Abstract
The GABA (γ-aminobutyric acid)-containing interneurons of the neocortex are largely derived from the ganglionic eminences in the subpallium. Numerous studies have previously defined the migratory paths travelled by these neurons from their origins to their destinations in the cortex. We review here results of studies that have identified many of the genes expressed in the subpallium that are involved in the specification of the subtypes of cortical interneurons, and the numerous transcription factors, motogenic factors and guidance molecules that are involved in their migration.
Keywords: gene expression, interneuron, migration, neocortex, neuronal specification, subpallium
Abbreviations: AEP, anterior entopeduncular; BDNF, brain-derived neurotrophic factor; CGE, caudal ganglionic eminence; CR, calretinin; CP, cortical plate; CXCR, CXC chemokine receptor; E, embryonic day; GABA, γ-aminobutyric acid; GABAR, GABA receptor; HGF/SF, hepatocyte growth factor/scatter factor; 5-HT, 5-hydroxytryptamine; IZ, intermediate zone; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; MZ, marginal zone; NGR, neuregulin; NPY, neuropeptide Y; Nrp, neuropilin; POA, preoptic area; PV, paravalbumin; Robo, Roundabout; SDF-1, stromal-derived factor 1; SHH, sonic hedgehog; SST, somatostatin; SVZ, subventricular zone; VZ, ventricular zone
INTRODUCTION
Interest in neuronal migration in the cerebral cortex has never been greater, because investigations into the mechanisms that regulate neuronal movement pointed to migration abnormalities in several naturally occurring genetic defects in humans (Ross and Walsh, 2001). This is particularly the case for the GABA (γ-aminobutyric acid)-containing interneurons, since it was discovered in the late 1990s that they do not arise in the pallial ventricular zone but, instead, originate in distinct subpallial regions (Parnavelas, 2000; Wonders and Anderson, 2005). Specifically, fate-mapping experiments and loss-of-function analyses in rodents have shown that cortical interneurons arise predominantly from the medial (MGE) and caudal (CGE) ganglionic eminences (Tamamaki et al., 1997; Lavdas et al., 1999; Wichterle et al., 1999; Nery et al., 2002; Xu et al., 2004), and from the embryonic POA (preoptic area) (Gelman et al., 2009). Studies in avian and zebrafish brains have also demonstrated the subpallial origin of GABAergic interneurons (Cobos et al., 2001; Tuorto et al., 2003; Mione et al., 2008). However, recent observations in fetal human and monkey brains have suggested that a substantial proportion of cortical interneurons may arise from the lateral ventricular epithelium (Letinic et al., 2002; Rakic and Zecevic, 2003; Fertuzinhos et al., 2009).
Abundant evidence indicates that cortical interneurons comprise distinct neuronal subpopulations as defined by their morphological, neurochemical and electrophysiological properties (Ramón y Cajal, 1911; Lorente de Nó, 1922; Parnavelas et al., 1989; Kawaguchi, 1993; Markram et al., 2004; Butt et al., 2005). It has been suggested that the generation of the different subpopulations is linked to regional differences, defined by the expression of particular combinations of transcription factors, in the specification of progenitor cells in the subpallium (Wonders and Anderson, 2006; Flames et al., 2007; Wonders et al., 2008). However, it has yet not been firmly established what factors contribute to the generation of interneuron diversity in the cerebral cortex.
Numerous studies have traced in detail the three long and tortuous migratory paths that interneurons follow from their origins in the subpallium to the cortex (reviewed in Corbin et al., 2001; Marin and Rubenstein, 2003; Métin et al., 2006). However, there exists evidence for other streams. Specifically, Yozu et al. (2005) have documented the presence of a stream for the caudal migration of CGE interneurons, and Inta et al. (2008) identified a stream of migrating 5-HT3A (5-hydroxytryptamine) interneurons that arises in the SVZ (subventricular zone) and is postnatally directed towards the occipital cortex. Once in the cortex, interneurons display diverse migratory behaviours before settling in their positions in the developing CP (cortical plate) (Nadarajah et al., 2002; Ang et al., 2003; Tanaka et al., 2006). More recently, Tanaka et al. (2009) described multidirectional, long-distance and often prolonged movement of interneurons in the MZ (marginal zone) prior to descending radially to the CP. These authors speculated that these behaviours may contribute to the dispersion of these cells throughout the cortex. Furthermore, work by Yokota et al. (2007) has suggested that radial glia may provide a structural matrix for allocating interneurons within the developing cortex. Thus it appears that, once interneurons reach the cortex through one of the spatially confined streams, they adopt a radial trajectory to the final stage of their journey in the CP (Figure 1). The present review examines the molecules involved in the generation and specification of cortical interneurons, and in the mechanisms that regulate their migration.
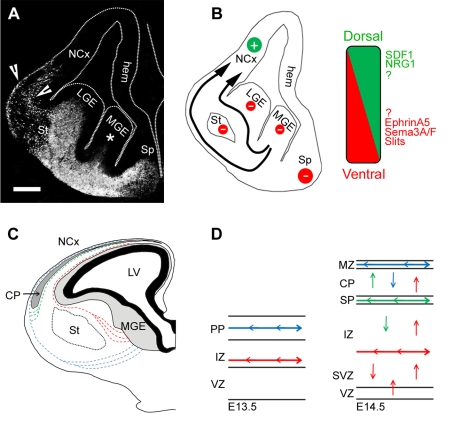
Figure 1. Tangential migration of cortical interneuron.
(A) Coronal section taken from the brain of an E13.5 GAD67–GFP (green fluorescent protein) transgenic mouse showing the tangential paths of migrating cortical interneurons (arrowheads) generated in the MGE (asterisk). Scale bar: 200 μm. (B) Schematic drawing of (A) illustrating chemorepulsive (red) and chemoattractive (green) gradients established from ventro-dorsal and dorso-ventral parts of the forebrain respectively. (C) Drawing of a coronal section of E15 mouse brain showing the tangential paths of early- (blue broken lines), and late (red broken lines)-born interneurons. Upon the emergence of the CP, an additional migratory path is formed within the subplate (green broken lines). (D) Schema showing the tangential and radial movements of interneurons within the cortical wall at E13.5 and E14.5. Abbreviations: hem, cortical hem; LV, lateral ventricle; PP, preplate; NCx, neocortex; SP, subplate; Sp, septum; St, striatum.
GENE EXPRESSION AND SPECIFICATION OF INTERNEURON PROGENITORS
The embryonic subpallium or ventral telencephalon is subdivided into the MGE, LGE (lateral ganglionic eminence), CGE and AEP (anterior entopeduncular)/POA domains. Ganglionic eminences appear as a sloping swelling at the telo-diencephalic junction, protruding into the lateral and third ventricles, at the early [E11 (embryonic day 11)] stages of embryonic mouse development. At E12, a second sloping swelling appears; these are respectively called MGE and LGE. The CGE is defined as the posterior region where the LGE and MGE fuse together. As for the AEP/POA domain, it is located in the telencephalic stalk, close to the pallidal domain (Puelles et al., 2000). The sulcus between the eminences begins to disappear at E14–E15 and, by the end of embryonic development, it has receded into the wall of the lateral ventricle. The LGE gives rise to the striatal domain (caudate nucleus and putamen) as well as parts of the septum and amygdala. The MGE gives rise to the globus pallidus and, partly, to the septum, but the CGE is the origin of neurons of the nucleus accumbens, the bed nucleus of the stria terminals, the hippocampus and specific nuclei of the amygdala (Nery et al., 2002).
These subpallial domains express specific genes, such as Dlx1, Dlx2, Gsh1, Mash1, Gsh2, Nkx2.1, Nkx5.1, Isl1, Six3 and Vax1, that define their identities and are involved in the specification of interneurons and oligodendrocytes (reviewed by Marin and Rubenstein, 2003). Dlx homeobox genes are transcription factors that act as critical molecular determinants in forebrain development (Panganiban and Rubenstein, 2002). They are specifically required to co-ordinate the timing of GABAergic interneuron migration and process formation (Anderson et al., 1997; Yun et al., 2002). In double Dlx1 and Dlx2 null mutants, migration is almost abolished and cells accumulate in the ganglionic eminences (Cobos et al., 2005, 2006, 2007). Ectopic expression of DLX has been shown to induce GAD65/67 expression in neuronal progenitors of the cerebral cortex normally committed to a glutamatergic phenotype (Stühmer at al., 2002). Interestingly, Dlx1 single null mutants show no migration defects. Recent studies suggest that DLX factors do not only have a role in migration, but also in cell specification. Thus abnormal dendritic morphology has been reported in subsets of SST- (somatostatin) and CR (calretinin)-expressing interneurons in Dlx1 and Dlx2 double-null mutants (Cobos et al., 2005). Moreover, CR-expressing interneurons are also absent from cortical cultures prepared from these animals.
Another homeobox transcription factor, Nkx 2.1, appears to play a pivotal role during the commitment of interneuron progenitors. In fact, Nkx 2.1 is fundamental for the correct MGE specification (Sussel et al., 1999), whereas LGE development is affected by the expression of Gsh2 (Hsieh-Li et al., 1995). Expression of Pax6 also distinguishes the LGE from the MGE and may contribute to the specification of the former. The AEP/POA territory, adjacent to the MGE, is also specified by Nkx2.1 expression, but, although the expression of common transcription factors indicates that MGE and AEP/POA may share some properties, progenitors arising from these structures appear molecularly distinct (Flames et al., 2007). These results suggest that the ganglionic eminences and AEP/POA are anatomically defined sites characterized by distinct gene expression domains. As such, they give rise to distinct populations of cortical interneurons (Xu et al., 2004; Butt et al. 2005).
Earlier in vitro and in vivo experiments on the origins of interneuron subtypes suggested that they arise from different subpallial progenitor pools (reviewed by Fishell, 2007). More recent in vitro experiments have shown that expression of Nkx2.1 in the MGE is required for the specification of MGE-derived interneurons. Accordingly, primary cultures prepared from cortices of Nkx2.1-null mutants were found to contain no PV- (paravalbumin) or SST-expressing interneurons, but included CR-expressing cells. This suggested that PV and SST subtypes originate primarily within the MGE, whereas CR-expressing interneurons are derived mainly from the CGE (Xu et al., 2004; Butt et al., 2005). NPY- (neuropeptide Y) positive interneurons have also been found to derive from the MGE (Wonders and Anderson, 2006). Moreover, in vivo loss-of-function experiments have confirmed that removal of Nkx2.1 at distinct developmental time points results in a switch of the MGE progenitor fate into LGE- and CGE-derived cells (Anderson et al., 2001). This phenotype has also been observed in conditional mutagenesis experiments using the Olig2 promoter to drive the expression of Cre-recombinase (Butt et al., 2008). Recent in utero transplantation experiments attempted to identify the presence of dorso-ventral genetic patterning in the ganglionic eminences, and indicated the existence of five different domains within the MGE. Specifically, these studies suggested that most SST-positive cortical interneurons originate from progenitors located in the dorsal aspect of the MGE, whereas PV-positive subtypes originate from more ventrally located domains (Flames et al., 2007; Wonders et al., 2008).
Upstream of Nkx2.1, the morphogen Shh (sonic hedgehog) appears to play a critical role in the establishment of Nkx2.1 expression in the MGE (Sussel et al., 1999; Fuccillo et al., 2004) and in the maintenance of its expression during neurogenesis (Xu et al., 2005). Thus, mice carrying mutations in Shh expression within the neural tube fail to express the interneuron fate-determining gene Nkx2.1 in the MGE. This effect was also reproduced by inhibiting SHH signalling in slice cultures (Gulacsi and Anderson, 2006). Downstream of Nkx2.1, the lim-homeodomain transcription factor Lhx6 may be directly activated by Nkx2.1. Lhx6 is expressed by cells from the MGE (Lavdas et al., 1999; Alifragis et al., 2004; Liodis et al., 2007) and, more specifically, it has been detected in all of the MGE-derived Nkx2.1-dependent subpopulations, whereas the CR-expressing subpopulation from the CGE is generally Lhx6 negative (Fogarty et al., 2007). Analysis of animals homozygous for the Lhx6 mutation has shown a similar number of GABA-positive interneurons in the neocortex, but lacking the PV and SST subpopulations (Liodis et al., 2007). It seems, then, that Lhx6 activity is not required for the specification of the GABAergic identity of cortical interneurons, in agreement with an earlier study that utilized RNAi (RNA interference) in dissociated MGE cell cultures (Alifragis et al., 2004), but is necessary for the specification of the Nkx2.1-dependent subgroups. Moreover, electroporation of Nkx2.1 cDNA into the ventral telencephalon of slice cultures prepared from Nkx2.1-null mouse embryos was found to induce Lhx6 expression (Xu et al., 2008).
IDENTIFYING INTERNEURON SUBTYPES IN THE CORTEX
The use of in vivo Cre-lox technology allows one to permanently label interneuron precursor populations with molecular markers of interest. This approach is not only useful to confirm previous in vivo and in vitro data about the existence of various interneuron populations, but also to identify their final positions in the cortex. Cre-mediated recombination fate-mapping, which uses Cre-recombinase driven by different factors such as Lhx6, Nkx2.1 and Nkx6.2, produced results consistent with loss of function and transplantation analyses (Fogarty et al., 2007; Xu et al., 2008). Interestingly, Fogarty et al. (2007) have described a characteristic cell population positive for CR and SST located in the dorsal region of the MGE neuroepithelium and expressing Nkx6.2. In the ventral telencephalon, Nkx6.2 is expressed in a small subset of ventral neural progenitors at the border between the MGE and CGE (Stenman et al., 2003). In particular, these cells are present in deep cortical layers of adult mice and show features characteristic of Martinotti cells; their number is reduced in Nkx6.2-null mice. Instead, the majority of CR cells are generated outside the MGE, possibly within the CGE (Xu et al., 2004; Butt et al., 2005), have a bipolar morphology and reside in the outer layers of the cortex (Sousa et al., 2009).
It has been suggested that interneuron diversity may also arise as a consequence of a temporal change in the fate of progenitor cells (Miyoshi et al., 2007). Moreover, Miyoshi et al. (2007) have fate-mapped distinct cohorts of cells arising from progenitors expressing high levels of the bHLH (basic helix-loop-helix) gene Olig2. In the forebrain, Olig2 is expressed in the progenitors of the ventral embryonic eminences, with the highest levels of expression in the MGE Nkx2.1-positive domain. Consistent with previous studies (Butt et al., 2005), it was observed that populations labelled at early developmental time points (E9.5, E10.5) preferentially populate deep cortical layers, whereas cells labelled at later developmental stages (i.e. E15.5) mainly occupy superficial layers. The numbers of SST-positive, CR-negative interneurons fate-mapped at early developmental stages is high, but decrease at later time points and are almost absent from populations at E15.5. In conclusion, CR-positive, SST-negative interneurons and VIP (vasoactive intestinal peptide)-positive interneurons, often associated with bipolar morphologies, are mainly observed at E15.5 and found enriched within the superficial layers of cortex.
Previous in utero tracing experiments suggested that the embryonic POA is another source of cortical interneurons. Recent lineage experiments using the Cre/loxP system have shown that Nkx5-1 appears to be transiently expressed by a population of early post-mitotic POA-derived cells. Gelman et al. (2009) have generated a [BAC (bacterial artificial chromosome)] transgenic mouse line that expresses the Cre-recombinase protein under the transcriptional control of Nkx5-1 in order to permanently label cells generated from the POA. It seems that the murine POA is a source of a population of cortical GABAergic interneurons with uniform neurochemical, morphological, and electrophysiological properties. In addition, POA Nkx5-1-derived interneurons frequently express NPY, but not any other common markers of interneurons. Figure 2 provides a pictorial view of the expression patterns of genes in the subpallium, which play key roles in the specification of cortical interneuron subtypes.
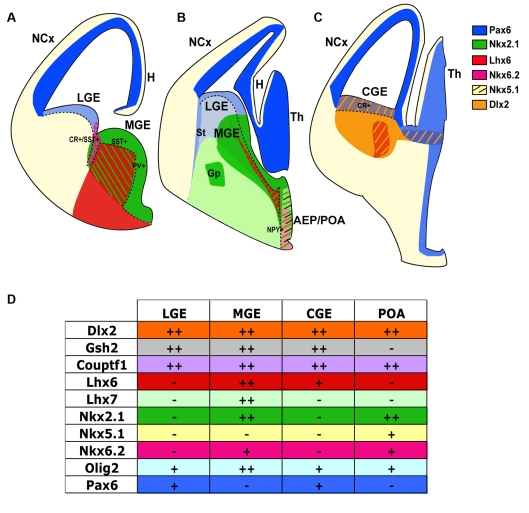
Figure 2. Gene expression patterns in the subpallium.
Drawings of medial (A), caudal-intermediate (B) and caudal (C) coronal sections of E13.5 mouse brain showing the expression of different genes in the subpallial domain which give rise to distinct interneuron subpopulations. Broken lines delineate the VZ. Sub-domains that express two or more genes are marked with stripes. (A and B) Pax6 is expressed at high levels in the pallial proliferative zone and thalamic territory, and at a lower level in the subpallial proliferative zone of the LGE. Nkx2.1 is specifically expressed in the VZ and mantle layer of the MGE. It is also expressed in the proliferative zone of the AEP/POA. Nkx6.2 expression is confined to the LGE/MGE border and overlaps with the expression of Pax6/Dlx2 in the LGE, and Nkx2.1/Dlx2 in the MGE. CR+ (CR-positive) and SST+ (SST-positive) interneuron precursors arise specifically from this Nkx6.2/Nkx2.1-expressing territory. Lhx6 expression is confined to the subventricular zone and the mantle layer of the MGE, and it specifies PV+ (PV-positive) interneurons. Its expression does not overlap with Nkx6.2 expression. AEP/POA, thought to give rise to NPY+ (NPY-positive) interneurons, expresses Nkx2.1, Dlx2 and Nkx5.1, as well as Nkx6.2 in its ventral domain. (C) The CGE is anatomically positioned at the most caudal part of the telencephalon where the LGE and MGE fuse. It remains a matter of debate which genes are specifically expressed in this territory. However, abundant evidence indicates that the CGE generates CR+ interneurons. Shown here is the expression of Dlx2, which characterizes all subpallial domains, Pax6 confined to the proliferative zone, and Lhx6. (D) A summary of the expression of different genes in the LGE, MGE, CGE and POA. ++, High level of expression: +, lower level of expression; -, lack of expression. Abbreviations: Gp, globus pallidus; H, hippocampus; NCx, neocortex; St, striatum; Th, thalamus.
EXPRESSION OF NOVEL GENES IN INTERNEURON PROGENITORS
Microarray analyses have also suggested that genes are differentially expressed in the dorso-ventral axis of the MGE. Dorsally enriched MGE genes are most likely downstream effectors of the Gli transcription factor family, part of the SHH signaling pathway (Xu et al., 2005), and transcriptional targets of Nkx6.2. In fact, Gli2, Nkx6.2 and Hhip1 are dorsally MGE-enriched genes, whereas ventrally MGE-enriched genes include sulfatase 1, sulfatase 2, brevican and FoxJ1, as well as Zic1, Zic3 and Lhx7/8 (Wonders et al., 2008).
Long et al. (2009) have analysed the differential expression of transcription factors in the MGE and CGE. Their studies have suggested that Mash1, and Dlx1 and Dlx2 are required to promote expression of several factors in MGE progenitors, including Arx, bMaf, Brn4, Cux2, Dlx1, Dlx2, Dlx5, Dlx6, ER81 (Etv1), Gli1, Lhx6, Lhx7, Pbx1, Peg3, Sox4, Sox11 and Vax1, and non-transcription factors, including CXCR4 (CXC chemokine receptor 4), CXCR7, CyclinD2, GAD67, Gucy1a3, Shb, Tiam2 and Thbs. Dlx1 and Dlx2 null mutants preserve the expression of Nkx2.1, but the expression of Cux2 and Lhx7(8) are clearly reduced. Cux2 is expressed in tangentially migrating interneurons (Cobos et al., 2006) and its function is linked to the development of reelin-expressing interneurons (Cubelos et al., 2008). Lhx7(8) is expressed in the SVZ of the ventral MGE (Flames et al., 2007) and its derivatives in the pallidal and striatal interneurons, where it is required for the expression of the cholinergic phenotype (Zhao et al., 2003; Fragkouli et al., 2005). On the other hand, some transcription factors show increased expression, including ATBF1, Ebf1, ESRG, Fez, FoxP2 and Islet1; this may be secondary to increased notch signalling, as reflected by increased Mash1 and Hes5 expression (Yun et al., 2002). Most interneuron precursors fail to migrate into the cortex in Dlx1 and Dlx2 double null mutants (Cobos et al., 2005) and remain as ectopic cells in the basal ganglia (Marin et al., 2001).
Dlx1 and Dlx2 also have a profound role in promoting differentiation of the dorsal domain of CGE, as exemplified by the reduced expression of Arx, Brn4, Dlx5, Dlx6, ESRG, FoxP4, Meis1, Meis2, Pbx1, Pbx3, Prox1, Six3, Sox4, Sox11, Sp8, Tle4, Tshz1 and Vax1 in null mutants. Several transcription factors continue to be expressed in the CGE of the triple null mutant Dlx1/Dlx2/Mash1, but at lower levels (Gsh1, Islet1, Olig2 and Sp9) and in the MGE (ER81, Islet1, Olig2 and Sp9); in addition, GAD67 expression is weakly maintained. Alterations in migration may be attributed to reduced expression of cytokine receptors (CXCR4 and CXCR7) and the neuregulin receptor, ErbB4. Migration defect may also be due to alterations in Gucy1a3, NP2, Robo2, Shb, Thbs and Tiam2 expression.
In their microarray analysis of cortical interneurons, Batista-Brito et al. (2008) observed the expression of numerous genes, including Cux2, Zfhx1b and Carhsp1, which are likely candidates for regulating the maturation and diversification of these cells. Expression of a number of genes encoding cell-surface proteins, including Neto1, Kitl, Chl1, Dscaml, Ncam and Astn1, have been observed in immature migrating interneurons. Neto1 shares some homology with the neuropilin family of cell-surface-receptor genes, and there are indications that, similar to Nrps (neuropilins), this receptor can bind to class 3 semaphorins (Kolodkin et al., 1997; Michishita et al., 2003). Class 3 semaphorins act as chemotactic signals for Nrps and mediate the directed migration of striatal and cortical interneurons (Marin et al., 2001). The observation that Nxph1, which has a crucial role in both GABAergic and glutamatergic synapses, is specifically enriched in cortical interneuron precursors suggests a potential role in neurotransmitter release at very early stages of cortical development.
A series of genes associated with neurological disorders are also expressed in cortical interneurons. For example, Npas3 and Npas1/3 compound mutants have behavioural deficits associated with mental disorders (Erbel-Sieler et al., 2004). Arx null mice have been implicated in autism and show impairment in interneuron migration (Colombo et al., 2007; Friocourt et al., 2008). Ncam1- and Chl1-encoding cell-surface molecules have been linked to schizophrenia (Irintchev et al., 2004; Atz et al., 2007). Moreover, interneurons in transgenic mice lacking Ncam1 function have compromised synaptic connectivity and exhibit abnormal behaviour (Pillai-Nair et al., 2005). Finally, disruption of genes encoding for channel proteins, such as Cacnb4, are also enriched in cortical interneuron precursors. Disruption of these genes has been associated with neurological disorders.
MOLECULAR MECHANISMS INVOLVED IN INTERNEURON MIGRATION
Motogenic factors
It has been shown in vitro that ganglionic eminence neurons have an astonishing intrinsic migratory capacity (Wichterle et al., 1999; Nery et al., 2002). In addition, a number of soluble factors have been proposed to play a role in cortical interneuron migration by acting as motogenic factors in vivo. One of these molecules, HGF/SF (hepatocyte growth factor/scatter factor), was first described as a promoter of cell motility for different cell lines (Birchmeier and Gherardi, 1998; Stella and Comoglio, 1999). Powell et al. (2001) first showed that expression of HGF/SF and its receptor MET are present in the proliferative zones and demarcate the migratory routes of migrating interneurons in the developing forebrain. These authors have also shown that, in slice cultures, HGF/SF enhances the migration of cells away from subpallial area, but exogenous addition of the factor disrupts the migration of interneurons. Moreover, analysis of u-PAR−/− (urokinase-type plasminogen activator receptor-deficient) mice (where the inactive pro-form of HGF/SF is not cleaved to its active form) revealed a reduction in the number of interneurons in the cortex, exhibited by counting of calbindin-positive cells, but an accumulation of such cells in the corticostriatal sulcus (Powell et al., 2001).
Members of the neurotrophin family have also been proposed as motogenic factors in the migration of interneurons. Thus evidence shows that tyrosine kinase receptors TrkB and TrkC, the cognate receptors for neurotrophins, are expressed in cortical interneurons (Klein et al., 1990; Gorba and Wahle, 1999). Furthermore, neurotrophins are widely expressed in the developing cortex (Maisonpierre et al., 1990; Timmusk et al., 1993; Friedman et al., 1998; Fukumitsu et al., 1998) and have been thought to have a pivotal role in neuronal migration (Behar et al., 1997, 2000; Brunstrom et al., 1997). Polleux et al. (2002) have provided evidence that BDNF (brain-derived neurotrophic factor) and NT4 (neurotrophin 4) stimulate interneuron migration, and analysis of TrkB-deficient mice revealed a significant reduction in cortical interneuron numbers, suggesting a role for these molecules as mitogenic factors. However, it has been suggested that disruption of BDNF signalling leads to down-regulation of calbindin and other neuropeptides expressed in interneurons (Jones et al., 1994; Arenas et al., 1996; Fiumelli et al., 2000), casting some doubt as to whether the reduction of interneuron numbers in the TrkB−/− animals reflects an actual defect in migration or simply a reduction of cell markers. It has also been suggested that BDNF signalling, in addition to modulating Reelin expression, regulates the distribution of both Cajal-Retzius cells and interneurons in the MZ and participates in the final phase of interneuron migration within the cerebral cortex (Alcantara et al., 2006). Thus analysis of nestin–BDNF transgenic mice has revealed that over-expression of BDNF leads to an inappropriate integration of interneurons within the CP, as well as segregation of Cajal-Retzius cells and interneurons in the MZ (Alcantara et al., 2006). Another neurotrophin, GDNF (glial-cell-line-derived neurotrophic factor), has also been shown to affect cortical interneurons by stimulating their migration (Pozas and Ibañez, 2005).
Chemotactic molecules
Once interneurons have initiated their migration, guidance systems impart directionality to channel them into appropriate migratory routes. It has been thought that an exquisite co-ordination of chemoattractive and chemorepulsive cues, expressed within the developing brain, allow cortical interneurons to reach the cerebral cortex and avoid subcortical areas (reviewed by Parnavelas, 2000; Marin and Rubenstein, 2003; Métin et al., 2006; Andrews et al., 2007). Thus slice culture assays have revealed that the cerebral cortex indeed provides attractive cues and the subpallial preoptic area produces repulsive factors (Marin et al., 2001, 2003; Polleux et al., 2002; Wichterle et al., 2003).
The chemokine SDF-1 (stromal-derived factor 1; also known as CXCL12) is a well known chemoattractant for leucocytes, germ cells and neurons (Tashiro et al., 1993; Bleul et al., 1996; Lazarini et al., 2000; Klein et al., 2001; Doitsidou et al., 2002; Li et al., 2008; Liapi et al., 2008). SDF-1 is highly expressed in the leptomeninges and the IZ (intermediate zone)/SVZ of the developing cortex (Tham et al., 2001; Zhu et al., 2002; Tiveron et al., 2006). Stumm et al. (2003, 2007) identified the CXC chemokine receptor 4 (CXCR4; a receptor for SDF-1) in Cajal-Retzus cells and tangentially migrating interneurons within the developing cortex, and showed that SDF-1 serves as a chemoattractant for the latter. Interestingly, these authors also showed that late-generated, but not early-generated, interneurons failed to integrate into their appropriate cortical layers in the absence of SDF-1 signalling (Stumm et al., 2003). Further work indicated that SDF-1 expression by projection neuron progenitors in the SVZ is crucial for the recognition of the IZ/SVZ path of migrating interneurons (Tiveron et al., 2006; Stumm et al., 2007). In addition, Liapi et al. (2008) have provided evidence suggesting that SDF-1 signalling is essential for both radial (projection neurons) and tangential (interneurons) migration within the cortical wall. Moreover, it has been suggested that this chemokine is not required for interneuron migration from the subpallium to the cortex, but it is pivotal to maintain interneurons migrating tangentially within the cortical wall (Stumm et al., 2003; Tiveron et al., 2006; Li et al., 2008; Liapi et al., 2008; López-Bendito et al., 2008).
NGRs (neuregulins), a family of growth factors encoded by four structurally related genes (NRG-1, NRG-2, NRG-3 and NRG-4), have been related to important events in the developing nervous system (Falls, 2003a; Anton et al., 2004; Xu et al., 2009). They are ligands for receptor tyrosine kinases of the ErbB family and activate a wide spectrum of intracellular signalling cascades, resulting in the induction of cellular responses in different organs (Buonanno and Fischbach, 2001; Falls, 2003a, 2003b; Anton et al., 2004; Britsch, 2007; Birchmeier, 2009). Several lines of evidence suggest that NRG-1 acts as a chemoattractant for interneurons (Yau et al., 2003; Flames et al., 2004). First, ErB4 is expressed in tangentially migrating neurons and co-localises with the interneuron marker DLX2 (Yau et al., 2003). Secondly, soluble NGR1-Ig is expressed in the cortical proliferative zones, and has been hypothesized to attract migrating interneurons to the IZ/SVZ path (Flames et al., 2004; Ghashghaei et al., 2006). Thirdly, secreted NGR1 is a potent chemoattractant for MGE-derived cells in vitro (Flames et al., 2004). Fourthly, loss-of-function assays have demonstrated that the migration of cortical interneurons depends on ErB4 signalling, and their number is significantly decreased in conditional ErB4 mutants (Flames et al., 2004).
To date, the chemorepulsive molecules expressed in the preoptic area remain largely unknown. Zhu et al. (1999) suggested that Slit proteins might repel interneurons from the subpallium to the cerebral cortex; their chemorepulsive activity is mediated by members of the Robo (Roundabout) receptor family (Kidd et al., 1998). During brain development, Slit1 is strongly expressed throughout the VZ (ventricular zone) and SVZ of the ganglionic eminences, as well as at the ventral midline and in basal regions of the forebrain (Yuan et al., 1999; Bagri et al., 2002; Marillat et al., 2002; Whitford et al., 2002). Robo receptors (Robo1, Robo2 and Robo3) show distinct, but complementary expression patterns to slit expression (Yuan et al., 1999; Bagri et al., 2002; Marillat et al., 2002; Whitford et al., 2002). Robo1 expression corresponds to subpallial regions through which early born interneurons migrate, and overlaps with their migratory path at the level of IZ/SVZ in the cortex (Andrews et al., 2006, 2007, 2008; Barber et al., 2009). Moreover, it has been shown that Robo1, Robo2 and Robo3 are expressed in cortical interneurons, suggesting that Slit–Robo signalling might play a pivotal role in their migration (Andrews et al., 2006, 2007, 2008; Barber et al., 2009). In support of this notion, it has been reported that secreted Slits from the VZ of the LGE repel ganglionic eminence cells away from the SVZ (Zhu et al., 1999). However, cell tracing studies carried out on slice cultures prepared from Slit1/Slit2 double mutant mice (Slit1−/−/Slit2−/−) showed no defects in the tangential migration of interneurons and, furthermore, no differences in the number or distribution of GABAergic interneurons (GABA-, Lhx6- or Dlx2-positive cells) were detected in the cortices of Slit1−/−/Slit2−/− mice (Marin et al., 2003). Interestingly, our analysis of two different strains of Robo1-deficient transgenic mice (Robo1−/−) has shown a perceptible influx of calbindin-labelled cells within the endogenously chemorepulsive striatum (see below), as well as an increased number of such cells, presumably interneurons, within the embryonic cortex (Andrews et al., 2006, 2008). The significant increase of cortical interneurons within the cortex of Robo1−/− persists until adulthood (Andrews et al., 2008). Moreover, detailed analysis of Robo1-, Robo2- and Robo3-deficient transgenic mice (Robo1−/−, Robo2−/− and Robo3−/−) showed marked alterations in the morphology of these cells (Andrews et al., 2006, 2008; Barber et al., 2009).
Another group of chemorepulsive cues for cortical interneurons is the membrane-bound ephrin family and their Eph-receptor tyrosine kinases (Eph). The ephrin/Eph signalling system has been related to a vast number of events in the developing brain (Klein, 2004). Experimental evidence has revealed that ephrins can direct migration and enhance the motility of neurons in vitro and in vivo (Santiago and Erickson, 2002; Nomura et al., 2006; Zimmer et al., 2007). Zimmer et al. (2008) have recently reported that calbindin-positive cells isolated from the MGE expressed the EphA4 receptor. In addition, they showed that ephrinA5 and its receptor EphA4 are complementarily expressed in the VZ and SVZ of the ganglionic eminences respectively (Zimmer et al., 2008). In vitro stripe assays demonstrated that ephrinA5 is a potent chemorepellent for MGE-derived neurons (Zimmer et al, 2008). Taken together, these results suggest that ephrinA5/EphA4 signalling may contribute to the channelling of cortical interneurons to the migratory path that runs deep to the striatum (see below).
Several lines of evidence suggest that neurotransmitters such as GABA have an active role in controlling the migration of cortical neurons, including interneurons (Behar et al., 1996, 1998, 1999, 2000; Manent et al., 2006; Heng et al., 2007). First, chemotaxis and pharmacological experiments in vitro have demonstrated that cortical neurons respond to GABA in a concentration-dependent manner (Behar et al., 1996) Thus a low concentration of GABA promotes cell migration, whereas higher concentrations induce random movements (Behar et al., 1996). Secondly, disruption of the activity of GABARs (GABA receptors) leads to alterations in the migratory dynamics of cortical neurons in vitro (Behar et al., 1996, 1998). Thirdly, GABA expression is present in the migratory path of cortical interneurons and, furthermore, these cells express GABA receptors (López-Bendito et al., 2003; Morante-Oria et al., 2003; Cuzon et al., 2006). Fourthly, transplantation studies revealed that the type A GABAR (GABAAR) signalling is necessary for interneurons to traverse the cortical-striatal notch en route to the cortex (Cuzon et al., 2006). Finally, type B GABAR (GABABR) signalling is required for the correct navigation of interneurons within the developing cortex (López-Bendito et al., 2003). A number of investigators have also shown that GluRs (glutamate receptors), such as NMDA (N-methyl-d-aspartate), kinate and AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid), are expressed in cortical interneurons, and are thought to participate in the migration of cortical interneurons, possibly through an increase in intracellular calcium (Métin et al., 2000; Poluch et al., 2001; Soria and Valdeomillos, 2002). A recent investigation indicates that GABA, in addition to promoting interneuron migration, plays a role in the cessation of their movement (Bortone and Polleux, 2009). Specifically, Bortone and Polleux (2009) showed that interneurons up-regulate the potassium-chloride co-transporter, KCC2, after reaching the cortex. Up-regulation of KCC2 was observed to reduce the motility of interneurons through the activation of GABA receptors and the diminution of the membrane potential, which resulted in reduced intracellular calcium.
5-HT (5-hydroxytryptamine; also known as serotonin) is another neurotransmitter that is thought to affect cortical interneuron migration (Vitalis et al., 2007; Riccio et al., 2009). Pharmacological treatments of rodent embryos, using the specific inhibitor of 5-HT synthesis, PCPA (dl-p-chlorophenylalanine), have revealed alterations in the cytoarchitecture of the cerebral cortex (Vitalis et al., 2007). Interestingly, 5-HT depletion resulted in altered incorporation of cortical interneurons to the CP and compromised the differentiation of interneuron subpopulations expressing CR and/or cholecystokinin (Vitalis et al., 2007). Recent work by Riccio et al. (2009) suggests that 5-HT, through the activation of 5-HT6 receptors (expressed by cortical interneurons), reduces the migration of interneurons in the developing brain.
Channelling cortical interneurons to their proper migratory paths
Interneurons en route to the cerebral cortex from the ganglionic eminence encounter the developing striatum and avoid entering into it. It is thought that repulsive cues expressed within the developing striatum create an exclusion zone for cortical interneurons and participate in channelling them into appropriate adjacent paths (Marin et al., 2001; Métin et al., 2006). The molecules involved in maintaining cortical interneurons away from the striatum are thought to be Sema3A and Sema3F, members of the family of class 3 semaphorins, which are key regulators of a number of processes in the developing nervous system (Neufeld and Kessler, 2008; Roth et al., 2009). Semaphorin signalling is mediated by Nrp and Plexin receptors (Neufeld and Kessler, 2008; Roth et al., 2009), and in vitro and in vivo studies have shown that cortical interneurons express Nrp1 and Nrp2 receptors and respond to the chemorepulsive activity of Sema3A and Sema3F expressed in the developing striatum (Marin et al., 2001). These authors also showed that loss of Nrp function leads to an increased number of interneurons within the striatum, suggesting that semaphorin signalling is crucial to maintaining the striatum clear of cortical interneurons, in addition to channeling these cells in the appropriate migratory paths (Marin et al., 2001). Recent analysis of postnatal Nrp2-deficient mice (Nrp2−/−) also showed a significant reduction of interneurons in the hippocampus compared with control animals (Gant et al., 2009).
CONCLUDING REMARKS
Cortical interneuron types generated in the subpallium arrive in the cortex after a long and tortuous journey, disperse in all areas and all cortical layers where they assemble with their pyramidal counterparts into functional neuronal circuits, and contribute to a precise balance of synaptic excitation and inhibition in the cortex. Disruption of this balance is thought to result in neuropathological conditions, especially epilepsy (Sloviter, 1987; Cobos et al., 2005; Kumar and Buckmaster, 2006). In addition to epilepsy, a number of other neurological disorders in humans have been attributed to abnormalities in cortical GABAergic function (Baulac et al., 2001; Harkin et al., 2002; Francis et al., 2006; Friocourt et al., 2006; Friocourt and Parnavelas, 2010). Thus understanding the molecular mechanisms that control the generation, specification and migration of interneurons, and the roles these cells play in cortical function, is of significant clinical relevance and therapeutic importance.
Footnotes
Our work was supported by the Wellcome Trust [Programme Grant number 074549 (to J.G.P.)].
REFERENCES
- Alcantara S, Pozas E, Ibañez CF, Soriano E. BDNF-modulated spatial organization of Cajal-Retzius and GABAergic neurons in the marginal zone plays a role in the development of cortical organization. Cereb Cortex. 2006;16:487–499. doi: 10.1093/cercor/bhi128. [DOI] [PubMed] [Google Scholar]
- Alifragis P, Liapi A, Parnavelas JG. Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J Neurosci. 2004;24:5643–5648. doi: 10.1523/JNEUROSCI.1245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Andrews W, Barber M, Parnavelas JG. Slit-Robo interactions during cortical development. J Anat. 2007;211:188–198. doi: 10.1111/j.1469-7580.2007.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews W, Barber M, Hernadez-Miranda LR, Xian J, Rakic S, Sundaresan V, Rabbitts TH, Pannell R, Rabbitts P, Thompson H, Erskine L, Murakami F, Parnavelas JG. The role of Sli-Robo signalling in the generation, migration and morphological differentiation of cortical interneurons. Dev Biol. 2008;313:648–658. doi: 10.1016/j.ydbio.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Ang ES, Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Arenas E, Akerud P, Wong V, Boylan C, Persson H, Lindsay RM, Altar CA. Effects of BDNF and NT-4/5 on striatonigral neuropeptides or nigral GABA neurons in vivo. Eur J Neurosci. 1996;8:1707–1717. doi: 10.1111/j.1460-9568.1996.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr Genet. 2007;17:55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Marín O, Plump AS. Slit prevents midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Barber M, Di Meglio T, Andrews WD, Hernández-Miranda LR, Murakami F, Chédotal A, Parnavelas JG. The role of Robo3 in the development of cortical interneurons. Cereb Cortex. 2009;19:22–31. doi: 10.1093/cercor/bhp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex. 2008;18:2306–2317. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme JF, Baulac M, Brice A, Bruzzone R, Le Guern E. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Dugich-Djordjevic MM, Li YX, Ma W, Somogyi R, Wen X, Brown E, Scott C, McKay RD, Barker JL. Neurotrophins stimulate chemotaxis of embryonic cortical neurons. Eur J Neurosci. 1997;9:2561–2570. doi: 10.1111/j.1460-9568.1997.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, O’Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J Neurosci. 1998;18:6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu QY, Colton CA, Barker JL. Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci. 1999;19:4449–4461. doi: 10.1523/JNEUROSCI.19-11-04449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res. 2009;315:611–618. doi: 10.1016/j.yexcr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- Brunstrom JE, Gray-Swain MR, Osborne PA, Pearlman AL. Neuronal heterotopias in the developing cerebral cortex produced by neurotrophin-4. Neuron. 1997;18:505–517. doi: 10.1016/s0896-6273(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Puelles L, Martinez S. The avian telencephalic subpallium originates inhibitory neurons that invade tangentially the pallium (dorsal ventricular ridge and cortical areas). Dev Biol. 2001;239:30–45. doi: 10.1006/dbio.2001.0422. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Cobos I, Long JE, Thwin MT, Rubenstein JL. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex. 2006;16:82–88. doi: 10.1093/cercor/bhk003. [DOI] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, Collombat P, Colasante G, Bianchi M, Long J, Mansouri A, Rubenstein JL, Broccoli V. Inactivation of Arx, the murine ortholog of the X-linked lissencephaly with ambiguous genitalia gene, leads to severe disorganization of the ventral telencephalon with impaired neuronal migration and differentiation. J Neurosci. 2007;27:4786–4798. doi: 10.1523/JNEUROSCI.0417-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Nery S, Fishell G. Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci. 2001:1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Sebastián-Serrano A, Kim S, Redondo JM, Walsh C, Nieto M. Cux-1 and Cux-2 control the development of Reelin expressing cortical interneurons. Dev Neurobiol. 2008;68:917–925. doi: 10.1002/dneu.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Cheng Q, Yeh HH. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb Cortex. 2006;16:1377–1388. doi: 10.1093/cercor/bhj084. [DOI] [PubMed] [Google Scholar]
- Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- Erbel-Sieler C, Dudley C, Zhou Y, Wu X, Estill SJ, Han T, Diaz-Arrastia R, Brunskill EW, Potter SS, McKnight SL. Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc Natl Acad Sci USA. 2004;101:13648–13653. doi: 10.1073/pnas.0405310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003a;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins and the neuromuscular system: 10 years of answers and questions. J Neurocytol. 2003b;32:619–647. doi: 10.1023/B:NEUR.0000020614.83883.be. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin M-R, Kwan KY, Chen J-G, Judas M, Hayashi M, Sestan N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex. 2009;19:2196–2207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G. Perspectives on the developmental origins of cortical interneuron diversity. In Cortical Development: Genes and Genetic Abnormalities. Novartis Found Symp. 2007;288:21–35. [PubMed] [Google Scholar]
- Fiumelli H, Kiraly M, Ambrus A, Magistretti PJ, Martin JL. Opposite regulation of calbindin and calretinin expression by brain-derived neurotrophic factor in cortical neurons. J Neurochem. 2000;74:1870–1877. doi: 10.1046/j.1471-4159.2000.0741870.x. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marín O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marín O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkouli A, Hearn C, Errington M, Cooke S, Grigoriou M, Bliss T, Stylianopoulou F, Pachnis V. Loss of forebrain cholinergic neurons and impairment in spatial learning and memory in LHX7-deficient mice. Eur J Neurosci. 2005;21:2923–2938. doi: 10.1111/j.1460-9568.2005.04141.x. [DOI] [PubMed] [Google Scholar]
- Francis F, Meyer G, Fallet C, Moreno S, Kappeler C, Socorro AC, Beldjord C, Chelly J. Human disorders of cortical development: from past to present. Eur J Neurosci. 2006;23:877–893. doi: 10.1111/j.1460-9568.2006.04649.x. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Black IB, Kaplan DR. Distribution of the neurotrophins brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the postnatal rat brain: an immunocytochemical study. Neuroscience. 1998;84:101–114. doi: 10.1016/s0306-4522(97)00526-5. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Parnavelas JG. Mutations in ARX result in several defects involving GABAergic neurons. Frontiers Cell Neurosci. 2010;4:4. doi: 10.3389/fncel.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friocourt G, Poirier K, Rakić S, Parnavelas JG, Chelly J. The role of ARX in cortical development. Eur J Neurosci. 2006;23:869–876. doi: 10.1111/j.1460-9568.2006.04629.x. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Kanatani S, Tabata H, Yozu M, Takahashi T, Antypa M, Raguénès O, Chelly J, Férec C, Nakajima K, Parnavelas JG. Cell-autonomous roles of ARX in cell proliferation and neuronal migration during corticogenesis. J Neurosci. 2008;28:5794–5805. doi: 10.1523/JNEUROSCI.1067-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M, Rallu M, McMahon AP, Fishell G. Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development. 2004;131:5031–5040. doi: 10.1242/dev.01349. [DOI] [PubMed] [Google Scholar]
- Fukumitsu H, Furukawa Y, Tsusaka M, Kinukawa H, Nitta A, Nomoto H, Mima T, Furukawa S. Simultaneous expression of brain-derived neurotrophic factor and neurotrophin-3 in Cajal-Retzius, subplate and ventricular progenitor cells during early development stages of the rat cerebral cortex. Neuroscience. 1998;84:115–127. doi: 10.1016/s0306-4522(97)00505-8. [DOI] [PubMed] [Google Scholar]
- Gant JC, Thibault O, Blalock EM, Yang J, Bachstetter A, Kotick J, Schauwecker PE, Hauser KF, Smith GM, Mervis R, Li Y, Barnes GN. Decreased number of interneurons and increased seizures in neuropilin 2 deficient mice: implications for autism and epilepsy. Epilepsia. 2009;50:629–645. doi: 10.1111/j.1528-1167.2008.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nóbrega-Pereira S, Pierani A, Kessaris N, Marín O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, Eisenstat DD, Lai C, Anton ES. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci USA. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex and organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- Gulacsi A, Anderson SA. Shh maintains Nkx2.1 in the MGE by a Gli3-independent mechanism. Cereb Cortex. 2006;16(Suppl 1):i89–i95. doi: 10.1093/cercor/bhk018. [DOI] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S. Truncation of the GABAA-receptor γ2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng JI, Moonen G, Nguyen L. Neurotransmitters regulate cell migration in the telencephalon. Eur J Neurosci. 2007;26:537–546. doi: 10.1111/j.1460-9568.2007.05694.x. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li HM, Witte DP, Szucsik JC, Weinstein M, Li H, Potter SS. Gsh-2, a murine homeobox gene expressed in the developing brain. Mech Dev. 1995;50:177–186. doi: 10.1016/0925-4773(94)00334-j. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci USA. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Koch M, Needham LK, Maness P, Schachner M. Impairment of sensorimotor gating in mice deficient in the cell adhesion molecule L1 or its close homologue, CHL1. Brain Res. 2004;1029:131–144. doi: 10.1016/j.brainres.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Jones KR, Fariñas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1993;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionary conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Klein R, Martin-Zanca D, Barbacid M, Parada LF. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990;109:845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, Luster AD. SDF-1α induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006;26:4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Casanova P, Tham TN, De Clercq E, Arenzana-Seisdedos F, Baleux F, Dubois-Dalcq M. Differential signaling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–125. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Li G, Adesnik H, Li J, Long J, Nicoll RA, Rubenstein JL, Pleasure SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liapi A, Pritchett J, Jones O, Fujii N, Parnavelas JG, Nadarajah B. Stromal-derived factor 1 signalling regulates radial and tangential migration in the developing cerebral cortex. Dev Neurosci. 2008;30:117–131. doi: 10.1159/000109857. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb Cortex. 2009;19(Suppl 1):i96–i106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Luján R, Shigemoto R, Ganter P, Paulsen O, Molnár Z. Blockade of GABAB receptors alters the tangential migration of cortical neurons. Cereb Cortex. 2003;13:932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Sánchez-Alcañiz JA, Pla R, Borrell V, Picó E, Valdeolmillos M, Marín O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de Nó R. La corteza cerebral del raton. Trab Lab Invest Biol (Madrid) 1922;20:41–78. [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Manent JB, Jorquera I, Ben-Ari Y, Aniksztejn L, Represa A. Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. J Neurosci. 2006;26:5901–5909. doi: 10.1523/JNEUROSCI.1033-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chédotal A. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol. 2002;442:130–155. doi: 10.1002/cne.10068. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin/neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Marin O, Plump AS, Flames N, Sánchez-Camacho C, Tessier-Lavigne M, Rubenstein JL. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development. 2003;130:1889–1901. doi: 10.1242/dev.00417. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Métin C, Denizot JP, Ropert N. Intermediate zone cells express calcium-permeable AMPA receptors and establish close contact with growing axons. J Neurosci. 2000;20:696–708. doi: 10.1523/JNEUROSCI.20-02-00696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métin C, Baudoin JP, Rakić S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur J Neurosci. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- Michishita M, Ikeda T, Nakashiba T, Ogawa M, Tashiro K, Honjo T, Doi K, Itohara S, Endo S. A novel gene, Btcl1, encoding CUB and LDLa domains is expressed in restricted areas of mouse brain. Biochem Biophys Res Commun. 2003;306:680–686. doi: 10.1016/s0006-291x(03)01035-0. [DOI] [PubMed] [Google Scholar]
- Mione M, Baldessari D, Deflorian L, Nappo G, Santoriello C. How neuronal migration contributes to the morphogenesis of the CNS: insights from the zebrafish. Dev Neurosci. 2008;30:65–81. doi: 10.1159/000109853. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morante-Oria J, Carleton A, Ortino B, Kremer EJ, Fairén A, Lledo PM. Subpallial origin of a population of projecting pioneer neurons during corticogenesis. Proc Natl Acad Sci USA. 2003;100:12468–12473. doi: 10.1073/pnas.1633692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci. 2002;5:218–224. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- Nomura T, Holmberg J, Frisen J, Osumi N. Pax6-dependent boundary defines alignment of migrating olfactory cortex neurons via the repulsive activity of ephrin A5. Development. 2006;133:1335–1345. doi: 10.1242/dev.02290. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–4386. doi: 10.1242/dev.129.19.4371. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG. The origin and migration of cortical neurones: new vistas. Trends Neurosci. 2000;23:126–131. doi: 10.1016/s0166-2236(00)01553-8. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Dinopoulos A, Davies SW. The central visual pathways. In: Björklund A, Hökfelt H, Swanson LW, editors. In Handbook of Chemical Neuroanatomy, Vol. 7: Integrated Systems of the CNS, Part II. Elsevier: 1989. pp. 1–164. [Google Scholar]
- Pillai-Nair N, Panicker AK, Rodriguiz RM, Gilmore KL, Demyanenko GP, Huang JZ, Wetsel WC, Maness PF. Neural cell adhesion molecule-secreting transgenic mice display abnormalities in GABAergic interneurons and alterations in behavior. J Neurosci. 2005;25:4659–4671. doi: 10.1523/JNEUROSCI.0565-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Poluch S, Drian MJ, Durand M, Astier C, Benyamin Y, König N. AMPA receptor activation leads to neurite retraction in tangentially migrating neurons in the intermediate zone of the embryonic rat neocortex. J Neurosci Res. 2001;63:35–44. doi: 10.1002/1097-4547(20010101)63:1<35::AID-JNR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Pozas E, Ibañez CF. GDNF and GFRα1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–713. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cerb Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Paris: Maloine; 1911. Histologie du Systeme Nerveux de l’Homme et des Vertebres, Vol. 2. [Google Scholar]
- Riccio O, Potter G, Walzer C, Vallet P, Szabó G, Vutskits L, Kiss JZ, Dayer AG. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry. 2009;14:280–290. doi: 10.1038/mp.2008.89. [DOI] [PubMed] [Google Scholar]
- Ross ME, Walsh CA. Human brain malformations and their lessons for neuronal migration. Annu Rev Neurosci. 2001;24:1041–1070. doi: 10.1146/annurev.neuro.24.1.1041. [DOI] [PubMed] [Google Scholar]
- Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago A, Erickson CA. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Soria JM, Valdeolmillos M. Receptor-activated calcium signals in tangentially migrating cortical cells. Cereb Cortex. 2002;12:831–839. doi: 10.1093/cercor/12.8.831. [DOI] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–i10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella MC, Comoglio PM. HGF: a multifunctional growth factor controlling cell scattering. Int J Biochem Cell Biol. 1999;31:1357–1362. doi: 10.1016/s1357-2725(99)00089-8. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer T, Puelles L, Ekker M, Rubenstein JL. Expression from a Dlx gene enhancer marks adult mouse cortical GABAergic neurons. Cereb Cortex. 2002;12:75–85. doi: 10.1093/cercor/12.1.75. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Höllt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Schulz S, Kohtz JD, Höllt V. Patterns of SDF-1α and SDF-1γ mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. J Comp Neurol. 2007;502:382–399. doi: 10.1002/cne.21336. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17:8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka DH, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development. 2006;133:2167–2176. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Yanagida M, Zhu Y, Mikami S, Nagasawa T, Miyazaki J, Yanagawa Y, Obata K, Murakami F. Random walk behavior of migrating cortical interneurons in the marginal zone: time-lapse analysis in flat-mount cortex. J Neurosci. 2009;29:1300–1311. doi: 10.1523/JNEUROSCI.5446-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- Tham TN, Lazarini F, Franceschini IA, Lachapelle F, Amara A, Dubois-Dalcq M. Developmental pattern of expression of the alpha chemokine stromal cell-derived factor 1 in the rat central nervous system. Eur J Neurosci. 2001;13:845–856. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Belluardo N, Metsis M, Perrson H. Widespread and developmentally regulated expression of NT-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J, König N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuorto F, Alifragis P, Failla V, Parnavelas JG, Gulisano M. Tangential migration of cells from the basal to the dorsal telencephalic regions in the chick. Eur J Neurosci. 2003;18:3388–3393. doi: 10.1111/j.1460-9568.2003.03059.x. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci. 2007;26:331–344. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E. Regulation of cortical dendritic development by Slit-Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Alvarez-Dolado M, Erskine L, Alvarez-Buylla A. Permissive corridor and diffusible gradients direct medial ganglionic eminence cell migration to the neocortex. Proc Natl Acad Sci USA. 2003;100:727–732. doi: 10.1073/pnas.242721899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders C, Anderson SA. Cortical interneurons and their origins. Neuroscientist. 2005;11:199–205. doi: 10.1177/1073858404270968. [DOI] [PubMed] [Google Scholar]
- Wonders C, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1–lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li X, Zhou M. Neuregulin-1/ErbB signaling: a druggable target for treating heart failure. Curr Opin Pharmacol. 2009;9:214–219. doi: 10.1016/j.coph.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Gashghaei HT, Han C, Watson H, Campbell KJ, Anton ES. Radial glial dependent and independent dynamics of interneuronal migration in the developing cerebral cortex. PLoS One. 2007;2:e794. doi: 10.1371/journal.pone.0000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25:7268–7277. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;21:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- Yun K, Fischman S, Johnson J, Hrabe de Angelis M, Weinmaster G, Rubenstein JL. Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development. 2002;129:5029–5040. doi: 10.1242/dev.129.21.5029. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Marín O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JL, Westphal H. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci USA. 2003;100:9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Li H, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Kästner B, Weth F, Bolz J. Multiple effects of ephrin-A5 on cortical neurons are mediated by SRC family kinases. J Neurosci. 2007;27:5643–5653. doi: 10.1523/JNEUROSCI.0954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Garcez P, Rudolph J, Niehage R, Weth F, Lent R, Bolz J. Ephrin-A5 acts as a repulsive cue for migrating cortical interneurons. Eur J Neurosci. 2008;28:62–73. doi: 10.1111/j.1460-9568.2008.06320.x. [DOI] [PubMed] [Google Scholar]