Abstract
Glyceryl ether monooxygenase is a tetrahydrobiopterin-dependent membrane-bound enzyme which catalyses the cleavage of lipid ethers into glycerol and the corresponding aldehyde. Despite many different characterisation and purification attempts, so far no gene and primary sequence have been assigned to this enzyme. The seven other tetrahydrobiopterin-dependent enzymes can be divided in the family of aromatic amino acid hydroxylases - comprising phenylalanine hydroxylase, tyrosine hydroxylase, and the two tryptophan hydroxylases - and into the three nitric oxide synthases.
We tested the influences of different metal ions and metal ion chelators on glyceryl ether monooxygenase, phenylalanine hydroxylase, and nitric oxide synthase activity to elucidate the relationship of glyceryl ether monooxygenase to these two families. 1,10-Phenanthroline, an inhibitor of non heme iron dependent enzymes, was able to potently block glyceryl ether monooxygenase as well as phenylalanine hydroxylase but had no effect on inducible nitric oxide synthase. Two tetrahydrobiopterin analogues, N5-methyltetrahydrobiopterin and 4-aminotetrahydrobiopterin, had a similar impact on glyceryl ether monooxygenase activity as has already been shown for phenylalanine hydroxylase. These observations point to a close analogy of the role of tetrahydrobiopterin in glyceryl ether monooxygenase and in aromatic amino acid hydroxylases and suggest that glyceryl ether monooxygenase may require a nonheme iron for catalysis.
Keywords: 4-amino tetrahydrobiopterin, N5-methyltetrahydrobiopterin, nitric oxide synthase, phenylalanine hydroxylase
Introduction
Of the eight so far described tetrahydrobiopterin-dependent enzymes all but one have been extensively characterised. These seven enzymes can be classified into two prominent groups, the aromatic amino acid hydroxylases comprising phenylalanine hydroxylase, tyrosine hydroxylase, and the two tryptophan hydroxylases, as well as the nitric oxide synthase family consisting of three isoforms, the endothelial, neuronal, and inducible nitric oxide synthase (Thöny et al., 2000). The eighth enzyme depending on tetrahydrobiopterin is glyceryl ether monooxygenase [EC 1.14.16.5], a microsomal protein which is responsible for the catalytic cleavage of lipid ethers by a hydroxylation of the aliphatic carbon atom adjacent to the ether bond (Taguchi and Armarego, 1998). The primary hydroxylation product, the semiacetal, is unstable and rearranges into the aldehyde which is further oxidised to the acid by long chain fatty aldehyde dehydrogenase [EC 1.2.1.48] (Taguchi and Armarego, 1998).
Despite different attempts to characterise and purify glyceryl ether monooxygenase (Ishibashi and Imai, 1983, 1985) there is still no sequence or gene information available. The position hydroxylated by this enzyme is different from both above described enzyme families. Aromatic amino acid hydroxylases hydroxylate aromatic carbon atoms; nitric oxide synthases in contrast hydroxylate the arginine guanidium nitrogen. Glyceryl ether monooxygenase with its aliphatic carbon atom hydroxylation can be placed somewhere in between these two reaction schemes and neither belongs to one or the other family. Significant protein sequence homology is only detectable within the aromatic amino acid hydroxylases or within the nitric oxide synthases but not between these two families.
A difference between the aromatic amino acid hydroxylases and the nitric oxide synthases is the way of coordinating the iron crucial for the catalytic activity. In aromatic amino acid hydroxylases this metal ion is bound via two neighbouring histidines of the primary sequence and can therefore be removed by strong iron chelators like 1,10-phenanthroline whereas weak iron chelators like EDTA are not able to do so (Kaufman, 1993). In contrast, all nitric oxide synthases contain a heme-bound iron which is so tightly bound that it cannot be removed either by weak or strong metal chelators. Strong versus weak metal-ion chelators are therefore a means to differentiate between these two classes of enzymes. A second difference between the two enzymatic families is their dependence on the chemical nature of the essential cofactor tetrahydrobiopterin and its analogues. N5-methyltetrahydrobiopterin is a tetrahydrobiopterin analogue incapable of undergoing redox recycling by dihydropteridine reductase and is an inhibitor of phenylalanine hydroxylase activity (Werner et al., 2000). On the other hand, nitric oxide synthases can accept N5-methyltetrahydrobiopterin as cofactor (Werner et al., 2000) and do not depend on the two electron mechanism of cofactor recycling but use a one electron tetrahydrobiopterin cycle (for review, see Stuehr, 1999; Werner et al., 2003). A second cofactor analogue that differently affects phenylalanine hydroxylase and nitric oxide synthase is 4-aminotetrahydrobiopterin. This pterin is a potent inhibitor of nitric oxide synthase (Werner et al., 1996; Schmidt et al., 1999) but does not change phenylalanine hydroxylase activity (Schmidt et al., 1999).
Previously studied effects of chelators and metal ions on glyceryl ether monooxygenase yielded weak influences (Taguchi and Armarego, 1998) but they were obtained using assays with small sensitivities and high protein concentrations because the purified enzyme is still not available. Our novel assay enabled us to very sensitively test for glyceryl ether monooxygenase activity in diluted microsomal homogenates (Werner et al., 2007). This way, we were able to detect changes in activity caused by 1,10-phenanthroline, EDTA, various divalent metal ions, and N5-methyltetrahydrobiopterin. To validate our setting, we performed parallel experiments on inducible nitric oxide synthase from stimulated RAW264.7 cells (Lyons et al., 1992) as representative for the nitric oxide synthases, and on recombinant phenylalanine hydroxylase as representative for the aromatic amino acid hydroxylases and compared the findings to those obtained for glyceryl ether monooxygenase from solubilised rat liver microsomes.
Results
1,10-Phenanthroline inhibits glyceryl ether monooxygenase activity in rat liver microsomes
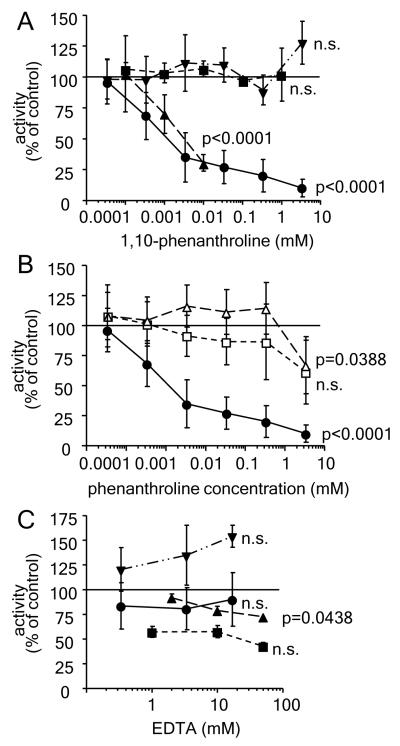
As shown in Figure 1A, 1,10-phenanthroline was able to significantly inhibit both phenylalanine hydroxylase and glyceryl ether monooxygenase activity in concentrations of 0.01 mm and 0.000338 mm, respectively, while nitric oxide synthase and fatty aldehyde dehydrogenase activity could not be significantly affected with concentrations of the chelator up to 1 mm. Control experiments on glyceryl ether monooxygenase with 1,7-phenatroline, an isomer of 1,10-phenanthroline that is not able to complex iron, as well as 1,10-phenanthroline pre-incubated with a 1.1 times molar amount of ferrous iron both resulted in a much reduced inhibition (Figure 1B). Similar control experiments on all four enzymes were also performed with EDTA in concentrations up to 50 mm (Figure 1C). While both, glyceryl ether monooxygenase and fatty aldehyde dehydrogenase were not inhibited by EDTA up to 16.9 mm, phenylalanine hydroxylase activity displayed a significant inhibition in concentrations of EDTA up to 10 mm. Nitric oxide synthase activity was reduced by up to 50% in all tested concentrations but the extent of inhibition did not change significantly with increasing EDTA concentrations (Figure 1C).
Figure 1.
Influence of chelators on the enzyme activity of glyceryl ether monooxygenase, nitric oxide synthase, phenylalanine hydroxylase, and fatty aldehyde dehydrogenase.
A. Solubilised microsomes displaying glyceryl ether monooxygenase (●) and fatty aldehyde dehydrogenase (▾) activity were incubated with 1,10-phenanthroline for 15 min at 25°C by vigorous shaking and assayed for the respective enzyme activity. Formation of pyrenedecanoic acid was detected by fluorescence detection after HPLC based product separation as described under “Materials and Methods”. 1,10-Phenatroline effect on nitric oxide synthase (∎) was tested in stimulated RAW264.7 cells and conversion of radioactively labelled L-arginine to L-citrulline was measured as indicated in the section “Materials and Methods”. Recombinant phenylalanine hydroxylase (▴) was incubated with 1,10-phenanthroline and the resulting enzyme activity was measured by fluorescence detection of the formed tyrosine after HPLC based separation. B. Glyceryl ether monooxygenase from solubilised rat liver microsomes was incubated for 15 min at 25°C by vigorous shaking with 1,10-phenatroline (●), 1,7-phenatroline (□), or 1,10-phenatroline pre-incubated with 1.1 fold molar excess of ferrous iron (Δ). Activity was tested by HPLC based separation of the pyrene-labelled acid and quantification by fluorescence detection. C. Solubilised microsomes displaying glyceryl ether monooxygenase (●) and fatty aldehyde dehydrogenase (▾) activity were incubated with in various concentrations of EDTA for 15 min at 25°C by vigorous shaking and assayed for the respective enzyme activity. Formation of pyrenedecanoic acid was detected by fluorescence detection after HPLC based product separation as described under “Materials and Methods”. EDTA effect on nitric oxide synthase (∎) was tested in stimulated RAW264.7 cells and conversion of radioactively labelled L-arginine to L-citrulline was measured as indicated in the section “Materials and Methods”. Recombinant phenylalanine hydroxylase (▴) was incubated with EDTA and the resulting enzyme activity was measured by fluorescence detection of the formed tyrosine after HPLC based separation. Data are all normalised to the enzyme activities obtained under standard conditions without chelator addition. Mean ± SD values for at least three independent experiments are shown. Levels of significance obtained by one-way ANOVA followed by Bonferroni’s post hoc test are indicated in the figure.
Selected divalent metal ions inactivate glyceryl ether monooxygenase
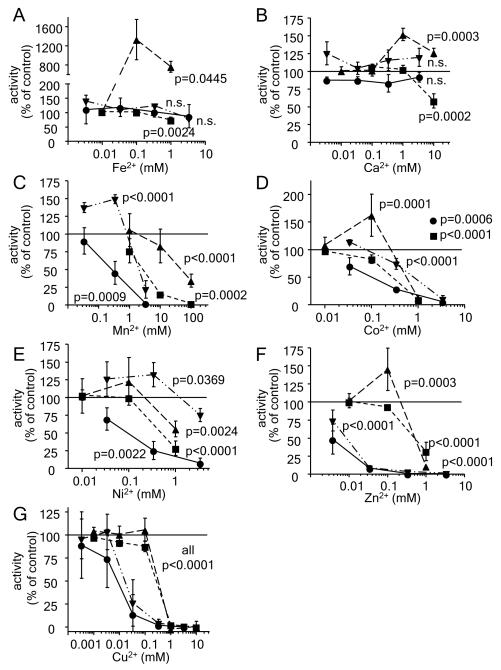
We incubated rat liver microsomes displaying glyceryl ether monooxygenase activity with different divalent cations in at least three different concentrations and compared the results to those obtained for fatty aldehyde dehydrogenase, nitric oxide synthase, and phenylalanine hydroxylase (Figure 2). As shown in panel A, ferrous iron was able to strongly activate recombinant phenylalanine hydroxylase whereas glyceryl ether monooxygenase, fatty aldehyde dehydrogenase, and nitric oxide synthase were slightly inhibited by this metal ion. This minor inhibition was significant only for nitric oxide synthase (p=0.0024), but not for fatty aldehyde dehydrogenase (p=0.354) and glyceryl ether monooxygenase (p=0.496) and can be explained by a decreased stability of tetrahydrobiopterin in the presence of ferrous iron (Howells et al., 1986). Glyceryl ether monooxygenase was not affected by calcium. In contrast, nitric oxide synthase activity was significantly inhibited at 10 mm (Figure 2B). While fatty aldehyde dehydrogenase was completely unaffected, phenylalanine hydroxylase was weakly activated by this metal ion (Figure 2B). Glyceryl ether monooxygenase was the enzyme that was most potently blocked by small manganese, cobalt and nickel concentrations, but these divalent cations were all able to significantly inhibit also the other investigated enzymes at the highest concentration tested (Figure 2C, 2D, and 2E). On the other hand, both divalent zinc and copper blocked fatty aldehyde dehydrogenase as well as glyceryl ether monooxygenase by nearly 100% at concentrations which left the other two enzymes unaffected. By further increasing the concentrations up to 1 mm and higher both zinc and copper did also inhibit nitric oxide synthase and phenylalanine hydroxylase (Figures 2F and 2G).
Figure 2.
Influence of divalent metal ions on the enzyme activity of glyceryl ether monooxygenase, nitric oxide synthase, phenylalanine hydroxylase, and fatty aldehyde dehydrogenase.
A. Glyceryl ether monooxygenase (●) from solubilised rat liver microsomes, nitric oxide synthase (∎) from stimulated RAW264.7, recombinant phenylalanine hydroxylase (▴), and fatty aldehyde dehydrogenase (▾) from solubilised rat liver microsomes were incubated with increasing concentrations of ferrous iron. For exact description of the enzyme activity assays see section “Materials and Methods”. The same experimental protocol was followed for Ca2+ (B), Mn2+ (C), Co2+ (D), Ni2+ (E), Zn2+ (F), and Cu2+ (G). Data are normalised to the enzyme activities obtained under standard conditions without metal ion addition. Mean ± SD values for at least three independent experiments are shown. Levels of significance obtained by one-way ANOVA followed by Bonferroni’s post hoc test are indicated in the figure.
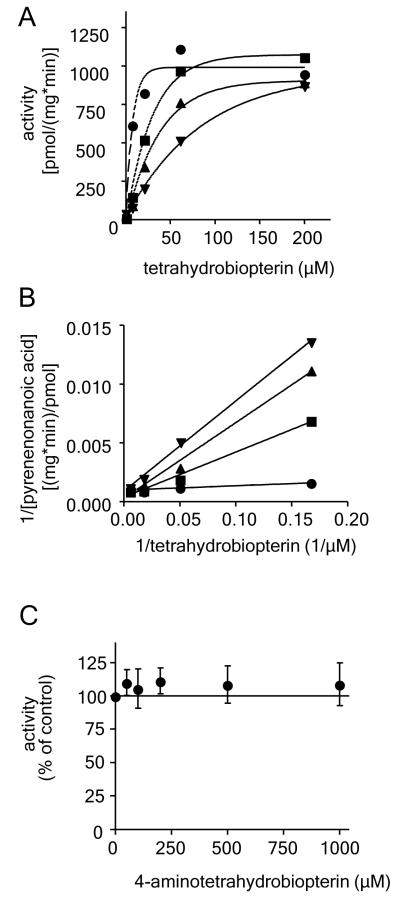
Glyceryl ether monooxygenase is competitively inhibited by N5-methyltetrahydrobiopterin while it remains unaffected by 4-aminotetrahydrobiopterin
N5-Methyltetrahydrobiopterin proved to be a competitive inhibitor of glyceryl ether monooxygenase. The extent of N5-methyltetrahydrobiopterin inhibition could be reversed by the natural cofactor tetrahydrobiopterin and a Ki value of 78.7 ± 46.2 μm for N5-methyltetrahydrobiopterin was calculated at a KM for tetrahydrobiopterin of 8.85 ± 3.88 μm (Figures 3A and 3B). 4-Aminotetrahydrobiopterin did not significantly change the activity of glyceryl ether monooxygenase in a concentration range from 50 μm to 1 mm when incubated in the assay together with the standard tetrahydrobiopterin concentration of 200 μm (Figure 3C).
Figure 3.
Influence of N5-methyltetrahydrobiopterin and 4-aminotetrahydrobiopterin on the enzyme activity of glyceryl ether monooxygenase.
A. Glyceryl ether monooxygenase activity was measured in the presence of five different concentrations of tetrahydrobiopterin (0 - 200 μm) and increasing amounts of the cofactor analogue N5-methyltetrahydrobiopterin (● 0 μm; ∎ 100 μm; ▴ 300 μM; ▾ 1 mm). One of three independent experiments is shown. B. Lineweaver-Burk plot of the data shown in panel A. Each data set was analysed by linear regression and Vmax and KM of tetrahydrobiopterin at each individual concentration of N5-methyltetrahydrobiopterin were calculated. C. Glyceryl ether monooxygenase was incubated with increasing concentrations (0 μm, 50 μm, 100 μm, 200 μm, 500 μm, and 1000 μm) of the cofactor analogue 4-aminotetrahydrobiopterin. Formation of pyrenedecanoic acid was detected by fluorescence detection after HPLC based product separation as described under “Materials and Methods” and activity was plotted normalised to the enzyme activity obtained under standard conditions without exceptional cofactor addition. Data are presented as means ± SD for 4 independent experiments.
Discussion
While the dependence of aromatic amino acid hydroxylases and nitric oxide synthases on a non heme and heme iron, respectively, is well documented, the metal requirement of glyceryl ether monooxygenase is still unclear. Here, we present for the first time a detailed analysis of the effects of two metal ion chelators and different divalent metal ions on glyceryl ether monooxygenase activity obtained from solubilised rat liver microsomes. We compare these data on one hand to those obtained for one representative of each of the two well described tetrahydrobiopterin-dependent enzyme families, the aromatic amino acid hydroxylases and the nitric oxide synthases. On the other hand, we also compare the results to those obtained for fatty aldehyde dehydrogenase, the enzyme that catalyses the oxidation of the fatty aldehyde, the product of the glyceryl ether monooxygenase reaction, to the corresponding fatty acid. Since our glyceryl ether monooxygenase assay detects the fatty acid which is the result of both reactions, the additional assay for the second step allows us to draw conclusions about the first step, the glyceryl ether monooxygenase reaction.
We show a clear inhibition of glyceryl ether monooxygenase by 1,10-phenanthroline, a very potent iron chelator. 1,10-Phenanthroline was able to also reduce phenylalanine hydroxylase activity whereas nitric oxide synthase was left untouched. Also the conversion of the long chain fatty aldehyde by fatty aldehyde dehydrogenase was unaffected by 1,10-phenanthroline when tested under the same conditions applied in the assay for glyceryl ether monooxygenase. This is in good agreement with the published finding that fatty aldehyde dehydrogenase does not need a metal ion for catalytic function (Lloyd et al., 2007). By this we can assign this inhibitory effect unambiguously to the glyceryl ether monooxygenase activity. Altogether this not only leads us to the conclusion that glyceryl ether monooxygenase depends on at least one sort of metal ion for its enzymatic function like it is for the other seven tetrahydrobiopterin-dependent enzymes, but it also gives us information about the nature of this metal ion binding within the enzyme. Whereas the heme bound iron of nitric oxide synthase could not be released with 1,10-phenanthroline, the non heme iron of phenylalanine hydroxylase was sensitive to this chelator and so was glyceryl ether monooxygenase activity. We therefore assume that glyceryl ether monooxygenase may also require a non heme iron for catalytic activity. This assumption is further strengthened by the lack of effect of carbonmonoxide on glyceryl ether monooxygenase compared to cytochrome P-450-dependent hydroxylase (Kaufman et al., 1990).
When we performed analogous experiments with EDTA, which is a less potent iron chelator, we did not detect any important changes below 10 mm neither on glyceryl ether monooxygenase, fatty aldehyde dehydrogenase, nor phenylalanine hydroxylase activity. In contrast, EDTA was able to decrease activity of nitric oxide synthase. This we attribute to the fact that the active form of nitric oxide synthase consists of a homodimer stabilised by a zinc ion coordinated tetrahedrally by two cysteine residues of each monomer (Raman et al., 1998; for review, see Ludwig and Marletta, 1999). Concentrations of 1 and 10 mm EDTA decreased nitric oxide activity about 40% and 50 mm EDTA by even more than 50%. The results obtained for EDTA further allow the conclusion that glyceryl ether monooxygenase is like phenylalanine hydroxylase not dependent on calcium.
Among the four enzymatic activities investigated here, glyceryl ether monooxygenase appeared to be most sensitive to metal ions. Except for iron and calcium, all divalent metal ions showed the highest inhibitory capacity on glyceryl ether monooxygenase. Only copper and zinc both displayed the same ability to block fatty aldehyde dehydrogenase and glyceryl ether monooxygenase reaction, rendering it impossible for us to draw conclusions about their effect on glyceryl ether monooxygenase, since our assay for glyceryl ether monooxygenase depends on aldehyde oxidation. All metal ions that were able to significantly inhibit glyceryl ether monooxygenase could also inhibit nitric oxide synthase and phenylalanine hydroxylase but the concentrations needed were at least one order of magnitude higher than for glyceryl ether monooxygenase. This is in agreement with published data on phenylalanine hydroxylase (Fisher et al., 1972), tyrosine hydroxylase (Haavik et al., 1991), and nitric oxide synthases (Perry and Marletta, 1998; Geyer et al., 2000). The slight activation detected for phenylalanine hydroxylase at low concentrations of some of the tested divalents can possibly be attributed to small iron contaminations of the salts used. In contrast, previous work on 1,10-phenanthroline and metal ions showed a smaller inhibitory capacity on glyceryl ether monooxygenase (Taguchi and Armarego, 1998). Our new and very sensitive assay allows us to detect much lower activities; therefore, we use a much more diluted microsomal sample as starting material. When repeating some of the experiments in higher concentrations of solubilised microsomes the inhibitory effects were dramatically reduced (data not shown).
N5-Methyltetrahydrobiopterin, a methylated analogue of the physiological cofactor tetrahydrobiopterin, is incapable of undergoing redox recycling by the dihydropteridine reductase system. This modified cofactor is a competitive inhibitor on phenylalanine hydroxylase (Werner et al., 2000). In contrast, the purified neuronal isoform of nitric oxide synthase (Werner et al., 2000) was not blocked but even activated by this cofactor analogue. Here we show that N5-methyltetrahydrobiopterin is also a competitive inhibitor of glyceryl ether monooxygenase. In addition, tetrahydroneopterin which is very similar to tetrahydrobiopterin has been shown to be able to promote both glyceryl ether monooxygenase (Werner et al., 2007) and phenylalanine hydroxylase activity (Kaufman, 1978) activity comparable to tetrahydrobiopterin, while nitric oxide synthase activity was stimulated at least two orders of magnitude less than by tetrahydrobiopterin (Werner et al., 1996). We also tested the influence of 4-aminotetrahydrobiopterin on glycerol ether monooxygenase in the presence of 200 μm of natural tetrahydrobiopterin cofactor and did not detect any changes in activity with concentrations up to 1 mm. 4-Aminotetrahydrobiopterin displays varying effects within the family of aromatic amino acid hydroxylases: While phenylalanine hydroxylase is almost not affected by this compound (Schmidt et al., 1999), tyrosine hydroxylase has been shown to be strongly inhibited (Almås et al., 2000). However, in clear contrast to glyceryl ether monooxygenase and phenylalanine hydroxylase, all three isoforms of nitric oxide synthases are strongly inhibited by 4-aminotetrahydrobiopterin (Werner et al., 1996; Schmidt et al., 1999). Altogether, these results indicate that glyceryl ether monooxygenase resembles the family of aromatic amino acid hydroxylases in regard to its cofactor handling characteristics.
A further difference between aromatic amino acid hydroxylases and nitric oxide sythases is the fate of the cofactor tetrahydrobiopterin. Tetrahydrobiopterin leaves the aromatic amino acid hydroxylase reaction as 4a-hydroxytetrahydrobiopterin in stoichiometric amounts (Fitzpatrick, 2003) and is subsequently recycled via quinoid dihydrobiopterin by carbinolamine dehydratase and NAD(P)H-dependent dihydropteridine reductase. The 4a-hydroxy intermediate could never be detected as by-product in the nitric oxide reaction where a tetrahydrobiopterin radical is formed instead (Hurshman et al., 2003). Similar to aromatic amino acid hydroxylases also glyceryl ether monooxygenase displays a coupling ratio of about one for the oxidation of cofactor to the conversion of the reducing agent NADH in the dihydropteridine reductase reaction (Kaufman et al., 1990; Kosar-Hashemi et al., 1994).
Our data provide evidence that glyceryl ether monooxygenase closely resembles the family of aromatic amino acid hydroxylases with respect to binding of the metal ion crucial for catalytic function and for the biochemistry of tetrahydrobiopterin-handling. Cofactor recycling also depends on dihydropteridine reductase and this again strengthens the relation to the family of aromatic amino acid hydroxylases. Furthermore, glyceryl ether monooxygenase is not calcium-dependent and does not need to form a zinc-stabilised dimer to be fully functional like the family of nitric oxide synthases. These data encourage a bioinformatic search for glyceryl ether monooxygenase candidates based on homology to tetrahydrobiopterin binding motifs of the aromatic amino acid hydroxylases.
Materials and Methods
Materials
Preparation of 1-O-pyrenedecyl-sn-glycerol is described elsewhere (Werner et al., 2000). Pyrenedecanal was obtained from Ramidus AB (Lund, Sweden). Dihydropteridine reductase from Physarum polycephalum was purified by DEAE chromatography after expression in Escherichia coli (Wild et al., 2003) and stored at −20°C in the presence of 50% glycerol. Bovine liver catalase (Sigma Chemical Company, St. Louis, MO, USA; purified on NAP-5 Sephadex G-25 columns, Amersham-Pharmacia, Uppsala, Sweden) and some buffer and metal salts were from Sigma. Methanol for HPLC and most buffer and metal salts were from Merck (Darmstadt, Germany). All pteridines were purchased at B. Schircks Laboratories (Jona, Switzerland).
Preparation of solubilised microsomes from rat liver
Livers were harvested from anesthetised and decapitated male Sprague-Dawley rats in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and approved by the respective committee of the Austrian Ministry of Science and Education. Tissues were frozen in liquid nitrogen and stored at −20°C. All further steps were performed at 4°C. For preparation of microsomes, 250 g rat livers were homogenised with a Omni Mixer (Sorvall Inc., Norwalk, CT, USA) in 375 ml homogenisation buffer (0.1 m TrisHCl, pH 7.6 containing 0.25 m sucrose and 1 mm PMSF) and subsequently centrifuged at 3000 g for 10 min. Supernatants were centrifuged at 13 000 g for 20 min and microsomal pellets were collected after a third centrifugation step at 40 000 g for 1 hour. Pellets were washed with homogenisation buffer and were re-suspended in 10 ml of homogenisation buffer yielding total protein concentrations around 10 mg/ml determined by Bradford protein quantification. Microsomes were solubilised by first washing them in an equal volume high salt buffer (20 mm phosphate buffer, pH 6.5 containing 1.5 m NaCl, 10% (v/v) glycerol, and 1 mm DTT), incubation for 30 min on a slowly rotating wheel, and centrifuging at 150 000 g for 30 min. The pellet was re-suspended in again the same volume of high salt buffer by usage of an ultraturrax (IKA, Germany), another volume of low salt buffer (20 mm phosphate buffer, pH 6.5 containing 0.5 m NaCl, 10% (v/v) glycerol, and 1 mm DTT) containing 0.3 % (v/v) Triton X-100 (reduced form) was added (final Triton X-100 concentration: 0.15 % (v/v)) and incubated for 30 min on a slowly rotating wheel. After a last centrifugation step at 150 000 g for 30 min, the supernatant was collected and protein content was determined by Bradford protein quantification. Solubilised microsomes were stored at −80°C until usage. After re-thawing them and prior to use in the different assays, Triton X-100 (reduced form) was added to a final concentration of 0.05% to the microsomal mix, shaken for 30 min at 8-12°C and centrifuged at 150 000 g to remove re-associated membrane compartments. Solubilised microsomes displayed a specific activity of about 2000 pmol/(mg*min). This represents a 1.6 fold enrichment when compared to the crude rat liver homogenate (1250 pmol/(mg*min), Werner et al., 2007).
Glyceryl ether monooxygenase assay
Analysis of pyrene-labelled long chain fatty acid was performed by reversed phase HPLC chromatography as previously published (Werner et al., 2007) with small adaptations. Shortly, for a typical glyceryl ether monooxygenase assay solubilised microsomes (1-12 μg of protein content) were incubated with 100 mm TrisHCl pH 8.5, 0.2 mm NADPH, 0.2 mm NAD+, 0.1 mg/ml catalase, 0.2 μg/ml dihydropteridine reductase, 0.2 mm 6R-5,6,7,8-tetrahydro-L-biopterin (tetrahydrobiopterin), and either 0.1 mm 1-O-pyrenedecyl-sn-glyerol (Werner et al., 2007) or 0.1 mm 1-O-pyrenenonyl-sn-glyerol (Zheng et al., 2006) in a final assay volume of 10 μl. Reaction was started by addition of protein, incubation was performed for 20 - 60 min in the dark, and termination achieved by addition of 30 μl methanol. Both lipid substrates stocks were diluted in methanol to a concentration of 4 mm. Reagent blanks with assay buffer added instead of microsomal homogenate were always run in parallel and were negative for the pyrene-labelled acid (<1 nm).
To test for the influences of chelators and metal ions, solubilised microsomes were diluted 1:10 in various dilutions of chelator/metal ion in an optimised buffer containing 50 mm triethanolamine/HCl pH 7.5, 150 mm NaCl, 10% glycerol, 10 mm ascorbic acid, and 20 μm tetrahydrobiopterin and vigorously shaken for 15 min at 25°C. For the inhibition experiments N5-methyltetrahydrobiopterin was added to the assay mix in concentrations ranging from 0 to 1000 μm combined with five tetrahydrobiopterin concentrations (0, 6, 20, 60, and 200 μm). 4-Aminotetrahydrobiopterin was added to the assay mix in concentrations of (in μm) 0, 50, 100, 200, 500, 1000 combined with the standard assay tetrahydrobiopterin concentration of 200 μm. All data were normalised to the activity of glyceryl ether monooxygenase tested under the same conditions without addition of metals, chelators, or cofactor analogues.
HPLC chromatography was performed on a 1200 HPLC system with Chemstation software (Agilent, Vienna, Austria). 10 μl of the stopped assay reaction were injected onto a Zorbax XDB-C8 Rapid resolution column (Agilent), eluted with 10 mm potassium phosphate buffer pH 6.0 containing 79% (v/v) of methanol at a flow rate of 1 ml/min for 4.5 min, followed by a gradient to 100% (v/v) methanol at 5 min. At 8 min the initial buffer/methanol (21:79) mix was re-established and the column equilibrated until 8.5 min to prepare for the next sample injection. Detection of the pyrene-labelled acid was performed by fluorescence reading at 340 nm excitation and 400 nm emission, with a detection limit of 1 nm.
Fatty aldehyde dehydrogenase assay
The assay for long chain fatty aldehyde dehydrogenase was performed in analogy of the glyceryl ether monooxygenase assay by replacing the pyrene-labelled glycerol with pyrenedecanal at a final concentration of 1.6 μm in the assay mix. Incubation time was always 10 min due to the quick turnover and loss of linearity at longer incubation periods.
The influence of chelators and metal ions on fatty aldehyde dehydrogenase was tested by diluting solubilised microsomes 1:10 in different dilutions of chelator/metal ion solved in buffer containing 50 mm triethanolamine/HCl pH 7.5, 150 mm NaCl, 10% glycerol, 10 mm ascorbic acid, and 20 μm tetrahydrobiopterin and vigorously shaken for 15 min at 25°C. Analysis of the synthesised pyrenedecanoic acid was performed on the same HPLC system and analogue fluorescence reading as for the glyceryl ether monooxygenase assay.
Induction of nitric oxide synthase (inducible isoform)
Mouse macrophage RAW264.7 cells (ATCC TIB-71, Rockville, MD, USA) were grown in 75 cm2 culture flasks in Dulbecco’s MEM medium (Biochrom AG, Berlin, Germany) supplemented with 10% heat-inactivated FCS (Pan Biotech GmbH, Aidenbach, Germany) and 2 mM L-glutamine (Sigma) at 37°C under a 5% CO2 atmosphere. Nitric oxide synthase expression was induced in confluent cell layers by treatment with 1 μg/ml LPS from E. coli (Sigma) and 250 U/ml murine γ-IFN (Strathmann Biotech GmbH, Hannover, Germany). Induction of nitric oxide synthase activity in cells was tested by measuring the content of nitrite in the supernatant by Griess reagent and photometric quantification of the red azo dye at 546 nm. Cells were harvested 24 hours after induction in 500 μl of aqua destillata containing 5 mm dithioerythreitol, shock frozen in liquid nitrogen and stored at −80°C until further usage.
Nitric oxide synthase assay
To 50 μl LPS stimulated RAW264.7 cell homogenate the following components were added (final concentration is indicated): 50 μm non-labelled L-arginine, 2 mm NADPH, 5 μm tetrahydrobiopterin, 25 μm FAD, 25 μm FMN, and radioactively labelled arginine (25000 cpm/50 μl). Chelators and metal ions were solved in aqua destillata and also added to the respective final concentration. The final assay volume of 200 μl was reached by filling up with sample buffer (50 mm Tris buffer with 10% glycerol, pH 8.0). All stock solutions were prepared in sample buffer. The reaction was incubated at 37°C for 30 min and was subsequently stopped by adding 800 μl stop buffer (20 mm sodium acetate, 1 mm L-citrulline, and 2 mm EDTA, pH 5.0). Each assay was then added onto an ion exchange column (Dowex 50 WX8, Sigma), washed with 1 ml aqua destillata. Radioactively labelled L-citrulline was quantified by radioactive counting after addition of 7 ml scintillation cocktail (Rotiscint Eco Plus, Carl Roth, Karlsruhe, Germany). A blank was determined by assay incubation without cell homogenate, stopping with 800 μl stop buffer, column separation and washing as indicated for the samples and radioactive counting after addition of scintillation mixture. To obtain the total amount of radioactivity added to the assay, the same procedure as for blank value was repeated but without column separation. To maintain the volumes, 1 ml aqua destillata was added prior to 7 ml of scintillation cocktail.
Phenylalanine hydroxylase assay
Phenylalanine hydroxylase was obtained by PCR from a rat liver cDNA, verified by sequencing, and was subsequently cloned into pMalc2 expression vector (NEB, Beverly, MD, USA) using standard molecular biology protocols. After expression of the fusion protein phenylalanine hydroxylase with maltose binding protein in E. coli, protein was purified by affinity chromatography for maltose binding protein. Recombinant protein (1.14 mg/ml) was preincubated for 15 min at room temperature in 0.1 M HEPES, pH 7.0 containing 1 mm phenylalanine, 1 mg/ml catalase and the final concentration of ion metal or chelator with a final incubation volume of 100 μl. Reaction was started by addition of 75 μm tetrahydrobiopterin in 5 mm dithioerythreitol and incubated for 2 min at 37°C. Reaction was stopped with 10 μl of 40% trichloroacetic acid. Analysis of 10 μl of reaction mixture with an RP-18 column (125 × 4, Lichrosphere, 5 μm particle size; Merck) was performed on a 1050 HPLC system with Chemstation software (Hewlett Packard, Agilent) and eluted with 40 mm sodium acetate/acetic acid, pH 3.5 at a flow rate of 1.0 ml/min. Reaction product tyrosine was quantified by fluorescence detection (excitation 275 nm, emission 310 nm).
Statistical analysis
All data are presented as means ± standard deviation (SD). Data were analysed for statistical significance by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test using GraphPad Prism 5.01 (GraphPad Software, Inc., San Diego, CA, USA).
Acknowledgements
The authors thank Renate Kaus, Petra Loitzl, Nina Madl, and Tamara Pfeifenberger for expert technical help, Helmut Prast for providing us with rat livers, Raphael A Zoeller for the kind donation of the 1-O-pyrenenonyl-sn-glyerol and the Austrian Research Funds “zur Förderung der wissenschaftlichen Forschung” (P-19764) for financial support.
References
- Almås B, Toska K, Teigen K, Groehn V, Pfleiderer W, Martinez A, Flatmark T, Haavik J. A kinetic and conformational study on the interaction of tetrahydropteridines with tyrosine hydroxylase. Biochemistry. 2000;39:13676–13686. doi: 10.1021/bi0011983. [DOI] [PubMed] [Google Scholar]
- Fisher DB, Kirkwood R, Kaufman S. Rat liver phenylalanine hydroxylase, an iron enzyme. J. Biol. Chem. 1972;247:5161–5167. [PubMed] [Google Scholar]
- Fitzpatrick PF. Mechanism of aromatic amino acid hydroxylation. Biochemistry. 2003;42:14083–14091. doi: 10.1021/bi035656u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer O, Podos SM, Oron Y, Mittag TW. The effect of divalent cations on bovine retinal NOS activity. Mol. Cell Biochem. 2000;204:11–16. doi: 10.1023/a:1007037307370. [DOI] [PubMed] [Google Scholar]
- Haavik J, Le BB, Martinez A, Flatmark T, Mallet J. Recombinant human tyrosine hydroxylase isozymes. Reconstitution with iron and inhibitory effect of other metal ions. Eur. J. Biochem. 1991;199:371–378. doi: 10.1111/j.1432-1033.1991.tb16133.x. [DOI] [PubMed] [Google Scholar]
- Howells DW, Smith I, Hyland K. Estimation of tetrahydrobiopterin and other pterins in cerebrospinal fluid using reversed-phase high-performance liquid chromatography with electrochemical and fluorescence detection. J. Chromatogr. 1986;381:285–294. doi: 10.1016/s0378-4347(00)83594-x. [DOI] [PubMed] [Google Scholar]
- Hurshman AR, Krebs C, Edmondson DE, Marletta MA. Ability of tetrahydrobiopterin analogues to support catalysis by inducible nitric oxide synthase: formation of a pterin radical is required for enzyme activity. Biochemistry. 2003;42:13287–13303. doi: 10.1021/bi035491p. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Imai Y. Solubilization and partial characterization of alkylglycerol monooxygenase from rat liver microsomes. Eur. J. Biochem. 1983;132:23–27. doi: 10.1111/j.1432-1033.1983.tb07320.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Imai Y. Affinity purification of alkylglycerol monooxygenase from rat liver microsomes by chimyl alcohol-Sepharose 4B column chromatography. J. Lipid Res. 1985;26:393–395. [PubMed] [Google Scholar]
- Kaufman S. Phenylalanine hydroxylase from rat liver. Methods Enzymol. 1978;53:278–286. doi: 10.1016/s0076-6879(78)53034-6. [DOI] [PubMed] [Google Scholar]
- Kaufman S, Pollock RJ, Summer GK, Das AK, Hajra AK. Dependence of an alkyl glycol-ether monooxygenase activity upon tetrahydropterins. Biochim. Biophys. Acta. 1990;1040:19–27. doi: 10.1016/0167-4838(90)90141-2. [DOI] [PubMed] [Google Scholar]
- Kaufman S. The phenylalanine hydroxylating system. Adv. Enzymol. Relat Areas Mol. Biol. 1993;67:77–264. doi: 10.1002/9780470123133.ch2. [DOI] [PubMed] [Google Scholar]
- Kosar Hashemi B, Taguchi H, Armarego WL. Glyceryl-ether Monooxygenase [EC 1.14.16.5] Part V: Some aspects of the Stoichiometry. Pteridines. 1994;5:1–7. [Google Scholar]
- Lloyd MD, Boardman KD, Smith A, van den Brink DM, Wanders RJ, Threadgill MD. Characterisation of recombinant human fatty aldehyde dehydrogenase: implications for Sjogren-Larsson syndrome. J. Enzyme Inhib. Med. Chem. 2007;22:584–590. doi: 10.1080/14756360701425360. [DOI] [PubMed] [Google Scholar]
- Ludwig ML, Marletta MA. A new decoration for nitric oxide synthase - a Zn(Cys)4 site. Structure. 1999;7:R73–R79. doi: 10.1016/s0969-2126(99)80047-1. [DOI] [PubMed] [Google Scholar]
- Lyons CR, Orloff GJ, Cunningham JM. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J. Biol. Chem. 1992;267:6370–6374. [PubMed] [Google Scholar]
- Perry JM, Marletta MA. Effects of transition metals on nitric oxide synthase catalysis. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11101–11106. doi: 10.1073/pnas.95.19.11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Werner-Felmayer G, Mayer B, Werner ER. Preferential inhibition of inducible nitric oxide synthase by the 4-amino analogue of tetrahydrobiopterin in cultured cells. Eur. J. Biochem. 1999;259:25–31. doi: 10.1046/j.1432-1327.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ. Mammalian nitric oxide synthases. Biochim. Biophys. Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Taguchi H, Armarego WL. Glyceryl-ether monooxygenase [EC 1.14.16.5]. A microsomal enzyme of ether lipid metabolism. Med. Res. Rev. 1998;18:43–89. doi: 10.1002/(sici)1098-1128(199801)18:1<43::aid-med3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- Werner ER, Pitters E, Schmidt K, Wachter H, Werner-Felmayer G, Mayer B. Identification of the 4-amino analogue of tetrahydrobiopterin as a dihydropteridine reductase inhibitor and potent pteridine antagonist of rat neuronal nitric oxide synthase. Biochem. J. 1996;320:193–196. doi: 10.1042/bj3200193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ER, Habisch HJ, Gorren ACF, Schmidt K, Canevari L, Werner-Felmayer G, Mayer B. Contrasting effects of N5-substituted tetrahydrobiopterin derivatives on phenlyalanine hydroxylase, dihydropteridine reductase and nitric oxide synthase. Biochem. J. 2000;348:579–583. [PMC free article] [PubMed] [Google Scholar]
- Werner ER, Gorren ACF, Heller R, Werner-Felmayer G, Mayer B. Tetrahydrobiopterin and nitric oxide: Mechanistic and pharmacological aspects. Exp. Biol. Med. 2003;228:1291–1302. doi: 10.1177/153537020322801108. [DOI] [PubMed] [Google Scholar]
- Werner ER, Hermetter A, Prast H, Golderer G, Werner-Felmayer G. Widespread occurrence of glyceryl ether monooxygenase activity in rat tissues detected by a novel assay. J. Lipid Res. 2007;48:1422–1427. doi: 10.1194/jlr.D600042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C, Golderer G, Gröbner P, Werner-Felmayer G, Werner ER. Physarum polycephalum expresses a dihydropteridine reductase with selectivity for pterin substrates with a 6(1′2′-dihydroxypropyl) substitution. Biol. Chem. 2003;384:1057–1062. doi: 10.1515/BC.2003.118. [DOI] [PubMed] [Google Scholar]
- Zheng H, Duclos RI, Jr., Smith CC, Farber HW, Zoeller RA. Synthesis and biological properties of the fluorescent ether lipid precursor 1-O-[9′-(1″-pyrenyl)]nonyl-sn-glycerol. J. Lipid Res. 2006;47:633–642. doi: 10.1194/jlr.M500493-JLR200. [DOI] [PubMed] [Google Scholar]