Summary
The complete consensus genome sequence was determined for avian paramyxovirus (APMV) serotype 3 strain Wisconsin. The genome is 16,182 nucleotides (nt) in length, consisting of six non-overlapping genes in the order of 3’-N-P/V/W-M-F-HN-L-5’, with a 55-nt leader at its 3’ end and a 681-nt trailer at its 5’ end. Comparison of the APMV-3 strain Wisconsin nt and the aggregate predicted amino acid (aa) sequences with those of APMV-3 strain Netherlands revealed 67 and 78%, identity, respectively. The nt and aa sequence identities between the two APMV-3 strains were lower than between the two antigenic subgroups of human respiratory syncytial virus (81 and 88% identity, respectively) and the two subgroups of human metapeumovirus (80 and 90% identity, respectively). Reciprocal cross-hemagglutination inhibition and cross-neutralization assays using post infection sera from chickens indicated that strains Wisconsin and Netherlands are highly related antigenically, with only a 2- to 4-fold difference in antibody reactivity between the homologous and heterologous strains. Taken together, our results indicate that the two APMV-3 strains represent a single serotype with two subgroups that differ substantially based on nt and aa sequences, but with only a modest antigenic difference.
Keywords: APMV, Avian paramyxovirus, Paramyxoviridae, Paramyxovirinae, Genome sequence, Serotype
Introduction
The family Paramyxoviridae includes viruses that are isolated from many species of avian, terrestrial and aquatic animals (Lamb and Parks, 2007; Nylund et al., 2008). Members of this family are characterized by pleomorphic enveloped particles that contain a nucleocapsid bearing a single-stranded, negative sense RNA genome (Lamb and Parks, 2007). The family is divided into two subfamilies, Paramyxovirinae and Pneumovirinae. Subfamily Paramyxovirinae is further divided into five genera: Rubulavirus (including mumps virus, human parainfluenza viruses [HPIV] 2 and 4, simian virus type 5 [SV5] and Tioman virus [TiV]), Respirovirus (including Sendai virus [SeV] and HPIV-1 and 3), Henipavirus (comprising Hendra virus [HeV] and Nipah virus [NiV]), Morbillivirus (including measles [MeV], rinderpest virus [RPV] and canine distemper [CDV] viruses), and Avulavirus (comprising avian paramyxovirus [APMV] serotypes 1 to 9). Subfamily Pneumovirinae is divided into two genera: Pneumovirus (comprising human respiratory syncytial virus [HRSV] and its animal counterparts including bovine respiratory syncytial virus [BRSV]), and Metapneumovirus (comprising human metapneumovirus [HMPV] and avian metapneumovirus [AMPV]) (Lamb et al., 2005; Mayo and Pringle, 1998; Pringle, 1998; Rima et al., 1995).
The genomes of members of family Paramyxoviridae range in length from 15-19 kb and contain six to ten genes in a linear array (Lamb and Parks, 2007). All members of subfamily Paramyxovirinae follow the “rule of six”, whereby efficient RNA replication depends on the genome length being an even multiple of six nucleotides (nt) (Calain and Roux, 1993; Kolakofsky et al., 1998; Samal and Collins, 1996). All members of family Paramyxoviridae encode a nucleocapsid protein (N), a phosphoprotein (P), a matrix protein (M), a fusion protein (F), an attachment protein called the hemagglutinin (H) or haemagglutinin-neuraminidase (HN) or glycoprotein (G), and a large polymerase protein (L).
Many paramyxoviruses encode additional proteins that play important roles in viral morphogenesis, RNA synthesis, and pathogenesis (Lamb and Parks, 2007). For example, most members of subfamily Paramyxovirinae encode additional proteins from the P gene by RNA editing. This involves the introduction of one or more G residues into a subset of the P transcripts by polymerase stuttering at a specific editing sequence motif. This shifts the reading frame to access alternative ORFs in one or both of the alternative frames, resulting in the expression of proteins in which the upstream domain is P-derived and the downstream domain is derived from the alternative frame. In the most significant of these chimeric proteins, called V, the downstream domain contains a cysteine-rich motif that is highly conserved across the subfamily. The V protein has been implicated in the regulation of viral RNA synthesis (Horikami et al., 1996; Lin et al., 2005) and in counteracting host antiviral responses (Goodbourn et al., 2000). Alternatively, the insertion of two G residues would shift the reading frame to access an internal ORF that leads to production of the W protein, whose significance is unclear (Steward et al., 1993).
The APMVs have been classified into nine different serotypes based on hemagglutination inhibition (HI) and neuraminidase inhibition (NI) assays (Alexander and Collins, 1984). Newcastle disease virus (NDV) composes serotype 1. Naturally-occurring strains of NDV include highly virulent strains capable of causing devastating disease in poultry, as well as strains of moderate or low virulence. NDV is the most highly characterized virus among all APMV serotypes (Alexander, 1980). APMV-2 has been isolated from chickens, turkeys and wild birds, and is responsible for decreases in egg production and fertility in turkeys (Lipkind et al., 1979; Tumova et al., 1979). The pathogenicity of APMV 4-9 is generally unknown except for a few isolated reports (Alexander, 1982; Warke et al., 2008). APMV-3 was first isolated from a turkey in 1968 in Wisconsin. Since then, many APMV-3 strains have been isolated from turkeys in different parts of the world including Canada, the United States (Tumova et al., 1979) and England (Macpherson et al., 1983). The APMV-3 strain parakeet/Netherlands/449/75, isolated from parakeets in the Netherlands, is considered the prototype for the entire serotype (Alexander and Chettle, 1978). APMV-3 has also been isolated from non-domesticated species such as Psittaciformes and Passeriformes (Alexander, 1980). APMV-3 has been associated with encephalitis and high mortality in caged birds, and with respiratory disease in turkeys (Tumova et al., 1979). The virus causes acute pancreatitis and central nervous system symptoms in psittacine and Passeriformes birds (Beck et al., 2003). APMV-3 also infects chickens at an early age, with evidence of stunting growth that may be more marked in broiler chicken breeds (Alexander and Collins, 1982). In terms of pathogenicity, APMV-3 probably is second in importance only to NDV.
As noted, APMV-3 strains have been isolated from a wide range of avian species and from different parts of the world. But very little is known about the genetic and antigenic relatedness among these strains. This information is important for epidemiological studies and for the development of vaccines against this virus. We recently described a complete consensus sequence of the APMV-3 prototype strain parakeet/Netherlands/449/75 (Kumar et al., 2008), but other strains remain to be characterized. As a first step towards understanding the genetic and serological relationship among APMV-3 strains, we now report the complete genome sequence of APMV-3 strain turkey/Wisconsin/68 and describe comparison with those of prototype strain parakeet/Netherlands/449/75 and other APMV serotypes. The genome of strain turkey/Wisconsin/68 is 16,182 nt long, which is smaller than that of strain parakeet/Netherlands/449/75 by 90 nt. There is 67 % nt and 78% amino acid (aa) sequence identity between the two strains. The cleavage site of F protein of strain turkey/Wisconsin/68 contains a monobasic residue, compared to a dibasic residue in strain parakeet/Netherlands/449/75. Our antigenic and sequence analysis suggested that the two strains represent a single serotype with two genetic subgroups.
Materials and Methods
Virus and cells
APMV-3 strain turkey/Wisconsin/68 was obtained from the National Veterinary Services Laboratory, Ames, Iowa. Nine-day-old specific pathogen free (SPF) embryonated chicken eggs and the chicken embryo fibroblast DF1 cell line were used to propagate APMV-3 strain Wisconsin. Hemagglutination (HA) titers of virus stocks were determined using 0.5% chicken RBC at room temperature. The ability of the virus to replicate in cell culture was examined in nine different established cell lines, each representing a different species of origin: DF1; MDBK, Madin Darby Bovine Kidney; MDCK, Madin Darby Canine Kidney; Vero, African green monkey kidney; HEp2, Human Epidermoid carcinoma; PK15, Pig Kidney, all the cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). All cells, except DF1 cells, were grown in Eagle s minimum essential media (MEM) with 10% fetal calf serum (FCS), while DF1 cells were grown in Dulbecco s MEM containing 10% FCS. All cells were grown in a 37°C incubator with 5% CO2. Cell monolayers were infected with a 10-3 dilution of 29 HA units of egg-grown APMV-3 strain Wisconsin and, after 1 h of adsorption, the viral inoculum was replaced with maintenance medium containing 2% FCS. The growth of virus in each cell type was observed with and without the presence of 10% allantoic fluid, or 1-5 μg/ml of acetyl trypsin/ml (Gibco), or 1-5 μg/ml of α-chymotrypsin/ml (Sigma), each of which provided a source of protease for the cleavage of F protein if necessary. The cells were observed daily for cytopathic effects (CPE) and HA titers were recorded every 24 h until the fifth day post-infection. The virus titer in each cell type was quantified by plaque assay in DF 1 cells as previously described (Krishnamurthy et al., 2000). The cells were overlaid with DMEM containing 2% FCS and 0.9% methylcellulose that were further supplemented with 10% allantoic fluid as a source of proteases. The plaques were visualized by staining with 1% crystal violet after 4 days of infection.
Serological analysis
Antisera against APMV-3 strains turkey/Wisconsin/68 and parakeet/Netherlands/449/75 were prepared at separate times to avoid cross infections. Briefly, groups of six two-week old SPF chicken were inoculated with infective allantoic fluid (29 HA units) of each virus through intranasal (IN) and intraocular (IO) routes. Two weeks after inoculation the birds were bleed and sera were collected. The sera were heat-inactivated at 56°C for 30 minutes and stored at -20°C. The antibody level of serum samples collected from chicken immunized with APMV-3 strains were evaluated by HI assay with chicken erythrocyte as previously described (Alexander, 1988). Cross reactivity of immunized chicken sera were determined by HI assay against the heterologous APMV-3 strains and other APMV serotypes. The ability of chicken sera to cross neutralize heterologous APMV-3 strains was determined by plaque reduction neutralization (PRN) assay in DF-1 cells and virus neutralization (VN) assay in embryonated chicken eggs using standard procedures (Alexander, 1995; Alexander, 2003).
Virus RNA isolation and sequence analysis
Total RNA from virus-infected DF1 cells was isolated using TRIZOL reagent, following the manufacturer s instructions (Invitrogen, USA). Viral RNA was also isolated from allantoic fluid collected from virus-infected eggs, using RNeasy kit according to the manufacturer s instruction (QIAGEN, USA).
Most of the APMV-3 genome, excepting the 3’ and 5’ termini, was copied and amplified into cDNAs using (i) primers designed from the published APMV-3 Netherlands genome sequence (Kumar et al., 2008), and (ii) consensus primers designed using published Avulavirus, Rubulavirus, Respirovirus, Morbillivirus, and Henipavirus genome sequences (Table 2). In addition, as APMV-3 Wisconsin sequence became available, it was used to design additional primers. N787 forward and N1022 reverse consensus primers were used to amplify a 235 base pair (bp) fragment that served as one starting point for downstream genome walking (Chang et al., 2001). A primer designed from the available APMV-3 Netherlands P gene sequence (GenBank Accession number EU403085) (P 780 forward) was used in conjunction with (i) a consensus sequence designed for the M gene, M350 reverse, to amplify a 800-bp product, and (ii) a second consensus sequence, M1066 reverse, to amplify a 1500-bp product that were used as a second starting point for downstream genome walking. The upstream 850 nt in the HN gene were amplified using the consensus GS forward (GE forward) primer and consensus HN840 reverse primer (Table 2). An overlapping region containing the rest of the HN gene was amplified using the consensus forward primer HN490 forward and the consensus gene-end reverse (GE reverse) primer. The upstream end of the L gene was amplified using the consensus primer HN1492 forward primer and consensus L669 reverse primer (Table 2). Downstream regions of L gene were amplified by L gene-specific forward and consensus L reverse primers listed in Table 2.
Table 2.
Primers used to amplify APMV-3 genome based on consensus sequences from Avulavirus, Rubulavirus, Respirovirus, Morbillivirus, and Henipavrus genome sequences. The primer name indicates the gene name, position from the gene-start, and the forward or reverse direction of priming. N=A/T/C/G, R= A/G, K= G/T, Y=C/T, S=G/C, W=A/T, D= A/G/T and M=A/C. Sequences used for alignment are noted in the Materials and Methods.
| Position in gene | Primer sequences |
|---|---|
| N787 forward | 5′AAYRCSGGKMTKRCWSCWTTCTT3′ |
| N1022 reverse | 5′GWWSCYAYWCCCATKGCAWA3′ |
| GS forward | 5’WWWWWWAGGGGCGGAA3’ |
| GE reverse | 5’WWWWWTTTTTTATTAAW3’ |
| P780forward | 5’ CAGGAACATGCAGGCGGC3’ |
| M350reverse | 5’CWTAKATTTYGSTTSAAACAAC3’ |
| M1066reverse | 5’CNTGDTATYGAAAMATCATGTG3’ |
| HN840reverse | 5’CAKTKCAGTSGDGSCTCTTGATC3’ |
| HN490forward | 5’GTCGATGATATATAGGAATTTC3’ |
| HN1492forward | 5’GCCCTTGACTGAGGGTACAACAC3’ |
| L487forward | 5’CATTAATGACTAAGCCTGGG3’ |
| L3020forward | 5’GACTCAGCAGGATGAAGAGG3’ |
| L4633forward | 5’ GATGTTGGATAGGAGTGATTTGAAG3’ |
| L669reverse | 5’AYATTCRGKTGTGGTWATAATTG3’ |
| L2015reverse | 5’CAATKGATCMAYTCAARACCATG3’ |
| L3784reverse | 5’CGDCRYDGSGCAWAGKGCCTGC3’ |
| L5377reverse | 5’GTATATWTWGTMRCTTCTAATG3’ |
The sequence of the 3’ terminus was determined from cDNA prepared by rapid amplification of cDNA ends (RACE) (Kumar et al., 2008; Li et al., 2005; Troutt et al., 1992) as described previously (Kumar et al., 2008). The sequence of the 5’ end of the genome was determined from cDNA obtained by 5’ RACE (Invitrogen) as described previously (Kumar et al., 2008).
To investigate RNA editing of the P gene, DF1 cells were infected with APMV-3 strain Wisconsin at a MOI of 0.1 PFU per cell. mRNAs were isolated from total RNA using an oligo-dT column (Oligotex mRNA mini kit, QIAGEN). RT was performed using a gene-specific reverse primer, P551 reverse (Table 2). A 414-bp cDNA fragment spanning the putative editing site in the P gene of APMV-3 strain Wisconsin was amplified using gene-specific primers P137 forward and P551 reverse (identified according to their position in the complete antigenome sequence). The PCR-amplified products were cloned and individual clones were sequenced spanning the putative P gene RNA editing site.
PCR-amplified products and plasmid DNAs were sequenced by using the BigDye terminator v 3.1 matrix standard kit (Applied Biosystems) and 3130xl genetic analyzer data collection software v3.0 (Applied Biosystems). The entire genome was sequenced at least three times, and at least once from uncloned PCR product, to ensure a consensus sequence.
Sequence and phylogenetic tree analysis
Sequence similarity searches were conducted using the basic length alignment search tool (BLAST) from the National Center for Biotechnology Information (NCBI). DNA pair-wise alignment was done using MegAlign (clustalW) in a Lasergene 8 software package. Prediction of phosphorylation sites, signal sequences and glycosylation sites in the complete genome sequence was determined by using NetPhos 2.0 software, SignalP 3.0 server and NetNGlyc program respectively (all from ExPASy Proteomics tools). To construct phylogenetic trees and determine divergence among different genera, aa sequences were aligned using clustalW multiple alignment algorithm. In addition, boot strap values were calculated for maximum parsimony using PHYLIP software.
Database accession numbers
The complete genome sequence of APMV-3 strain Wisconsin has been submitted in GenBank under accession number EU782025. Different sequences from GenBank were used for designing primers and comparing the APMV-3 genome. These include NC_002617 for NDV, EU338414 for APMV-2, EU403085 for APMV-3 Netherlands, FJ177514 for APMV-4, NC_003043 for APMV-6, FJ215863 for APMV-8, EU910942 for APMV-9, NC_003443 for hPIV2, NC_006430 for SV5, NC_004074 for TiV, NC_001552 for SeV, NC_001796 for hPIV3, NC_003461 for hPIV1, AY988601 for NiV, NC_001906 for HeV, NC_001498 for MeV, NC_001921 for CDV, NC_006383 for Peste des petits ruminants virus (PPRV), NC_006296 for RPV.
Results
In vitro growth characteristics of APMV-3 strain Wisconsin
APMV-3 strain Wisconsin produced a titer of 29-210 HA units per ml of allantoic fluid in 9-day-old embryonated SPF chickens eggs four days post inoculation. It was necessary to include 10% allantoic fluid for replication of the virus in cell culture, indicating a requirement of external proteases for efficient cleavage of the F protein. The virus grew most efficiently in DF1, with the next most efficient growth being in MDBK cells. Viral CPE involved rounding, detachment and syncytia formation. The growth kinetics and plaque morphology in DF-1 cells were similar for APMV-3 strains Wisconsin and Netherlands.
Antigenic relationship between APMV-3 strains Wisconsin and Netherlands
The antigenic relationship between APMV-3 strains Wisconsin and Netherlands was evaluated by reciprocal HI tests using strain specific convalescent sera raised by a single infection of chickens by the IN/IO route. Each of the antisera exhibited a four-fold differences in HI titer between the homologous and heterologous strains (Table 1A). As expected, the two APMV-3 strains were serologically distinct from representatives of the other APMV serotypes, although there was a low level of cross-reaction with APMV-8 (Table 1A).
Table 1.
Antigenic analysis of APMV-3 strains Wisconsin and Netherlands using antisera from chickens infected with the individual strains. (A) Homologous and heterologous hemaggutination-inhibition titers. (B) Homologous and heterologous neutralization titers based on plaque reduction assays in cell monolayers and serum neutralization assays in embryonated eggs.
| (A) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antiserum | virus |
||||||||
| APMV-1 | APMV-2 | APMV-3 Wisconsin | APMV-3 Netherlands | APMV-4 | APMV-6 | APMV-7 | APMV-8 | APMV-9 | |
| APMV-3 Wisconsin | 2 | 4 | 32 | 16 | 0 | 0 | 0 | 8 | 2 |
| APMV-3 Netherlands | 2 | 0 | 8 | 64 | 0 | 0 | 2 | 8 | 0 |
|
(B) | |||||||||
| Antiserum | Virus | Plaque reduction neutralization titerb | Serum neutralization titer (log2)a | ||||||
| APMV-3 (Wisconsin) serum | APMV-3 (Wisconsin) | 2048 | 7.75 | ||||||
| APMV-3(Netherlands) | 1024 | 7.5 | |||||||
| APMV-3 (Netherlands) serum | APMV-3 (Wisconsin) | 256 | 6.75 | ||||||
| APMV-3(Netherlands) | 512 | 7.5 | |||||||
HI titer are expressed as the reciprocal of the highest dilution of antisera inhibit 4HA units of the virus. Viruses used as antigen were NDV-LaSota, APMV-2/Ck/Yucaipa/56, APMV-3/PKT/Netherlands/449/75, APMV-3/Turkey/Wisconsin/68, APMV-4/DK/HK/D3/75, APMV-6/DK/HK/199/77, APMV-7/Dove/TN/4/75, APMV-8/Goose/DEL/1053/76, APMV-9/DK/NY/22/78.
The APMV-3 serum neutralizing titer against each strain was determined and is expressed as the reciprocal log2.
Neutralization titer was defined as the titer of serum that reduced plaque number by 60% compared to the positive control wells.
The ability of antisera to neutralize homologous and heterologous APMV-3 strains was assessed by PRN test in cell culture and by VN test in embryonated eggs. Our results showed that both the antisera cross neutralized the heterologous strain, but the neutralization titers were two-fold higher against the homologous strain than with the heterologous strain (Table 1B). These reactions indicated existence of a low level of antigenic difference between the two APMV-3 strains.
Determination of the complete genome sequence of APMV-3 strain Wisconsin
We determined the sequence of the entire genome of APMV-3 Wisconsin. A number of the initial cDNAs in this analysis were prepared using primers derived from consensus sequences identified by sequence alignment of multiple members of the Avulavirus, Rubulavirus, Respirovirus, Morbillivirus, and Henipavrus genera (Table 2). A few primers also were based on the published APMV-3 Netherlands sequence. Every nt in the complete sequence was confirmed in uncloned cDNA, indicating that it is a consensus sequence.
The genome of APMV-3 strain Wisconsin consists of 16,182 nt. Its genome organization is very similar to those of other members of family Paramyxoviridae. The genome codes for six open reading frames (ORF) corresponding to putative proteins N, P, M, F, HN and L, from the 3 to 5 end of the genome (Fig. 1A). In addition, there is evidence of a cysteine-rich V ORF in the P coding region. 84% of the genome codes for protein, which is less than the average coding percentage (92%) of other members of subfamily Paramyxovirinae (Miller et al., 2003). Comparison of complete genome sequence of APMV-3 strain Wisconsin with that of strain Netherlands showed minor changes in the length of the P, M, F, HN, and L genes as well as the trailer region, whereas the lengths of the N gene and leader region were the same in each strain (Fig. 1A and 1B).
Fig. 1.

Structure of the APMV-3 genome. (A) Gene maps of APMV-3 strains Wisconsin and Netherlands. Individual genes are indicated by boxes, with gene lengths given in nt together with the predicted amino acid length of the unmodified protein. The nt lengths of the extragenic 3’ leader, 5’ trailer, and intergenic regions are underlined. (B) Alignment of the sequences (genome-sense) of the leader region (i) or the downstream end of the trailer region (ii) of APMV-3 strains Wisconsin and Netherlands. Shading denotes identity between the strains.
The 3 leader sequence of APMV-3 strain Wisconsin consists of 55 nt, a length that is conserved among almost all the members of subfamily Paramyxovirinae (Krishnamurthy and Samal, 1998). The nt sequences of the leader region of the Wisconsin and Netherlands strains differed at 16 out of 55 positions, with the 3’-terminal 12 nt being identical between the two strains (Fig. 1B). The length of the 5’ trailer region of APMV-3 strain Wisconsin is 681 nt, which is 26 nt shorter than that of APMV-3 strain Netherlands. The sequences of the 3’ leader and 5’ trailer termini of strain Wisconsin showed a high degree of complementarity, with only a single mismatch in the terminal 16 positions (94% complementarity), suggestive of conserved elements in the 3’ promoter regions of the genome and antigenome. The sequence of the genomic termini of APMV-3 strain Wisconsin showed 59% identity (aggregate of the complete 3’ leader and 5’ trailer sequence) with that of strain Netherlands.
The boundaries of the individual genes were putatively identified based on the presence of conserved putative gene-start and gene-end transcription signals. The sequence of the APMV-3 strain Wisconsin putative gene-start signal is 3’UCCU/CCGCCUU and is highly conserved among the six genes (Table 3). The gene-end signal is slightly less well-conserved: the consensus gene-end sequence is 3–AAU/AUA/U(U)6, but among four of the six signals there is a total of four nt differences involving positions 3 and 5 (Table 3). The intergenic sequences of APMV-3 Wisconsin range in length from 37 to 63 nt. The lengths of the intergenic sequences are similar to those in strain Netherlands, although the length is exactly the same only for the P-M gene junction (Fig. 1A)
Table 3.
Putative gene-start, gene-end and intergenic sequences of APMV-3 strain Wisconsin (sequences are mRNA sense). Differences relative to the most conserved sequence are underlined.
| Gene | Gene start | Gene end | Intergenic sequence |
|---|---|---|---|
| N | AGGAGCGGAA | TTAAGAAAAAA | AGACCTGATGTGTACGAGGAGAAAAATAATTGATGACAAGCGGAGAAAAT |
| P | AGGAGCGGAA | TTTATAAAAAA | CCAAAATGATTATAACTAAACAATCTCAACAATTTGCAATGATAACAACACCATACGATCACT |
| M | AGGGGCGGAA | TTAATTAAAAA | TATTAATAATCATTAGCAACATCCGATCGGAATCTTC |
| F | AGGGGCGGAA | TTTATAAAAAA | ATGCCATGATACTCGTGCGAGTGTAACATAGTAACT |
| HN | AGGGGCGGAA | TTAAGAAAAAA | CTTCACTATCACTCTTTGAGTCGCTGAAGTGAGATTTCAGAAAGGTATGCATCTAAGAAGT |
| L | AGGAGCGGAA | TTAATAAAAAA | |
The nucleoprotein (N) gene
The N gene of APMV-3 strain Wisconsin is 1604 nt long and encodes an N protein of 457 amino acids. The predicted N protein contains a highly-conserved sequence motif, 322-FAPGNYALLYSYAMG-336 (F-X4-Y-X3-α-S- α-AMG, where X is any aa and α is any aromatic aa), that corresponds to a motif previously identified in the central region of the N protein of other members of Paramyxovirinae and which has been implicated in the self assembly of N with RNA or with other N monomers (Lamb and Parks, 2007; Yu et al., 1998). The APMV-3 strain Wisconsin N protein has 88.6% aa sequence identity with that of strain Netherlands (Table 4).
Table 4.
Amino acid identity between APMV-3 strain Wisconsin versus strain Netherlands and representatives of the other APMV serotypes. Individual values show the percent identity of N, P, M, F, HN and L proteins among different members of genus Avulavirus. The identity was determined by DNASTAR using MegAlign (clustalW) in a Lasergene8 software package.
| Protein | APMV-1 | APMV-2 | APMV- 3(Netherlands) | APMV-4 | APMV-6 | APMV-7 | APMV-8 | APMV-9 |
|---|---|---|---|---|---|---|---|---|
| N | 36.8 | 36.6 | 88.6 | 51 | 37.6 | 36.8 | 34.4 | 36.5 |
| P | 20.8 | 21.6 | 59.1 | 25.5 | 18.4 | 26.2 | 21.1 | 21 |
| M | 28.4 | 31.3 | 93.3 | 35.1 | 29.9 | 31.2 | 29.9 | 25.6 |
| F | 30.8 | 28.9 | 70.1 | 33.1 | 29.8 | 30.6 | 29.7 | 28.3 |
| HN | 34.7 | 30.6 | 72.6 | 39.3 | 29.7 | 35.9 | 31.5 | 37.2 |
| L | 33 | 34.9 | 83 | 40.5 | 35.9 | 37 | 34.6 | 33.5 |
The phosphoprotein (P) gene
The P gene of APMV-3 strain Wisconsin is 1389 nt long. The unedited version of the P mRNA encodes a P protein of 390 amino acids. The predicted P protein contains an abundance of phosphorylation sites, with 29 serine, 9 threonine and 1 tyrosine residues identified as potential phosphorylation sites using NetPhos 2.0 software. The APMV-3 strain Wisconsin P protein has 59.1% aa sequence identity with that of strain Netherlands (Table 4).
The P gene contains a putative editing site, 5’- TTTTTAAAGGGGG (mRNA sense), at positions 374-386 in the P gene (positions 2083-2095 in the complete antigenome sequence), which has a high degree of sequence identity with the editing sites in other members of subfamily Paramyxovirinae. The insertion of a single G residue (mRNA sense) at the editing site would shift the reading frame to encode a 252-aa V protein in which the N-terminal 115 amino acids are derived from P, and the downstream 137 amino acids include seven cysteine residues and one tryptophan residue that are highly conserved within Paramyxovirinae (Fig. 2). Alternatively, the insertion of two G residues would shift the reading frame to access an internal ORF that is open for only 12 additional amino acids, yielding a 127-aa W protein. Sequence analysis of cloned cDNAs of mRNAs isolated from infected cell culture showed mRNA with incorporation of one and two G residues at the predicted RNA editing site (Fig. 4). The APMV-3 strain Wisconsin V and W proteins have 63% and 55% aa sequence identity respectively with those of strain Netherlands.
Fig. 2.

Sequence alignment of C terminal end of the V proteins of different members of genus Avulavirus. Stars indicate conserved cysteine and tryptophan residues. Numbers indicate the amino acid position.
Fig. 4.
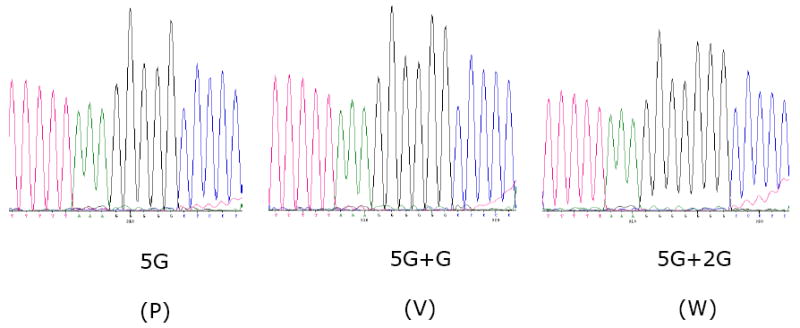
Sequence analysis of the P gene editing site in cDNAs made from polyadenylated intracellular mRNA. This shows sequencing electropherograms across the editing site region of transcripts that were unedited (5G, encoding the P protein), or contained the insertion of one (5G+G, encoding the V protein) or two (5G+2G, encoding the W protein) G residues. The sequence is mRNA-sense.
The matrix (M) protein gene
The M gene of APMV-3 strain Wisconsin is 1418 nt long and encodes a 375-aa M protein. The protein is highly basic with an isoelectric point of 9.1, which may be important for ionic interaction with the acidic N protein (Lamb and Parks, 2007). The bipartite cluster, which comprises clusters of basic amino acids either ‘single cluster’, containing three to five basic amino acids, or ‘bipartite’ sequence, containing two interdependent clusters of such amino acids separated by an intervening sequence is clearly seen in the positions 257-272 (257 KQRRRTPSEITQKVRR 272), which was different in two aa at position 267 and 272 with strain Netherlands (Kumar et al., 2008). The APMV-3 strain M protein has 93.3% aa sequence identity with that of strain Netherlands (Table 4).
The fusion (F) protein gene
The F gene of APMV-3 strain Wisconsin is 1966 nt long and codes for a 538-aa F protein. The protein is predicted to be a type I transmembrane protein, similar to the F proteins of other members of family Paramyxoviridae. The N-terminus of the F protein contains a predicted signal sequence that would mediate translocation of the nascent protein into the lumen of the endoplasmic reticulum and is predicted by the SignalP 3.0 server to be cleaved between residues 22 and 23 (SQS↓AN). The predicted C-terminal transmembrane domain (residues 484–506) would anchor the protein in the host cell membrane leaving a short cytoplasmic tail of 32 amino acids. The predicted cleavage-activation site of the APMV-3 F protein is R-P-S-G-R↓L (corresponding to 95-102 amino-acid position), which differed at two amino acids with the cleavage site of strain Netherlands (Fig. 3). The APMV-3 stain Wisconsin F protein has 70.1% aa sequence identity with that of strain Netherlands (Table 4).
Fig. 3.

Comparison of the F protein cleavage sites of different members of genus Avulavirus. Basic residues (R= arginine, K= lysine) are underlined and in bold. Numbers indicate the amino acid position.
The hemagglutinin-neuraminidase (HN) protein gene
The HN gene of APMV-3 strain Wisconsin is 1987 nt long and encodes a polypeptide of 577 amino acids. The Paramyxovirinae HN protein is a type II integral membrane protein that spans the membrane once: the predicted HN protein of APMV-3 Wisconsin has a predicted hydrophobic signal anchor domain spanning residues 23-45. Five potential N-linked glycosylation sites were identified at positions 122, 155, 312, 325 and 342, using the Expasy, NetNGlyc program. The HN protein contains a high amount of acidic amino acids with an isoelectric point (pI) of 6.3. The HN proteins of subfamily Paramyxovirinae and the HA proteins of influenza viruses contain a conserved sequence, N-R-K-S-K-S, which is thought to contribute to the sialic acid binding site (Varghese et al., 1983). The aa sequence of the APMV-3 HN protein contains a similar motif, N-R-K-S-C-S (positions 237-242, difference underlined). The APMV-3 strain Wisconsin HN protein has 72.6% aa sequence identity with that of strain Netherlands (Table 4).
The large polymerase protein (L) gene
The L gene of APMV-3 strain Wisconsin is 6835 nt long and codes for a 2198-aa L protein. Alignment of the L protein aa sequence of APMV-3 Wisconsin with other members of Paramyxoviridae demonstrated six linear conserved domains, which have been described earlier for these family members and represent catalytic domains (Poch et al., 1989). The conserved QGDNQ sequence that is found within domain III involved in transcription activity (Schnell and Conzelmann, 1995) was also found in the L protein of APMV-3 at aa positions 744-748. The APMV-3 strain Wisconsin L protein has 83% aa sequence identity with that of strain Netherlands (Table 4).
Phylogenetic analysis
Phylogenetic trees were generated from alignments of the complete nt sequence of the genome of APMV-3 strain Wisconsin with the sequences of representative viruses of the other serotypes of APMV excluding only APMV-5, which remains to be sequenced (Fig. 5). The resulting phylogenic tree indicates that APMV-3 strains Wisconsin and Netherlands cluster together on a branch that is distinct from those of the other available serotypes.
Fig. 5.
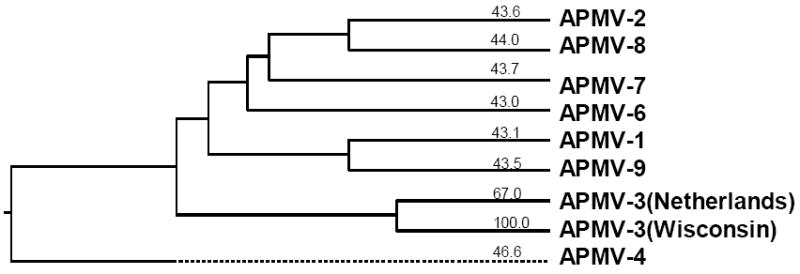
Phylogenetic tree depicting evolutionary relationships between the members of the family Paramyxoviridae based on complete genome nt sequences.
Discussion
Avian paramyxoviruses are classified into nine serotypes based on their serological relationships in HI and NI tests. Of these serotypes, a great deal of information is available on antigenic and genetic relationships among APMV-1 (NDV) strains isolated from different parts of the world (Alexander, 1988). Also, we and others have recently published complete genome sequences for representative strains of APMV-2, -3, -4, -6, -7, -8, and -9 (Chang et al., 2001; Kumar et al., 2008; Nayak et al., 2008; Paldurai et al., 2009; Samuel et al., 2009; Subbiah et al., 2008; Xiao et al., 2009). However, very little is known about the antigenic and genetic relationships among strains within APMV-2 through -9 (Alexander, 2003). In this study we compared APMV-3 strain Wisconsin, a virus isolated from a turkey in Wisconsin, and the prototype APMV-3 strain Netherlands, isolated from parakeets in the Netherlands. These two strains differ in their virulence for chickens (our unpublished data). APMV-3 strain Wisconsin is avirulent for chickens, whereas APMV-3 strain Netherlands is moderately virulent for chickens leading to paralysis and death occasionally. Therefore, it was of interest to know the extent of antigenic and genetic variation between these two strains. This information might have implications for studies in pathogenesis and epidemiology and for the development of vaccines.
To evaluate the antigenic relationship between the two APMV-3 strains, we raised chicken antiserum against each virus separately by respiratory infection, mimicking a natural route of infection. Since serological responses tend to broaden over time and with repeated antigenic exposure, we (i) limited the immunization to a single infection, and (ii) collected the serum samples at an early time point (14 days post infection). The antigenic relatedness between strains Wisconsin and Netherlands was examined by using reciprocal cross- HI and cross neutralization assays based on primary infection sera from chickens. The two APMV-3 strains were found to have a close antigenic relationship in each assay. The differences in homologous and heterologus HI titers between the two strains were 4- fold higher against the homologous virus versus the heterologous virus, indicating a minor antigenic difference. The difference in homologous and heterologous virus neutralization titers between the two strains was 2-fold higher for the homologous virus versus the heterologous virus, similar to the results of the HI tests. These results indicate that the two APMV-3 strains are somewhat more closely related antigenically then the two antigenic subgroups of human respiratory syncytial virus (HRSV). The two serogroups of HRSV exhibit a 3- to 4- fold reciprocal difference in neutralization by polyclonal convalescent serum for homologous versus heterologous strains (Biacchesi et al., 2003; Johnson et al., 1987a; Johnson et al., 1987b). Our results suggested that the two APMV-3 strains certainly represent a single serotype, but do not unambiguously represent two antigenic subgroups. In this regard, it will be of interest to evaluate additional strains.
In order to analyze the genetic relationship between the two APMV-3 strains, we determined the complete genome sequence of strain Wisconsin and compared it with the sequence of strain Netherlands that we previously reported (Kumar et al., 2008). The genome lengths differ slightly: strain Wisconsin was 16,182 nt compared to 16,272 nt for strain Netherlands. Both viruses have identical genome organizations and highly conserved genome terminal sequences and gene start and stop sequences. The two genomes shared 67% nt identity, compared to 80% nt identity between the two subgroups of human metapneumovirus (HMPV) (Biacchesi et al., 2003) and 81% nt identity between the two subgroups of HRSV (Johnson et al., 1987a). This divergence was the greatest in regions that did not encode protein or constitute cis-acting RNA signals: for example, the intergenic regions between the two APMV-3 strains were 48% identical, similar to the value of 48% identity between two HPMV subgroups and 42% identity between two HRSV subgroups (Biacchesi et al., 2003). When compared with the other serotypes of APMV, the genome of APMV-3 Wisconsin had a nt sequence identity of 43%, 44%, 47%, 43%, 44%, 44%, and 44% versus APMV-1, -2, -4, -6, -7, -8, and -9, respectively. Thus, the divergence of nt sequence between the two APMV-3 strains was substantially more than between the subgroups of HRSV or HMPV, but was substantially less than the divergence between APMV serotypes.
Comparison of nt sequence relatedness of ORFs between the two APMV-3 strains showed 72 to 75% nt identity except for P, F, and HN genes which were more divergent (66.2, 66.7 and 65.7 % nt identity respectively). This pattern is similar to those of the two subgroups of HMPV and HRSV, in which the genes of internal virion proteins are highly conserved, while the genes of outer surface proteins are more divergent (Biacchesi et al., 2003; Johnson et al., 1987b). The most notable exception in the pattern was observed for the P gene of APMV-3 strains. The P gene of the two subgroups of HMPV and HRSV are highly conserved (81 and 85% nt identity respectively), while the P genes are more divergent (66% nt identity) between the two APMV-3 strains.
The total proteome of the two APMV-3 strains shared 78% aa sequence identity compared to 90% aa sequence identity between the HMPV subgroups and 88% aa sequence identity between the HRSV subgroups (Biacchesi et al., 2003). In comparison, the APMV-3 proteome had an aa sequence identity of 31%, 31%, 38%, 30%, 33%, 31%, and 31% when compared to APMV-1, -2, -4, -6, -7, -8, and -9, respectively. Thus, the aa sequence divergence between the two APMV-3 strains was substantially more than between the subgroups of HRSV or HMPV, but was substantially less than the divergence between APMV serotypes.
The aa sequence identity between the cognate proteins of the two APMV-3 strains varied from 59% for P protein to 93% for M protein (Table 4). The envelope proteins (F and HN) were more divergent between the two APMV-3 strains (Table 4). It was surprising to find that the F protein of two APMV-3 strains exhibited more divergence (70% identity) than the F protein of the HMPV subgroups (95% identity) and the HRSV subgroups (89% identity) (Biacchesi et al., 2003). The HN protein of the two APMV-3 strains had 73% identity, which was higher than between the G proteins of the two HMPV subgroups (55% identity) and the two HRSV subgroups (37% identity). In comparison, the APMV-3 F protein has 31%, 29%, 33%, 30%, 31%, 30%, and 28% when compared to the F protein of APMV-1, -2, -4, -6, -7, -8, and -9, respectively, and the APMV-3 HN protein has 35%, 31%, 40%, 30%, 36%, 32%, and 37% when compared to APMV-1, -2, -4, -6, -7, -8, and -9, respectively (Table 4). The P, V and W proteins were more divergence between the two APMV-3 strains (59, 63 and 55% aa identity, respectively) compared to the P proteins of HMPV subgroups (85% aa identity) and HRSV subgroups (90% aa identity) (Biacchesi et al., 2003). Whether the high degree of divergence in P, V and W proteins is responsible for the difference in the pathogenicity of APMV-3 strains needs further studies.
In conclusion, the complete genome sequence was determined for APMV-3 strain Wisconsin. Comparison of the nt and predicted aa sequences of strains Wisconsin and Netherlands showed that the divergence between the two strains was substantially greater overall than between the two HMPV and HRSV subgroups. This indicated that the two APMV-3 strains represent two distinct genetic subgroups. Given the rather substantial differences in the aa sequences between the APMV-3 strains, it was somewhat surprising that the extent of antigenic difference was modest. This is particularly evident when compared with the HRSV subgroups, where there was substantially greater aa sequence identity overall and yet greater antigenic difference. One factor may be that, while one of the HRSV neutralization antigens (F) was more highly conserved than for the APMV-3 strains, the other HRSV neutralization antigen (G) was much more divergent than its APMV-3 counterpart (HN) (Johnson et al., 1987a). Additional antigenic and genetic analysis involving additional APMV-3 strains is needed to further define the antigenic and genetic variability of APMV-3.
Acknowledgments
We thank Daniel Rockemann and all our laboratory members for their excellent technical assistance and help. “This research was supported by NIAID contract no.N01A060009 (85% support) and NIAID, NIH Intramural Research Program (15% support). The views expressed herein do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DJ. Avain paramyxoviruses. Vet Bull. 1980;50:737–752. [Google Scholar]
- Alexander DJ. Avain paramyxoviruses-other then Newcastle disease virus. World’s Poul Sci J. 1982;38:97–104. [Google Scholar]
- Alexander DJ. Newcastle disease diagnosis. In: Alexander JD, editor. Newcastle Disease. Kluwer Academic Publishers; Boston: 1988. pp. 147–160. [Google Scholar]
- Alexander DJ. The epidemiology and control of avian influenza and Newcastle disease. J Comp Pathol. 1995;112(2):105–26. doi: 10.1016/s0021-9975(05)80054-4. [DOI] [PubMed] [Google Scholar]
- Alexander DJ. Avian paramyxoviruses 2–9. In: Saif YM, editor. Diseases of Poultry. 11. Iowa State University Press; Ames: 2003. pp. 88–92. [Google Scholar]
- Alexander DJ, Chettle NJ. Relationship of parakeet/Netherland/449/75 virus to other avian paramyxovirus. Res Vet Sci. 1978;25:105–106. [PubMed] [Google Scholar]
- Alexander DJ, Collins MS. Pathogenecity of PMV-3/Parakeet/Netherland/449/75 for chickens. Avian Pathol. 1982;11:179–185. doi: 10.1080/03079458208436091. [DOI] [PubMed] [Google Scholar]
- Alexander DJ, Collins MS. Characterization of avain paramyxoviruses of serotype PMV-3 isolated from commercial turkey in Great Britain. Avian Pathol. 1984;13:215–221. doi: 10.1080/03079458408418525. [DOI] [PubMed] [Google Scholar]
- Beck I, Gerlach H, Burkhardt E, Kaleta EF. Investigation of several selected adjuvants regarding their efficacy and side effects for the production of a vaccine for parakeets to prevent a disease caused by a paramyxovirus type 3. Vaccine. 2003;21(910):1006–22. doi: 10.1016/s0264-410x(02)00552-2. [DOI] [PubMed] [Google Scholar]
- Biacchesi S, Skiadopoulos MH, Boivin G, Hanson CT, Murphy BR, Collins PL, Buchholz UJ. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315(1):1–9. doi: 10.1016/s0042-6822(03)00528-2. [DOI] [PubMed] [Google Scholar]
- Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67(8):4822–30. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Hsieh ML, Shien JH, Graham DA, Lee MS, Shieh HK. Complete nucleotide sequence of avian paramyxovirus type 6 isolated from ducks. J Gen Virol. 2001;82(Pt 9):2157–68. doi: 10.1099/0022-1317-82-9-2157. [DOI] [PubMed] [Google Scholar]
- Goodbourn S, Didcock L, Randall RE. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81(Pt 10):2341–64. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- Horikami SM, Smallwood S, Moyer SA. The Sendai virus V protein interacts with the NP protein to regulate viral genome RNA replication. Virology. 1996;222(2):383–90. doi: 10.1006/viro.1996.0435. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Jr, Olmsted RA, Prince GA, Murphy BR, Alling DW, Walsh EE, Collins PL. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987a;61(10):3163–6. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Spriggs MK, Olmsted RA, Collins PL. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A. 1987b;84(16):5625–9. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72(2):891–9. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S, Huang Z, Samal SK. Recovery of a virulent strain of newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology. 2000;278(1):168–82. doi: 10.1006/viro.2000.0618. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Samal SK. Nucleotide sequences of the trailer, nucleocapsid protein gene and intergenic regions of Newcastle disease virus strain Beaudette C and completion of the entire genome sequence. J Gen Virol. 1998;79(Pt 10):2419–24. doi: 10.1099/0022-1317-79-10-2419. [DOI] [PubMed] [Google Scholar]
- Kumar S, Nayak B, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus type 3 reveals an unusually long trailer region. Virus Res. 2008;137(2):189–97. doi: 10.1016/j.virusres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R, Parks G. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1449–1496. [Google Scholar]
- Lamb RA, Collins PL, Kolakofsky D, Melero JA, Nagai Y, Oldstone MBA, Pringle CR, Rima BK. Family Paramyxoviridae. In: Fauquet CM, editor. Virus Taxonomy: The Classification and Nomenclature of Viruses. The Eighth Report of the International Committee in Taxonomy of Viruses. E.A. Press; 2005. [Google Scholar]
- Li Z, Yu M, Zhang H, Wang HY, Wang LF. Improved rapid amplification of cDNA ends (RACE) for mapping both the 5’ and 3’ terminal sequences of paramyxovirus genomes. J Virol Methods. 2005;130(12):154–6. doi: 10.1016/j.jviromet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Lin Y, Horvath F, Aligo JA, Wilson R, He B. The role of simian virus 5 V protein on viral RNA synthesis. Virology. 2005;338(2):270–80. doi: 10.1016/j.virol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Lipkind MA, Weisman Y, Shihmanter E, Shoham D, Aronovici A. The isolation of yucaipa-like paramyxoviruses from epizootics of a respiratory disease in turkey poultry farms in Israel. Vet Rec. 1979;105(2526):577–8. [PubMed] [Google Scholar]
- Macpherson I, Watt RG, Alexander DJ. Isolation of avian paramyxovirus other than Newcastle disease virus from commercial poultry in Great Britain. Vet Rec. 1983;112(20):479–80. doi: 10.1136/vr.112.20.479. [DOI] [PubMed] [Google Scholar]
- Mayo MA, Pringle CR. Virus taxonomy--1997. J Gen Virol. 1998;79(Pt 4):649–57. doi: 10.1099/0022-1317-79-4-649. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Boyle DB, Eaton BT, Wang LF. Full-length genome sequence of Mossman virus, a novel paramyxovirus isolated from rodents in Australia. Virology. 2003;317(2):330–44. doi: 10.1016/j.virol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Nayak B, Kumar S, Collins PL, Samal SK. Molecular characterization and complete genome sequence of avian paramyxovirus type 4 prototype strain duck/Hong Kong/D3/75. Virol J. 2008;5:124. doi: 10.1186/1743-422X-5-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund S, Karlsen M, Nylund A. The complete genome sequence of the Atlantic salmon paramyxovirus (ASPV) Virology. 2008;373(1):137–48. doi: 10.1016/j.virol.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Paldurai A, Subbiah M, Kumar S, Collins PL, Samal SK. Complete genome sequences of avian paramyxovirus type 8 strains goose/Delaware/1053/76 and pintail/Wakuya/20/78. Virus Res. 2009;142(12):144–53. doi: 10.1016/j.virusres.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O, Sauvaget I, Delarue M, Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. Embo J. 1989;8(12):3867–74. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle CR. Virus taxonomy--San Diego 1998. Arch Virol. 1998;143(7):1449–59. doi: 10.1007/s007050050389. [DOI] [PubMed] [Google Scholar]
- Rima B, Alexander DJ, Billeter MA, Collins PL, Kingsbury DW, Lipkind MA, Nagai Y, Orvell C, Pringle CR, ter Meulen V. Family Paramyxoviridae. In: Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, Martelli GP, Mayo MA, Summers MD, editors. Sixth Report of the International Committee on Taxonomy of Viruses. 1995. pp. 268–274. [Google Scholar]
- Samal SK, Collins PL. RNA replication by a respiratory syncytial virus RNA analog does not obey the rule of six and retains a nonviral trinucleotide extension at the leader end. J Virol. 1996;70(8):5075–82. doi: 10.1128/jvi.70.8.5075-5082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel AS, Kumar S, Madhuri S, Collins PL, Samal SK. Complete sequence of the genome of avian paramyxovirus type 9 and comparison with other paramyxoviruses. Virus Res. 2009;142(12):10–8. doi: 10.1016/j.virusres.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Conzelmann KK. Polymerase activity of in vitro mutated rabies virus L protein. Virology. 1995;214(2):522–30. doi: 10.1006/viro.1995.0063. [DOI] [PubMed] [Google Scholar]
- Steward M, Vipond IB, Millar NS, Emmerson PT. RNA editing in Newcastle disease virus. J Gen Virol. 1993;74(Pt 12):2539–47. doi: 10.1099/0022-1317-74-12-2539. [DOI] [PubMed] [Google Scholar]
- Subbiah M, Xiao S, Collins PL, Samal SK. Complete sequence of the genome of avian paramyxovirus type 2 (strain Yucaipa) and comparison with other paramyxoviruses. Virus Res. 2008;137(1):40–8. doi: 10.1016/j.virusres.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt AB, McHeyzer-Williams MG, Pulendran B, Nossal GJ. Ligation-anchored PCR: a simple amplification technique with single-sided specificity. Proc Natl Acad Sci U S A. 1992;89(20):9823–5. doi: 10.1073/pnas.89.20.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumova B, Robinson JH, Easterday BC. A hitherto unreported paramyxovirus of turkey. Res in Vet Sci. 1979;27:135–140. [PubMed] [Google Scholar]
- Varghese JN, Laver WG, Colman PM. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Warke A, Stallknecht D, Williams SM, Pritchard N, Mundt E. Comparative study on the pathogenicity and immunogenicity of wild bird isolates of avian paramyxovirus 2, 4, and 6 in chickens. Avian Pathol. 2008;37(4):429–34. doi: 10.1080/03079450802216645. [DOI] [PubMed] [Google Scholar]
- Xiao S, Paldurai A, Nayak B, Subbiah M, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus type 7 (strain Tennessee) and comparison with other paramyxoviruses. Virus Res. 2009;145(1):80–91. doi: 10.1016/j.virusres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Hansson E, Shiell B, Michalski W, Eaton BT, Wang LF. Sequence analysis of the Hendra virus nucleoprotein gene: comparison with other members of the subfamily Paramyxovirinae. J Gen Virol. 1998;79(Pt 7):1775–80. doi: 10.1099/0022-1317-79-7-1775. [DOI] [PubMed] [Google Scholar]


