Abstract
The mechanisms through which cytokine signals prevent the activation and mitochondrial targeting of the pro-apoptotic Bcl-2-associated X protein (Bax) are unclear. Here we showed, using primary human eosinophils, that in the absence of the pro-survival cytokines granulocyte macrophage-colony stimulating factor (GM-CSF) or interleukin 5, Bax spontaneously undergoes activation and initiates mitochondrial disruption. Bax inhibition reduced eosinophil apoptosis, even in the absence of cytokines. GM-CSF induced activation of Erk1/2, which phosphorylated Thr167 of Bax, which facilitated de novo interaction of Bax with the prolyl isomerase Pin1. Pin1 blockade led to Bax cleavage, mitochondrial translocation and caspase activation, irrespective of the presence of cytokines. Our findings indicate that Pin1 is a key mediator of pro-survival signaling and a regulator of Bax function.
INTRODUCTION
Pulmonary eosinophilic inflammation is a defining feature of asthma. Within a few days of allergen challenge, airway eosinophils increase by 20-150-fold in number in humans and other animals1,2. Depletion of eosinophils by systemic steroid3 or anti-interleukin 54 treatment in humans, or by genetic ablation in animal models5, markedly attenuates submucosal matrix deposition, airway smooth muscle hyperplasia and in some cases, airway hyperresponsiveness, suggesting a critical role of these cells in asthmatic lung pathology.
Eosinophils, like neurons, are terminally differentiated, non-dividing cells. Normally short-lived (approximate half life of 1.5 days), peripheral blood eosinophils exhibit prolonged survival and activation after exposure to the anti-apoptotic cytokines granulocyte macrophage-colony stimulating factor (GM-CSF), interleukin 5 (IL-5) and IL-3, which increase in the plasma and the lung allergen challenge6. These cytokines exert overlapping effects on hematopoietic cells (e.g. neutrophils, eosinophils, monocytes and early progenitor cells), and share a common receptor β-subunit, which plays a major role in recruiting intracellular adapters, scaffolds and kinases7. Signaling from the receptors of these cytokines is initiated by recruitment and activation of JAK2 and Lyn tyrosine kinases, which activate the signal transducer and activator of transcription (STAT) and Ras-Raf1-MAP kinase pathways, respectively8,9. The JAK-STAT cascade induces the transcription of the gene encoding the pro-survival protein Bcl-xL10 which can inhibit proapoptotic BH3-only Bcl-2 family members (e.g. Bad, Bid and Bim) and prevent downstream cytochrome c release from mitochondria11. However, eosinophils express undetectable quantities of anti-apoptotic Bcl-2 and very low amounts of Bcl-xL, even after treatment with cytokines10. Nevertheless, activation of the Ras-Raf1-MAP kinase cascade by pro-survival cytokines inhibited spontaneous eosinophil apoptosis by preventing mitochondrial translocation of Bax (http://www.signaling-gateway.org/molecule/query?afcsid=A000364)12. Thus, it remains unknown how these signaling cascades regulate Bax function.
Previously we showed that Pin1 (http://www.signaling-gateway.org/molecule/query?afcsid=A002516), a peptidyl-prolyl isomerase (PPIase), was necessary for GM-CSF production as well as pro-survival signaling in cytokine-treated eosinophils13. Pin1 blockade antagonized GM-CSF anti-apoptotic signaling and rapidly induced caspase 3 activation and subsequent cell death. Pin1 consists of an N-terminal WW domain and a carboxy-terminal isomerase domain14. The WW domain binds to serine–proline (Ser–Pro) or threonine–proline (Thr–Pro) motifs often, but not exclusively, after phosphorylation mediated by proline directed protein kinases, such as cyclin-dependent kinases (CDKs), glycogen synthase kinase (GSK3) 3, protein kinase C (PKC) and MAPKs15. Growing evidence suggests that Pin1 plays a significant role in apoptosis in neurons and tumor cells16. Pin1 binds to phosphorylated Bcl-2 in cancer cells arrested in M phase18, and to p53, which regulates Bax gene expression17. Depletion of nuclear Pin1 accelerated neuronal cell death through excessive tau phosphorylation18 or by enhancing the expression and function of BimEL19, a pro-apoptotic Bcl-2 family member. In the immune system, Bax and/or Bak are required for the induction of mitochondria-dependent apoptotic pathways. Cells lacking both Bax and Bak are resistant to cell death induced by a variety of stimuli including DNA damage, growth-factor withdrawal and nutrient starvation20. Bax and Bak can be antagonized by pro-survival cytokines21 but the mechanisms mediating this antagonism are not well understood.
Using pro-survival factor-dependent primary human eosinophils, here we demonstrated that IL-5 or GM-CSF signaling triggers Erk1/2-mediated phosphorylation of Bax Thr167. Phosphorylation at this Thr-Pro site enhanced Bax-Pin1 interactions and prevented exposure of the pro-apoptotic N-terminal domain of Bax, as well as Bax cleavage and mitochondrial targeting. Conversely, Pin1 blockade accelerated Bax activation, mitochondrial localization, caspase 9 and 3 activation and cell death. Our study indicates that Pin1 is a critical mediator of pro-survival signaling in eosinophils and clarifies how Bax is regulated during programmed eosinophil death.
RESULTS
Pin1 facilitates eosinophil survival
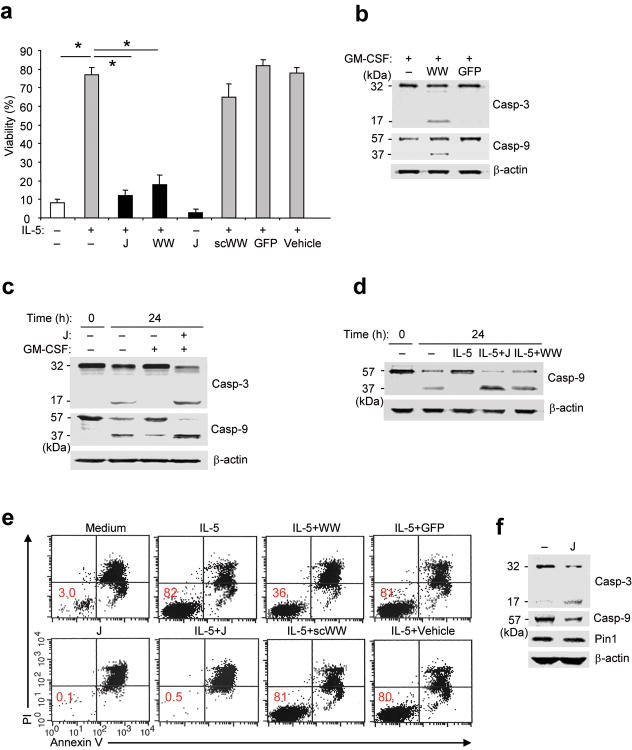
Recently, we showed that Pin1 blockade both reduced the production of and pro-survival signaling of GM-CSF, and accelerated eosinophil death in vitro13 and in vivo2,22. IL-5, like GM-CSF, prolongs eosinophil survival12, possibly through a Bax-dependent mechanism(s). To identify these mechanism(s), eosinophils were untreated or incubated with IL-5 or GM-CSF. Untreated eosinophils obtained from normal donors showed very low Pin1 isomerase activity2 but exhibited activation of caspase 3 and caspase 9 and reproducibly underwent apoptosis (80-90%) within 3-4 days in vitro (Fig. 1a,c-e, 5f). IL-5 or GM-CSF treatment markedly enhanced survival, suppressed caspase activation and increased Pin1 activity (Fig. 1a,c-e, 2d, 5f). Juglone (5-hydroxy-1,4-naphthoquinone), a selective and irreversible Pin1 inhibitor23, or a dominant negative Pin1 peptide composed of the WW domain tethered to a TAT penetratin tag (TAT-WW-Pin1), induced caspase 9 and 3 activation (Fig. 1b-e) and eosinophil apoptosis (Fig. 1a,e) in a dose-dependent fashion, even after treatment with IL-5 or GM-CSF. Even in the absence of IL-5 and GM-CSF, Pin1 blockade accelerated eosinophil apoptosis (Fig. 1a,e, 2c). These effects were not seen in the cells treated with TAT-GFP or a TAT-control peptide (TAT-scWW) composed of the same amino acid composition and length as TAT-WW-Pin1 but with a scrambled sequence. These results indicate that Pin1 is required for GM-CSF– or IL-5–induced eosinophil survival and suggest that Pin1 is a downstream target of JAK2 or other kinases activated by cytokine signaling in eosinophils.
Figure 1. Pin1 blockade suppresses cytokine-induced survival.
(a) Viability of purified eosinophils left untreated (–) or incubated for 3 days with IL-5 (200 pM) and/or with juglone (J; 1 μM), TAT-WW-Pin1 (WW; 300 nM), scrambled WW peptide (scWW; 300 nM), TAT-GFP (GFP; 300 nM), or vehicle (ethanol). Cell viability was assessed in triplicate cultures from 3 different donors by trypan blue exclusion, and expressed as a percentage of the viability at time 0. *, P < 0.05 by Student’s t-test in a two-tailed analysis. (b-d, f) Immunoblots of total Eos lysates. Anti-caspase 3 detects the “pro-form” (p32) and the cleaved, active form (p17). Anti-caspase 9 detects the “pro-form” (p57) and the cleaved, active form (p37). (b) Eosinophils were incubated for 24 h with GM-CSF (100 pg/ml) alone or together with TAT-WW-Pin1 or TAT GFP. (c,d) Cells were left untreated (–) and lysed immediately (0 h) or incubated for 24 h in culture alone (–), or with GM-CSF or IL-5 alone or together with juglone or TAT-WW-Pin1. (e) Representative flow cytometric analysis of eosinophil viability 72 h after exposure to the indicated treatment. Eosinophils were stained with PI and annexin V. Percentage of viable cells is shown in the lower left quadrant. (f) Purified eosinophils from BAL fluid were obtained from subjects after allergen challenge. Cells were left untreated (–) or incubated with juglone for 24 h prior to immunoblot with antibodies specific for the indicated proteins. Immunoblots are representative of results from at least 3 different donors.
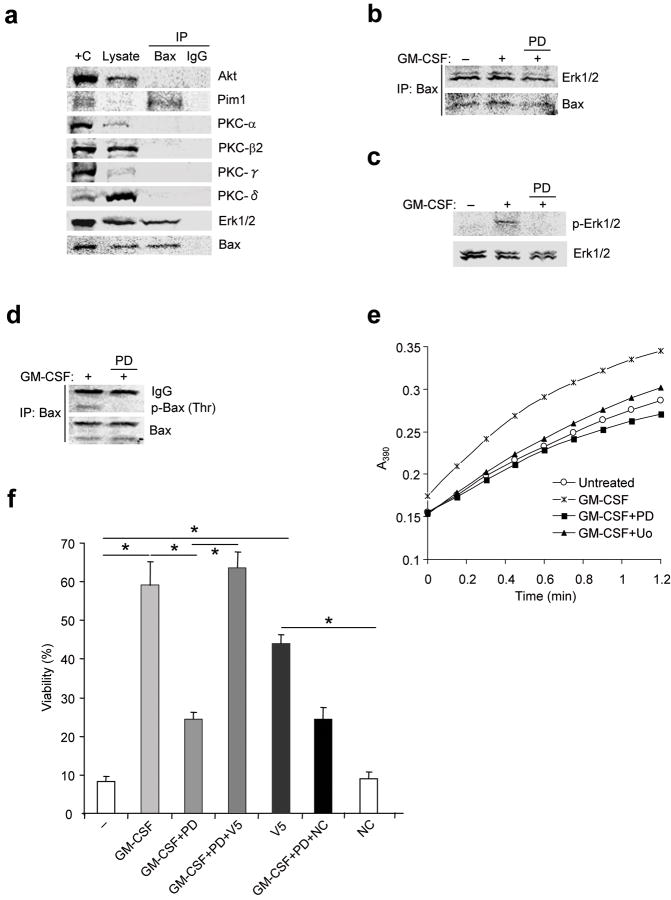
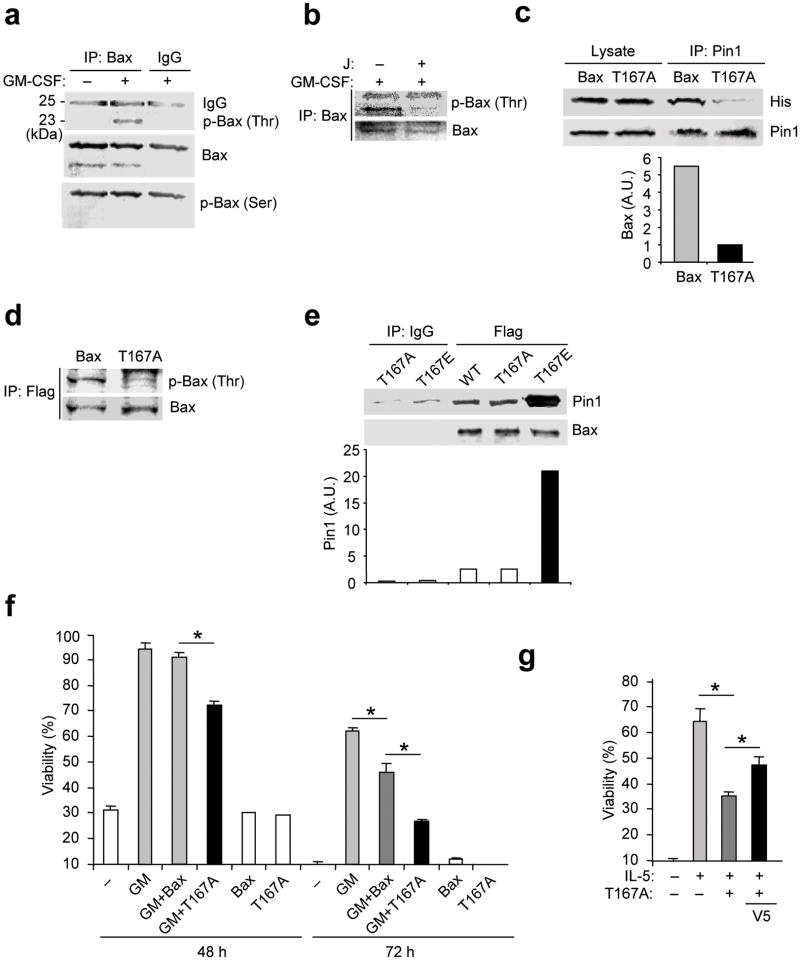
Figure 5. Bax associates with and is phosphorylated by Erk1/2.
(a) Eosinophils were treated with GM-CSF for 1 h. Cells were lysed in CHAPS buffer and pre-cleared with nonimmune IgG prior to immunoprecipitation with anti-Bax (total) or nonimmune IgG followed by immunoblot as shown. +C: positive control (mouse brain lysate), ; L: 10% of initial lysate. (b-e) Eosinophils were pre-incubated with the MEK1 inhibitors PD98059 (PD; 50 μM) or U0126 (Uo; 10 μM) for 30 min before stimulation with GM-CSF for 1 h. (b-d) Cells were lysed in CHAPS buffer and the lysates were pre-cleared with nonimmune IgG and immunoprecipitated with anti-Bax followed by immunoblot (b), or lysates were immunoblotted with antibodies specific for p-Erk1/2 and total Erk1/2 (c) or anti-p-Thr and anti-Bax (d). (e) PPIase activity in lysates was measured (See Methods). Uo: U0126 (10 μM). (f) Cells were pre-incubated with PD98059 for 1 h before incubation with GM-CSF (50 pg/ml) with or without the V5 Bax inhibitory peptide (V5, 500 μM) or a control peptide (NC) for 72 h. Cell viability was determined by trypan blue exclusion, and viability was expressed as a percentage of viability at time 0. *, P < 0.05 by Student’s t-test in a two-tailed analysis. Immunoblot and isomerase assays are representative results from at least 3 different donors. Cell viability was assessed in triplicate cultures from 3 different donors.
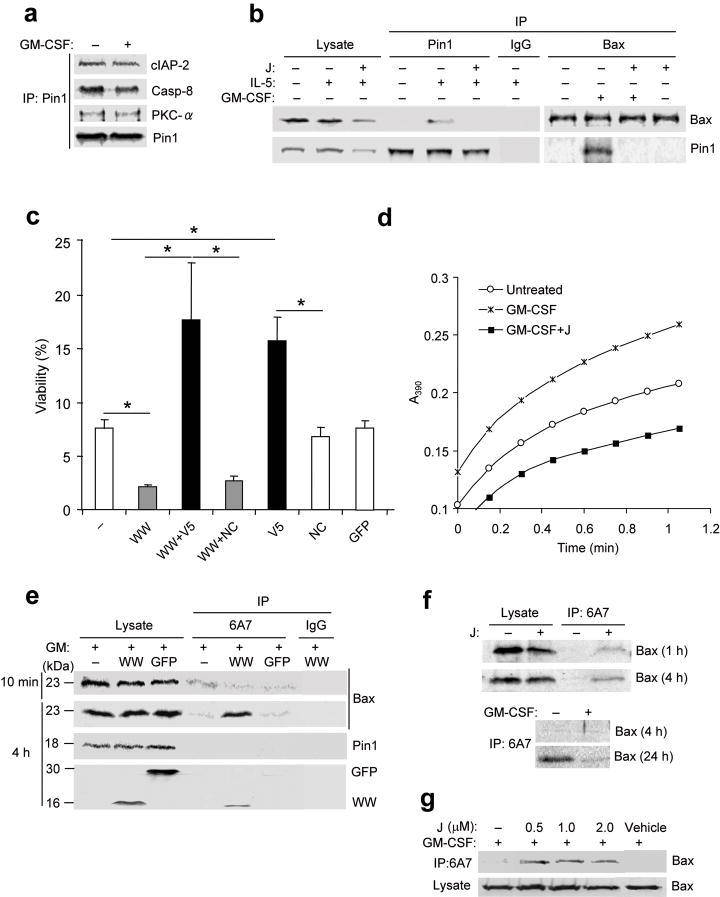
Figure 2. Pin1 interacts with Bax and regulates its activation.
(a-b) Eosinophils were left untreated (–) or incubated for 4 h with cytokine alone or with juglone (J). Cells were lysed in zwitterionic detergent (CHAPS) buffer and the lysates (10% used for immunoblot) were pre-cleared with nonimmune IgG and immunoprecipitated (IP) followed by immunoblot as shown. (c) Eosinophils were left untreated (–) or incubated for 3 days with TAT-WW-Pin1 (WW) or TAT-GFP (GFP), with or without V5 (500 μM) or a negative control peptide (NC). Cell viability of triplicate cultures from 3 different donors was determined by trypan blue exclusion, and expressed as a percentage of the viability at time 0. *, P < 0.05 by Student’s t-test in a two-tailed analysis. (d) Pin1 isomerase assay of cytoplasmic lysates from eosinophils left untreated or after treatment for 10 min with GM-CSF alone or together with juglone. (e) Eosinophils were treated with GM-CSF alone or together with His-TAT-WW-Pin1 or His-TAT-GFP for 10 min or 4 h. Lysates were subjected to immunoprecipitation with the antibody 6A7 or nonimmune IgG, followed by immunoblot. Anti-His was used to detect His-GFP-TAT and His-TAT-WW-Pin1. (f) Cells were incubated without (top) or with GM-CSF (bottom), with or without juglone for the times shown. Lysates were subjected to immunoprecipitation with 6A7 and immunoblot with anti-Bax. (g) Cells were treated with GM-CSF alone or together with juglone at the indicated dose. Cell lysates were immunoprecipitated with 6A7 followed by immunoblot with anti-Bax. Immunoblots and isomerase assay are representative of at least 3 experiments with different donors.
To address the relevance of Pin1 to asthma, we obtained bronchoalveolar lavage (BAL) eosinophils from donors 2 days after segmental allergen challenge. Typically, more than 50% of BAL cells from these patients are activated eosinophils that exhibit prolonged GM-CSF–dependent survival ex vivo and elevated Pin1 isomerase activity2,13. Despite the activated status of these BAL eosinophils, Pin1 blockade ex vivo triggered caspase 9 and 3 cleavage without affecting Pin1 expression (Fig. 1f). Caspase 8, which mediates death receptor-dependent apoptosis, was unaffected by Pin1 inhibitors (data not shown). Thus, Pin1 likely functions as a downstream effector of GM-CSF and IL-5 signaling, and regulates cell death through the intrinsic (mitochondria- and caspase 9-dependent) apoptotic pathway.
Pin1 prevents activation of Bax
The data above suggested that Pin1 regulates mitochondria-dependent eosinophil apoptosis. Cytochrome c release from mitochondria and subsequent caspase 9 cleavage is influenced by the relative expression and activity of pro- and anti-apoptotic proteins. GM-CSF and IL-5 can induce expression of the anti-apoptotic proteins Bcl-xL, cIAP2 and survivin10,12, and can prevent the translocation of the pro-apoptotic Bax to mitochondria12. Eosinophils also express variable amounts of other anti-apoptotic (Mcl-1, PKC-α, Pim1, PI3K, MAPK2,24) and pro-apoptotic (caspases) effectors25, many of which contain Pin1 recognition sites (Ser/Thr-Pro).
To identify pro- and/or anti-apoptotic protein(s) influenced by Pin1 activity, we treated eosinophils with GM-CSF or IL-5 prior to lysis and immunoprecipitation with anti-Pin1. cIAP2, caspase 8 and PKC-α were reproducibly pulled down with Pin1 in both resting and activated cells (Fig. 2a) whereas Bax was associated with Pin1 only in eosinophils treated with GM-CSF or IL-5 (Fig. 2b). Bad and Bak were not detected by immunoblot of cell lysates under any conditions. Treatment of cytokine activated eosinophils with the Pin1 inhibitor juglone markedly attenuated the interaction between Bax and Pin1 (Fig. 2b), suggesting that Pin1 PPIase activity is required for stable interaction between Pin1 and Bax.
To establish that Bax activation was a prelude to death in this system, unstimulated eosinophils were incubated with the Bax inhibitor V5, a cell-permeable pentapeptide derived from the Ku70-Bax interaction domain26. V5 blocks the mitochondrial targeting of active Bax26. Eosinophils treated with V5, but not those incubated with a control peptide of identical length, showed significantly less, but not complete resistance to, apoptosis (Fig. 2c). Interestingly, V5 also reduced but did not completely eliminate cell death induced by TAT-WW-Pin1 (Fig. 2c). The lack of complete rescue likely reflects the heterogeneity of the freshly purified eosinophil populations used for these studies, which contain a spectrum of young, middle-aged and old eosinophils, approximately 30% of which are at or beyond the point of mitochondrial disruption at the time of isolation (data not shown). Thus, it is expected that a substantial percentage of cells should be resistant to V5-mediated rescue. In aggregate, these results suggest Bax activation is a critical step leading to eosinophil apoptosis after cytokine withdrawal or Pin1 inhibition.
Given the dependence of apoptosis on Pin1 and Bax, we hypothesized that Pin1-mediated isomerization prevents the activation of Bax. To test this, eosinophils were exposed to GM-CSF or IL-5 alone, Pin1 inhibitors alone, or both cytokines and inhibitors. Bax conformation was probed with the antibody 6A7, which recognizes the N-terminal activation domain of Bax27. Brief exposure (10 min) to GM-CSF or IL-5 resulted in increased PPIase activity; juglone or TAT-WW-Pin1 reduced PPIase activity but did not alter Pin1 expression (Fig. 2d and data not shown). Cytokine treatment also induced interaction between Pin1 and Bax; this interaction was also blocked by juglone (Fig. 2b). In eosinophils treated for 10 min with either GM-CSF or IL-5, 6A7 epitope exposure was detectable but low (Fig. 2e and data not shown). The small amount of activated Bax likely reflects the presence of a small percentage of aged eosinophils undergoing irreversible apoptosis despite cytokine treatment. 6A7 immunoreactivity was markedly increased after brief (1h - 4h) exposure to TAT-WW-Pin1 but not to TAT-GFP or vehicle. In the absence of cytokine signaling, 6A7 reactivity spontaneously increased over 24 hours, an effect which was accelerated by Pin1 inhibitors (Fig. 2f). These results strongly suggest that Bax activation occurs by default in the absence of GM-CSF or IL-5, or when Pin1 is inhibited. In addition, these data show that Pin1 activity is required to prevent the exposure of the N-terminal activation domain of Bax. Notably, treatment with juglone—at a dose as low as 0.5μM for 10 min–resulted in Bax activation, even in the presence of GM-CSF (Fig. 2g). Thus, Pin1 interacts with, and likely suppresses activation of, Bax in response to pro-survival cytokine signaling.
Pin1 prevents Bax translocation to mitochondria
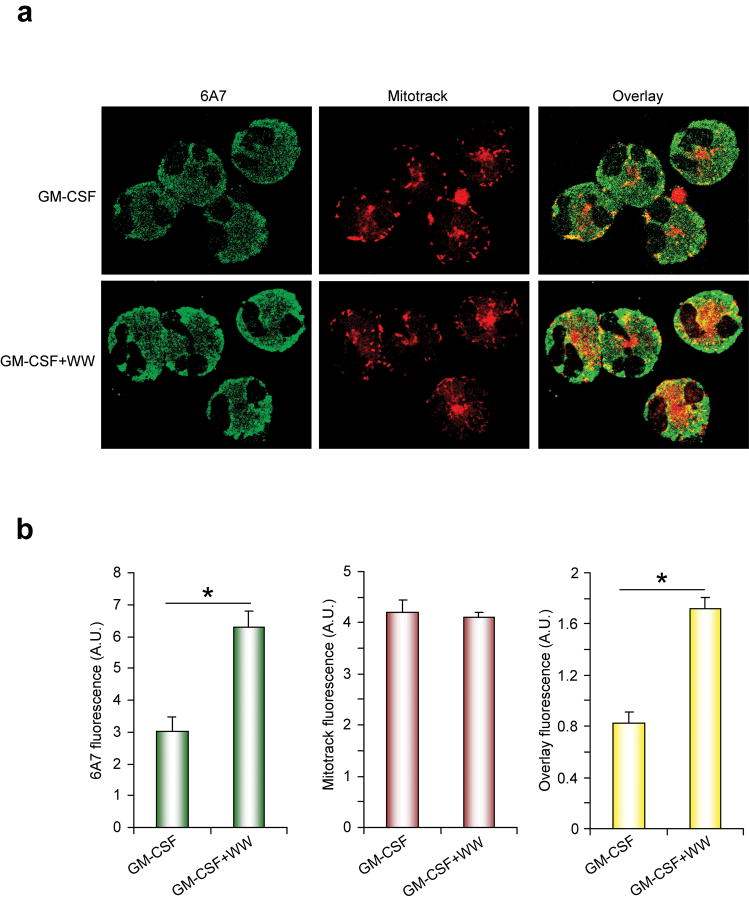
Activated Bax mediates cell death by translocation to and disruption of the mitochondrial outer membrane leading to the release of cytochrome c and Smac20. To determine if Pin1 prevents Bax localization to and disruption of the mitochondria, we used immunofluorescence to track Bax localization in eosinophils after treatment with GM-CSF alone or together with Pin1 inhibitors. As populations of freshly isolated, unstimulated eosinophils contain cells at all stages of apoptosis, untreated eosinophils were not imaged. In the presence of GM-CSF alone, Bax was diffuse and cytosolic with occasional localization to mitochondria (Fig. 3a). These findings are consistent with those shown in Fig. 2e and likely reflect a minor population of aged eosinophils that are irreversibly committed to apoptosis and express activated Bax12. Eosinophils treated with GM-CSF and WW-Pin1-TAT showed markedly increased amounts of activated Bax that extensively overlapped with mitochondria in most cells (Fig. 3a). Quantification of several hundred eosinophils confirmed that Bax activation and mitochondrial localization were significantly increased after Pin1 blockade (Fig. 3b). Despite the colocalization of activated Bax with mitochondria, these cells did not yet exhibit morphological features of apoptosis including changes in mitochondrial morphology, blebbing or nuclear condensation (data not shown), indicating Bax had yet to initiate downstream caspase activation. Our data strongly suggest that GM-CSF or IL-5, through Pin1, prevent Bax activation. In the absence of cytokines or in the presence of Pin1 inhibitors, Bax efficiently translocates to mitochondria in the absence additional stimuli.
Figure 3. Pin1 blockade causes mitochondrial translocation of Bax.
(a) Eosinophils were incubated for 4 h with GM-CSF alone or with TAT-WW-Pin1. Mitotracker (red; 75 nM) was added to culture medium 45 min before harvest. Cytospins were prepared and stained with 6A7 antibody (green). (b) The density of fluorescence staining for >50 cells was quantified as described in Methods. *, P < 0.05 by Student’s t-test in a two-tailed analysis. Results are representative of at least 3 experiments with different donors.
Cytokines induce Bax phosphorylation
The cell cycle-dependent phosphorylation of MPM-2 antigens enhances Pin1 binding in tumor cells28. The finding that Pin1 interacted with Bax after cytokine treatment prompted us to assess whether cytokines induce Bax phosphorylation. Freshly purified eosinophils were left untreated or treated with GM-CSF. Cell lysates were prepared with nondenaturing detergents and were subjected to immunoprecipitation with an antibody recognizing activated as well as resting Bax proteins. GM-CSF induced Bax phosphorylation on Thr but not Ser residues (Fig. 4a); this Thr phosphorylation was blocked by Pin1 inhibition (Fig. 4b). Bax residues Ser163-Tyr164, Thr167-Pro168 and Ser184-Lys185 are phosphorylated in cancer cells by GS3K, JNK and p38 MAPK29 and Akt30, respectively. Thr167-Pro168, a potential Pin1 recognition site, likely plays a critical role in Bax activation by influencing C-terminal and N-terminal conformation31.
Figure 4. Phosphorylation of Bax Thr167 facilitates cell survival.
(a) Eosinophils were left untreated (–) or incubated for 4 h with GM-CSF. Cells were lysed in CHAPS buffer and immunoprecipitated (IP) with anti-Bax or nonimmune IgG, followed by immunoblot with anti-p-Thr, anti-p-Ser and anti-Bax. (b) Eosinophils were treated with GM-CSF alone or together with juglone as in (a). Cell lysates were immunoprecipitated with anti-Bax prior to immunoblot with anti-p-Thr and anti-Bax. (c) Eosinophils were incubated with His-tagged TAT-WT-Bax (Bax) or TAT-T167A-Bax (T167A) for 10 min in the presence of GM-CSF. Cell lysates were pre-cleared and immunoprecipitated with anti-Pin1 followed by immunoblot with anti-His and anti-Pin1. Top, representative immunoblot. Bottom, the density of Bax and T167A bands, normalized to the Pin1 bands. (d) Eosinophils were incubated with Flag-tagged TAT-WT-Bax or TAT-T167A-Bax. After treatment with GM-CSF, cell lysates were immunoprecipitated with anti-Flag followed by immunoblot with anti-p-Thr and anti-Bax. (e) WT, T167A and T167E Bax proteins were incubated in vitro with GST-Pin1 in CHAPS buffer prior to immunoprecipitation with anti-Flag or nonimmune IgG followed by immunoblot with anti-Pin1 or anti-Bax. Top, representative immunoblot. Bottom, the density of Pin1 bands, normalized to the Bax bands. (f) Eosinophils were incubated with or without Flag-tagged TAT-WT-Bax or TAT-T167A-Bax in the presence or absence of GM-CSF (50 pg/ml). Cell viability was determined after 48 and 72 h. (g) Cells were incubated with or without IL-5 (100 pM) alone or together with TAT-Bax-T167A or V5. Cell viability was determined after 72 h). *, P < 0.05 Student’s t-test in a two-tailed analysis. Immunoblots are representative of at least 3 experiments with different donors.
To determine if cytokine-induced phosphorylation of Bax Thr167 is required for Bax interaction with Pin1, we mutated Thr167 to alanine (T167A) and generated His-tagged, recombinant WT or T167A Bax fused to TAT. Eosinophils were transduced with equal amounts of TAT-His-WT Bax, TAT-His-T167A Bax or TAT-His-GFP 15 min after treatment with GM-CSF. Lysates were immunoprecipitated with anti-Pin1 followed by immunoblot with anti-His. TAT-His-WT-Bax, but not TAT-His-T167A Bax co-precipitated with endogenous Pin1 (Fig. 4c). Consistent with the data shown in Fig. 2b, this interaction was not observed after Pin1 blockade (data not shown). The small amount of T167A Bax detected is likely nonspecific absorption to agarose beads during Pin1 immunoprecipitation. Of note, transduced TAT-His-T167A-Bax showed markedly reduced threonine phosphorylation compared to TAT-WT-Bax (Fig. 4d), strongly suggesting that GM-CSF stimulation induces Thr167 phosphorylation.
It remained possible that Pin1 and Bax are part of a larger, macromolecular complex but do not directly interact. Based on the immunoblot results above (Fig. 4a-c), we further hypothesized Pin1 and Bax would preferentially interact when Bax was phosphorylated at Thr167. To test this, we generated recombinant Flag-tagged versions of WT and Bax mutants where Thr167 is mutated to alanine (T167A) or to glutamate (T167E). The former can not be phosphorylated while the latter mimics phosphor-Thr. We incubated each Flag-tagged Bax construct with GST-Pin1 in vitro in a cell-free assay. Small amounts of WT or T167A Bax bound to GST-Pin1, but the amount of GST-Pin1-bound T167E was much higher (Fig. 4e). These data demonstrate that Pin1 and Bax directly interact and preferentially do so if Thr167 is phosphorylated.
The above results showed that Bax T167A mutants bind poorly to Pin1. If Pin1 binding is a prelude to isomerization which prevents Bax activation, Bax T167A mutants should block GM-CSF-mediated survival. To test this hypothesis, freshly isolated eosinophils were transduced with equal amounts of TAT-His-WT-Bax or TAT-His-T167A-Bax in the presence or absence of GM-CSF and survival and sensitivity to V5-mediated inhibition of Bax was assessed. TAT-His-WT-Bax had no effect on GM-CSF induced survival at day 2, whereas TAT-His-T167A-Bax significantly reduced survival compared to TAT-His-WT-Bax (Fig. 4f). At day 3, both TAT-His-WT-Bax and TAT-His-T167A-Bax significantly reduced survival compared to untransduced control cells, but T167A was significantly more deadly (Fig. 4f). Similar results were observed in IL-5–treated cells. Notably, apoptosis of IL-5–treated cells induced by T167A was partially rescued by V5 treatment (Fig. 4g). In aggregate, these data suggest that Bax Thr167 is phosphorylated in response to cytokine treatment, and that Thr167 phosphorylation facilitates Bax binding to Pin1. Bax binding to Pin1 is essential for inhibition of Bax activation. In the absence of either Bax Thr167 phosphorylation or Pin1 interaction, Bax is activated and orchestrates eosinophil death.
Erk1/2 interacts with and phosphorylates Bax
Multiple kinases have been implicated in Bax regulation in diverse cellular systems. Whereas Akt protected tumor cells from apoptosis by phosphorylating Bax Ser18432, Erk1/2 has also been implicated in similar events33. PKC may also play a role in Bax activation although the mechanism has not been established. As Akt, Erk1/2 and PKC are serine/threonine directed kinases expressed by eosinophils2,24, cell lysates from GM-CSF–treated cells were subjected to immunoprecipitation with anti-Bax followed by immunoblotting with antibodies specific for Akt, Erk1/2 or PKC. Bax reproducibly co-precipitated with Erk1/2, but not with Akt or PKC (Fig. 5a). We also irregularly observed an interaction between Pim1 and Bax which varied among donors (Fig. 5a and data not shown). Erk1/2 was associated with Bax in resting cells, in cells stimulated with GM-CSF and in cells treated with the MEK1 inhibitor PD98059 (Fig. 5b). GM-CSF rapidly induced MEK1-dependent phosphorylation of Erk (Fig. 5c) and Bax (Fig. 5d). In the absence of MEK1 activity GM-CSF failed to induce Pin1 PPIase activity (Fig. 5e). Taken together, these results indicate that Bax interacts with the proline-directed threonine/serine kinase Erk1/2 which, in response to cytokine signaling, phosphorylates Bax on Thr167.
The above data also suggested that Erk1/2 activation would be required for cytokine-induced eosinophil survival. Confirming this notion, GM-CSF–induced survival was suppressed by the MEK1 inhibitors PD98059 and U0126; this impaired survival was reversed by treatment with the V5 Bax inhibitor (Fig. 5f and data not shown).
Pin1 prevents Bax cleavage by calpain
As Pin1 shows >1,000 fold greater isomerase activity towards phospho-Thr-Pro than Thr-Pro bonds28, Bax proteins bearing phosphorylated Thr167 residues may be efficiently isomerized by Pin1. Such isomerization may prevent cleavage of full-length Bax (p23) at Asp33 to p18, a modification that has been implicated in Bax mitochondrial targeting and pro-apoptotic activity34. Confirming this possibility, the large amounts of p23 Bax detected in freshly isolated eosinophils, decreased substantially within 24 h and were replaced by p18 Bax (Fig. 6a, b). Treatment with GM-CSF or IL-5, which promoted eosinophil viability, prevented p23 disappearance and p18 appearance (Fig. 1a, 6a,b,d). However, Pin1 blockade antagonized the protective effects of GM-CSF or IL-5, and accelerated Bax cleavage (Fig. 6a, b) and activation (Fig. 2e,g, 3).
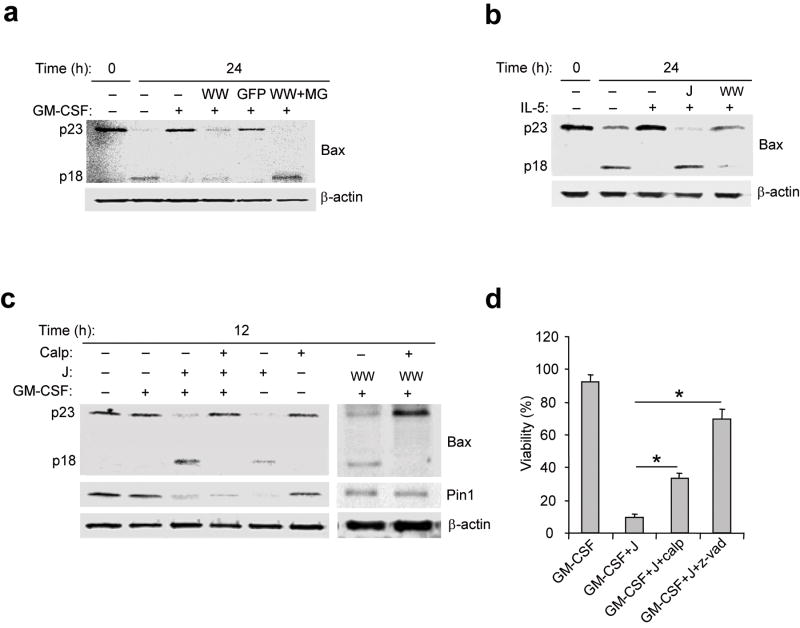
Figure 6. Bax activity and cleavage are modified by calpain inhibitors and cytokine signaling.
(a-c) Immunoblots of total eosinophil lysates. Anti-Bax (clone 3) detects the “pro-form” (p23) and the cleaved form (p18). (a) Eosinophils were left untreated (–) and immediately lysed (0) or incubated for 24 h with GM-CSF alone or together with TAT-WW-Pin1 (WW), TAT-GFP (GFP) or TAT-WW-Pin1 and MG132 (MG; 40 μM). (b) Freshly purified eosinophils were immediately lysed (0) or left untreated for 24 h or incubated with IL-5 alone or together with juglone (J) or TAT-WW-Pin1. (c) Eosinophils were left untreated or incubated with GM-CSF alone or together with juglone or TAT-WW or calpeptin (Calp, 20 μM). (d) Eosinophils were treated with GM-CSF alone or with juglone or calpeptin or with the broad spectrum caspase inhibitor z-Vad-fmk (z-vad; 50 μM). Viability was determined after 72 h incubation. Cell viability was determined by trypan blue exclusion, and viability was expressed as a percentage of viability at time 0. *, P < 0.05 Student’s t-test in a two-tailed analysis. Immunoblots are representative of at least 3 experiments with different donors while cell viability was assessed in triplicate cultures from 3 different donors.
Next we sought to identify the protease(s) responsible for cleaving Bax in this system. We focused on calpains, which are calcium-dependent cysteine proteases and targets of Erk1/235 that have been implicated in drug-induced tumor cell death34,36. Bax remained full-length for 12 h in the absence or presence of GM-CSF (Fig. 6c). However, Pin1 blockade with juglone or TAT-WW-Pin1 induced Bax cleavage within 12 h of GM-CSF treatment. This Bax cleavage was blocked by the calpain inhibitor calpeptin (Fig. 6c), but not by a proteasome inhibitor (Fig. 6a). Cell death induced by Pin1 inhibitors was also partially suppressed by calpeptin and completely suppressed by broad spectrum caspase inhibitors (Fig. 6d).
Based on present results, we propose the following model for Pin1-mediated regulation of Bax (Supp. Fig. 1). In response to cytokine signaling, Pin1 is activated while Bax is phosphorylated at Thr167 by Erk1/2. This phosphorylation enhances Pin1 binding to, and likely isomerization of, the phospho-Thr167-Pro168 peptide bond. This isomerization constrains Bax in an inactive conformation that is resistant to calpain-mediated cleavage. As eosinophils can survive for weeks in the asthmatic lung, this process can persist for extended time periods. In the absence of cytokine or after loss of Pin1 function, Bax spontaneously exposes the 6A7 epitope and is cleaved at the N-terminus by calpain-like proteases. Both conformationally altered p23 Bax and cleaved p18 Bax translocate to mitochondoria, causing cytochrome c release and activation of caspase 9 and 3.
DISCUSSION
In this study, we identified a heretofore unknown role for Pin1 as a mediator of GM-CSF and IL-5 signaling through the direct regulation of Bax. These results clarify how Bax function is regulated by cytokine signaling in terminally differentiated, growth factor-dependent eosinophils and suggest Pin1 could be a new therapeutic target for the treatment of asthma as well as other eosinophilic diseases.
Eosinophilic airway inflammation is a hallmark of asthma which often culminates in subepithelial fibrosis with variable airway obstruction. Drugs such as steroids that reduce airway and parenchymal eosinophilic accumulation are the mainstay of therapy for asthmatics. A variety of monoclonal antibody based therapies (anti-IL-5, anti-selectin and anti-chemokine receptor)37 can block eosinophil development, trafficking and survival. Multiple doses of anti-IL-5 substantially depleted peripheral blood eosinophils, but had modest effects on pulmonary eosinophils38. This may reflect both the loss of IL-5 receptor β-subunit from pulmonary eosinophils39 and persistently high expression of other anti-apoptotic cytokines such as GM-CSF, which is induced by allergen exposure22. Previously, we showed that Pin1 is essential for the expression of GM-CSF13,40, which along with IL-5, contributes to eosinophil survival in vitro. The data here suggest that Pin1 blockade in vivo would not only reduce GM-CSF production13,40 but would also activate the intrinsic apoptosis machinery to accelerate eosinophil death.
Numerous pro- and anti-apoptotic molecules are expressed by and implicated in eosinophil death25. However, it has been unclear how pro-survival cytokines attenuate the eosinophil apoptosis. GM-CSF and IL-5 induce expression of Bcl-2 and Bcl-xL10, but neither protein are detectable in eosinophils by immunoblotting even after treatment with IL-5 or GM-CSF (data not shown). Therefore, it is unlikely that the transcriptional induction of these proteins is of a sufficient magnitude or rapidity to account for GM-CSF or IL-5 prolonged survival. On the other hand, freshly purified human eosinophils from every donor evaluated expressed high amounts of Pin1 and Bax. After cell activation, Pin1 reproducibly interacted with Bax, in a manner prevented by inhibition of Pin1 PPIase activity. As Bax inhibition prevented cell death after GM-CSF withdrawal or Pin1 blockade, Bax, rather than Bak and Bad, is the dominant proapoptotic multidomain Bcl-2 family protein responsible for orchestrating eosinophil death25. In support of this conclusion, allergen challenged Bax-deficient mice accumulated nearly 2-fold more airway eosinophils after allergen challenge than wild-type mice41. Similarly, Bax was reduced in bronchial mucosal biopsies from active asthmatics42. These results suggest that the normal, rapid turnover of peripheral blood eosinophils likely reflects Bax activation in the absence of adequate pro-survival signaling or Pin1 activity.
Increasing evidence suggests that Pin1 plays an important role in neuronal and tumor cell apoptosis16. Pin1 accelerated neuronal apoptosis by enhancing the expression and function of proapoptotic Bcl-2 family member BimEL19. Moreover, Pin1 physically interacted with Bcl-2 in cancer cells arrested in M phase. Cell death was associated with increased amounts of hyper-phosphorylated Bcl-2 and p53, with the latter transactivating Bax gene expression17. In contrast, in Alzheimer’s disease, neuronal apoptosis was associated with the absence of nuclear Pin118. These and our data suggests that Pin1 plays a complex role in apoptosis of dividing as well as quiescent, terminally differentiated cells. Unlike neurons, tumor cell lines and neutrophils, which express abundant quantities of several Bcl-2 family proteins43-45, eosinophils expressed high quantities of Bax but very low amounts of other pro- or anti-apoptotic Bcl-2 family proteins25. Thus eosinophils employ a seemingly less complex apoptotic signaling pathway. Whether Pin1 regulates other pro-apoptotic Bcl-2 family members has not been investigated. Our preliminary results showing interactions with c-IAP2 and caspase 8 suggest Pin1 may play a broader role, perhaps in a cell type-specific manner, in the overall regulation of apoptotic decisions.
Despite the important role of Bax in apoptosis, the mechanisms controlling Bax conformational modifications, activation and mitochondrial translocation are not completely understood. Ku70, which interacts with the N-terminus of Bax26, or humanin, which binds to the C-terminus of Bax46, can both antagonize Bax activation. However, deficiency of Ku70 or humanin failed to induce Bax translocation to mitochondria or apoptosis in the absence of appropriate stimuli. Mitochondria-associated Bax interacts with clusterin, which prevents Bax oligomerization and cytochrome c release47. In contrast, Pin1 selectively interacted with Thr-phosphorylated Bax in cytokine-stimulated cells. In the absence of Pin1 interactions or PPIase activity, irrespective of cytokine signaling, the 6A7 epitope became exposed and Bax translocated to the mitochondria. These data strongly suggest that at least in cytokine-dependent primary human eosinophils, Bax is suppressed by Pin1, likely through isomerization after Erk-mediated threonine phosphorylation.
Bax can be phosphorylated at three sites (Ser163-Tyr164, Thr167-Pro168 and Ser184-Lys185) which confer distinct phenotypes. Whereas phosphorylation of Ser163 induced cell death in neurons by promoting Bax translocation to mitochondria, phosphorylation of Ser184 reversed this phenotype32. Ser184-phosphorylated Bax can hetero-dimerize with Mcl-1, Bcl-xL and A1, and thereby increase neutrophil survival32. JNK- and p38-mediated Thr167 phosphorylation accelerated apoptosis in human tumor cells29, whereas Erk1/2 protected melanoma cells from apoptosis by inhibiting Smac/DIABLO release from mitochondria33. Thus, depending on the cell type, different MAP Kinases can modify Bax on Thr167 with disparate apoptotic endpoints.
Despite GM-CSF signaling, Bax was hypo-phosphorylated at Thr167 if Pin1 was inhibited. These results suggest that Pin1-mediated isomerization is required to stabilize phospho-Ser/Thr-Pro sites, possibly by preventing phosphatase access to these sites. Consistent with this hypothesis, Pin1 interacts with and is itself regulated by PP2A2.
In primary human eosinophils, mutagenesis of Bax Thr167 to alanine increased Bax activity and reduced its interaction with Pin1 and sensitivity to pro-survival signaling. Mutagenesis of Bax Pro168 prevented Bax translocation to the mitochondria31. The Thr167-Pro168 site is located in a hinge immediately adjacent to the Bax C-terminal transmembrane domain. Our results suggest Pin1 isomerizes phosphorylated Bax, and that this isomerization constrains the C- and N-termini within Bax. This notion is consistent with our inability to immunoprecipitate Bax with the active conformation-specific 6A7 antibody during pro-survival signaling. Upon cessation of cytokine signaling or blockade of Pin1 PPIase activity, the N-terminal domain of Bax is spontaneously exposed and Bax is activated.
Terminally differentiated eosinophils express high amounts of p23 Bax which is cleaved to p18 Bax during apoptosis or after Pin1 blockade. This process was completely blocked by GM-CSF or IL-5, or by a calpain inhibitor. p18 Bax is a more potent inducer of apoptosis than p23 Bax37. p23 cleavage occurred subsequent to 6A7 epitope exposure, approximately 12 h after cytokine withdrawal. Similar events occurred in tumor cell lines treated with chemotherapeutic agents36. The toxicity of p18 Bax may reflect exposure of the hydrophobic BH3 domain (amino acids 59-73), which facilitates oligomerization and death pore formation in the mitochondrial outer membrane48. Consistent with these results, p18 Bax is detected exclusively in the mitochondrial fraction and is associated with increased calpain activation. An attractive hypothesis is that Pin1 maintains Bax in a calpain-resistant conformation, thereby reducing p18 generation.
METHODS
Reagents
Recombinant GM-CSF and IL-5, anti-Bad, and anti-survivin were purchased from R&D; juglone, anti-phospho-threonine, anti-phospho-serine and proteasome inhibitor z-LLnV were from Sigma. QIAexpress anti-His Kit was from Qiagen, proteasome inhibitor MG132, protease inhibitor Cocktail Set-III, Bax inhibiting peptide (V5) and control peptide (NC) were from Calbiochem. Anti-Active MAPK (pTEpY), anti-ERK1/2, PD98059 and U0126 were from Promega. Anti-caspase 3 (clone H-277), anti-Pin1 (clone H-123), anti-Bax (N20) and anti-Mcl-1 (Clone S-19) were from Santa Cruz Biotechnology, Inc. Mitotracker Red CNXRos was from Molecular Probes. Anti-caspase 9 and 8 were from Lab Vision. Anti-PKCs, anti-Akt and anti-Bcl-xL were from Cell Signaling. Anti-cIAP2 was from Novus Biological, Inc. Anti-Bim (clone 5E5) was from Chemicon. Anti-Pim1 (clone 2181) was from Novus Biologicals. Monoclonal anti-β-actin (clone Ab-1) was from Oncogene Research Products. Anti-Bax (clone 6A7 and clone 3) were from BD Bioscience. Monoclonal Anti-Flag was from Abcam. Horseradish peroxidase–conjugated anti-rabbit (secondary antibody) and the ECL immunoblot detection system were from Amersham-Pharmacia.
Subjects and eosinophil preparation
Peripheral blood was obtained by venipuncture from healthy or mildly atopic donors. Peripheral blood or BAL fluid eosinophils were purified with a negative immunomagnetic procedure as described49. Cells were used only when more than 99% pure. After isolation, eosinophils were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air at a density of 1×106 cells/ml in RPMI 1640 medium, 10% FBS and 50 μg/ml of gentamycin (all from Life Technologies). All participants have a clinical record at the University of Wisconsin Hospital and informed consent was obtained according to an approved University of Wisconsin Hospital Institutional Review Board protocol.
Immunocytochemistry and confocal analysis
Cells (1×106) were treated with cytokines or together with Pin1 inhibitors for 4 h at 37°C. 75 nM Mitotracker Red CMXRos was incubated with cells for last 45 min. Cells were washed in pre-warmed RPMI medium without serum, and cytospins were performed (300 r.p.m. for 3 min). Slides were fixed in 2% paraformaldehyde in PBS for 15 min at room temperature and washed 5 times in PBS. Cells were permeabilized for 10 min at room temperature in blocking buffer (3% BSA in PBS) plus 0.1% Triton X-100 followed by blocking of nonspecific binding in blocking buffer for 1 h at room temperature47. Cells were incubated overnight at 4°C with 5 μg/mL primary antibody diluted in blocking buffer. Cells were then incubated for 20 min at room temperature with 0.2% chromotrope-2R, which binds to highly basic eosinophil granules thereby reducing nonspecific binding of the secondary antibody. Cells were washed 5 times in PBS then incubated with FITC-conjugated secondary antibody diluted 1:200 in blocking buffer for 50 min at room temperature in the dark. Cells were washed 5 times prior to mounting with ProLong gold antifade (Dako). Images were collected by confocal laser microscopy (Nikon C1 Laser Scanning Confocal). The 488- and 568-nm lines of the krypton/argon laser were used for the excitation of FITC and Mitotracker Red CMXRos, respectively. The area and intensity of fluorescence staining was outlined and quantified using ImageJ software. At least 100 cells were counted in a blinded fashion in random visual fields. Results are expressed as the intensity of signals per cell.
Cell viability and flow cytometry
Eosinophils (1×106 cells/ml) were cultured in 96-well tissue culture plates (BD Biosciences). Cell viability was assessed by trypan blue exclusion on a hemocytometer. Alternatively, cells (0.5 × 106) were stained with annexin V-FITC and propidium iodide (PI) (BD Bioscience) followed by flow cytometry using a BD Biosciences FACSCalibur System.
Immunoprecipitation and immunoblot
After activation, eosinophils were ‘snap-frozen’ at - 80°C and cell lysates were prepared in CHAPS buffer (150 mM NaCl, 10 mM HEPES, pH 7.4, and 1% CHAPS). For immunoprecipitation, 2–5 μg of antibody was added to each sample, followed by incubation for 2-4 h at 4 °C. Protein G–agarose beads (Sigma-Aldrich) were added and the incubation was continued overnight. Pellets were washed five times with lysis buffer and the beads were dissolved in SDS-PAGE loading buffer for immunoblot.
Recombinant TAT proteins
The cDNA encoding enhanced green fluorescent protein (GFP), the WW domain of Pin1, scrambled WW peptide (scWW) containing identical amino acid composition to wild-type WW but scrambled in sequence, or the human Bax-α were cloned in-frame into pHisTAT50 (provided by S. Dowdy, Washington University, St. Louis, Missouri). T167A and T167E Bax mutants were generated by QuikChange Site-Directed Mutagenesis Kit (Stratagene). Proteins were expressed in Escherichia coli and were purified on a Ni2+ chelate column (Qiagen) as described by the manufacturer. Both TAT-linked proteins were more than 95% pure, based on Coomassie blue staining of SDS gels.
Pin1 activity assay
Activity was measured as described23 with slight modifications13,40. Briefly, cytoplasmic lysates (1 μg) were incubated in buffer with a glutamine-proline containing pentapeptide target modified with a C-terminal nitroanaline. After isomerization from cis to trans, the nitroanaline is cleaved by chymotrypsin and resulting absorbance measured at 390 nm.
Supplementary Material
Acknowledgments
We thank J. Sedgwick for eosinophils; Dr. Kun Ping Lu (Harvard University, Boston, Massachusetts) for the Pin1 WW domain cDNA; N. Jarjour for bronchoscopy samples; and members of the lab and the UW-Asthma group for suggestions. Supported by the National Institutes of Health (R01HL087950, P01HL088594 and P30HD03352 to J.S.M). C.B. is funded by the Jose Carreras Leukemia Foundation, Germany, the Deutsche Forschungsgesellschaft (DFG) (GRK1004, Graduate School GSC-4 and Excellence Cluster BIOSS) and the Bundesministerium fur Bildung und Forschung (BMBF) (Hepatosys). A.S. was supported by the Swiss National Science Foundation.
Footnotes
Competing interests statement. The authors declare no competing financial interests.
References
- 1.Brightling CE, et al. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax. 2003;58:528–532. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen ZJ, et al. Pin1 regulates TGF-beta1 production by activated human and murine eosinophils and contributes to allergic lung fibrosis. J Clin Invest. 2008;118:479–490. doi: 10.1172/JCI32789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson PG, Saltos N, Borgas T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J Allergy Clin Immunol. 2000;105:752–759. doi: 10.1067/mai.2000.105319. [DOI] [PubMed] [Google Scholar]
- 4.Flood-Page P, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humbles AA, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 6.Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol. 1992;51:323–382. doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- 7.Hirai K, Miyamasu M, Takaishi T, Morita Y. Regulation of the function of eosinophils and basophils. Crit Rev Immunol. 1997;17:325–352. doi: 10.1615/critrevimmunol.v17.i3-4.40. [DOI] [PubMed] [Google Scholar]
- 8.Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183:1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazdrak K, Olszewska-Pazdrak B, Stafford S, Garofalo RP, Alam R. Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. J Exp Med. 1998;188:421–429. doi: 10.1084/jem.188.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dibbert B, et al. Role for Bcl-xL in delayed eosinophil apoptosis mediated by granulocyte-macrophage colony-stimulating factor and interleukin-5. Blood. 1998;92:778–783. [PubMed] [Google Scholar]
- 11.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 12.Dewson G, Cohen GM, Wardlaw AJ. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood. 2001;98:2239–2247. doi: 10.1182/blood.v98.7.2239. [DOI] [PubMed] [Google Scholar]
- 13.Shen ZJ, Esnault S, Malter JS. The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils. Nat Immunol. 2005;6:1280–1287. doi: 10.1038/ni1266. [DOI] [PubMed] [Google Scholar]
- 14.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 15.Lu KP, Liou YC, Zhou XZ. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002;12:164–172. doi: 10.1016/s0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 16.Wulf G, Finn G, Suizu F, Lu KP. Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol. 2005;7:435–441. doi: 10.1038/ncb0505-435. [DOI] [PubMed] [Google Scholar]
- 17.Perfettini JL, Kroemer RT, Kroemer G. Fatal liaisons of p53 with Bax and Bak. Nat Cell Biol. 2004;6:386–388. doi: 10.1038/ncb0504-386. [DOI] [PubMed] [Google Scholar]
- 18.Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999;399:784–788. doi: 10.1038/21650. [DOI] [PubMed] [Google Scholar]
- 19.Becker EB, Bonni A. Pin1 mediates neural-specific activation of the mitochondrial apoptotic machinery. Neuron. 2006;49:655–662. doi: 10.1016/j.neuron.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 21.Plas DR, Rathmell JC, Thompson CB. Homeostatic control of lymphocyte survival: potential origins and implications. Nat Immunol. 2002;3:515–521. doi: 10.1038/ni0602-515. [DOI] [PubMed] [Google Scholar]
- 22.Esnault S, et al. A critical role for Pin1 in allergic pulmonary eosinophilia in rats. J Allergy Clin Immunol. 2007;120:1082–1088. doi: 10.1016/j.jaci.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Hennig L, et al. Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by juglone. Biochemistry. 1998;37:5953–5960. doi: 10.1021/bi973162p. [DOI] [PubMed] [Google Scholar]
- 24.Esnault S, Malter JS. Extracellular signal-regulated kinase mediates granulocyte-macrophage colony-stimulating factor messenger RNA stabilization in tumor necrosis factor-alpha plus fibronectin-activated peripheral blood eosinophils. Blood. 2002;99:4048–4052. doi: 10.1182/blood.v99.11.4048. [DOI] [PubMed] [Google Scholar]
- 25.Simon HU. Molecules involved in the regulation of eosinophil apoptosis. Chem Immunol Allergy. 2006;91:49–58. doi: 10.1159/000090229. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, et al. Bax-inhibiting peptide protects cells from polyglutamine toxicity caused by Ku70 acetylation. Cell Death Differ. 2007;14:2058–2067. doi: 10.1038/sj.cdd.4402219. [DOI] [PubMed] [Google Scholar]
- 27.Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe MB, et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- 29.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 30.Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- 31.Schinzel A, et al. Conformational control of Bax localization and apoptotic activity by Pro168. J Cell Biol. 2004;164:1021–1032. doi: 10.1083/jcb.200309013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardai SJ, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XD, et al. Activation of ERK1/2 protects melanoma cells from TRAIL-induced apoptosis by inhibiting Smac/DIABLO release from mitochondria. Oncogene. 2003;22:2869–2881. doi: 10.1038/sj.onc.1206427. [DOI] [PubMed] [Google Scholar]
- 34.Wood DE, et al. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- 35.Glading A, et al. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci U S A. 2000;97:3850–3855. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 38.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 39.Liu LY, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor αin the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 40.Esnault S, Shen ZJ, Whitesel E, Malter JS. The peptidyl-prolyl isomerase Pin1 regulates granulocyte-macrophage colony-stimulating factor mRNA stability in T lymphocytes. J Immunol. 2006;177:6999–7006. doi: 10.4049/jimmunol.177.10.6999. [DOI] [PubMed] [Google Scholar]
- 41.Tesfaigzi Y, et al. Bax is crucial for IFN-gamma-induced resolution of allergen-induced mucus cell metaplasia. J Immunol. 2002;169:5919–5925. doi: 10.4049/jimmunol.169.10.5919. [DOI] [PubMed] [Google Scholar]
- 42.Schwalm K, et al. Expression of the proapoptotic protein Bax is reduced in bronchial mucous cells of asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1102–9. doi: 10.1152/ajplung.00424.2007. [DOI] [PubMed] [Google Scholar]
- 43.Pinon LG, Middleton G, Davies AM. Bcl-2 is required for cranial sensory neuron survival at defined stages of embryonic development. Development. 1997;124:4173–4178. doi: 10.1242/dev.124.20.4173. [DOI] [PubMed] [Google Scholar]
- 44.Daniel PT, et al. Expression of the death gene Bik/Nbk promotes sensitivity to drug-induced apoptosis in corticosteroid-resistant T-cell lymphoma and prevents tumor growth in severe combined immunodeficient mice. Blood. 1999;94:1100–1107. [PubMed] [Google Scholar]
- 45.Iwai K, et al. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–1208. [PubMed] [Google Scholar]
- 46.Guo B, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, et al. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 49.Hansel TT, et al. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 50.Ignatovich IA, et al. Complexes of plasmid DNA with basic domain 47-57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J Biol Chem. 2003;278:42625–42636. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.