Abstract
Vaccines are needed to prevent the oculogenital diseases of Chlamydia trachomatis. Infected hosts develop immunity, although temporary, and experimental vaccines have yielded significant protective immunity in animal models, fueling the impetus for a vaccine. Because infections cause sequelae, the functional relationship between infection- and vaccine-induced immunity is unclear. We hypothesized that infection- and vaccine-induced immunity are functionally distinct, particularly in the ability to prevent sequelae. Chlamydia-immune mice, with immunity generated by either a previous infection or vaccination, exhibited a significant degree of protective immunity, marked by a lower-intensity, abbreviated course of infection. However, vaccinated mice were protected from infertility, whereas preinfected mice were not. Thus, infection-induced immunity does not prevent the pathologic process leading to infertility. Furthermore, T cell subsets, especially CD8 T cells, play a major role in Chlamydia-induced infertility. The results have important implications for the immunopathogenesis of chlamydial disease and new vaccine strategies.
Genital infection by the obligate intracellular bacterium Chlamydia trachomatis is the most common bacterial sexually transmitted disease worldwide and a major public health concern. The United States spends >$3 billion annually on an estimated 4 million reported cases [1, 2]. Pelvic inflammatory disease and tubal factor infertility are complications of genital chlamydial infection in women [3, 4]. Because the increasing prevalence of infections [1, 5, 6] could predispose persons to human immunodeficiency virus–related AIDS [7] and human papillomavirus–associated cervical dysplasia [8], urgent efforts are underway to develop a prophylactic vaccine, which would be the most reliable and cost-effective means to achieve the greatest control [8, 9]. The focus of contemporary vaccine research on the pathogenesis and immunobiology of chlamydial infection is to clarify the contribution of the pathogen and host factors to sequelae. Specific studies will define the correlates of protective immunity for vaccine evaluation and elucidate the antimicrobial mechanisms. Other studies will identify stable vaccine candidates, the factors that regulate immunity in mucosal infection sites and boost protective immunity via immunomodulation, effective delivery systems, and potent adjuvants. In this respect, studies in animal models and corroborating clinical findings in humans have revealed that an infected host develops immunity against C. trachomatis [10-12], which correlates with a strong T helper type 1 (Th1) response and a complementary antibody response [13-17]. Thus, a vaccine that elicits this profile of specific immune effectors may be protective. However, infection-induced immunity is temporary and wanes with time, leading to reinfections [11, 12]. In addition, recent epidemiologic data from screening and prevention programs have led to the “arrested immunity” hypothesis [6], suggesting that rapid treatments of incident infections prevent the development of protective immunity after an infection. Consequently, arrested immunity leads to susceptibility to reinfections, and pathologies may increase in future if there is an increase in silent reinfections. In addition, the incidence of sequelae increases with repeated genital Chlamydia infection [18]. Thus, it is uncertain whether infection-induced immunity can protect against the development of tubal pathologies, such as pelvic inflammatory disease, hydrosalpinx, and infertility.
Meanwhile, promising chlamydial antigens that could form the basis of single or multiple subunit vaccines have been identified [19, 20]. Dendritic cells are also key regulatory cells for vaccine regimens inducing chlamydial immunity [21], functioning as primary antigen-presenting cells with a proclivity for activating Th1 response, which is attributable to the potent costimulatory function [22-24]. Furthermore, the design of chlamydial vaccines benefits from appropriate routes of administration that optimize mucosal immune response at targeted sites, using the tenets of the common mucosal immune system [16, 25]. Thus, intranasal immunization effectively targets antigen-specific immune effectors to the genital mucosa, a demonstration of the favorable cooperation between nasal mucosa as an inductive site and genital mucosa as a mucosal effector site [25, 26]. Applying these immunologic paradigms, several experimental antichlamydial vaccine regimens have been produced that yielded significant levels of protective immunity in animal models. They include adjuvanted membrane and nonmembrane proteins, vectors expressing chlamydial proteins or the immunodominant epitopes, antibody complexes, and Fc fusion proteins [17, 27-29].
The demonstration of vaccine- and infection-induced immunity has fueled enthusiasm for developing a vaccine to control Chlamydia in humans; however, the functional and immunologic similarity between the 2 types of immunity is unclear. We hypothesized that, unlike vaccine-induced immunity, infection-induced immunity would not confer protection against complications because of the differential profile of immune effectors induced. We used protective experimental vaccines to compare the functional and immunologic properties of vaccine- and infection-induced immunity in a murine model of Chlamydia-induced infertility. We verified that infection-induced immunity could not protect against hydrosalpinx and infertility and that T cell–mediated immune responses, especially those mediated by the CD8 T cell subset, are major contributors to pathology. The results have important implications for our understanding of the pathogenesis of chlamydial disease and new strategies for designing a future vaccine.
MATERIALS AND METHODS
Chlamydia stocks, antigens, and animals
Stocks of the C. trachomatis agent of mouse pneumonitis (also known as Chlamydia muridarum) were grown in McCoy cells or HeLa cells, and titers were expressed as inclusion-forming units per mL, as determined by standard procedures. Chlamydial antigen was prepared by growing mouse pneumonitis in HeLa cells and purifying elementary bodies over renografin gradients, followed by inactivation under ultraviolet light for 3 h. Wild-type and genetically engineered knockout (KO) female C57BL/6 mice (H2b) were obtained from Taconic Farms at 5–8 weeks of age. Animals received food and water ad libitum in laminar flow racks, under pathogen-free conditions with alternating 12-h periods of light and darkness.
Immunizations, challenge infection, and analysis of immunity
Infection-induced immunity was established by infecting female mice intravaginally with 1 × 105 inclusion-forming units of mouse pneumonitis per mouse in a volume of 20 μL of phosphate-buffered saline, with the mice under phenobarbital anesthesia. The course of the infection was monitored by isolation of chlamydiae from cervicovaginal swabs in tissue culture and immunofluorescence staining of inclusions. Mice in which the primary infection resolved acquired a significant level of immunity for ≥6 weeks, although lower-intensity reinfection is possible after this period [12].
Vaccine-induced immunity was achieved by 2 immunization protocols that produce significant protective immunity against genital chlamydial infection [29-31]. In the first approach, mice were immunized intranasally or genitally (twice at 3-week intervals; 20 μg per mouse in 25 μL of phosphate-buffered saline) with the Fc fusion protein of the chlamydial major outer membrane protein (Fc-MOMP). The controls were the Fc fusion protein of the protective glycoprotein D2 antigen of herpes simplex virus type 2 (Fc-gD2) and the phosphate-buffered saline carrier. The construction and production of Fc-MOMP fusion protein has been described elsewhere [29, 30]. Briefly, the Fc-fusion proteins, Fc-MOMP and Fc-gD2, were generated using the plasmid pFUSE-mFc2 (IL2ss) (InvivoGen) and the coding sequences of ompA of C. trachomatis agent of mouse pneumonitis and herpes simplex virus type 2 gD2 genes, respectively. Plasmid pFUSE-mFc2 (IL2ss) is specifically designed for the construction of Fc-fusion proteins containing a murine immunoglobulin G2a Fc portion. These fusion proteins were purified using protein G affinity columns from transfected Chinese hamster ovary cell cultures. The Fc portion of the fusion protein targets the antigen (MOMP) to antigen-presenting cells, such as Fc receptor (FcR)–positive dendritic cells in the nasal or genital mucosa, eliciting an enhanced immune response. The enhanced immunogenicity of Fc-MOMP was established by the potent immunostimulatory activity of Fc-MOMP–pulsed dendritic cells for T cell activation against Chlamydia in vitro and in vivo. Genital challenge of Fc-MOMP–immunized animals revealed significant protective immunity, characterized by a shorter, lower-intensity course of infection, as measured by shedding of chlamydiae into the cervicovaginal vault [29, 31, 32]. Vaccine-immune mice were also produced by a cellular vaccine strategy that used Chlamydia-pulsed interleukin (IL)10 KO dendritic cells in adoptive transfer immunization [24]. Challenged animals exhibited a sterilizing and long-term protective immunity.
Analysis of Chlamydia-induced sequelae
Chlamydia-immune female C57BL/6 mice that were generated by preinfection or vaccination were mated and scored for fertility, hydrosalpinx, and uterine abnormalities, according to standard procedures [33-35], as were control mice. Briefly, groups of infection- and vaccine-immune mice were reinfected to enhance infertility [35], mated with proven-fertile males after 4–6 weeks of challenge infection, and subsequently weighed daily for 19–21 days to determine pregnancy. Three days of consistent weight gain by female mice was considered to be evidence of pregnancy. The numbers of pregnant mice and the average numbers of pups in the different groups were recorded. Animals were also scored for the presence of hydrosalpinx (unilateral or bilateral) in the reproductive system. Experiments were repeated ≥3 times.
Fluorescence-activated cell sorting analysis
For fluorescence-activated cell sorting analysis, single cell preparations were costained with fluorochrome-labeled monoclonal antibodies to murine cell surface antigens and analyzed on a FAC-Scan Flow Cytometer (Becton Dickinson) [36]. Controls were stained with isotype-matched irrelevant antibodies. Binding of biotinylated monoclonal antibodies were detected with streptavidin-red 613 (Life Technologies). Results were expressed as the percentage of positively stained cells [37, 38]. Experiments were repeated ≥3 times.
Measurement of frequency of Chlamydia-specific Th1 cells
A modified procedure of the limiting dilution technique [39] was used to assess Chlamydia-specific Th1 cell frequency in immune mice. Briefly, T cells are seeded in a serial doubling dilution (105–102 cells/well) and stimulated with antigen-presenting cells (5 × 105 cells/well) and chlamydial antigen (10 μg/mL). Background cultures contained antigen-presenting cells and antigen. After incubation, supernatants were assayed for interferon-γ by a sensitive enzyme-linked immunosorbent assay [37]. The mean of the background plus 3 times the standard deviation was taken as the baseline for positive experimental wells. The number of positive and negative wells per dilution of each T cell preparation was calculated, and the data were analyzed by a limiting dilution computer program (LIDIA) [39, 40], which provided both the Th1 cell frequency and the conformity of input data with a single-hit Poisson model [24].
Functional analysis of dendritic cells pulsed with Fc-MOMP
Dendritic cells were isolated according to the standard procedure (using IL-4 and granulocyte-macrophage colony-stimulating factor in culture). For fusion protein internalization, dendritic cells were first dyed with phycoerythrin-conjugated anti-CD11c antibody and then incubated with Fc-MOMP fusion protein for 5, 15, 30, or 60 min. The secondary antibody (fluorescein isothiocyanate–conjugated anti-mouse Fab) was used for 30 min, and DRAQ5 (blue color) was used as the nuclear dye. Stained cells were visualized and analyzed by confocal microscopy. T cell stimulatory function was evaluated by coculturing Fc-MOMP–pulsed dendritic cells with purified immune T cells for 5 days, and supernatants were assayed for interferon-γ by Luminex assay, according to a standard procedure [24].
Statistical analysis
The data derived from different experiments were analyzed and compared by means of a 1- or 2-tailed t test, and the relationship between different experimental groupings was assessed by analysis of variance. Differences were considered statistically significant at P < .05.
RESULTS
Infection- or vaccine-induced immunity and clearance of genital chlamydial infection
Our initial experiments confirmed findings of other studies showing that immunity developed after an infection and that certain experimental vaccine regimens produced significant levels of protective immunity [10-14, 16]. Thus, when Chlamydia-immune mice—generated by genital infection (infection-induced immunity) or by vaccination with either Fc-MOMP or Chlamydia-pulsed IL-10KO dendritic cells (vaccine-induced immunity)—were challenged genitally, they exhibited enhanced clearance of the infection and less intense infection, compared with nonimmune mice (figure 1). It is noteworthy that infection-induced immunity and Fc-MOMP–based vaccination conferred comparable levels of protection and that challenge infection cleared in the immune mice within 2 weeks; however, the IL-10KO dendritic cell–based cellular vaccine conferred a superior level of immunity, with the infection resolving within 6 days (P > .010). As established elsewhere for other protective experimental vaccine regimens, including the IL-10KO dendritic cell–based vaccine [24], the protective immunity conferred by Fc-MOMP was correlated with a relatively high frequency of Chlamydia-specific Th1 cells in the genital mucosa (figure 2) and associated with the ability of mucosal FcR-positive dendritic cells to internalize Fc-MOMP rapidly via the FcRs and with up-regulation of key costimulatory molecules, such as CD40, CD197 (CCR7), and major histocompatibility complex class II antigens [29, 30]. Thus, Fc-MOMP is a safe (administered intranasally or intravaginally) and reliable protective subunit vaccine whose efficacy in mice is equivalent to the level of immunity induced after genital chlamydial infection. The Fc-MOMP vaccine regimen is therefore suitable for investigating the functional and immunologic profiles of infection- and vaccine-induced immunity.
Figure 1.
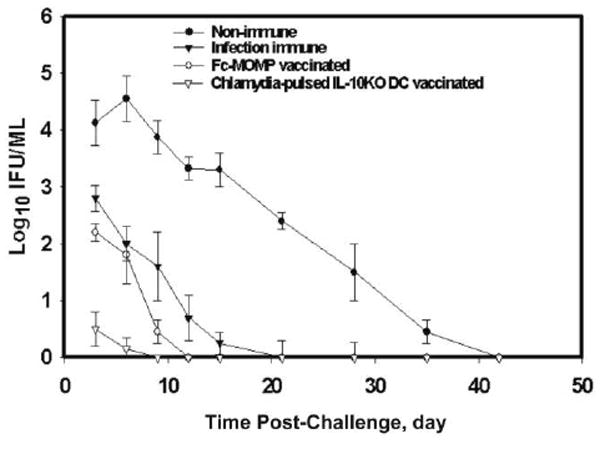
Infection- or vaccine-induced immunity enhance clearance of genital chlamydial infection. Groups of C57BL/6 mice with immunity to chlamydial genital infection due to preinfection (infection-induced immunity) or vaccination with either the Fc fusion protein of the chlamydial major outer membrane protein (Fc-MOMP) or Chlamydia-pulsed interleukin-10 knockout dendritic cells (IL-10KO DCs) (vaccine-induced immunity), as well as control mice, were challenged genitally with mouse pneumonitis (1 × 105 inclusion-forming units [IFUs] per mouse). The course of infection was monitored by periodic swabbing of cervicovaginal vaults, and chlamydiae were isolated in tissue culture, as described in the Materials and Methods. Experiments were repeated 3 times, with 6 mice per experimental group, and Chlamydia IFU values represent means ± standard deviations.
Figure 2.
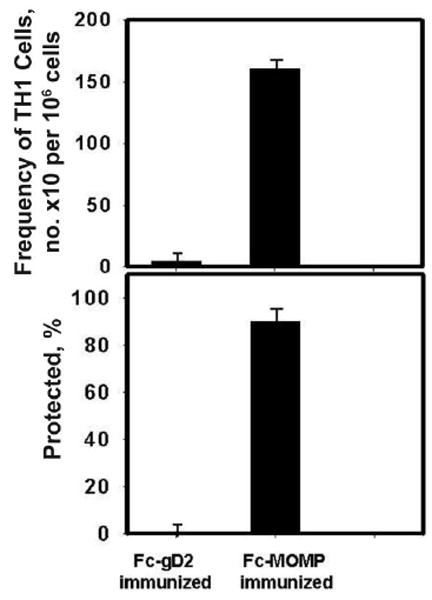
Protective immunity induced by the Fc fusion protein of the chlamydial major outer membrane protein (Fc-MOMP) is related to Chlamydia-specific T helper type 1 (Th1) cells in the genital mucosa. Two groups of C57BL/6 mice were vaccinated with either Fc-MOMP or the control, Fc fusion protein of the protective glycoprotein D2 antigen of herpes simplex virus type 2 (Fc-gD2), and then challenged with mouse pneumonitis (1 × 105 inclusion-forming units per mouse). Two weeks after challenge, the infection status was assessed by swabbing the cervicovaginal vaults, and chlamydiae were isolated in tissue cultures, as described in the Materials and Methods. Data are expressed as the percentage of animals in which infection resolved within 2 weeks. At the same time, T cells were isolated from the reproductive tissues of each group, and the frequency of Chlamydia-specific Th1 cells was determined by a limiting dilution technique, as described in the Materials and Methods. T cells from naive mice had a Chlamydia-specific Th1 frequency of 15 (range, 9–21), similar to the frequency measured in the control group that received Fc-gD2. The experiment was repeated twice, with 6 mice per experimental group.
Infection-induced immunity and the development of Chlamydia-induced pathologies
The acquired immunity conferred by preinfection or vaccine administration, as measured by the kinetics of clearance of chlamydial shedding into the cervicovaginal vault, may suggest a functional and immunologic similarity between infection- and vaccine-induced immunity. However, it is uncertain whether infection-induced immunity can protect against the development of tubal pathologies such as pelvic inflammatory disease, hydrosalpinx, or infertility, the incidences of which are increased by repeated genital Chlamydia infection [18]. We hypothesized that infection-induced immunity would not protect against the development of sequelae, possibly because of the differential profile of immune effectors induced, which are mostly pathogenic. To test this hypothesis, groups of infection- and vaccine-immune mice were evaluated after mating for Chlamydia-induced sequelae, specifically infertility and hydrosalpinx formation. Results presented in figure 3 show that immune mice generated by vaccination with either Chlamydia-pulsed IL-10KO dendritic cells or Fc-MOMP were protected from infertility, whereas infection-immune mice suffered a decrease in fertility of ~40% (P > .021). Thus, vaccine-induced immunity protected against the subsequent development of infertility but infection-induced immunity did not (ie, 35%–45% fertility for infection-induced vs 95%–100% fertility for vaccine-induced immunity; P > .006). Bilateral or unilateral hydrosalpinx was also observed in almost 40% of the mice with infection-induced immunity; in contrast, only ~4% of mice vaccinated with Fc-MOMP and 1%–2% of cellular vaccine recipients showed evidence of hydrosalpinx; these rates were not significantly different from those in uninfected controls (P > .35). Thus, unlike vaccine-induced immunity, infection-induced immunity does not prevent the onset of the pathologic process leading to infertility or hydrosalpinx associated with chlamydial genital infection.
Figure 3.
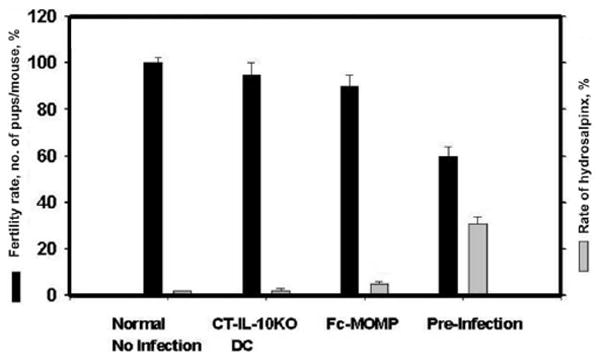
Infection-induced immunity does not protect against the development of Chlamydia-induced pathologies. Female C57BL/6 mice were challenged twice genitally with mouse pneumonitis (1 × 105 inclusion-forming units per mouse); groups included control mice and mice with immunity to chlamydial genital infection due to preinfection (infection-induced immunity) or vaccination with either the Fc fusion protein of the chlamydial major outer membrane protein (Fc-MOMP) or Chlamydia trachomatis–pulsed interleukin-10 knockout dendritic cells (CT-IL-10KO DCs) (vaccine-induced immunity); mice in each group were genitally infected twice. After 4 weeks, the mice were mated with proven-fertile males and subsequently weighed daily for 19–21 days to determine pregnancy, as described in the Materials and Methods. Animals were also scored for the presence of hydrosalpinx (unilateral or bilateral) in the reproductive system, also as described in the Materials and Methods. Results are expressed as the fertility rate percentage (no. of pregnant mice/total no. of mice) (black bars) and the percentage of mice with unilateral or bilateral hydrosalpinx (gray bars). The difference in pathology (infertility or hydrosalpinx) between vaccinated and preinfected groups was statistically significant (P < .01). Experiments were repeated 3–5 times with 8 mice per experimental group, and the values represent means ± standard deviations.
Role of specific immune response in the pathogenesis of Chlamydia-induced infertility
We tested the hypothesis that the increase in the incidence and intensity of pathologies after multiple genital chlamydial infections is due to the activation and subsequent expansion of pathogenic specific antichlamydial immune responses in the host. In initial studies to verify this hypothesis, we demonstrated that multiple genital infections or pre-exposure to live chlamydiae by the intranasal route may increase the incidence of infertility on subsequent genital infection (figure 4). In addition, the transfer of immune lymph node cells from infection-immune mice to naive recipients, followed by a genital infection, reduced fertility in the recipient mice significantly, by ~25%, (P > .031) (figure 5).
Figure 4.
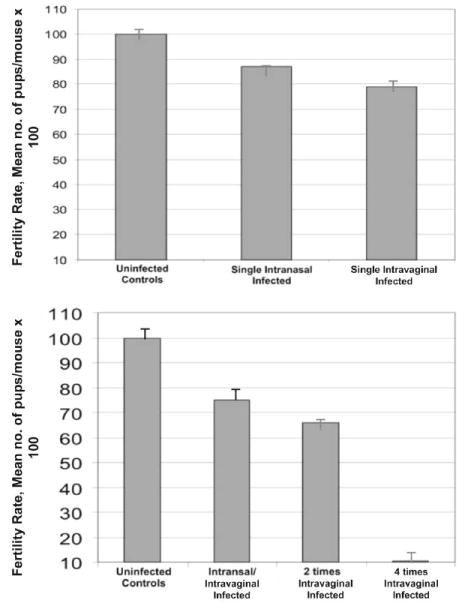
Effect of pre-exposure to Chlamydia on fertility rate. Groups of female C57BL/6 mice were infected either intravaginally or intranasally with mouse pneumonitis (1 × 105 inclusion-forming units per mouse), such that each group was genitally infected as indicated. Four weeks after the last infection, the mice were mated with proven-fertile males and subsequently weighed daily for 19–21 days to determine pregnancy, as described in the Materials and Methods. Results are expressed as the fertility rate percentage (no. of pregnant mice/total no. of mice). Experiments were repeated 3 times with 8 mice per experimental group, and values represent means ± standard deviations.
Figure 5.
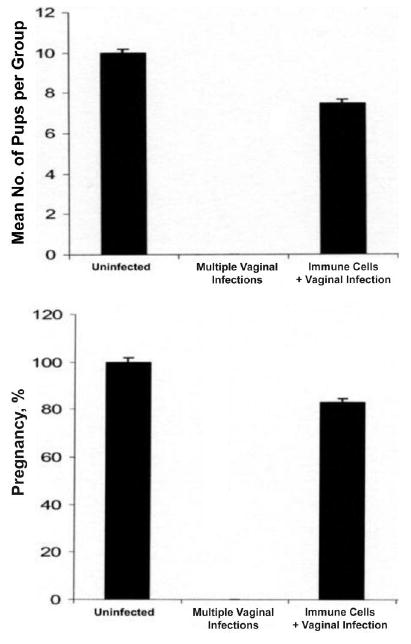
Transfer of pathogenic immune cells from preinfected animals. Purified Iliac lymph node cells were adoptively transferred into naive female C57BL/6 mice (25 × 106 per mouse) from other C57BL/6 mice that were infected twice intravaginally with mouse pneumonitis. The recipient mice were subsequently infected genitally. After 4 weeks, the mice were mated with proven-fertile males and weighed daily for 19–21 days to determine pregnancy, as described in the Materials and Methods. Control animals were age matched. Results are expressed as the average number of pups per group (total no. of pups/total no. of mice) (top) and the percentage pregnant (no. of pregnant mice/total no. of mice) (bottom). The difference between recipients of immune cells and noninfected mice was statistically significant (P > .021). Experiments were repeated twice with 6 mice per experimental group.
Lymphocyte population and T cell subsets mediating Chlamydia-induced pathologies
To investigate whether specific lymphocyte populations and T cell subsets were involved in the pathogenesis of Chlamydia-induced pathologies, genetically engineered mice lacking B cells, CD4, CD8, and IL-10 were infected and analyzed for fertility. Results presented in figure 6 revealed that infected IL-10KO mice did not become drastically infertile (P > .006). However, B cell–deficient mice, as well as CD8KO mice, suffered a decrease in fertility of ~70%, whereas CD4KO mice were 100% infertile. The data suggested that T cells of both CD4 and CD8 subsets are involved in the pathogenic process leading to infertility associated with genital chlamydial infection; however, the absence of CD4 enhances the process leading to infertility.
Figure 6.
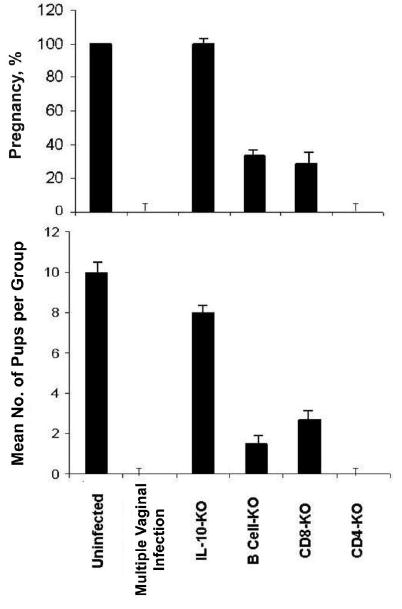
Involvement of T cells and subsets in the pathogenesis of Chlamydia-induced infertility. Groups of genetically engineered, specific gene knockout (KO) female C57BL/6 mice were infected twice intravaginally with mouse pneumonitis (1 × 105 inclusion-forming units per mouse) at 6-week intervals. The multiply infected group was infected 4 times. Four weeks after the last infection, the mice were mated with proven-fertile males and then weighed daily for 19–21 days to determine pregnancy, as described in the Materials and Methods. Results are expressed as the percentage pregnant (no. of pregnant mice/total no. of mice) (top) and the average number of pups per group (total no. of pups/total no. of mice) (bottom). The difference between groups was statistically significant (P < .006). Experiments were repeated twice with 6 mice per experimental group. IL-10, interleukin-10.
DISCUSSION
There is increasing evidence that chlamydial disease has an immunopathogenic basis, with both acute and chronic inflammatory responses implicated in the pathologic process leading to tubal scarring, which culminates in infertility [41]. Other attempts to link chlamydial components (eg, heat shock proteins and homologues of human elongation factors) as provocateurs of hypersensitivity or autoimmune reactions have had inconclusive results. Moreover, polymorphisms in certain host cytokine, chemokine, and Toll-like receptor activities during chlamydial infection may underlie susceptibility to chlamydial disease [42, 43]. However, other than measurement of nonspecific inflammatory responses, no studies to date have investigated the role of specific immune effectors, including the Th17 pathway [44], in the pathogenesis of the infection sequelae. Furthermore, a recent report revealed that despite microbiologic evidence that chlamydiae cured of the cryptic plasmid retained infectivity in mice, the infected animals did not develop pathologies [45]. This observation may corroborate a testable hypothesis that the host’s specific immune responses directed against Chlamydia-encoded components could play a key role in the development of pathologies after infection.
Other studies in animal models and supporting clinical observation in humans have established that prior infection and certain experimental vaccine regimens can produce significant levels of protective immunity against C. trachomatis [10-14, 16]. In this study, we confirmed that both infection-induced and vaccine-induced immunity (produced using either Fc-MOMP or Chlamydia-pulsed IL-10KO dendritic cells) lead to enhanced clearance of a genital challenge infection, compared with nonimmune mice. The acquired immunity conferred by preinfection or vaccination, as measured by the kinetics of clearance of chlamydial shedding into the cervicovaginal vault, could suggest a functional and immunologic similarity between infection- and vaccine-induced immunity. However, because the incidence of Chlamydia-induced tubal pathologies such as pelvic inflammatory disease increases with repeated genital Chlamydia infection [18], we hypothesized that infection-induced immunity would not protect against the development of hydrosalpinx or infertility. The prediction is that vaccine- and infection-induced immunity may induce differential profiles of functional immune effectors, such that the profile of the latter is mostly pathogenic.
A better understanding of the functional properties of vaccine- and infection-induced immunity in relation to the key pathologic outcomes of chlamydial infection helps clarify the likely effect of an efficacious vaccine. We demonstrated in this study that vaccine-induced immunity protected against the development of infertility and hydrosalpinx, whereas infection-induced immunity did not. Unlike vaccine-induced immunity, infection-induced immunity does not prevent onset of the pathologic process leading to infertility or hydrosalpinx associated with chlamydial genital infection. In addition, the onset of pathology leading to infertility appears to be more likely after multiple live infections, including nasal infection, suggesting that pre-exposure to C. trachomatis by other routes could constitute a risk factor for developing sequelae after a genital infection. In this respect, we do not know the effects of pre-exposure to Chlamydia pneumoniae by the respiratory route or ocular infection by other C. trachomatis serovars on the severity and incidence of reproductive tract sequelae. At a minimum, our key findings provide some interesting insights into the benefit of administering a future chlamydial vaccine before exposure to genital chlamydial infection.
The inability of infection-induced immunity to protect against the development of tubal pathologies on subsequent reinfection suggested that an apparently irreversible pathologic process is initiated in the genital tract after exposure to live chlamydiae, despite the relatively rapid clearance of a reinfection. Alternatively, the initial infection induces deleterious immune responses against specific chlamydial antigens, and subsequent exposure to genital chlamydial infection leads to the expansion of disease-causing (pathogenic) memory immune effectors that aggravate disease progression. Our results favor the latter scenario, suggesting that T cell–mediated immune responses involving both CD4 and CD8 subsets are involved in Chlamydia-induced sequelae, although the CD8 T cell subset is the major contributor. We concluded that CD8 T cells play a greater role in pathology development than CD4 T cells because a predominant CD8 condition (ie, CD4KO) resulted in greater infertility than did a predominant CD4 condition (ie, CD8KO). Because the protective immunity against Chlamydia in the murine model is primarily mediated by CD4, we concluded that the protective effect of some of the CD4 T cell clonotypes may modulate the pathogenic clonotypes in predominant CD4 conditions.
The existence of protective and pathogenic immune effectors may imply that infection-induced immunity includes both pathogenic and protective components, with the latter being responsible for microbial clearance, although the extent of their involvement in pathogenesis is unclear. The activation of immunopathogenic effectors may also be a slow process, because rapid T cell response in IL-10KO mice protected them against pathologies. Furthermore, the involvement of specific T cells in protective immunity and in the pathogenesis of chlamydial disease, along with the apparent paradoxical coexistence of protective and immunopathogenic immune responses, support the suggestion that C. trachomatis harbors protective and immunopathogenic antigens. If such antigens are identified, efforts to design a vaccine against Chlamydia will involve distinguishing conditions for inducing protective immunity from those that help elicit deleterious effectors. In this respect, Bachmaier et al [46] found that omcB (OMP2) of several C. trachomatis serovars, C. pneumoniae and Chlamydia psittaci harbor a sequence homologous to the pathogenic epitope of the human a-myosin heavy chain, which can induce autoimmune myocarditis in mice. This sequence was not found in ompA or OMP3. Moreover, the likely pathogenic components encoded by the C. trachomatis cryptic plasmid [47] are unknown. The possibility that intact chlamydiae may harbor immunopathenic antigens has supported the focus on a subunit vaccine, which should be screened for toxicity as well. Finally, we have shown that specific immune effectors are involved in the pathogenesis of chlamydial disease, but their mechanism of action and the antigens they recognize are unknown. Identifying the immunopathogenic components of Chlamydia and the deleterious immune effectors they induce, as well as the biochemical mechanisms of tissue injury, should be research priorities in efforts to develop an efficacious vaccine against Chlamydia.
Acknowledgments
Financial support: National Institutes of Health and Centers for Disease Control and Prevention (Public Health Service grants AI41231, GM 08248, and RR03034).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Johnson RE, Newhall WJ, Papp JR, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections—2002. MMWR Recomm Rep. 2002;51:1–40. [PubMed] [Google Scholar]
- 2.Groseclose SL, Zaidi AA, DeLisle SJ, Levine WC, St Louis ME. Estimated incidence and prevalence of genital Chlamydia trachomatis infections in the United States, 1996. Sex Transm Dis. 1999;26:339–44. doi: 10.1097/00007435-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Paavonen J, Wolner-Hanssen P. Chlamydia trachomatis: a major threat to reproduction. J Hum Reprod. 1989;4:111–24. doi: 10.1093/oxfordjournals.humrep.a136855. [DOI] [PubMed] [Google Scholar]
- 4.Westrom L, Joesoef R, Reynolds G, Hadgu A, Thompson SE. Pelvic inflammatory inflammatory disease and infertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopy results. Sex Transm Dis. 1992;19:185–92. [PubMed] [Google Scholar]
- 5.Nieuwenhuis RF, Ossewaarde JM, Gotz HM, et al. Resurgence of lymphogranuloma venereum in Western Europe: an outbreak of Chlamydia trachomatis serovar L2 proctitis in The Netherlands among men who have sex with men. Clin Infect Dis. 2004;39:996–1003. doi: 10.1086/423966. [DOI] [PubMed] [Google Scholar]
- 6.Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of Chlamydia control. Sex Transm Dis. 2008;35:53–4. doi: 10.1097/OLQ.0b013e31815e41a3. [DOI] [PubMed] [Google Scholar]
- 7.Kilmarx PH, Mock PA, Levine WC. Effect of Chlamydia trachomatis coinfection on HIV shedding in genital tract secretion. Sex Transm Dis. 2001;28:347–8. doi: 10.1097/00007435-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Mahdi OS, Byrne GI, Kalayoglu M. Emerging strategies in the diagnosis, prevention and treatment of chlamydial infections. Expert Opin Ther Patents. 2001;11:1253–65. [Google Scholar]
- 9.De la Maza MA, De la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–27. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]
- 10.Katz BP, Batteiger BE, Jones RB. Effect of prior sexually transmitted disease on the isolation of Chlamydia trachomatis. Sex Transm Dis. 1987;14:160–4. doi: 10.1097/00007435-198707000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Brunham R, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 12.Rank RG. Models of immunity. In: Stephens RS, editor. Chlamydia: intracellular biology, pathogenesis and immunity. Washington, DC: ASM Press; 1999. pp. 239–95. [Google Scholar]
- 13.Igietseme JU, Eko FO, Black CM. Contemporary approaches to designing and evaluating vaccines against Chlamydia. Expert Rev Vaccines. 2003;2:129–46. doi: 10.1586/14760584.2.1.129. [DOI] [PubMed] [Google Scholar]
- 14.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loomis PW, Starnbach MN. T cell responses to Chlamydia trachomatis. Curr Opinion Microbiol. 2002;5:87–91. doi: 10.1016/s1369-5274(02)00291-6. [DOI] [PubMed] [Google Scholar]
- 16.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Murthy A, Guentzel M, et al. Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008;180:3375–82. doi: 10.4049/jimmunol.180.5.3375. [DOI] [PubMed] [Google Scholar]
- 18.Hillis SD, Owens LM, Marchbanks PA, Amsterdam LF, Mac Kenzie WR. Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol. 1997;176:103–7. doi: 10.1016/s0002-9378(97)80020-8. [DOI] [PubMed] [Google Scholar]
- 19.Stephens RS, Lammel CJ. Chlamydia outer membrane protein discovery using genomics. Curr Opin Microbiol. 2001;4:16–20. doi: 10.1016/s1369-5274(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 20.Stephens RS. Chlamydial genomics and vaccine antigen discovery. J Infect Dis. 2000;181:S521–3. doi: 10.1086/315631. [DOI] [PubMed] [Google Scholar]
- 21.Stagg AJ, Tuffrey M, Woods C, Wunderink E, Knight SC. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue. Infect Immun. 1998;66:3535–44. doi: 10.1128/iai.66.8.3535-3544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins MK, DeSilva DR, Johnson JG, Norton SD. Costimulating factors and signals relevant for antigen presenting cell function. Adv Exp Med Biol. 1993;329:87–92. doi: 10.1007/978-1-4615-2930-9_15. [DOI] [PubMed] [Google Scholar]
- 23.Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J Exp Med. 1998;188:809–18. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igietseme JU, Ananaba GA, Bolier J, et al. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for enhanced specific Th1 induction: potential for cellular vaccine development. J Immunol. 2000;164:4212–9. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 25.Wu HY, Russell MW. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 26.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch–mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–9. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Q, Martinez-Sobrido L, Eko FO, et al. Live-attenuated influenza viruses as delivery vectors for Chlamydia vaccines. Immunology. 2007;122:28–37. doi: 10.1111/j.1365-2567.2007.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igietseme J, He Q, Eko F, et al. Enhanced chlamydial antigen presentation by Fc receptor bearing dendritic cells using Fc-fusion proteins. Proc Annu Meet Am Soc Microbiol; Toronto, Ontario. 2007. [Google Scholar]
- 30.Igietseme JU, He Q, Eko FO, et al. Development of vaccines to prevent chlamydial STDs. Mucosal Immunol Update. 2005;13:12–17. [Google Scholar]
- 31.Moore T, Ekworomadu C, Eko F, et al. Fc receptor–mediated antibody regulation of T cell immunity against intracellular pathogens. J Infect Dis. 2003;188:617–24. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- 32.Igietseme J QH, Joseph K, et al. Involvement of specific immune response in the pathogenesis of Chlamydia trachomatis disease. In: Christiansen G, editor. Chlamydia research. Vol. 6. Aarhus, Denmark: University of Aarhus; 2008. p. 290. [Google Scholar]
- 33.Barr EL, Ouburg S, Igietseme JU, et al. Host inflammatory response and development of complications of Chlamydia trachomatis genital infection in CCR5-deficient mice and subfertile women with the CCR5delta32 gene deletion. J Microbiol Immunol Infect. 2005;38:244–54. [PubMed] [Google Scholar]
- 34.Pal S, Hui W, Peterson EM, de la Maza LM. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital infection. J Med Microbiol. 1998;47:599–605. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- 35.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil. 1992;95:31–8. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]
- 36.Perry LL, Feilzer K, Portis JL, Caldwell HD. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–14. [PubMed] [Google Scholar]
- 37.Igietseme JU, Uriri IM, Kumar SN, et al. Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030–5. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsey KH, Newhall WJ, Rank RG. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect Immun. 1989;57:2441–6. doi: 10.1128/iai.57.8.2441-2446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kees U, Kynast G, Weber E, Krammer PH. A method for testing the specificity of influenz A virus–reactive memory cytotoxic T lymphocytes (CTL) clones in limiting dilution cultures. J Immunol Methods. 1984;69:215–27. doi: 10.1016/0022-1759(84)90320-x. [DOI] [PubMed] [Google Scholar]
- 40.Krammer PH, Dy M, Hultner L, et al. Production of lymphokines by murine T cells grown in limiting dilution and long-term cultures. In: Fathman CG, Fitch FW, editors. Isolation, characterization, and utilization of T lymphocyte clones. New York: Academic Press; 1982. pp. 252–62. [Google Scholar]
- 41.Cohen CR, Brunham RC. Pathogenesis of Chlamydia induced pelvic inflammatory disease. Sex Transm Infect. 1999;75:21–4. doi: 10.1136/sti.75.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darville T, O’Niell JM, Andrews CW, J, Nagarajan UM, Ojcius DM. Toll-like receptor-2, but not toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187–97. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 43.Cohen CR, Gichui J, Rukaria R, Sinei SS, Gaur LK, Brunham RC. Immunogenetic correlates for Chlamydia trachomatis–associated tubal infertility. Obstet Gynecol. 2003;101:438–44. doi: 10.1016/s0029-7844(02)03077-6. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–47. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 45.O’Connell C, Ingalls R, Andrews CJ, Scurlock A, Darville T. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol. 2007;179:4027–34. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 46.Bachmaier K, Neu N, dl Maza LM, Pal S, Hessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–9. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–28. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


