Abstract
Cholera toxin (CT) travels from the plasma membrane of intestinal cells to the endoplasmic reticulum (ER) where a portion of the A-subunit, the A1 chain, crosses the membrane into the cytosol to cause disease. A related toxin, LTIIb, binds to intestinal cells but does not cause toxicity. Here, we show that the B-subunit of CT serves as a carrier for the A-subunit to the ER where disassembly occurs. The B-subunit binds to gangliosides in lipid rafts and travels with the ganglioside to the ER. In many cells, LTIIb follows a similar pathway, but in human intestinal cells it binds to a ganglioside that fails to associate with lipid rafts and it is sorted away from the retrograde pathway to the ER. Our results explain why LTIIb does not cause disease in humans and suggest that gangliosides with high affinity for lipid rafts may provide a general vehicle for the transport of toxins to the ER.
INTRODUCTION
Most toxins must cross a cellular membrane into the cytosol to cause disease. One strategy, used by cholera toxin (CT), ricin, Shiga toxin, and others, is to cross the endoplasmic reticulum (ER) membrane, hijacking the cellular machinery that allows misfolded proteins to cross the ER membrane for degradation in the cytosol (Hazes and Read, 1997). Obviously, these toxins must first move from the plasma membrane to the ER, a process that is the reversal of the normal secretory pathway. Although retrograde transport from the Golgi back to the ER can occur for some proteins, cellular proteins do not go back from the plasma membrane to the ER. For example, plasma membrane glycoproteins are internalized and their oligosaccharide moiety is modified by trans-Golgi enzymes, but not by cis-Golgi enzymes, much less by ER enzymes (Volz et al., 1995; Porwoll et al., 1998). How then are the toxins able to travel all the way from the plasma membrane to the ER? Here, we have addressed this question, using as a model CT and the related Escherichia coli toxin LTIIb.
CT consists of noncovalently associated A- and B-subunits. Five identical polypeptides assemble into a pentameric ring to form the B-subunit that binds to a membrane lipid ganglioside GM1 at the cell surface. The A-subunit is cleaved into A1- and A2 chains, which are linked by a disulfide bond and extensive noncovalent interactions (Sixma et al., 1993; Zhang et al., 1995). The A2 chain protrudes with its C terminus through the central pore in the B-ring and has a KDEL motif facing the membrane. The A1 chain enters the cytosol and causes disease. It ADP-ribosylates a trimeric G protein to activate adenylyl cyclase, which induces intestinal chloride secretion that causes the massive secretory diarrhea seen in cholera.
The toxin LTIIb is highly related to CT. Its two subunits are structurally and functionally similar, the major difference being that the B-subunit binds to the ganglioside GD1a (Fukuta et al., 1988; van den Akker et al., 1996). In many cells CT and LTIIb elicit the same activation of adenyl cyclase (Lee et al., 1991). However, in human intestinal cells LTIIb is not active, even though it can bind to GD1a on the cell surface (Wolf et al., 1998). The reason for the lack of activity of LTIIb in human intestinal cells is unclear.
The best evidence that the A1 chain of the toxins crosses the ER membrane comes from in vitro experiments with CT. The holotoxin is disassembled and the A1 chain is unfolded by the ER chaperone protein disulfide isomerase (PDI). PDI binds in its reduced form to the A1 chain, targets the chain to the lumenal side of the ER membrane, and releases it upon oxidation by the enzyme Ero1 (Tsai et al., 2001; Rodighiero et al., 2002; Tsai and Rapoport, 2002). The A1 chain may then be transported through the Sec61 channel into the cytosol (Schmitz et al., 2000; Teter et al., 2002). In vivo, brefeldin A blocks toxin action, but the drug affects several pathways and not only Golgi-to-ER transport (Lencer et al., 1993; Orlandi et al., 1993).
Cholera toxin starts its journey into the cell by binding to the GM1 ganglioside in the plasma membrane. CT is associated with detergent-insoluble membrane microdomains, termed lipid rafts. Depletion of cholesterol, which disrupts rafts, inhibits toxin action (Orlandi and Fishman, 1998; Wolf et al., 1998, 2002), but the precise role of gangliosides or lipid rafts has not yet been determined. Endocytosis can occur by both clathrin-dependent and -independent mechanisms (Parton, 1994; Nichols et al., 2001; Nichols, 2003), but it is unclear whether both pathways lead to a functional response. CT can be found in early and recycling endosomes as well as the Golgi apparatus (Nichols et al., 2001; Richards et al., 2002). Trafficking through the Golgi apparatus may be a necessary step in the induction of toxicity (Richards et al., 2002).
How the toxin travels to the ER is unclear. In the current model, the A-subunit is key. The KDEL sequence at its C terminus is thought to mediate the transport of the toxin from the Golgi to the ER. According to this model, the A- and B-subunits would travel together from the plasma membrane to the Golgi apparatus, where they dissociate. The A-subunit and A1 and A2 chains together would then continue its journey on its own by association with the KDEL-receptor ERD2 that normally retrieves ER proteins from the Golgi. The released B-subunit would recycle from the Golgi into late endosomes and lysosomes (Bastiaens et al., 1996; Majoul et al., 1996, 1998). How in this model the A-subunit, including the A2 chain, would be released from the pore of the B-ring is difficult to understand. Furthermore, inactivating mutations in the KDEL motif of CT do not ablate toxin function (Lencer et al., 1995).
In an alternative model, the B-subunit would be the transport vehicle for the A-subunit. In this case, however, an explanation is needed for how the B-subunit has the ability to go back into the ER. One possibility is that the gangliosides in lipid rafts to which it binds at the plasma membrane carry it back to the ER; there may be an endogenous retrograde transport pathway for gangliosides that is hijacked by the toxin. Such a model has been proposed for Shiga toxin that also can bind a ceramide glycolipid in lipid rafts (Falguieres et al., 2001). However, the experiments are not conclusive because a KDEL sequence was appended to the toxin and the KDEL receptor may have been responsible for transport to the ER and for binding to ER membranes. Here, we show that the B-subunit of CT is indeed the transport vehicle for the toxin. It travels on gangliosides that associate with lipid rafts all the way to the ER. In most cells, the toxin LTIIb uses a very similar pathway, but in human intestinal cells, it binds a ganglioside that does not associate with lipid rafts, explaining why it cannot reach the ER and cause diarrheal disease.
EXPERIMENTAL PROCEDURES
Materials
Antibodies directed against CT-A and B-subunit and LTIIb were described previously (Wolf et al., 1998). Recombinant LTIIb and CT were prepared and purified as described previously (Rodighiero et al., 2002). GM1 and GD1a were obtained from Matreya (Pleasant Gap, PA).
Cell Culture, Electrophysiology, Toxin Binding and Endocytosis, and Cell Rounding Assays
Cell culture, electrophysiology, toxin binding, and cAMP-induced cell rounding assays were as described previously (Wolf et al., 1998; Richards et al., 2002; Wolf et al., 2002).
Preparation of Variant CT and LTIIb
A clone, pYF4, was constructed that produced a CT B-subunit variant containing a C-terminal extension, SSSGGGGSSHPNNTSNNTSSAEDYEYPS. Sequences encoding four partly overlapping N-glycosylation concensus motifs (Gavel and von Heijne, 1990; in bold) and for tyrosine sulfation (Leitinger et al., 1994; underlined) followed by a termination codon were introduced at 3′ end of the CTB gene by cloning a SacI-BglII digested oligonucleotide linker into SacI-BamHI cut pCTB53, producing pYF4. Plasmid pCTB53 was made by cloning a 0.6-kb EcoRI fragment, made by polymerase chain reaction (PCR) with pLMP1 as template (Jobling et al., 1997), into pUC18 (Yanisch-Perron et al., 1985). This added an EcoRI site overlapping the terminal asn103 codon and removed the termination codon. Plasmid pYF4 encoding the C-terminally extended CTB was transformed into E. coli XLI-blue, to produce CTB-GS homopentamer, or into E. coli TX1 harboring the wild-tyle (wt) CTA-subunit expressing vector pLMP3 (Jobling et al., 1997) to produce CT-GS holotoxin as described previously (Wolf et al., 1998). Single point mutation L241V in the A-subunit and denoted CT(KDEV) or CT(KDEV)-GS were prepared by site-directed mutagenesis by using pLMP3 as a template and recombined with the corresponding wild-type or GS-variant B-subunits, respectively.
To prepare LTIIb-GS, the LTIIb A- and B-subunit genes were PCR amplified from wt LTIIb expressing clone pMGJ176 by using primers LTIIbF (5′-gatctgtatcaaccttcttttg-3′) and LTIIbR (5′-caaagaatagagctcgattctgcctc-3′, containing a SacI site). Plasmid pMGJ176 was constructed by cloning a ClaI-BamHI fragment from pTC100 (van den Akker et al., 1996) into AccI-BamHI cut pUC19 (Yanisch-Perron et al., 1985). The PCR product was digested with SacI and ligated to an EcoRV-SacI fragment of pYF4 that encoded only the tag domain indicated above. The resulting plasmid, pYF5, was transformed into E. coli TX1. LTIIb-GS and CT-GS holotoxins were prepared in periplasmic extracts. The coding regions of toxin genes were verified by dideoxy sequencing.
Retrograde Golgi and ER Transport Assay
T84 monolayers or Vero cells were incubated with sulfate-free Hanks' balanced salt solution (HBSS) at 37°C for 1.5 h before transfer to 0.5 mCi/ml Na235SO4 in the same buffer for 30 min. CT-GS, LTII-GS, or CTB-GS (20 nM final concentration) was added and incubated for 50 min to 3 h. For pulse-chase studies, cells were further incubated in a DMEM/Ham's F12. At the indicated times, cells were washed with ice-cold HBSS and lysed in SDS-containing buffer, and toxins were immunoprecipitated using polyclonal anti-B-subunit antibody as described previously (Lencer et al., 1995). Samples were treated or not treated with N-glycanase (PNGaseF) or endoglycosidase H (EndoH) as indicated and analyzed by SDS-PAGE and phosphorimaging (Amersham Biosciences, Piscataway, NJ) or by immunoblot. All images were processed for publication using Adobe Photoshop.
Double Affinity Precipitation of N-glycosylated B-Subunit
CT-GS or recombinant wt CT (12 μg) was incubated with 6 μg of trypsin at 37°C for 30 min followed by 600 μg of soybean trypsin inhibitor. Vero cells were then incubated with trypsin treated or not treated toxin (10 nM) in HBSS at 4°C for 45 min. After washing, cells were incubated at 37°C for 5 h in fresh HBSS. After washing again, cells were lysed in 1 ml of TN lysis buffer containing 1% Triton X-100, 1.75% n-octyl-β-d-glucopyranoside, 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.2% gelatin, 0.1% sodium azide, and protease inhibitors cocktail (Complete, EDTA-free; Roche Diagnostics, Mannheim, Germany) and clarified by centrifugation before incubation with concanavalin A (Con A)-Sepharose 4B (Amersham Biosciences) in a 1:1 mix solution of TN lysis buffer and Con A binding buffer (10 mM Tris-HCl, pH 7.4, 6 mM CaCl2, 6 mM MnCl2, 6 mM MgCl2, 0.5M NaCl, and 0.2% gelatin), and protease inhibitors cocktail at 4°C for 1 h. Beads were washed with washing buffer (1% Triton X-100, 1.75% n-octyl-β-d-glucopyranoside, 10 mM Tris-HCl, pH 7.4, 0.1% azide, 1 mM CaCl2, 1 mM MnCl2, 1 mM MgCl2, 0.5 M NaCl, and protease inhibitors cocktail), and bound substrates were eluted with 0.2 M d-methylmannoside. The eluate was incubated with GM1-coupled polystyrene beads (Tsai et al., 2001) overnight at 4°C. After washing with washing buffer (containing only 0.15 M NaCl), N-glycosylated CTB-subunits were released by boiling in sample buffer and analyzed by SDS-PAGE and immunoblot.
Sucrose Equilibrium Density Centrifugation and Binding Assay
Fractionation of toxins with lipid rafts were as described previously (Wolf et al., 1998). Binding of CT to isolated dog pancreatic membranes was assessed in a 10-μl reaction volume, by using 15 μg of ER proteoliposomes or membranes (Gorlick and Rapoport, 1993), or bovine serum albumin incubated with 10 ng of CTB (Calbiochem, San Diego, CA) for 30 min at 30°C. In some experiments, microsomes were incubated with puromycin (1.5 mM) in 350 mM KOAc (acetate), 250 mM sucrose, 2 mM Mg(OAc)2, 50 mM HEPES, pH 7.4, before the addition of CTB. The reaction volume was adjusted to 60% sucrose by adding 20 μl of an 85.5% sucrose solution and mixing thoroughly. This was placed in the bottom of a Beckman centrifuge tube (7 × 20 mm) and overlaid with 34 μl of a 45% sucrose solution, 68 μl of a 35% sucrose solution, and 68 μl of a 25% sucrose solution. The samples were centrifuged in a Beckman TLA 100 rotor at 100,000 rpm for 60 min. Then 20-μl aliquots were removed sequentially from the top of the centrifuge tube and analyzed by SDS-PAGE with an anti-CTB.
Digitonin Permeabilization of Microsomes
Microsomes were isolated from Vero cells pretreated at 37°C with CT-GS for 3 h, washed, and resuspended in 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, and 2 mM MgCl2, containing protease inhibitors. Membrane and soluble fractions of the microsomal preparation were separated by centrifugation at 100,000 × g and analyzed by SDS-PAGE and immunoblot for CT B-subunit and for PDI. Where indicated, microsomes were permeabilized by 0.2% digitonin before analysis.
RESULTS
Transport of the CT B-Subunit from the Plasma Membrane into the Golgi and ER
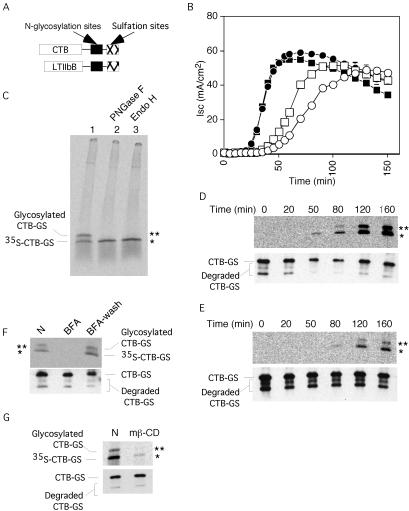
To test whether the CT B-subunit is transported from the plasma membrane to the ER, we prepared a mutant with consensus motifs for tyrosine sulfation and N-glycosylation (Figure 1A) (Gavel and von Heijne, 1990; Bundgaard et al., 1997; Rapak et al., 1997). Modification by sulfation, detected by incorporation of radioactive sulfate, indicates arrival of the B-subunit in the Golgi, whereas N-glycosylation, detected by N-glycanase–sensitive increase in molecular mass, indicates transport to the ER. Initial studies showed that the B-subunit mutant correctly assembled with the CT A-subunit to form a functional holotoxin (CT-GS). When applied to basolateral membranes of polarized human intestinal epithelial T84 cells, CT-GS induced a cAMP-dependent Cl– secretory response identical to that of wt CT (Figure 1B, compare wt CT, filled squares, with CT-GS, filled circles). When applied to the apical cell surface, a slight delay in toxin action, compared with wt CT, was observed (Figure 1B, compare wt CT, open squares, with CT-GS, open circles).
Figure 1.
Transport of CT B-subunit into the Golgi and the ER. (A) N-Glycosylation and tyrosine sulfation sites added to the C terminals of CTB-subunit and LTIIbB-subunit. (B) Time course of Cl– secretion measured as short circuit current (Isc) induced by 20 nM wt CT (squares) or CT-GS (circles) after application at 0 min to basolateral (filled symbols) or apical membranes (open symbols) of T84 monolayers. Representative of three independent experiments. (C) Phosphorimage showing sulfation (marked by *) and N-glycosylation (marked by **) of CT-GS B-subunit 3 h after apical and basolateral application to T84 cells. Immunoprecipitates were divided into three equal samples and treated with PNGaseF (lane 2), EndoH (lane 3), or mock treated (lane 1) before analysis by SDS-PAGE. Representative of two independent experiments. (D and E) Top, phosphorimages showing time course of 35S sulfation (*) and N-glycosylation (**) of B-subunit of CT-GS applied at 0 min to basolateral (D) or apical membranes (E) of T84 cells. Bottom, immunoblot for CT B-subunit to show equal loading for each lane. (F) Phosphorimage (top) showing effect of 10 μM BFA on sulfation and N-glycosylation of CT-GS B-subunit 3 h after application to T84 cells. T84 cells were treated (lane BFA) or not treated with BFA (lane N), or treated with BFA for 2 h, washed to remove BFA, and incubated again for an additional 3 h (lane BFA-wash). Bottom, immunoblot for CT B-subunit. (G) Phosphorimage (top) showing effect of methyl-β-cyclodextrin on sulfation and N-glycosylation of CT-GS B-subunit 3 h after application to T84 monolayers (lane mβ-CD) or mock-treated control (lane N). Bottom, immunoblot for CT B-subunit. Representative of two independent experiments.
Next, we added the CT-GS B-subunit to T84 cells, incubated them with Na235SO4, immunoprecipitated the toxin, and analyzed the radiolabeled material by SDS-PAGE and autoradiography (Figure 1C, lane 1). Within 3 h of incubation, two radiolabeled bands of apparent 17 and 21 kDa were observed. The lower band represents sulfated, but nonglycosylated B-subunit. The upper band is N-glycosylated, as shown by its sensitivity to N-glycanase that cleaves all N-linked oligosaccharides (Figure 1C, lane 2) and to endoglycosidase H that cleaves only the ER form of high mannose containing N-linked oligosaccharides (Figure 1C, lanes 3). These data demonstrate that the B-subunit travels from the cell surface to the Golgi and that a significant fraction (∼50%) of the Golgi-modified protein moves to the ER and becomes N-glycosylated.
We next determined the time course of toxin transport to test whether it corresponds to the time course of toxin function. When toxin was applied to the basolateral surface of T84 cells, Golgi-modified material occurred after 50 min and increased thereafter (Figure 1D, bottom, shows a loading control). ER-modified material occurred with a further delay of ∼30 min, as expected for a Golgi-to-ER pathway. The rate of transport from the cell surface to the ER was only slightly delayed compared with the appearance of toxin-induced chloride secretion (Isc; Figure 1B, filled circles). A similar correlation between ER transport and toxin function was seen when the toxin was applied to the apical cell surface (Figure 1E). The slightly slower transport compared with basolateral application of the toxin is consistent with the difference seen in the functional assay (Figure 1B).
Because brefeldin A (BFA) is known to inhibit toxin-induced chloride secretion (Lencer et al., 1993), we tested its effect on toxin transport. Interestingly, addition of BFA blocked even the appearance of sulfated B-subunit (Figure 1F, lane 2), suggesting that the drug affects plasma membrane-to-trans-Golgi transport in addition to its well-characterized effect on Golgi-to-ER transport. The effect was fully reversible (lane 3). These data are consistent with the inhibitory effects of BFA on endosome to trans-Golgi transport of CT in Vero cells (Richards et al., 2002) and BSC1 cells (Massol, Lencer, and Kirchhausen, unpublished data). In this experiment, however, we cannot exclude the possibility that BFA may inhibit the sulfotransferase as well as delivery of the toxin to the Golgi and ER. Cholesterol depletion by methyl-β-cyclodextrin also inhibits toxin function in T84 and other cells (Orlandi and Fishman, 1998; Shogomori and Futerman, 2001b; Wolf et al., 2002) and correspondingly reduced toxin transport into the Golgi and ER by fivefold (Figure 1G). Sulfation of endogenous proteins in the same cells was reduced by less than twofold (40–45% reduction, n = 2). The reduction in toxin transport caused by cholesterol depletion is consistent with the loss of toxicity seen in the functional assay (Wolf et al., 2002). Together, these data show that the B-subunit travels from the plasma membrane through the Golgi to the ER and that this correlates with toxin function.
Disassembly of the Holotoxin Occurs in the ER
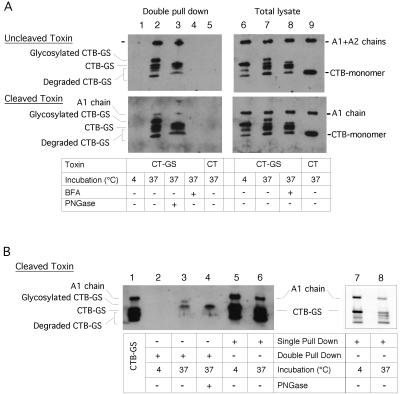
We tested whether the A- and B-subunits remain associated until entry into the ER. For these experiments, we used monkey kidney Vero cells that internalize a large amount of toxin. A CT-GS toxin preparation was used in which the A-subunit was not proteolytically cleaved into A1 and A2 chains; the uncleaved toxin is not recognized by PDI in vitro (Tsai et al., 2001) and should remain assembled after arrival in the ER. To isolate the ER-modified fraction of the B-subunit, we used concanavalin beads to capture glycosylated proteins, followed by purification of the B-subunit with GM1-linked beads and immunoblotting for the A- and B-subunits. A significant percentage of the A-subunit was coprecipitated with the glycosylated B-subunit (Figure 2A, top, lanes 2 and 3). In addition, the ratio of A to B chain in the glycosylated fraction was identical to that in the total cell lysate (lanes 7 and 8), indicating that there was little disassembly of the holotoxin. As expected from the isolation procedure, the majority of the glycosylated B-subunit was sensitive to N-glycanase (lane 3). Controls show that no toxin chains were recovered when the cells were incubated at low temperature or with brefeldin A to inhibit toxin transport (lanes 1 and 4). In addition, no toxin chains were precipitated when native toxin, lacking the sulfation and glycosylation sites were used (lane 5). These results show that the toxin moves into the ER as a fully assembled protein complex.
Figure 2.
Disassembly of the holotoxin occurs in the ER. (A) Immunoblot for A- and B-subunits after double affinity precipitation (by using concanavalin A then GM1-linked beads) for the N-glycosylated B-subunit or total cell lysate from Vero cells treated with CT-GS at 4°C (lanes 1 and 6), at 37°C for 5 h in buffer alone (lanes 2, 3, and 7) or at 37°C with BFA to inhibit retrograde transport (lanes 4 and 8). Lanes 5 and 9 show results for cells treated with wt CT that lacks the N-glycosylation motif. Lane 3 shows same sample as in lane 2 treated with PNGaseF. Both uncleaved (top two panels) and cleaved CT-GS (bottom two panels) were used. Both unglycosylated and degraded forms of CTB-GS monomers were pulled down in the pentameric B-subunit together with full-length glycosylated CT-GS monomers. Representative of two independent experiments. (B) Immunoblot for A- and B-subunits after double affinity precipitation as in A (lanes 2–4) or single precipitation by GM1-beads alone (lanes 5 and 6) as described in A. Lane 4 shows same sample as in lane 3 treated with PNGaseF. Lane 1 shows purified CT-GS as control. Lanes 7 and 8 show repeat immunoblot by using less material of the same samples shown in lanes 5 and 6. Representative of three independent experiments.
To test for disassembly of the holotoxin, we used a CT-GS toxin preparation in which the A-subunit was proteolytically cleaved into A1 and A2 chains, which allows interaction of the A1 chain with PDI in the ER. In this case, the ER-modified form of the B chain only brought down a very small fraction of the A1 chain (Figure 2A, bottom, lanes 2 and 3). The ratio of the A1 to B chain in the glycosylated fraction was much less than that found in the total cell lysate (Figure 2A, bottom, compare lane 2 with lane 7), indicating that the ER form of the B-subunit is not associated with the A1 chain. In contrast, the nonglycosylated fraction of the B-subunit was associated with the A1 chain (Figure 2B, lanes 5 and 6). The ratio of A1 chain to B chain in the nonglycosylated fraction was similar to that of the purified holotoxin (Figure 2B, compare lanes 6 and 1 and lanes 8 and 7). Thus, the A1 and B chains dissociate in the ER, in agreement with in vitro experiments demonstrating disassembly and unfolding of the toxin by PDI (Tsai et al., 2001).
The A Chain Is Not Required for Transport to the ER
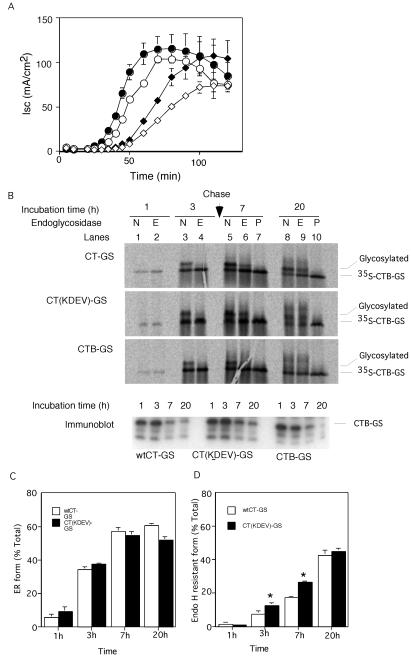
One explanation for how the toxin has the ability to go back from plasma membrane to ER predicts that the A chain is required and that the KDEL sequence plays an important role. To test this idea, we prepared a variant of CT-GS lacking the KDEL-motif [CT(KDEV)-GS]. When added to either the basolateral or apical cell surface, the mutant toxin showed a delayed induction of chloride secretion compared with the wild-type protein (Figure 3A, compare filled and open symbols). To study transport, the cells were incubated with the toxin mutant and 35S sulfate for 3 h and then for an additional 4 or 17 h with unlabeled sulfate. After the pulse period, sulfation and glycosylation of the mutant was equal to that of the wild-type protein, and the majority of the modified material was sensitive to endoglycosidase H (Figure 3B, top and middle, lanes 3 and 4; quantification shown in C). Thus, a functional ER retrieval signal in the A chain is not required for transport to the ER. After chase incubation, a large fraction of both wild-type and mutant toxin became resistant to endoglycosidase H digestion (lanes 6 and 9), indicating that the B-subunit can return from the ER to the Golgi. The KDEL mutant reproducibly had a higher level of resistance to endoglycosidase H (see quantification in D). The mutant may cycle less efficiently between the ER and cis-Golgi than the wild-type protein, which may explain the delay observed in the functional assay (Figure 3A).
Figure 3.
The A chain is not required for transport to the ER. (A) Time course of Isc induced by 20 nM wt CT-GS (filled symbols) or the KDEL-mutant CT(KDEV)-GS (open symbols) applied at 0 min to apical (diamonds) or basolateral membranes (circles) of polarized T84 monolayers, mean ± SD, n = 3. (B) T84 cells were loaded with Na235SO4 and exposed to 20 nM CT-GS (top), CT (KDEV)-GS (middle), or B-subunit only CTB-GS (bottom) for 3 h. At 3 h, cells were chased (arrow) with fresh medium. Sulfation and glycosylation were analyzed by SDS-PAGE and autoradiography at the indicated times of incubation. Samples were treated with EndoH (lanes 2, 4, 6, and 9, labeled E), PNGaseF (lanes 7 and 10, labeled P), or mock treated (lanes 1, 3, 5, and 8, labeled N) before analysis. Parallel samples were analyzed by immunoblot for CT B-subunit (bottom, labeled immunoblot). The total cellular content of B chains decreases in the chase period. Representative of three independent experiments. (C and D) Quantification of the N-glycosylated and EndoH-resistant forms of CTB-GS measured as percentage of total sulfated toxin as described in B [wt CT-GS, open bars, and CT(KDEV)-GS mutant, filled bars; mean ± SE, n = 3, *p < 0.05].
Because the KDEL sequence was not required for transport to the ER, we tested whether the A-subunit was needed at all. Indeed, a preparation containing only the B-subunit (CTB-GS) was transported from the plasma membrane via the Golgi to the ER as efficiently as the holotoxin (Figure 3B, compare top and bottom). Thus, transport of the toxin to the ER requires only the B-subunit.
The B Subunit Is Bound to GM1 in the ER
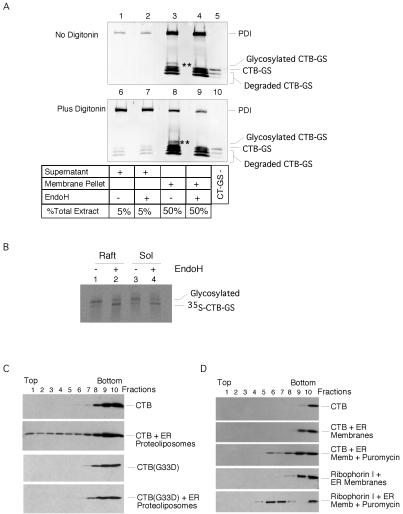
If GM1 provides the vehicle for transport of toxin to the ER, one would expect the B-subunit to remain membrane associated after entry into the ER. To test this possibility, we isolated microsomal membranes from Vero cells incubated with toxin. These were permeabilized with a low concentration of digitonin to release luminal content proteins. The bulk of the toxin (73%), including the population of glycosylated B-subunit that is sensitive to endoglycosidase H digestion, was associated with the membrane fraction, whereas the majority of the luminal ER protein PDI (97%) was released into the supernatant (Figure 4A, compare top and bottom). Identical results were obtained with the KDEL-mutant CT-GS(KDEV) (our unpublished data). These data suggest that after entry into the ER, CT remains associated with the membrane in a manner independent of its KDEL sequence, presumably bound to GM1.
Figure 4.
The B subunit is bound to GM1 in the ER. (A) Immunoblots of membrane and supernatant fractions for CT B-subunit and protein disulfide isomerase. Microsomes were prepared from Vero cells after incubation with CT-GS for 5 h at 37°C and membrane (lanes 3–4 and 8–9) and supernatant fractions (lanes 1–2 and 6–7) analyzed by immunoblot for CT B subunit or protein disulfide isomerase. Lanes 6–9 show microsomes permeabilized with low dose of digitonin. Lanes 4 and 9 are the same samples as in lanes 3 and 8 treated with endoglycosidase H. Lanes 5 and 10 show purified CT-GS as control. ** marks the EndoH-sensitive glycosylated form of GT-GS that always fractionates with the membrane pellet. (B) Phosphorimage of sulfation and N-glycosylation of CT-GS B-subunit immunoprecipitated from Triton X-100-insoluble (lipid raft) and -soluble fractions of T84 cells 5 h after application of toxin. Samples were treated or not treated with endoglycosidase H as indicated. (C) CT or the CT G33D mutant that cannot bind GM1 were incubated with proteoliposomes prepared from ER membranes isolated from canine pancreas, layered under a sucrose step gradient (lanes 8–10), and analyzed after equilibrium centrifugation by immunoblot. Toxin binding to the ER proteoliposomes is indicated by flotation into the gradient (lanes 1–7). Representative of two independent experiments. (D) CT was incubated with ER membranes isolated from canine pancreas, layered under a sucrose step gradient (lanes 8–10), and analyzed after equilibrium centrifugation by immunoblot for the CT B-subunit or the ER membrane protein ribophorin I. Toxin binding to the ER membranes is indicated by flotation into the gradient (lanes 1–7). Some ER membranes were also treated with puromycin to strip ribosomes. These membranes floated higher into the gradient after puromycin treatment and contained binding sites for CT showing the presence of GM1 in rough ER. A similar shift in density after treatment with puromycin was seen for the ER membrane protein ribophorin I. Representative of two independent experiments.
Because GM1 is a raft glycolipid, we examined whether the membrane-associated toxin in the ER is bound to lipid rafts. T84 cells were incubated with CT-GS in the presence of [35S]sulfate. The cells were lysed with Triton X-100 at 4°C, and the detergent-insoluble fraction was floated in a sucrose gradient to enrich for lipid rafts. Much of the sulfated and glycosylated toxin (∼50% for each form) was found in this fraction (Figure 4B, lane 1 versus 3). The material was sensitive to endoglycosidase H treatment, indicating that it was located in the ER or cis-Golgi (lane 2). These data suggest that a significant fraction of CT remains bound to GM1 in lipid rafts, or possibly in a raft domain induced by the B subunit cross-linking GM1, after arrival in the ER.
We also examined the distribution of CT in raft domains when the toxin is bound to the plasma membrane of T84 cells. When CT is bound to the apical membrane, 50% of the toxin localizes to the detergent-insoluble fraction. When bound to the basolateral membrane, 80% of the toxin is found in the detergent-insoluble raft fraction (Badizadegan and Lencer, unpublished data). Studies in hippocampal neurons (Shogomori and Futerman, 2001a) suggest that the CT-GM1 raft complex may become progressively more detergent soluble as it moves retrograde from the plasma membrane to the ER. In our ER transport assay, with CT added to both apical and basolateral membranes, a small decrease in detergent insolubility may have occurred as toxin moved from plasma membrane to ER, consistent with the results in hippocampal neurons, but the effect was not dramatic.
Finally, we examined whether ER membranes contain the ganglioside. ER proteoliposomes were prepared from ER microsomal membranes derived from canine pancreas (Gorlick and Rapoport, 1993) and incubated with the B-subunit. Binding was assessed by flotation of the proteoliposomes in a sucrose gradient, followed by immunoblotting of the fractions. A significant percentage of the B-subunit floated with the proteoliposomes (Figure 4C, second versus first panel). A mutant B-subunit (CTB-G33D), in which the substitution of Asp33 for Gly abolishes GM1 binding (Jobling and Holmes, 1991), did not float with the ER proteoliposomes (fourth versus third panel), indicating specificity for binding to GM1. To rule out that the binding occurred to GM1 from contaminating membranes, rather than ER, we used ER membranes treated or not treated with puromycin and high salt to strip off bound ribosomes, before adding the B-subunit. The ER microsomes are prepared in such a way that a significant fraction of the membranes are open. In the subsequent flotation, the B-subunit fractionated with the ribosome-stripped membranes at a significantly lower density than that of the original membranes (Figure 4D). This was not observed to the same extent when ER membranes were treated with high salt alone (our unpublished data). The shift in density of the ER membranes was confirmed by immunoblotting for the ER membrane protein ribophorin I (Figure 4D, bottom two panels). Thus, the B-subunit must be able to bind to rough ER membranes, providing evidence for GM1 in the ER. Together, the results of these studies suggest that the toxin remains bound to GM1 and associated with lipid rafts all the way from the plasma membrane to the ER.
Toxin Binding to Gangliosides in Lipid Rafts Is Required for Transport to the ER
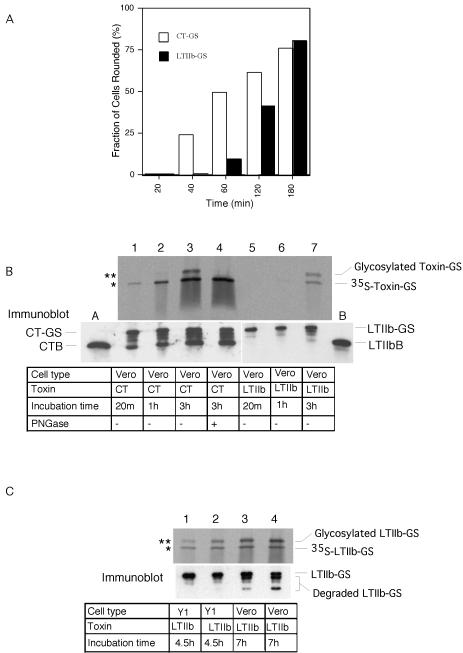
We next tested whether GM1 is specific for this pathway and examined whether the toxin LTIIb, which binds ganglioside GD1a instead of GM1, is transported from the plasma membrane to the ER. A similar construct as for CT was made, containing both sulfation and glycosylation signals (LTIIB-GS). When added to Vero cells, it caused a functional response that was slower in onset than that induced by CT (Figure 5A). In the transport assay, sulfated and glycosylated material was first seen after 3 h (Figure 5B, lane 7), again significantly later than for CT-GS (lanes 1–3). These data show that LTIIb travels from the plasma membrane to the ER in Vero cells. Similar results were obtained for mouse Y1 cells (Figure 5C and our unpublished data).
Figure 5.
LTIIb-GS traffics retrograde into the ER of Y1 and Vero cells. (A) Time course of cAMP-dependent shape change induced by CT (open bars) or LTIIb (filled bars) incubated with Vero cells for the indicated times at 37°C. Fraction of rounded cells in 400 total cells counted (n = 2). (B) Phosphorimage (top) showing time course of sulfation (*) and N-glycosylation (**) of CT-GS (lanes 1–4) or LTIIb-GS B-subunits (lanes 5–7) after incubation with Vero cells for the indicated times at 37°C. Sample in lane 4 was treated with PNGaseF before analysis. Parallel samples were analyzed by Immunoblot for CT or LTIIb B-subunits (bottom). Lanes labeled A and B contain 3 ng of purified CT or LTIIb to provide single point calibration for mass of toxin loaded in each lane (nondegraded top band labeled CT-GS or LTIIb-GS). Representative of two independent experiments. (C) Phosphorimage (top) showing sulfation (*) and N-glycosylation (**) of LTIIb-GS B-subunits after incubation with mouse adrenal Y1 (lanes 1 and 2) or monkey kidney Vero cells (lanes 3 and 4) for 4.5 or 7 h at 37°C. Parallel samples were analyzed by immunoblot for LTIIb B-subunit to demonstrate equal loading of all lanes (bottom).
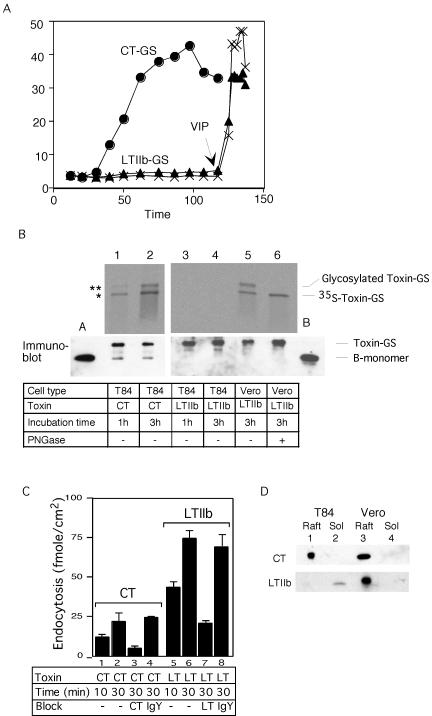
As reported before for the unmodified LTIIb toxin (Wolf et al., 1998), LTIIb-GS did not elicit toxicity in human intestinal T84 cells (Figure 6A). In the transport assay, no sulfated material was observed, even after long incubation times at which in Vero cells a clear signal was obtained (Figure 6B, compare lanes 3 and 4 with lane 5). Thus, LTIIb does not even reach the Golgi in the T84 intestinal cells, explaining why it cannot induce toxicity.
Figure 6.
Specificity for GM1 in retrograde traffic (A) Time course of Isc induced by 20 nM CT-GS (circles) or 100 nM LTIIb-GS (triangles), or buffer alone (crosses) in T84 cells. The viability of monolayers exposed to LTIIb was demonstrated by application of the cAMP-agonist vasoactive intestinal peptide, VIP, at 120 min (arrow). (B) Phosphorimage (top) showing sulfation (*) and N-glycosylation (**) of CT-GS or LTIIb-GS B-subunits after incubation with T84 (lanes 1–4) or Vero cells (lanes 5 and 6) for 1 or 3 h at 37°C. Parallel samples analyzed by immunoblot for CT B-subunit show equal loading of all lanes (bottom). Lanes labeled A and B contain 3 ng of purified CT or LTIIb toxin to provide single point calibration for the mass of toxin loaded. Representative of two independent experiments. (C) Receptor-mediated endocytosis of 20 nM 125I-CT or 125I-LTIIb after 10- or 30-min incubations with T84 cells in the presence of 120 μM CT (column 3) or LTIIb (column 4) as competitive inhibitors, or 10 μg of chicken IgY (columns 4 and 8) or 0.5% albumin (lanes 1, 2, 5, and 6) as nonspecific controls. Mean ± SE, n = 3–5 independent studies. (D) Immunoblot for CT (top) or LTIIb (bottom) showing association of CT and LTIIb with lipid raft (Raft) and triton-soluble fractions (Sol) isolated from T84 (lanes 1 and 2) and Vero cells (lanes 3 and 4) preincubated with the indicated toxins at 4°C.
LTIIb could, however, bind to intestinal cells and be endocytosed (Figure 6C). In fact, significantly more LTIIb was internalized by receptor-mediated endocytosis than CT (compare lane 2 with lanes 3 and 4, also lane 6 with lanes 7 and 8). In addition, binding of LTIIb to T84 cells could be prevented by competition with excess of the free ganglioside GD1a but not GM1 (60–70% of specific binding is blocked by GD1a and no block by GM1). Thus, the internalization of LTIIb is caused by the binding of LTIIb to the ganglioside GD1a. Indeed, all binding to cells of LTIIb at physiological concentrations seems to be mediated by GD1a, because minimal toxicity was observed in rat glioma C6 cells that are deficient in this ganglioside, and toxicity was restored after incubation with GD1a (our unpublished data). Why then is binding of LTIIb to GD1a not sufficient for transport to the Golgi and ER?
To test whether association with lipid rafts may provide the explanation, we added CT and LTIIb to T84 intestinal and Vero cells, isolated the Triton-insoluble material by flotation in a sucrose gradient, and tested the association of the toxins by immunoblotting. Whereas both toxins were found in lipid rafts in Vero cells, only CT fractionated with rafts in intestinal cells (Figure 6D). Similar results were obtained with mouse adrenal Y1 cells (Wolf et al., 1998 and our unpublished data). These data show that the ability of the ganglioside to associate with lipid rafts and to transport toxin to the ER are closely correlated and explain why the toxin LTIIb does not induce a functional response in T84 intestinal cells.
DISCUSSION
Our results show that cholera toxin travels as an intact molecule consisting of both A- and B-subunits from the plasma membrane all the way to the ER. Disassembly and unfolding occur in the ER because only the ER form of the B chain was found nonassociated with the A chain. Disassembly required cleavage of the A chain into the A1 and A2 fragments. These data are fully consistent with our previous in vitro experiments that demonstrated disassembly and unfolding of CT by the ER luminal chaperone PDI once the A chain has been cleaved (Tsai et al., 2001). PDI only releases the A1 chain from the rest of the toxin and probably leaves the A2 portion inside the ring formed by the five B chains. A role for PDI in vivo was indicated by the effect of redox reagents on the action of CT that lacked a disulfide bond in the A chain (Tsai et al., 2001). Together, these results show that dissociation of the holotoxin does not occur in the Golgi as previously claimed (Bastiaens et al., 1996; Majoul et al., 1996), but only upon arrival in the ER.
We demonstrate that the A chain does not play an essential role for targeting the toxin to the ER. Inactivation of the C-terminal KDEL sequence from the A chain or even removal of the A chain entirely had no dramatic effect on the transport of the B subunit to the ER. The B-subunit can reexit the ER for the Golgi, because a portion of the ER-modified form became endoglycosidase H resistant. This portion was higher without a KDEL sequence, suggesting that the KDEL sequence may function to retain the toxin in the ER more efficiently, allowing a larger fraction of the A1 chain to move into the cytosol. A small effect of the KDEL sequence in ER-to-Golgi transport may thus be multiplied during repetitive transport cycles between the organelles. This may explain the small delay in the physiological response that was observed for the toxin bearing a KDEL mutation. Our observation that the KDEL sequence is not essential for ER transport of CT is in agreement with the lack of a KDEL sequence in other toxins, such as Shiga and Vero toxin, which follow a similar pathway and require transport to the ER to induce toxicity (Sandvig and van Deurs, 1996; Johannes et al., 1997).
Our results show that the B-subunit is responsible for transporting CT all the way from the plasma membrane to the ER. It binds to GM1 gangliosides in lipid rafts at the plasma membrane and remains bound to GM1 until arrival in the ER, as demonstrated by the fact that CT is bound to the ER membrane, rather than soluble in the ER lumen, that a significant fraction of the ER form of the B-subunit is found in lipid rafts, and that the ER membrane contains GM1 gangliosides that allow CT binding. Binding to the ganglioside at the plasma membrane is not sufficient for ER transport. Rather, the ganglioside also needs to be associated with lipid rafts. This is indicated most clearly by our studies with the related toxin LTIIb. In intestinal cells, this toxin was able to bind to its receptor, the ganglioside GD1a, and enter the endosomal compartment, but it was not transported to the Golgi or ER, even though it contains a KDEL sequence in its A chain. In these cells, the LTIIb–GD1a complex does not fractionate with lipid rafts. In other cells, however, in which GD1a associates with lipid rafts, the ganglioside allows LTIIb to sort into the Golgi and go all the way to the ER.
Shiga toxin lacks a KDEL sequence in the A chain and may also be transported back to the ER by an association of the B-subunit with a glycolipid (Sandvig and van Deurs, 1996; Johannes et al., 1997). This toxin also fractionates with lipid rafts both in the plasma membrane and Golgi (Falguieres et al., 2001). Although it has been claimed that the glycolipid-containing rafts take Shiga toxin back to the ER, this conclusion was based on studies with a construct that also contained an artificial KDEL sequence at the C terminus of the B chain (Falguieres et al., 2001). It is thus possible that transport to the ER and association with ER membranes was mediated by the KDEL retrieval receptor, particularly because each toxin molecule may bind to up to five receptor molecules simultaneously. Nevertheless, based on our results we consider it very likely that Shiga toxin is indeed transported to the ER by binding to a glycolipid in rafts.
It seems very likely that the toxins hijack a preexisting transport pathway in the cell. Indeed, we found that the ER contains GM1 ganglioside even when the cells have not been exposed to toxins. This glycolipid must have been transported back from the Golgi where it received its carbohydrate moiety. In Saccharomyces cerevisiae, there is also evidence for the existence of lipid rafts in the ER in the absence of added toxins (Bagnat et al., 2000). The toxins seem to coopt a lipid, and not a protein, transport pathway, in agreement with the fact that cellular proteins do not normally go back from the plasma membrane to the ER (Volz et al., 1995; Porwoll et al., 1998). Although at steady-state glycolipids are predominately found in compartments beyond the Golgi (van Meer and Lisman, 2002), there seems to be some flux in the retrograde direction back into the ER. It remains unclear, however, why the gangliosides are transported back to the ER. Perhaps, they are part of a raft structure whose actual function is to transport cholesterol, so that its plasma membrane concentration can be sensed in the ER.
In principle, the lipid transport from the plasma membrane to the ER could be caused by promiscuity in lipid sorting between different compartments. Different organelles often contain different concentrations of certain lipids but rarely show the same high degree of compartmentalization seen with proteins. However, our results show that the lipid transport pathway is specific for gangliosides that associate stably with lipid rafts at least for the transport step from the plasma membrane to the Golgi; unlike GM1, the GD1a ganglioside in T84 intestinal cells does not carry toxin into the Golgi and ER. We therefore propose that there is a lipid transport pathway, hijacked by the toxins, that allows gangliosides with high affinity for lipid rafts to go back from the plasma membrane to the ER. Sorting of raft-associated from nonraft-associated gangliosides, and delivery of raft gangliosides into the retrograde pathway, may occur either at the level of the plasma membrane by different mechanisms of endocytosis, or after arrival in the endosomal compartment, or both (Parton, 1994; Nichols et al., 2001; Shogomori and Futerman, 2001a). CT enters several cell types by both clathrin and nonclathrin-dependent mechanisms, and in some cell types clathrin-mediated endocytosis leads to delivery of the toxin to the Golgi apparatus. GPI-anchored proteins are also associated with lipid rafts, but there are conflicting reports on whether they are transported only to endosomes or also retrograde to the Golgi (Fivaz et al., 2002; Nichols, 2002; Sabharanjak et al., 2002). Thus, perhaps raft association alone is not sufficient for Golgi targeting.
Lipid raft association of a ganglioside may be caused by its lipid anchor (Brown and London, 2000). Gangliosides consist of two major domains, an extracytoplasmic oligosaccharide and an intramembrane ceramide lipid. Both can exhibit heterogeneity in structure. In human intestinal cells, in contrast to other cells, GM1 but not GD1a ganglioside, is associated with lipid rafts even though both are present (Wolf et al., 1998). The simplest explanation is that in these intestinal cells, GM1 and GD1a have different ceramide structures. For example, raft association may require a certain chain length or saturation of the fatty acid or sphingosine. Even in cells where specific gangliosides partition into lipid rafts, there is a sizable population of those gangliosides that remains nonraft associated (up to 50% of GM1 in T84 cells), perhaps due to differences in their ceramide domains.
Finally, our results provide a plausible explanation why LTIIb, in contrast to the related toxin CT, has not been associated with diarrheal disease in humans. Human intestinal cells can bind LTIIb through its receptor ganglioside and can even endocytose it, but the toxin cannot reach the ER because the ganglioside does not associate with lipid rafts and does not move to the Golgi or ER. Thus, colonization of the human intestine by toxigenic E. coli expressing LTIIb cannot cause disease, in contrast to infection by E. coli expressing cholera-like toxin LTI or infection by Vibrio cholerae. In other animal species, LTIIb may be transported to the ER by GD1a and therefore cause toxicity.
Acknowledgments
We thank D. Kelleher and R. Gilmore for helpful discussions and assay of the CT-GS construct; E. Barclay for technical assistance; and M. Neutra, A. Osborne, and K. Solomon for critical reading of the manuscript. These studies were supported by research grants DK48106, DK57827, and DK34854 (to W.I.L.), AI31940 (to R.K.H.), the Damon Runyon Cancer Research Foundation (DRG1579 to B.T.). T.A.R. is a Howard Hughes Medical Institute investigator.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–06–0354. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-06-0354.
Abbreviations used: CT, cholera toxin; LTIIb, E. coli heat-labile toxin type II.
References
- Bagnat, M., Keranen, S., Shevchenko, A., and Simons, K. (2000). Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97, 3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens, P.I.H., Majoul, I.V., Verveer, P.J., Söling, H.-D., and Jovin, T.M. (1996). Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO J. 15, 4246–4253. [PMC free article] [PubMed] [Google Scholar]
- Brown, D., and London, E. (2000). Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275, 17221–17224. [DOI] [PubMed] [Google Scholar]
- Bundgaard, J.R., Vuust, J., and Rehfeld, J.F. (1997). New consensus features for tyrosine O-sulfation determined by mutational analysis. J. Biol. Chem. 272, 21700–21705. [DOI] [PubMed] [Google Scholar]
- Falguieres, T., Mallard, F., Baron, C., Hanau, D., Lingwood, C., Goud, B., Salamero, J., and Johannes, L. (2001). Targeting of shiga toxin b-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 12, 2453–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivaz, M., Vilbois, F., Thurnheer, S., Pasquali, C., Abrami, L., Bickel, P.E., Parton, R.G., and van der Goot, F.G. (2002). Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J. 21, 3989–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta, S., Magnani, J.L., Twiddy, E.M., Holmes, R.K., and Ginsburg, V. (1988). Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 56, 1748–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavel, Y., and von Heijne, G. (1990). Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 3, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlick, D., and Rapoport, T.A. (1993). Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell 75, 615–630. [DOI] [PubMed] [Google Scholar]
- Hazes, B., and Read, R.J. (1997). Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36, 11051–11054. [DOI] [PubMed] [Google Scholar]
- Jobling, M.G., and Holmes, R.K. (1991). Analysis of structure and function of the B subunit of cholera toxin by the use of site-directed mutagenesis. Mol. Microbiol. 5, 1755–1767. [DOI] [PubMed] [Google Scholar]
- Jobling, M.G., Palmer, L.M., Erbe, J.L., and Holmes, R.K. (1997). Construction and characterization of versatile cloning vectors for efficient delivery of native foreign proteins to the periplasm of Escherichia coli. Plasmid 38, 158–173. [DOI] [PubMed] [Google Scholar]
- Johannes, L., Tenza, D., Antony, C., and Goud, B. (1997). Retrograde transport of KDEL-bearing B-fragment of shiga toxin. J. Biol. Chem. 272, 19554–19561. [DOI] [PubMed] [Google Scholar]
- Lee, C.-M., Chang, P.P., Tsai, S.-C., Adamik, R., Price, S.R., Kunz, B.C., Moss, J., Twiddy, E.M., and Holmes, R.K. (1991). Activation of Escherichia coli heat-labile enterotoxins by native and recombinant adenosine diphosphateribosylation factors, 20-kD guanine nucleotide-binding proteins. J. Clin. Investig. 87, 1780–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger, B., Brown, J.L., and Spiess, M. (1994). Tagging secretory and membrane proteins with a tyrosine sulfation site. Tyrosine sulfation precedes galactosylation and sialylation in COS-7 cells. J. Biol. Chem. 269, 8115–8121. [PubMed] [Google Scholar]
- Lencer, W.I., de Almeida, J.B., Moe, S., Stow, J.L., Ausiello, D.A., and Madara, J.L. (1993). Entry of cholera toxin into polarized human intestinal epithelial cells: identification of an early brefeldin A sensitive event required for A1-peptide generation. J. Clin. Investig. 92, 2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer, W.I., Constable, C., Moe, S., Jobling, M., Webb, H.M., Ruston, S., Madara, J.L., Hirst, T., and Holmes, R. (1995). Targeting of cholera toxin and E. coli heat labile toxin in polarized epithelia: role of C-terminal KDEL. J. Cell Biol. 131, 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul, I., Sohn, K., Wieland, F.T., Pepperkok, R., Pizza, M., Hillemann, J., and Söling, H.-D. (1998). KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from Golgi involves COPI, p23, and the COOH terminus of Erd2p. J. Cell Biol. 143, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul, I.V., Bastiaens, P.I.H., and Söling, H.-D. (1996). Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells. J. Cell Biol. 133, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B.J. (2002). A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol 4, 374–378. [DOI] [PubMed] [Google Scholar]
- Nichols, B.J. (2003). GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr. Biol. 13, 686–690. [DOI] [PubMed] [Google Scholar]
- Nichols, B.J., Kenworthy, A.K., Polishchuk, R.S., Lodge, R., Roberts, T.H., Hirschberg, K., Phair, R.D., and Lippincott-Schwartz, J. (2001). Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi, P.A., Curran, P.K., and Fishman, P.H. (1993). Brefeldin A blocks the response of cultured cells to cholera toxin: implications for intracellular trafficking in toxin action. J. Biol. Chem. 268, 12010–12016. [PubMed] [Google Scholar]
- Orlandi, P.A., and Fishman, P.H. (1998). Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton, R.G. (1994). Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42, 155–166. [DOI] [PubMed] [Google Scholar]
- Porwoll, S., Loch, N., Kannicht, C., Nuck, R., Grunow, D., Reutter, W., and Tauber, R. (1998). Cell surface glycoproteins undergo postbiosynthetic modification of their N-glycans by stepwise demannosylation. J. Biol. Chem. 273, 1075–1085. [DOI] [PubMed] [Google Scholar]
- Rapak, A., Falnes, P.Ø., and Olsnes, S. (1997). Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to the cytosol. Proc. Natl. Acad. Sci. USA 94, 3783–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, A.A., Stang, E., Pepperkok, R., and Parton, R.G. (2002). Inhibitors of COP-mediated transport and cholera toxin action inhibit simian virus 40 infection. Mol. Biol. Cell 13, 1750–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodighiero, C., Tsai, B., Rapoport, T.A., and Lencer, W.I. (2002). Role of ubiquitination in retro-translocation of cholera toxin and escape of cytosolic degradation. EMBO Rep. 3, 1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharanjak, S., Sharma, P., Parton, R.G., and Mayor, S. (2002). GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2, 411–423. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (1996). Endocytosis, intracellular transport, and cytotoxic action of Shiga toxin and ricin. Physiol. Rev. 76, 949–966. [DOI] [PubMed] [Google Scholar]
- Schmitz, A., Herrgen, H., Winkeler, A., and Herzog, V. (2000). Cholera toxin is exported from microsomes by the sec61p complex. J. Cell Biol. 148, 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogomori, H., and Futerman, A.H. (2001a). Cholera toxin is found in detergent-insoluble rafts/domains at the cell surface of hippocampal neurons but is internalized via a raft-independent mechanism. J. Biol. Chem. 276, 9182–9188. [DOI] [PubMed] [Google Scholar]
- Shogomori, H., and Futerman, A.H. (2001b). Cholesterol depletion by methyl-beta-cyclodextrin blocks cholera toxin transport from endosomes to the Golgi apparatus in hippocampal neurons. J. Neurochem. 78, 991–999. [DOI] [PubMed] [Google Scholar]
- Sixma, T.K., Kalk, K.H., van Zanten, B.A., Dauter, Z., Kingma, J., Witholt, B., and Hol, W.G. (1993). Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J. Mol. Biol. 230, 890–918. [DOI] [PubMed] [Google Scholar]
- Teter, K., Allyn, R.L., Jobling, M.G., and Holmes, R.K. (2002). Transfer of the cholera toxin A1 polypeptide from the endoplasmic reticulum to the cytosol is a rapid process facilitated by the endoplasmic reticulum-associated degradation pathway. Infect. Immun. 70, 6166–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, B., and Rapoport, T. (2002). Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J. Cell Biol. 159, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, B., Rodighiero, C., Lencer, W.I., and Rapoport, T. (2001). Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 104, 937–948. [DOI] [PubMed] [Google Scholar]
- van den Akker, F., Sarfaty, S., Twiddy, E.M., Connell, T.D., Holmes, R.K., and Hol, W.G.J. (1996). Crystal structure of a new heat-labile enterotoxin, LTIIb. Structure 4, 665–678. [DOI] [PubMed] [Google Scholar]
- van Meer, G., and Lisman, Q. (2002). Sphingolipid transport: rafts and translocators. J. Biol. Chem. 277, 25855–25858. [DOI] [PubMed] [Google Scholar]
- Volz, B., Orberger, G., Porwoll, S., Hauri, H.P., and Tauber, R. (1995). Selective reentry of recycling cell surface glycoproteins to the biosynthetic pathway in human hepatocarcinoma HepG2 cells. J. Cell Biol. 130, 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, A.A., Fujinaga, Y., and Lencer, W.I. (2002). Uncoupling of the cholera toxin-G(M1) ganglioside receptor complex from endocytosis, retrograde Golgi trafficking, and downstream signal transduction by depletion of membrane cholesterol. J. Biol. Chem. 277, 16249–16256. [DOI] [PubMed] [Google Scholar]
- Wolf, A.A., Jobling, M.G., Wimer-Mackin, S., Madara, J.L., Holmes, R.K., and Lencer, W.I. (1998). Ganglioside structure dictates signal transduction by cholera toxin in polarized epithelia and association with caveolae-like membrane domains. J. Cell Biol. 141, 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron, C., Vieira, J., and Messing, J. (1985). Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33, 103–119. [DOI] [PubMed] [Google Scholar]
- Zhang, R.-G., Scott, D.L., Westbrook, M.L., Nance, S., Spangler, B.D., Shipley, G.G., and Westbrook, E.M. (1995). The three-dimensional crystal structure of cholera toxin. J. Mol. Biol. 251, 563–573. [DOI] [PubMed] [Google Scholar]