (see Figure 2). After the midday peak, light exposure levels fell with a steep downward slope until approximately 18:00, and reached levels below 100 lux after 19:00 in both groups.
Figure 2.
Light exposure during waking hours with respect to absolute clock time in older and young subjects. Light exposure levels (in lux) are shown on a logarithmic scale. Mean light exposure data for each waking clock hour were averaged per subject, and then averaged across subjects (± SEM) in each age group. Only those clock hours with at least 3 subjects are presented. Older subjects: filled symbols; younger subjects: open symbols.
We also found a significant main effect of ‘age group’, with the older subjects getting higher levels of light exposure compared to the young subjects (average ± SEM older: 982 ± 264 lux; younger: 517 ± 121 lux; F(1,566)=7.54; p=0.0062, see Figure 2). There was also a significant main effect of ‘season’, with all subjects being exposed to higher levels of light in summer and fall, and lower levels of light in spring and winter (F(3,566)=5.84; p=0.0006). No significant main effect of gender was observed (F(1,566)=0.00; p=0.97).
When we examined the two-way interactions between the significant main effects [factors ‘absolute clock hour’, ‘age group’, and ‘season’], we found a significant ‘clock hour‘ × ‘season’ interaction (F(53,566)=3.65; p<0.0001), whereby light exposure in the 5 hours from 14:00–18:00 was about 10 times lower in winter and spring compared to summer and fall (post-hoc analysis adjusted for multiple comparisons, p=0.002 at each of these clock hour bins). There were no other significant two–way interactions.
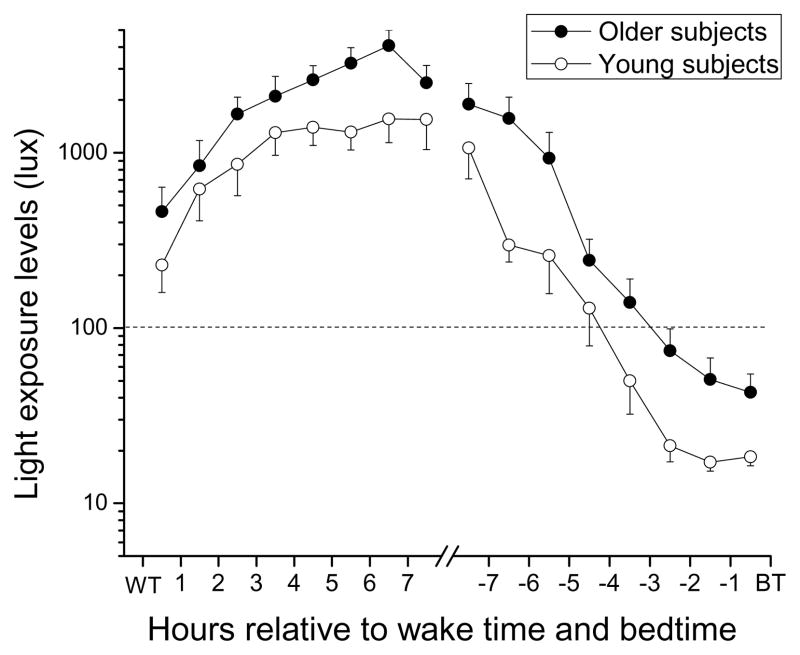
Light exposure in the first 8 hours after wake time
When examining light exposure relative to each individual’s wake time, we found a significant main effect of ‘wake hour bin’, with light levels rising steadily across the eight ‘wake hour bins’ (F(7, 34)=16.31; p<0.0001, see Figure 3). There was also a significant main effect of ‘age group’, with the older group being exposed to significantly higher light levels across the 8 hours following wake time (average ± SEM older subjects: 2186 ± 422 lux; younger subjects: 1102 ± 170 lux; F(1, 34)=6.7; p=0.01, see Figure 3). There was also a significant main effect of ‘season’, whereby overall light exposure in the first eight hours after wake time was higher in spring, summer, and fall relative to winter (F(3, 34)=10.46; p<0.0001). There were no significant two-way interactions [‘age group’ × ‘season’ interaction: (F(3, 34)=2.56; p=0.07; ‘wake hour bin’ by ‘age group’ interaction: (F(7, 34)=0.59; p=0.76)].
Figure 3.
Light exposure pattern relative to the 8 hours after wake time and relative to the 8 hours before bedtime in older and young subjects. Light exposure levels (in lux) are shown on a logarithmic scale. Mean light exposure data for each hour in the 8 hours following wake time and in the 8 hours preceding bedtime were averaged per subject, and then averaged across subjects (± SEM) in each age group. Older subjects: filled symbols; younger subjects: open symbols.
Light exposure levels in the final 8 hours before bedtime
When examining the patterns of light exposure in the last eight hours before bedtime, we found a significant main effect of ‘bedtime hour bin’, with light levels decreasing steadily in the last few hours before bedtime (F(7, 37)=40.19; p<0.0001; see Figure 3). We also found a significant main effect of ‘age group’, with the older group being exposed to significantly higher levels of light than the young group (average ± SEM older: 617 ± 264 lux; younger: 232 ± 125 lux; F(1, 37)= 14.92; p=0.0004, see Figure 4). There was no main effect of ‘gender’ (F(1, 37)=0.67; p=0.42), and no significant main effect of ‘season’ (F(3, 37)=2.64; p=0.064). There was a significant ‘bedtime hour bin‘ × ‘season’ interaction (F(21, 37=3.11; p=0.0013), whereby light levels during summer and fall were significantly higher in both groups in the midday hours (BT-8-BT-7 bin) compared to other seasons (post-hoc analysis adjusted for multiple comparisons: p=0.007 at this bin). There was no significant ‘bedtime hour bin’ × ‘age group’ interaction (F (7, 37)= 0.94 ; p= 0.49).
DISCUSSION
The older subjects in the study woke up and went to bed about an hour earlier compared to their young counterparts, as has been reported previously (Ancoli-Israel, 1997; Bliwise, 1993; Czeisler et al., 1992; Dijk et al., 2000; Kawinska et al., 2005; Kripke et al., 2007; Prinz, 1995).
We found that both age groups spent most of their waking day in indoor levels of light, which is consistent with previous studies of natural light exposure (Espiritu et al., 1994; Hébert et al., 1998; Jean-Louis et al., 2000; Savides et al., 1986). In one prior study of young adults living in San Diego, subjects spent only about 15% of their waking day in light above 1,000 lux (Savides et al., 1986). Similarly, in a sample of 53 women and 53 men between ages 40 and 64 residing in San Diego, the authors reported their population averaged less than one hour per day in light above 1,000 lux (Espiritu et al., 1994). In a study carried out in Montréal, (~45.5° N) (Kawinska et al., 2005), both young and middle-aged subjects spent about 1.5 hours per day in light levels above 1,000 lux. Two prior studies including healthy older subjects also reported that older subjects spend only about an hour per day in outdoor light (Campbell et al., 1988; Staples et al., 2009).
In the present study, when we examined the amount of time subjects spent in various light exposure threshold levels, we found that the older subjects spent a significantly greater amount of their waking day in light levels above 100 lux and above 1,000 lux compared to their younger counterparts, and this finding was confirmed when we examined actual light exposure levels on an hour-by-hour basis across the waking day. Although the percentage of time spent within each category of light exposure (from dim to bright light) varied with season, the older subjects spent more time in levels of light suggestive of outdoors across all seasons compared to the young subjects. This finding that the older subjects had earlier wake times and were exposed to higher levels of light during the day parallels a finding in a group of 500 adolescents and young adults in which greater self-reported outdoor light exposure was associated with earlier self-reported sleep timing (Roenneberg et al., 2003).
Prior studies of light exposure with respect to age have found that older adults spend little time in outdoor levels of light and that this is the same or less than that of young subjects: in a study of community-dwelling women, older women were reported to have the same illumination parameters compared to younger women throughout the 24 h day (Jean-Louis et al., 2000); in another study, institutionalized older subjects were exposed to lower levels of light than young control subjects (Mishima et al., 2001); and a study of young and middle-aged subjects found both groups to be exposed to the same percentages of very dim, moderately dim, moderately bright, and very bright levels of light during the day, with similar patterns of light exposure with respect to habitual wake timing (Kawinska et al., 2005). In fact, only one prior study (Campbell et al., 1988) found that seven of their ten healthy older subjects were exposed to more bright light than five of their ten young subjects but it is not indicated in that paper whether the two groups were studied at the same times of year.
One explanation for why we found greater durations of exposure to outdoor levels of light in the older subjects in our study, in contrast to other studies, may be that our subjects (both older and young) were selected because they were healthy and living independently. Because most of the older subjects in our study were retired and most of our young subjects were either working or students, the older people may have had more opportunity to spend time outdoors than the young subjects. However, because of the retrospective aspect of our analysis, we did not have detailed information on the day-to-day behavior of our subjects to understand whether this was the case.
Another important difference between our study and prior studies of older and young subjects may be the matching of the subjects for time of year in our study. Time of year is an important factor contributing to overall light exposure in most locations, and therefore an important covariate in examining the relationship between light exposure and age group. In the present study, we found a main effect of season, whereby both groups were exposed to significantly higher levels of light in summer and fall compared to spring and winter. This seasonal variation in light exposure level is consistent with findings from a study by Cole et al. (Cole et al., 1995), who found that seasonal variations in light exposure accounted for most of the total variability in light exposure, even when adjusting for latitude [Rochester, Minnesota (~44°N) and San Diego, California (~32.7°N)] or age. Similarly, a study conducted in Montreal (~45.5°N) found that young adults spent less than a half hour per day in light above 1,000 lux in winter, but more than 2.5 hours per day in summer (Hébert et al., 1998). In our study, by carefully matching the two age groups for season, we found that the older subjects were exposed to higher levels of light than younger subjects consistently across all seasons.
We also found a difference in the pattern of light exposure across the waking day between the two age groups: in the first eight hours after wake time and in the last eight hours before bedtime, older subjects were exposed to significantly higher levels of light than their young counterparts. This finding is in contrast to that from a study of young and middle-aged subjects, where increasing age was associated with higher light levels in the morning relative to absolute clock time, but not relative to wake time (Kawinska et al., 2005).
How our current finding of differences in self-selected light exposure patterns between young and older adults relates to previously-reported age-related differences in timing of circadian phase and differences in phase angle of entrainment (Duffy et al., 1998; Duffy et al., 2002; Yoon et al., 2003; Youngstedt et al., 2001) cannot be determined from these data. Because of the retrospective nature of this study, we did not have phase data from all the subjects and therefore could not analyze whether the observed light exposure differences were associated with age differences in circadian phase or phase angle of entrainment. In the study by Kawinska et al, the observed change in the patterns of light exposure with age did not explain entirely the age-related advance in circadian phase (Kawinska et al., 2005).
In previous laboratory studies, significant phase shifts have been obtained using levels of light similar to those our subjects received in the hour or two after awakening (in the range of 100–1,000 lux) (Duffy et al., 2007; Zeitzer et al., 2000). Furthermore, recent laboratory and field studies in humans have demonstrated that prior exposure to dim light or darkness sensitizes the human circadian system, making it more responsive to subsequent light exposure (Hébert et al., 2002; Owen and Arendt, 1992; Smith et al., 2004). Therefore, ambulatory subjects would be expected to be more sensitive to morning light, having spent the prior ~8 hours in darkness, and perhaps less sensitive to evening light, having been exposed to greater amounts of light throughout the waking day. If this is indeed the case, then it suggests that the differences in self-selected morning light exposure between the two age groups in our study would have greater biological importance than the differences in evening light exposure.
In addition, those studies have found that older adults not only go to bed and wake at an earlier clock time, they also go to bed and wake at an earlier biological time than younger adults. That suggests that less of the phase delay region and more of the phase advance region of the phase response curve (PRC) (Khalsa et al., 2003) would be exposed to light in older subjects. For both these reasons, the differences in self-selected light exposure relative to wake time between the two age groups in our study may have greater biological importance than the differences in light exposure relative to bedtime. However, this would not necessarily translate in larger phase advances in older subjects. Indeed, normally-entrained young adults wake at a circadian phase just before the maximal phase advances to light occur (Khalsa et al., 2003) but the shape of the human PRC to light is such that light exposure just prior to that time will result in smaller phase advances. Thus, if the shape of the PRC does not change with age, higher levels of light exposure received by older subjects waking at an earlier biological time might not translate into larger phase advances. Also, there is evidence from both animal (Rosenberg et al., 1991; Zhang et al., 1996) and human (Duffy et al., 2007; Klerman et al., 2001) studies that there are age-related changes in light sensitivity, and evidence of age-related changes in transmission of light to the circadian system (Brainard et al., 1997; Charman, 2003; Herljevic et al., 2005), either of which could produce in older subjects a decreased phase shifting response of the circadian pacemaker to greater morning light exposure.
The higher levels of light throughout the waking day that we observed in older subjects could also contribute to age-related differences in phase angle of entrainment described in previous studies. In both rodents (Pittendrigh and Daan, 1976) and humans (Gronfier et al., 2007; Wright Jr. et al., 2005), it has been shown that phase angle decreases as the strength of the circadian synchronizer (light) increases.
The light exposure levels recorded in this study may not reflect the effective light exposure at the level of the cornea. Although an earlier study found that wrist measurements correlate highly (r=0.7) with light levels recorded at the angle of gaze (Okudaira et al., 1983), wrist measurements are unlikely to measure exactly the levels of light that reach the eye. On the other hand, it is unlikely that the difference between the recorded levels of light at the wrist and the actual levels of light that reach the eye would be systematically different between the older and the young subjects. Secondly, because this is a retrospective analysis with subjects in both age groups coming from a number of different studies, the light monitors used for this study were calibrated by the manufacturer but not tested or re-calibrated by us prior to use in the studies. Because there were so many different devices used in both groups, we have no reason to believe that the between-group variability would be greater than the within-group variability due to a calibration bias. Similarly, it is possible that some recorded light levels above 1 lux could have been artifactual due to a sleeve covering the sensor, thus underestimating the true levels of light to which the subjects were exposed. It is also possible that subjects wore sunglasses on bright days, and thus the levels measured at the wrist could overestimate the levels of light reaching the eye. In general, we have no reason to believe that one group wore long sleeves that would cover their Actiwatch or wore sunglasses more than the other. In fact, the close matching in time of older and young subjects would control for such issues as much as possible. Although the average values reported in this study may represent an approximation of the actual levels of light received at the cornea, we believe the limitations described above would have affected both age groups in a similar manner, and therefore should not affect the significance of the comparisons between the two groups.
In this analysis, we found that older subjects were exposed to significantly higher levels of light than their young counterparts, across the waking day, with respect to absolute clock time, and relative to wake times and bedtimes. Additional prospective studies where ambulatory light exposure patterns and measures of circadian phase and phase angle are assessed in older and young subjects studied at the same time of year should be conducted to extend our current observation and determine how habitual patterns of light exposure may affect entrainment in older compared with young adults.
Acknowledgments
The authors wish to thank the study subjects; the subject recruitment staff of the Division of Sleep Medicine; JM Ronda for technical support; K Huard for assistance preparing the manuscript; Drs. AM Chang, JJ Gooley, SW Lockley, and FAJL Scheer who served as Project Leaders for some of the studies; and Dr. CA Czeisler for overall support.
GRANTS: The studies were supported by NIH grants AG18578, AG09975, AG06072, MH45130, NS41886, HL77453, AT002129, and NASA cooperative agreement NCC 9–58 with the National Space Biomedical Research Institute. Screening procedures for the studies were conducted in the BWH GCRC supported by grant RR02635. KS was supported by NIH Institutional Training Grant T32 HL 07901 and an Individual Fellowship F32 AG03910.
References
- Ancoli-Israel S. Sleep problems in older adults: Putting myths to bed. Geriatrics. 1997;52:20–30. [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: Ocular and neural signal transduction. Journal of Biological Rhythms. 1997;12:537–546. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Browman KE, Monk TH, Reynolds CF, III, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. Journal of the American Geriatrics Society. 1992;40:779–786. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Munch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiology International. 2006;23:461–474. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Kripke DF, Gillin JC, Hrubovcak JC. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiology and Behavior. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- Carrier J, Paquet J, Morettini J, Touchette E. Phase advance of sleep and temperature circadian rhythms in the middle years of life in humans. Neuroscience Letters. 2002;320:1–4. doi: 10.1016/s0304-3940(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Charman WN. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthal Physiol Opt. 2003;23:181–187. doi: 10.1046/j.1475-1313.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Wisbey J, Mason WJ, Gruen W, Hauri PJ, Juarez S. Seasonal variation in human illumination exposure at two different latitudes. Journal of Biological Rhythms. 1995;10:324–334. doi: 10.1177/074873049501000406. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sánchez R, Ríos CD, Ronda JM. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiology International. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. American Journal of Physiology. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, Klauber MR, Kaplan OJ. Low illumination experienced by San Diego adults: association with atypical depressive symptoms. Biol Psychiatry. 1994;35:403–407. doi: 10.1016/0006-3223(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. John Wiley & Sons, Inc; 2004. [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24h days. Proc Natl Acad Sci USA. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette J, Hébert M, Paquet J, Dumont M. Natural bright light exposure in the summer and winter in subjects with and without complaints of seasonal mood variations. Biological Psychiatry. 1998;44:622–628. doi: 10.1016/s0006-3223(97)00543-x. [DOI] [PubMed] [Google Scholar]
- Hébert M, Dumont M, Paquet J. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiology International. 1998;15:59–70. doi: 10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. Journal of Pineal Research. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herljevic M, Middleton B, Thapan K, Skene DJ. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Experimental Gerontology. 2005;40:237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 1988;42:167–168. [Google Scholar]
- Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS, Mowen MA, Assmus JD, Langer RD. Circadian sleep, illumination, and activity patterns in women: Influences of aging and time reference. Physiology and Behavior. 2000;68:347–352. doi: 10.1016/s0031-9384(99)00186-9. [DOI] [PubMed] [Google Scholar]
- Kawinska A, Dumont M, Selmaoui B, Paquet J, Carrier J. Are modifications of melatonin circadian rhythm in the middle years of life related to habitual patterns of light exposure? Journal of Biological Rhythms. 2005;20:451–460. doi: 10.1177/0748730405280248. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol (Lond) 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. J Investig Med. 2001;49:30–40. doi: 10.2310/6650.2001.34088. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Singer CM, Minkunas DV, Sack RL. Later circadian phase of plasma melatonin relative to usual waketime in older subjects. Sleep. 2000;23:A188–A189. [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neuroscience Letters. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Mishima K, Okawa M, Shimizu T, Hishikawa Y. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. Journal of Clinical Endocrinology and Metabolism. 2001;86:129–134. doi: 10.1210/jcem.86.1.7097. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) Journal of Physiology. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggemyer KA, Begley A, Monk T, Buysse DJ. Circadian and homeostatic modulation of sleep in older adults during a 90-minute day study. Sleep. 2004;27:1535–1541. doi: 10.1093/sleep/27.8.1535. [DOI] [PubMed] [Google Scholar]
- Okudaira N, Kripke DF, Webster JB. Naturalistic studies of human light exposure. American Journal of Physiology. 1983;245:R613–R615. doi: 10.1152/ajpregu.1983.245.4.R613. [DOI] [PubMed] [Google Scholar]
- Owen J, Arendt J. Melatonin suppression in human subjects by bright and dim light in Antarctica: Time and season-dependent effects. Neuroscience Letters. 1992;137:181–184. doi: 10.1016/0304-3940(92)90399-r. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol [A] 1976;106:291–331. [Google Scholar]
- Prinz PN. Sleep and sleep disorders in older adults. Journal of Clinical Neurophysiology. 1995;12:139–146. doi: 10.1097/00004691-199503000-00004. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Rosenberg RS, Zee PC, Turek FW. Phase response curves to light in young and old hamsters. American Journal of Physiology. 1991;261:R491–R495. doi: 10.1152/ajpregu.1991.261.2.R491. [DOI] [PubMed] [Google Scholar]
- Savides TJ, Messin S, Senger C, Kripke DF. Natural light exposure of young adults. Physiol Behav. 1986;38:571–574. doi: 10.1016/0031-9384(86)90427-0. [DOI] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Staples VS, Archer SN, Arber S, Skene DJ. Daily light exposure profiles in older non-resident extreme morning and evening types. Journal of Sleep Research. 2009 doi: 10.1111/j.1365-2869.2009.00762.x. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Cao V, Heller HC. Circadian system of mice integrates brief light stimuli. American Journal of Physiology. 1998;275:R654–R657. doi: 10.1152/ajpregu.1998.275.2.R654. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. Journal of Biological Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. Journal of the American Geriatrics Society. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Kripke DF, Elliot JA, Klauber MR. Circadian abnormalities in older adults. Journal of Pineal Research. 2001;31:264–272. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol (Lond) 2000;526.3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, FOS expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]